Abstract
Cardiac fibrosis is involved in adverse cardiac remodeling and heart failure, which is the leading cause of deteriorated cardiac function. Accumulative evidence has elucidated that microRNAs (miRNAs) play important roles in the pathogenesis of cardiac fibrosis. However, the exact molecular mechanism underlying miR-144 in cardiac fibrosis remains unknown. In the present study, a transverse aortic constriction (TAC) mouse model and angiotensin II (Ang II)-induced cardiac fibroblasts (CFs) were constructed in order to investigate the expression levels of miR-144. It was demonstrated that miR-144 was significantly downregulated following pathological stimuli. CFs infected with miR-144 mimics were then used to test the effect of miR-144 on CF activation in vitro. The results revealed that overexpression of miR-144 led to a dramatically decreased proliferation and migration ability in CFs, as well as the transformation from fibroblasts to myofibroblasts, which was characterized by the decreased expression of collagen-I, collagen-III, CTGF, fibronectin and α-SMA. By contrast, such effects could be reversed by miR-144 knockdown. Mechanistically, the bioinformatics analysis and luciferase reporter assay in the present study demonstrated that cAMP response element-binding protein (CREB) was a direct target of miR-144, and the expression of CREB was attenuated by miR-144. The results of the present study demonstrated that miR-144 played a key role in CF activation, partially by targeting CREB, which further suggested that the overexpression of miR-144 may be a promising strategy for the treatment of cardiac fibrosis.
Keywords: microRNA-144, cardiac fibroblasts, angiotensin II, cAMP response element-binding protein, myofibroblasts
Introduction
Cardiac fibrosis, characterized as excessive deposition of extracellular matrix (ECM) in the perivascular and interstitial region of the heart, is a common pathological manifestation involved in multiple cardiac diseases, including hypertension, myocardial infarction and valvular heart disease (1-3). Cardiac fibroblasts (CFs) are the predominant cell type in the myocardium, and are the primary producers of ECM (2,4). During cardiac remodeling, the important effect of CFs on cardiac fibrosis is the differentiation to a myofibroblast phenotype in response to various stimuli, which results in a markedly decreased proliferation, migration and secretory ability, and notably distinguished expression of α-smooth muscle actin (α-SMA) (5,6). Cardiac fibrosis contributes to the excessive activation of myofibroblasts, leading to myocardial stiffness, cardiac hypertrophy, and the destruction of physiological cardiac tissue and impaired cardiac functions, which causes ventricular remodeling, heart failure and even cardiac death (3,7).
MicroRNAs (miRNAs) are endogenous and highly conserved RNA sequences, that are 20-23 nucleotide in length, which act as negative regulators of gene expression by promoting the degradation or inhibiting the translation of target mRNAs (8-10). Notably, miRNAs act as important modulators in the development and progression of cardiac fibrosis, particularly regarding cell proliferation, migration of CFs and the transformation to myofibroblasts (11-13). Angiotensin II (Ang II) plays a critical role in cardiac fibrosis through inducing the production of collagens, other ECM proteins and activation of the pro-fibrogenic cascade (14,15). Notably, Ang II is involved in the pathogenesis of cardiac fibrosis with an array of gene expression changes (16,17). Among them, miRNAs may provide new insights. Previous studies have demonstrated that miR-144 was involved in multiple types of human malignancy through comprehensive meta-analyses of miRNA expression microarrays (18-21). There is a accumulating evidence to suggest that miR-144 plays an important role in attenuating the proliferation and migration of various cancer cells (22-24). Recently, miR-144 has been reported to mediate the effect of ROCK1 inhibition on the decreased lung endothelial hyperpermeability induced by LPS (25). miR-144 deficiency interrupts ECM remodeling characterized by increased cardiac collagen content associated with changes in Zeb1/LOX1 axis (26) and decreases left ventricular remodeling following myocardial infarction leading to worsened cardiac function (27). However, the potential role and precise molecular mechanism underlying miR-144 in the process of cardiac fibrosis remains unknown.
In the present study, it was observed that miR-144 is downregulated both in the heart of TAC-induced mouse models and in the CFs administered Ang II. The proliferation and migration ability of CFs was attenuated by miR-144 overexpression. Furthermore, miR-144 inhibited the differentiation of CFs into myofibroblasts, which was characterized by the decreased expression of collagen-I, collagen-III, CTGF, fibronectin and α-SMA. The results from the present study further demonstrated that miR-144 directly targeted CREB and decreased its expression levels. By contrast, such effects could be reversed by miR-144 knockdown. The present study implied that overexpression of miR-144 could be a novel therapeutic strategy for the treatment of cardiac fibrosis.
Materials and methods
Mouse model of TAC
Cardiac hypertrophy and fibrosis was induced by pressure overload via TAC in accordance with previously described methods (28). Briefly, male C57BL/6 mice aged 8-10 weeks were anesthetized with pentobarbital sodium (50 mg/kg) via intraperitoneal injection. After the chest was opened and the thoracic aorta was exposed, TAC was performed by inducing a 7-0 silk suture placed around the thoracic aorta and tied around a 26-gauge blunt needle. Subsequently, the needle was immediately removed and the thoracic cavity was closed. Sham surgery animals underwent the same procedure but without the tying of the suture around the aorta. The total number of mice used in our study is 15, 6 for sham group and 9 for the surgery procedure; meanwhile, the number of rat for CFs isolation is 12. All the procedures involving animals experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and were approved by the Animal Care and Use Committee of the Dezhou People's Hospital.
Tissue collection and Histological analysis
Hearts from mice subjected to sham or TAC for 4 weeks were excised after anesthetization with pentobarbital sodium (50 mg/kg) via intraperitoneal injection and arrested in diastole with 10% potassium chloride solution, fixed by 10% paraformaldehyde and embedded in paraffin. Subsequently, these hearts were sectioned transversely close to the apex to visualize the left and right ventricles at 5 µm. Sections of each heart at the mid-papillary muscle level were stained with hematoxylin-eosin (HE) for histopathology or with picrosirius red (PSR) for the collagen deposition.
CF isolation and culture
Neonatal rat CFs were isolated from 1-3-day-old Sprague-Dawley (SD) rats. The rats were anesthetized with pentobarbital sodium via intraperitoneal injection (50 mg/kg) and the hearts were removed and quickly dissected. The heart samples were washed in phosphate-buffered saline to remove blood and impurities and then digested with pancreatic enzyme and collagenase type II. Subsequently, pooled cell suspensions were centrifuged and resuspended in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc) for 60 min at 37˚C in humidified air with 5% CO2, which allowed for preferential attachment of fibroblasts to the bottom of the culture flasks. Flasks were washed twice with PBS in order to remove the weakly attached and non-adherent cells, whereas the CFs had attached onto the culture plates. CFs were passaged when the cell confluence achieved 70-80%, and the second or third passages were used in the present study. In addition, CFs were starved for 24 h in serum-free medium prior to treatment.
Cell transfection
The synthetic miR-144 analogs and antagomir were synthesized by GenePharma. CF transfection was performed using a riboFEC CP Transfection kit (Guangzhou RiboBio Co., Ltd.), according to the manufacturer's protocol. CFs were transfected with miR-144 mimic and antagomir, as well as the corresponding controls at a concentration of 50 nM. After 6 h, the transfected CFs were treated with Ang II (100 nM; Sigma-Aldrich; Merck KGaA) in serum-free medium for 24 h in order to induce cardiac fibrosis in vitro.
Cell proliferation and migration
A Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc.) was used to assay CF proliferation. CFs transfected with the miR-144 mimic were serum-starved in serum-free media for 24 h. The media was then placed with a mixture of fresh serum-free DMEM and CCK-8 reagent. The CCK-8 assay was performed after 12 h of incubation. The optical density was determined using a microplate reader (Bio-Rad) at a wavelength of 450 nm. Cell migration ability was analyzed using the Transwell chamber assay. CFs were starved for 24 h and then the cells were placed in the upper chamber of an insert (pore size, 8 µm). After 24 h of incubation, the cells on the underside were fixed with 4% paraformaldehyde for 20 min, and stained with 0.1% crystal violet in 20% ethanol for 10 min. Images were captured using a phase contrast microscope.
Reverse transcription-quantitative PCR and western blotting
Total RNA from cultured CFs was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and then reverse-transcribed using a Transcriptor First Strand cDNA synthesis kit (Roche Diagnostics) as previously described (29). The expression levels of target genes were quantified via real-time PCR using a LightCycler 480 SYBR Green 1 Master Mix and a Light-Cycler 480 qPCR system (Roche Diagnostics). The relative transcription levels of the target genes were normalized GAPDH, while the level of miR-144 was normalized to U6 level. The primers for miR-144 (cat. no. HmiRQP0190) were obtained from GeneCopoeia and the other primers are listed in the Table I. Total protein was extracted from the cultured CFs and the concentration of the proteins was determined using a BCA assay. Equal concentrations of proteins were separated via SDS-PAGE (10% gel) and then transferred onto PVDF membranes. After blocking with 5% non-fat dried milk in TBS for 1 h, the membranes were incubated with primary antibodies. Membranes were then incubated with a secondary antibody and treated with enhanced chemiluminescence reagent. Images were captured using a Molecular Imager ChemiDoc™ XRS+ and quantified with Image Lab™ Software (version 5.1). The expression levels of specific proteins were normalized against GAPDH. The associated antibodies are listed in Table II.
Table I.
Antibody for immunofluorescence.
| Antibody | Cat. no. | Manufacturer | Sources of species |
|---|---|---|---|
| Primary | |||
| α-SMA | ab7817 | Abcam | Mouse |
| Secondary | |||
| Alexa Fluor 568-conjugated donkey anti-mouse IgG | A10037 | Invitrogen; Thermo Fisher Scientific, Inc. | Mouse |
SMA, smooth muscle actin.
Table II.
Antibodies used for immunoblot analysis.
| Antibody | Cat. no. | Manufacturer | Sources of species |
|---|---|---|---|
| Primary | |||
| CREB | ab32515 | Abcam | Rabbit |
| GAPDH | 2118 | Cell Signaling Technology, Inc. | Rabbit |
| Secondary | |||
| IRDye800CW Conjugated Goat (polyclonal) Anti-Rabbit IgG (H+L) | bs-40295G-IRDye8 | LI-COR Biosciences | Rabbit |
Immunofluorescence staining
The cells were fixed with 3.7% formaldehyde in PBS for 15 min at room temperature, and permeabilized with 0.1% Triton X-100 in PBS for 40 min. Subsequently, the cell slides were incubated overnight with the α-SMA (1:100 dilution) at 4˚C. After rewarming at 37˚C for 1 h, the associated secondary antibody was used. The nuclei were stained with DAPI and the cell size was measured using Image Pro Plus software (version 6.0). The antibodies used are listed in Table III.
Table III.
The primers for reverse transcription PCR.
| Primer | Sequence (5'-3') |
|---|---|
| α-SMA-F | ACGATGGAAACTACCGTGGAG |
| α-SMA-R | TTGAAGGCCAATGACGTGCT |
| Collagen I-F | CCTCAAGGGCTCCAACGAG |
| Collagen I-R | TCAATCACTGTCTTGCCCCA |
| Collagen III-F | ACGTAGATGAATTGGGATGCAG |
| Collagen III-R | GGGTTGGGGCAGTCTAGTC |
| CTGF-F | TGACCCCTGCGACCCACA |
| CTGF-R | TACACCGACCCACCGAAGACACAG |
| Fibronectin-F | CCGGTGGCTGTCAGTCAGA |
| Fibronectin-R | CCGTTCCCACTGCTGATTTATC |
| GAPDH-F | GGTGGACCTCATGGCCTACA |
| GAPDH-R | CTCTCTTGCTCTCAGTATCCTTGCT |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
SMA, smooth muscle actin; F, forward; R, reverse.
Luciferase assay
For the luciferase assays, HEK293 cells purchased from Type Culture Collection of the Chinese Academy of Sciences were cultured in 24-well culture plates and co-transfected with a pEZX-MT01 vector containing wild-type or mutant CREB 3'-UTR reporters and 50 nm of miR-144 using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Luciferase activities were measured 48 h after transfection with the application of the Dual luciferase Reporter Assay System (Promega Corporation) according to the manufacturer's protocol.
Statistical analysis
All statistical data were analyzed using SPSS software (version 19.0; IBM) and are presented as the mean ± standard deviation. Differences between two groups were analyzed using Student's t-tests, while differences among multiple groups were analyzed by one-way ANOVA followed by a Bonferroni post hoc test or Tamhane's T2 post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-144 is closely associated with cardiac fibrosis
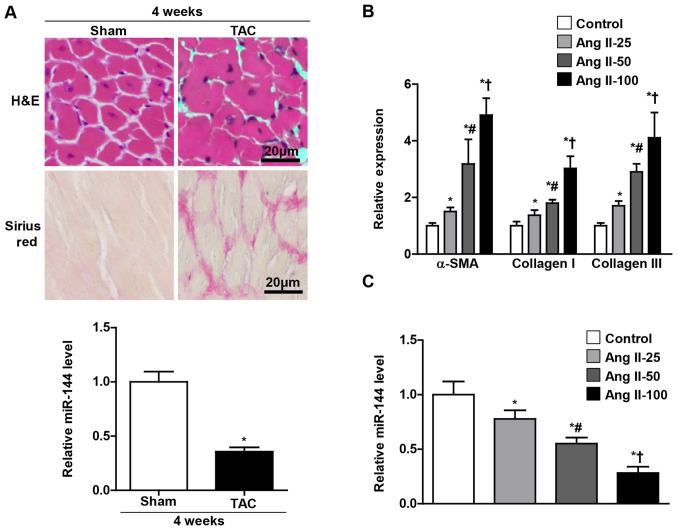
In order to investigate the potential role of miR-144 in the pathogenesis of cardiac fibrosis, the mice were subjected to TAC and cultured CFs administered with Ang II in the present study. As presented in Fig. 1A, the miR-144 expression levels were significantly decreased in the heart of the TAC group, which exhibited a larger cell size and accumulation of fibrosis than the sham group. Furthermore, it was observed in the present study that the miR-144 expression in the cultured CFs progressively decreased as the concentration of Ang II increased (Fig. 1C), and the level of α-SMA, collagen 1 and collagen III were reversely increased (Fig. 1B).
Figure 1.
Downregulation of miR-144 in cardiac fibrosis. (A) Histological analyses of the hematoxylin and eosin and picrosirius red (Red) staining on the tissue section of left ventricle. The expression levels of miR-144 in the left ventricles of TAC and sham mouse. *P<0.05 vs. sham group. n=3-5. Scale bar=20 µm. (B) Markers for extracellular matrix proteins (α-SMA, Collagen I and Collagen III) in CFs with increased Ang II stimulation. n=3. (C) Reverse transcription-PCR analysis of miR-144 expression in CFs upon increased Ang II stimulation. n=3. *P<0.05 vs. control group; #P<0.05 vs. 25 nm Ang II group; †P<0.05 vs. 50 nm Ang II group. miR, microRNA; TAC, transverse aortic constriction; α-SMA, α-smooth muscle actin; CF, cardiac fibroblasts; Ang II, angiotensin II.
Overexpression of miR-144 attenuates the proliferation and migration of CFs
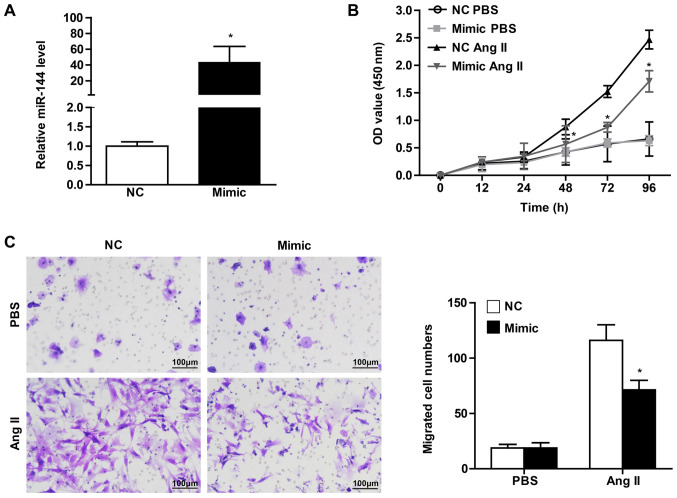
The CF activation underlying cardiac fibrosis is characterized by enhanced proliferation and migration ability, as well as the differentiation into myofibroblasts positive for α-SMA expression (1,6). In order to verify the effect of significant miR-144 change on CF activation, the present study transfected the CFs with miR-144 mimic and the corresponding normal control. It was observed that the CFs infected with miR-144 mimic demonstrated a dramatically increased expression level, by ~40-fold (Fig. 2A). CCK8 assay was performed in order to evaluate the effect of miR-144 on the proliferation of CFs. The results revealed that cell vitality of CFs infected with miR-144 mimics was significantly attenuated upon Ang II stimulation, whereas there was no difference in the groups treated with PBS (Fig. 2B). Meanwhile, the effect of miR-144 on the migration of CFs demonstrated results that were consistent with Ang II administration (Fig. 2C).
Figure 2.
The decreased proliferation and migration of CFs mediated by miR-144. (A) The transfection efficiency of miR-144 confirmed using quantitative PCR. Proliferation of CFs and migration of CFs transfected with miR-144 mimic or NC upon PBS and Ang II stimulation detected using (B) cell counting-kit 8 assay and (C) transwell migration assay, respectively. n=4-5. Scale bar=100 µm. *P<0.05 vs. NC group treated with Ang II. CFs, cardiac fibroblasts; miR, microRNA; NC, negative control; Ang II, angiotensin II.
miR-144 inhibits Ang II-induced myofibroblast differentiation into CFs
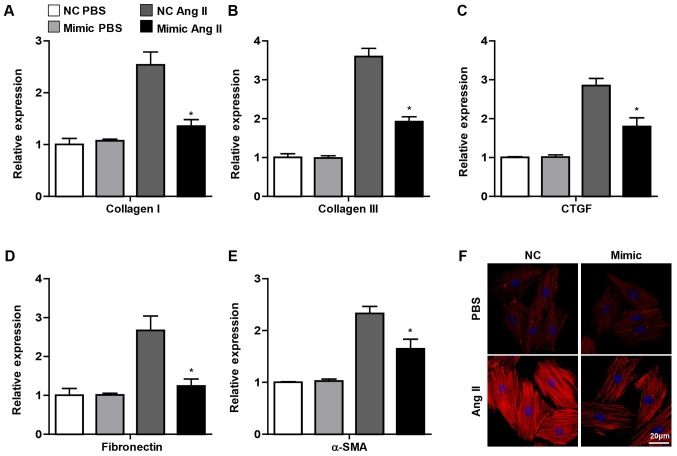
As aforementioned, the important change in CFs activation underlying cardiac fibrosis was the differentiation into myofibroblasts, which exhibit characteristics of smooth muscle cell contraction and the ability to synthesize ECM (2). It was observed in the present study that the pro-fibrotic genes, including collagen I, collagen III, CTGF, fibronectin and α-SMA, were decreased in CFs transfected with miR-144 mimic upon Ang II administration, whereas no significant difference was observed in the PBS group (Fig. 3A-E). Furthermore, the immunofluorescence staining demonstrated that overexpression of miR-144 attenuated the α-SMA expression in CFs treated with Ang II (Fig. 3F). Overall, these results demonstrated that miR-144 ameliorated the activation of CFs.
Figure 3.
Overexpression of miR-144 attenuates myofibroblast differentiation. mRNA expression of (A) Collagen I, (B) Collagen III, (C) CTGF, (D) Fibronectin and (E) α-SMA in CFs infected with miR-144 mimic and NC with Ang II and PBS administration. N=3. *P<0.05 vs. NC group treated with Ang II. (F) α-SMA expression of CFs infected with miR-144 mimic and NC with Ang II and PBS administration detected using immunofluorescence staining. n=4. Scale bar=20 µm. miR, microRNA; CFs, cardiac fibroblasts; NC, negative control; Ang II, angiotensin II; α-SMA, α-smooth muscle actin.
miR-144 knockdown accelerates myofibroblast differentiation into CFs, as well as the proliferation and migration of CFs
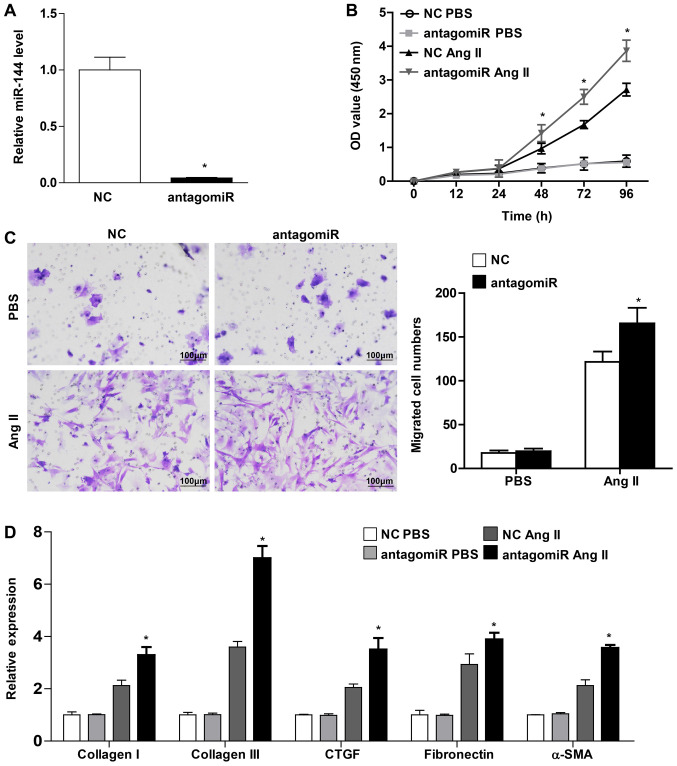
The present study transfected the CFs with miR-144 antagomiR in order to confirm the protective role of miR-144 on cardiac fibroblast activation. miR-144 expression exhibited a significant decrease, by 25-fold (Fig. 4A). The CFs exhibited a markedly enhanced proliferation and migration ability in those that were infected with miR-144 antagomiR upon Ang II stimulation (Fig. 4B and C). Furthermore, the present study demonstrated that the pro-fibrotic genes, including collagen I, collagen III, CTGF, fibronectin and α-SMA, were increased in CFs transfected with miR-144 antagomiR upon Ang II administration (Fig. 4D). Overall, these results revealed that miR-144 knockdown accelerated the activation of CFs.
Figure 4.
miR-144 knockdown promotes activation of cardiac fibroblast. (A) The transfection efficiency of miR-144 antagomiR confirmed using quantitative PCR. Proliferation of CFs and migration of CFs transfected with antagomiR or control upon PBS and Ang II stimulation detected using (B) cell counting kit-8 assay and (C) transwell migration assay, respectively. n=4-5. Scale bar=100 µm. (D) mRNA expression of Collagen I, Collagen III, CTGF, Fibronectin and α-SMA in CFs infected with miR-144 antagomiR and NC with Ang II and PBS administration. n=3. *P<0.05 vs. NC treated with Ang II. miR, microRNA; CFs, cardiac fibroblasts; α-SMA, α-smooth muscle actin; NC, negative control; Ang II, angiotensin II.
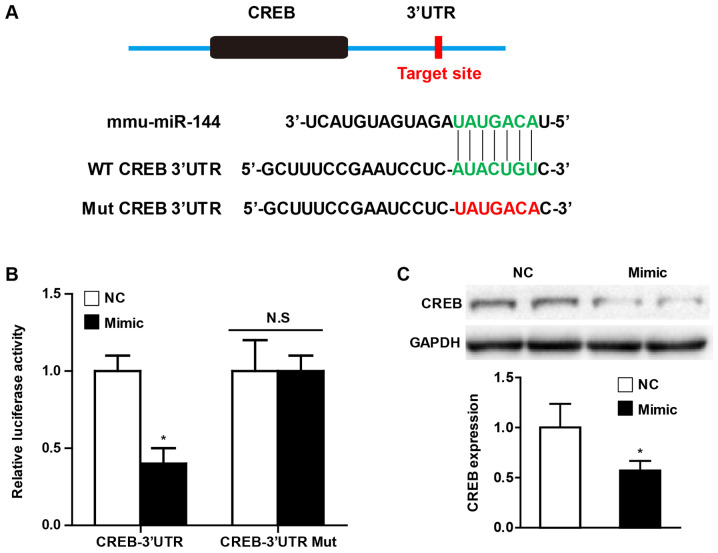
miR-144 directly targets CREB
Universally, the effects of miRNA on various pathophysiological processes depend on the direct regulation of their downstream targets. In order to further elucidate the potential molecular mechanism by which miR-144 affects CF activation, the present study performed a bioinformatics analysis using the prediction algorithms TargetScan (version 7.0) in order to identify the multiple target genes of miR-144. Of these potential targets, CREB has been demonstrated to play an important role in cardiac fibrosis, and the putative binding site for miR-144 in the 3'UTR is presented in Fig. 5A. Using luciferase reporter assays, the present study observed that miR-144 mimic transfection decreased CREB luciferase activity, and the effect was abolished when the predicted binding sites within the CREB 3'UTR were mutated (Fig. 5B). Furthermore, the western blot analysis demonstrated that overexpression of miR-144 significantly attenuated the CREB protein level of CFs upon Ang II administration (Fig. 5C).
Figure 5.
CREB is a target of miR-144. (A) Potential target sites for miR-144 in the 3'UTR of mouse CREB mRNA (Green). The sequence in the binding region that is highlighted in red was mutated. (B) Luciferase reporter assays in HEK293 cells treated with a miR-144 mimic or negative control using a pEZX-MT01 vector containing the CREB-3'UTR or the CREB-3'UTR with mutations in the predicted miR-144 binding site. *P<0.05 vs. NC group. n=3. (C) Western blot analysis of CREB expression in CFs transfected with miR-144 mimic upon Ang II stimulation. n=3. *P<0.05 vs. NC group. CREB, cAMP response element-binding protein; miR, microRNA; UTR, untranslated region; NC, negative control; CFs, cardiac fibroblasts; WT, wild-type; Mut, mutant.
Discussion
Accumulating evidence has demonstrated that cardiac fibrosis is implicated in multiple cardiac diseases, including hypertension, myocardial infarction and valvular heart disease (2). Furthermore, the severity of cardiac fibrosis is partially responsible for the progressive morbidity, mortality, and healthcare expenditure caused by heart failure (7,30). Cardiac fibrosis is characterized by excessive extracellular matrix accumulation, which leads to the destruction of normal heart tissue architecture, ventricular remodeling and accelerated heart dysfunction (3). Despite improvements in the knowledge surrounding cardiac fibrosis, there remains to be a lack of effective treatment strategies. Aberrant miRNA expression has emerged that links the pathophysiology of cardiac fibrosis with heart failure, which may act as a potential novel therapeutic target for the treatment of cardiac fibrosis (12,13).
miRNAs are endogenous, non-coding small RNA molecules, which can be recruited to the RNA-induced silencing complex and affect the target genes via diverse mechanisms (8). Recently, the importance of miRNAs in various cardiac disease has been well documented, particularly the role of miRNAs in cardiac fibrosis (13). miR-26a has an effect on cardiac fibrosis mediated by the NF-κB signaling pathway (31). miR-133a regulates collagen 1A and connective tissue growth factor in myocardial fibrosis of cardiac hypertension induced by Ang II stimulation (32). Let-7i negatively regulates cardiac inflammation and fibrosis (33). miR-122 suppresses fibrogenesis by targeting TGFβR1 in cardiac fibroblasts. miR-21 promotes cardiac fibrosis following myocardial infarction via targeting smad7(34). miR-155 accelerates cardiac fibrosis in the process of Ang II-induced cardiac remodeling (35). miR-150 inhibits the activation of CFs by regulating c-Myb (36). The previous miRNA arrays demonstrated that miR-144 is associated with hypertrophic cardiac tissues and cardiac diseases. However, the role and underlying molecular mechanism of miRNAs in cardiac fibrosis remains unclear.
miR-144 has been demonstrated to be involved in multiple types of human malignancy and is downregulated in colon cancer, lung cancer, prostate cancer and hepatocellular carcinoma, as revealed by a comprehensive meta-analysis of miRNA expression microarrays. Increasing evidence has verified that miR-144 acts as a novel suppressor of the development of various tumors via inhibition of the proliferation and migration of the cancer cells (18-24). Recently, it has been revealed that miR-144 plays an important role in cardiac diseases. Specifically, miR-144 attenuates cardiac ischemia/reperfusion injury by targeting FOXO1(37). miR-144 deficiency interrupts ECM remodeling and decreases left ventricular remodeling following myocardial infarction leading to worsened cardiac function (26,27). Notably, bleomycin-induced pulmonary fibrosis has been demonstrated to be associated with miR-144 expression (38), while miR-144 induces hepatic stellate cell activation in the human fibrotic liver by targeting TGF-β1(39) and regulates relaxin/insulin-like family peptide receptor 1 expression in lung fibroblasts (40). Overall, these results indicate that miR-144 may play an important role in the fibrotic progression underlying various diseases. Previous studies have demonstrated that cardiac fibrosis plays a critical role in the regulation of heart function, ventricular remodeling and the development of heart failure following cardiomyocyte hypertrophy and myocardial infarction (1). In the present study, it was observed that miR-144 expression was dramatically decreased in heart tissue subjected to TAC surgery, and a gradual decrease in CFs was observed as the concentration of Ang II increased. Furthermore, the critical event in cardiac fibrosis is the transformation of CFs to myofibroblasts, which is characterized by irreversible acquisition of expression of α-SMA (6). The present study clarified that miR-144 overexpression significantly inhibited the proliferation and migration of CFs upon Ang II administration. The multiple ECM proteins expressed in CFs were attenuated by miR-144 which included minimal α-SMA positive expression. By contrast, such effects could be reversed by downregulation of miR-144. Therefore, the present study indicated that miR-144 may play an important role in pathologies of cardiac fibrosis.
miRNAs play important roles in the regulation of cell development, differentiation, proliferation and apoptosis through acting as negative regulators of target gene expression by promoting the degradation or inhibiting the translation of downstream mRNAs (10). In silico target prediction analyses were performed in the present study in order to investigate the underlying molecular mechanism by which miR-144 regulated CF activation. Notably, CREB demonstrated a conserved putative binding site for miR-144 in the 3'UTR in different species, which is well recognized as being involved in the multiple crucial signaling pathways implicated in cardiac fibrosis and cardiac hypertrophy (41). Furthermore, several previous studies have suggested that the activation of CREB was essential for cardiomyocyte hypertrophy subjected to TAC surgery and Ang II administration (42). Meanwhile, during ventricular remodeling induced by pressure overload, cardiac fibrosis was the hallmark pathological manifestation characterize by excessive ECM deposition. In fact, the luciferase-reporter assays demonstrated that the CREB luciferase activity was largely attenuated by miR-144 mimic, and that this process was blocked when the predicted binding sites within the CREB 3'UTR were mutated. Notably, it was observed that CREB demonstrated a remarkable decrease in expression of CFs upon Ang II administration. Overall, it can be concluded that the detrimental role of miR-144 in pathological CF activation is partially dependent on the negative activation of CREB.
In conclusion, we first demonstrated that miR-144 showed a downregulation in heart tissue subjected to TAC and CFs upon Ang II stimulation. The in vitro experiment further clarified the miR-144 was largely implicated in the process of cardiac fibrosis, which characterized by the decreased proliferation and migration of CFs transfected with miR-144 induced by Ang II treatment, as well as the attenuated myofibroblasts transformation. Mechanistically, miR-144 directly targeted and downregulated the CREB expression in CFs with Ang II stimulation. Our current study suggests that miR-144-CREB axis could be an important novel target for the treatment of cardiac fibrosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FHL and QL designed the study and wrote the manuscript. ZYL ML and JL performed the animal experiments. JL and LLX performed the statistical analysis. ZNW and WWW performed the RT-PCR and western blot analysis. YL and ZJS performed the cell experiments.
Ethical approval and consent to participate
All the procedures involving animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and were approved by the Animal Care and Use Committee of the Dezhou People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5(15) doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leask A. Getting to the heart of the matter: New insights into cardiac fibrosis. Circ Res. 2015;116:1269–1276. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 4.Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res. 2007;74:207–212. doi: 10.1016/j.cardiores.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 7.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: Ultimate and proximate causes. J Clin Invest. 2014;124:4673–4677. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trujillo RD, Yue SB, Tang Y, O'Gorman WE, Chen CZ. The potential functions of primary microRNAs in target recognition and repression. EMBO J. 2010;29:3272–3285. doi: 10.1038/emboj.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Lima J Jr, Batty JA, Sinclair H, Kunadian V. MicroRNAs in ischemic heart disease: From pathophysiology to potential clinical applications. Cardiol Rev. 2017;25:117–125. doi: 10.1097/CRD.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 12.Quiat D, Olson EN. MicroRNAs in cardiovascular disease: From pathogenesis to prevention and treatment. J Clin Invest. 2013;123:11–18. doi: 10.1172/JCI62876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thum T, Lorenzen JM. Cardiac fibrosis revisited by microRNA therapeutics. Circulation. 2012;126:800–802. doi: 10.1161/CIRCULATIONAHA.112.125013. [DOI] [PubMed] [Google Scholar]
- 14.Schellings MW, Vanhoutte D, van Almen GC, Swinnen M, Leenders JJ, Kubben N, van Leeuwen RE, Hofstra L, Heymans S, Pinto YM. Syndecan-1 amplifies angiotensin II-induced cardiac fibrosis. Hypertension. 2010;55:249–256. doi: 10.1161/HYPERTENSIONAHA.109.137885. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM, Lan HY. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol Ther. 2014;22:974–985. doi: 10.1038/mt.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider MD. Serial killer: Angiotensin drives cardiac hypertrophy via TGF-beta1. J Clin Invest. 2002;109:715–716. doi: 10.1172/JCI15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwata M, Cowling RT, Yeo SJ, Greenberg B. Targeting the ACE2-Ang-(1-7) pathway in cardiac fibroblasts to treat cardiac remodeling and heart failure. J Mol Cell Cardiol. 2011;51:542–547. doi: 10.1016/j.yjmcc.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwaya T, Yokobori T, Nishida N, Kogo R, Sudo T, Tanaka F, Shibata K, Sawada G, Takahashi Y, Ishibashi M, et al. Downregulation of miR-144 is associated with colorectal cancer progression via activation of mTOR signaling pathway. Carcinogenesis. 2012;33:2391–2397. doi: 10.1093/carcin/bgs288. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Ying L, Tian Y, Yang P, Zhu Y, Wang Z, Qiu F, Lin J. miR-144 downregulation increases bladder cancer cell proliferation by targeting EZH2 and regulating Wnt signaling. FEBS J. 2013;280:4531–4538. doi: 10.1111/febs.12417. [DOI] [PubMed] [Google Scholar]
- 20.Zha W, Cao L, Shen Y, Huang M. Roles of Mir-144-ZFX pathway in growth regulation of non-small-cell lung cancer. PLoS One. 2013;8(e74175) doi: 10.1371/journal.pone.0074175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao M, Huang J, Gui K, Xiong M, Cai G, Xu J, Wang K, Liu D, Zhang X, Yin W. The downregulation of miR-144 is associated with the growth and invasion of osteosarcoma cells through the regulation of TAGLN expression. Int J Mol Med. 2014;34:1565–1572. doi: 10.3892/ijmm.2014.1963. [DOI] [PubMed] [Google Scholar]
- 22.Cui SQ, Wang H. MicroRNA-144 inhibits the proliferation, apoptosis, invasion, and migration of osteosarcoma cell line F5M2. Tumour Biol. 2015;36:6949–6958. doi: 10.1007/s13277-015-3396-0. [DOI] [PubMed] [Google Scholar]
- 23.Bao H, Li X, Li H, Xing H, Xu B, Zhang X, Liu Z. MicroRNA-144 inhibits hepatocellular carcinoma cell proliferation, invasion and migration by targeting ZFX. J Biosci. 2017;42:103–111. doi: 10.1007/s12038-016-9662-5. [DOI] [PubMed] [Google Scholar]
- 24.Gu J, Liu X, Li J, He Y. MicroRNA-144 inhibits cell proliferation, migration and invasion in human hepatocellular carcinoma by targeting CCNB1. Cancer Cell Int. 2019;19(15) doi: 10.1186/s12935-019-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui MR, Akhtar S, Shahid M, Tauseef M, McDonough K, Shanley TP. miR-144 mediated inhibition of ROCK1 protects against LPS induced lung endothelial hyperpermeability. Am J Respir Cell Mol Biol. 2019;61:257–265. doi: 10.1165/rcmb.2018-0235OC. [DOI] [PubMed] [Google Scholar]
- 26.He Q, Wang F, Honda T, James J, Li J, Redington A. Loss of miR-144 signaling interrupts extracellular matrix remodeling after myocardial infarction leading to worsened cardiac function. Sci Rep. 2018;8(16886) doi: 10.1038/s41598-018-35314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Cai SX, He Q, Zhang H, Friedberg D, Wang F, Redington AN. Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction. Basic Res Cardiol. 2018;113(36) doi: 10.1007/s00395-018-0694-x. [DOI] [PubMed] [Google Scholar]
- 28.Jiang DS, Bian ZY, Zhang Y, Zhang SM, Liu Y, Zhang R, Chen Y, Yang Q, Zhang XD, Fan GC, Li H. Role of interferon regulatory factor 4 in the regulation of pathological cardiac hypertrophy. Hypertension. 2013;61:1193–1202. doi: 10.1161/HYPERTENSIONAHA.111.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang DS, Wei X, Zhang XF, Liu Y, Zhang Y, Chen K, Gao L, Zhou H, Zhu XH, Liu PP, et al. IRF8 suppresses pathological cardiac remodelling by inhibiting calcineurin signalling. Nat Commun. 2014;5(3303) doi: 10.1038/ncomms4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 31.Wei C, Kim IK, Kumar S, Jayasinghe S, Hong N, Castoldi G, Catalucci D, Jones WK, Gupta S. NF-κB mediated miR-26a regulation in cardiac fibrosis. J Cell Physiol. 2013;228:1433–1442. doi: 10.1002/jcp.24296. [DOI] [PubMed] [Google Scholar]
- 32.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM, Dorn GW II. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106:166–175. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Wang HX, Li YL, Zhang CC, Zhou CY, Wang L, Xia YL, Du J, Li HH. MicroRNA Let-7i negatively regulates cardiac inflammation and fibrosis. Hypertension. 2015;66:776–785. doi: 10.1161/HYPERTENSIONAHA.115.05548. [DOI] [PubMed] [Google Scholar]
- 34.Yuan J, Chen H, Ge D, Xu Y, Xu H, Yang Y, Gu M, Zhou Y, Zhu J, Ge T, et al. Mir-21 promotes cardiac fibrosis after myocardial infarction via targeting Smad7. Cell Physiol Biochem. 2017;42:2207–2219. doi: 10.1159/000479995. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y, Yan X, Yan L, Hu F, Ma W, Wang Y, Lu S, Zeng Q, Wang Z. Inhibition of microRNA155 ameliorates cardiac fibrosis in the process of angiotensin II induced cardiac remodeling. Mol Med Rep. 2017;16:7287–7296. doi: 10.3892/mmr.2017.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng P, Chen L, Liu Z, Ye P, Wang S, Wu J, Yao Y, Sun Y, Huang X, Ren L, et al. MicroRNA-150 inhibits the activation of cardiac fibroblasts by regulating c-Myb. Cell Physiol Biochem. 2016;38:2103–2122. doi: 10.1159/000445568. [DOI] [PubMed] [Google Scholar]
- 37.E L, Jiang H, Lu Z. MicroRNA-144 attenuates cardiac ischemia/reperfusion injury by targeting FOXO1. Exp Ther Med. 2019;17:2152–2160. doi: 10.3892/etm.2019.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie T, Liang J, Guo R, Liu N, Noble PW, Jiang D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol Genomics. 2011;43:479–487. doi: 10.1152/physiolgenomics.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z, Yi J, Ye R, Liu J, Duan Q, Xiao J, Liu F. miR-144 regulates transforming growth factor-β1 iduced hepatic stellate cell activation in human fibrotic liver. Int J Clin Exp Pathol. 2015;8:3994–4000. [PMC free article] [PubMed] [Google Scholar]
- 40.Bahudhanapati H, Tan J, Dutta JA, Strock SB, Sembrat J, Alvarez D, Rojas M, Jäger B, Prasse A, Zhang Y, Kass DJ. MicroRNA-144-3p targets relaxin/insulin-like family peptide receptor 1 (RXFP1) expression in lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Biol Chem. 2019;294:5008–5022. doi: 10.1074/jbc.RA118.004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan EC, Dusting GJ, Guo N, Peshavariya HM, Taylor CJ, Dilley R, Narumiya S, Jiang F. Prostacyclin receptor suppresses cardiac fibrosis: Role of CREB phosphorylation. J Mol Cell Cardiol. 2010;49:176–185. doi: 10.1016/j.yjmcc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 42.El Jamali A, Freund C, Rechner C, Scheidereit C, Dietz R, Bergmann MW. Reoxygenation after severe hypoxia induces cardiomyocyte hypertrophy in vitro: Activation of CREB downstream of GSK3beta. FASEB J. 2004;18:1096–1098. doi: 10.1096/fj.03-1054fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.