Abstract
Purpose
To analyze the role of microglial and Müller cells in the formation of rings of photoreceptor degeneration caused by phototoxicity.
Methods
Two-month-old Sprague-Dawley rats were exposed to light and processed 1, 2, or 3 months later. Retinas were dissected as whole-mounts, immunodetected for microglial cells, Müller cells, and S- and L/M-cones and analyzed using fluorescence, thunder imaging, and confocal microscopy. Cone populations were automatically counted and isodensity maps constructed to document cone topography.
Results
Phototoxicity causes a significant progressive loss of S- and L/M-cones of up to 68% and 44%, respectively, at 3 months after light exposure (ALE). One month ALE, we observed rings of cone degeneration in the photosensitive area of the superior retina. Two and 3 months ALE, these rings had extended to the central and inferior retina. Within the rings of cone degeneration, there were degenerating cones, often activated microglial cells, and numerous radially oriented processes of Müller cells that showed increased expression of intermediate filaments. Between 1 and 3 months ALE, the rings coalesced, and at the same time the microglial cells resumed a mosaic-like distribution, and there was a decrease of Müller cell gliosis at the areas devoid of cones.
Conclusions
Light-induced photoreceptor degeneration proceeds with rings of cone degeneration, as observed in inherited retinal degenerations in which cone death is secondary to rod degeneration. The spatiotemporal relationship of cone death microglial cell activation and Müller cell gliosis within the rings of cone degeneration suggests that, although both glial cells are involved in the formation of the rings, they may have coordinated actions and, while microglial cells may be more involved in photoreceptor phagocytosis, Müller cells may be more involved in cone and microglial cell migration, retinal remodeling and glial seal formation.
Keywords: cones, retinal degeneration, phototoxicity, microglia, Müller cells
Light is necessary for vision but, paradoxically, can also damage in the retina, an effect known as phototoxicity.1–4 Light exposure causes well-documented apoptotic cellular death, mostly to the outer retinal layers.2,5,6 Furthermore, light is also a known risk factor for some retinal degenerations7,8 and exacerbates photoreceptor degeneration in some inherited9–12 or induced13,14 retinal degenerations.
Light-induced retinal degeneration has been widely used to study the mechanisms of cell death in retinal degenerations.1–4,11,13,15–17 This model has several advantages compared with inherited retinal degenerations because it is independent of the genetic defect and the age of the animals. Furthermore, because the degeneration is induced, the degree and duration of the insult can be manipulated, allowing the researcher to control the rate and the onset of retinal damage.
The role of the visual pigment rhodopsin, contained in rods,18 is essential for the initiation of the death signal of light-induced retinal degeneration,6 suggesting that rods are primarily affected in this degeneration. Why cones become vulnerable and die following rod degeneration is still a source of discussion, and there are several theories that try to explain this cone-rod survival dependence.10,19–21 We have previously documented in rats that light exposure causes rapid photoreceptor death2 that proceeds with the appearance of ring-shaped areas lacking rods15 and cones.22 These rings of photoreceptor degeneration have also been observed in animal models of inherited retinal degeneration such as the P23H-1-23,24 or the S334ter-line-325–30 rat strains. These rings have been proposed to be caused by the Müller cell glial seal30 (see the following section). Because rods are lost at earlier ages in the degenerations,15,28,29,31 the reorganization of the surviving cones in rings could be related to a rod-cone dependent survival mechanism,22,23 but it has also been proposed to depend on other factors such as the influence of the retinal glia32–34 and specifically to the activity and expression of zonula occludens by Müller cells.30
The mammalian retina contains three types of glial cells: astrocytes, Müller cells, and microglial cells, and the last two may be involved in the pattern of photoreceptor death in photoreceptor degenerations.19,34,35 The Müller cells, neuron-supporting macroglial cells, span the complete thickness of the retina, thus being potentially in close contact with all retinal neurons. These cells can play an active role in the process of retinal regeneration,36 in the survival and death of neurons,24,34,37 and in the regulation of synaptic transmission and the extracellular space volume.38,39 During mammalian retinal degenerations, Müller cells suffer reactive gliosis,40,41 leading to the formation of a glial seal or glial scar at the level of the outer limiting membrane.42–45 Glial scars are also seen in injuries to other areas of the central nervous system, usually around the lesion site probably in an attempt to isolate the intact part of the central nervous system.46 The glial seal is observed also in other retinal diseases and its role is not clear but it has been proposed to have a mechanic function, filling the spaces left by the dead neurons and conferring the retina some stability.
Müller cells may also contribute to photoreceptor death because they phagocytose apoptotic cell bodies during development.47 Recently, it has been shown also that Müller cells phagocytose dead photoreceptors in the healthy rodent retina, in a rodent model of inherited photoreceptor degeneration,48 and after light damage in the teleost retina.49
The role of microglial cells in photoreceptor degenerations is still poorly understood: it can be both neuroprotective and neurodestructive.19,34,50–52 In any case, previous works have shown that microglial cells become activated and travel through the retina in animal models of retinal degeneration,34,35,50,52–58 and are also present in the center of the rings of rod-cone degeneration.29,59
There is also evidence that there is an interaction between microglial and Müller cells that affects trophic factor release by Müller cells60 and therefore this interplay may also be important to regulate the responses of Müller cells in different scenarios, such as the regenerating zebrafish retina,61 in animal models of retinal degenerations62 and also in cocultures of activated microglia and Müller cells.60
In this study, we have used our previously described model of light-induced retinal degeneration2,3,13,15 and immunodetection of cones, Müller and microglial cells to investigate the role of the macro- and microglial cells in the rings of photoreceptor degeneration. To our knowledge, this is the first time that the relationship between cone degeneration, and microglial and macroglial cell reactivity has been analyzed in this model. We document a striking spatiotemporal relationship between cone degeneration, microglial activation, and Müller cell gliosis that suggests a coordinated intervention of these cells in the progression of disease.
Material and Methods
Animal Handling
Two-month-old albino Sprague-Dawley female rats (150–180 g body weight) were obtained from the University of Murcia breeding colony (n = 24) and exposed to 3000 lux of cold fluorescent white light continuously for 48 hours following previously described methods.2,3,13,15 Another group of animals (n = 8) was used as control group. Experimental animals were processed 1, 2, or 3 months after light exposure (ALE). Although we know from previous studies of our group that the numbers of S- and L/M- cones per retina remain unaltered in control animals for up to 6 months,23 we used control animals age-matched with the experimental group that survived longer (5 months).
All animals were housed in an environmentally controlled room with a 12-hour:12-hour light-dark cycle and had food and water ad libitum. The light intensity within the cages in our animal care facilities ranged from 5 to 30 lux. All experiments were carried out in accordance with the Spanish and the European Community Council Directives (86/609/EEC), and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were previously approved by the Ethics and Animal Studies Committee of the University of Murcia.
Tissue Processing and Immunohistofluorescence
Tissue fixation was performed by transcardial perfusion of the rats, first with saline and then with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4), following standard protocols of our laboratory.3,56,57,63 The retinas were then dissected as whole-mounts and double immunodetection was performed following previously described methods.13,23,56,57,64
Primary antibodies were used to immunodetect different retinal cell populations: (1) microglial cells (rabbit anti-Iba1; Ionized Calcium-Binding Adapter Molecule 1; AB_839504; 1:1000, 019-19741; Wako Chemicals, Neuss, Germany), (2) astrocytes and Müller cells (goat anti-GFAP; Glial Fibrillary Acidic Protein; AB_641021; 1:250, C-19, sc-6170; Santa Cruz Biotechnology, Heidelberg, Germany, or Guinea Pig anti-GFAP; AB_10641162; 173-004 Synaptic System, Goettingen, Germany), (3) Müller cells (goat anti-vimentin; AB_793998; 1:100, C-20, sc-7557; Santa Cruz Biotechnology), (4) L/M-cones (rabbit anti-L/M-opsin; AB_177456; 1:1200; Chemicon-Millipore Iberica, Madrid, Spain), and (5) S-cones (goat anti-S-opsin; N-20; anti-OPN1SW; AB_2158332; 1:1000; Santa Cruz Biotechnologies).
Secondary detection was performed using four different antibodies: donkey anti-goat Alexa 488 (AB_2534102), donkey anti-goat Alexa 594 (AB_142540), donkey anti-rabbit Alexa 594 (AB_141637), and goat anti-Guinea-Pig Alexa 647 (AB_2535867), all from Molecular Probes (Invitrogen, ThermoFisher, Madrid, Spain) and diluted at 1:500. Finally, the retinas were washed in phosphate-buffered saline, mounted on subbed slides, and covered with antifading mounting media (Vectashield mounting medium; Vector Laboratories, Palex Medical, Barcelona, Spain).
Retinal Image Analysis, Quantification, and Distribution of Retinal Populations
Retinal multiframe acquisitions (generally 154 frames per retina) of the whole retinas were acquired using an epifluorescence microscope (Axioscop 2 Plus; Zeiss Mikroskopie, Jena, Germany) equipped with a digital high-resolution camera (ProgRes C10; Jenoptik), and a computer-driven motorized stage (ProScan H128 Series; Prior Scientific Instruments, Cambridge, UK) controlled by the software Image-Pro Plus (IPP 5.1 for Windows; Media Cybernetics, Silver Spring, MD) following previously described methods.13,23,56,63–65 Acquired frames were tiled using IPP to create retinal photomontages.13,23,56,63 Reconstructed images were further processed using Adobe Photoshop CC 2018 (Adobe Systems, Inc., San Jose, CA) when needed.
The total numbers of S- and L/M- opsin+ cones per frame were automatically quantified using standard routines previously develop by our group using IPP macro language.13,17,23,64,66 Briefly, IPP applied a sequence of filters and transformations to each individual frame to clarify cell boundaries and separate individual cells, thus allowing automatic cell counting. Later, every frame was divided into 25 sampling areas and the number of S- or L/M- opsin+ cones in each of those areas were converted into cell densities that were used to represent S- or L/M-cone topography in isodensity maps using the software Sigmaplot13,23,64 (Systat Software, Inc., Hounslow, London, UK).
Some magnifications were also taken with a confocal microscope Leica SP8 (Spectral confocal and multiphoton system Leica TCS SP2; Leica, Jena, Germany) and a Leica DM6 B (Leica) microscope equipped with a Thunder imaging system that allowed us to acquire blur-free images.
Statistical Analysis
Data are shown as the mean ± standard deviation. Statistical analysis was performed using SigmaStat 3.1 for Windows (3.11 version, Systat Software, Inc.). The analysis of variance (ANOVA) test followed by Tukey post hoc test was used. Differences were considered significant when P < 0.05.
Results
Analysis of the Whole Population of S- and L/M-cones
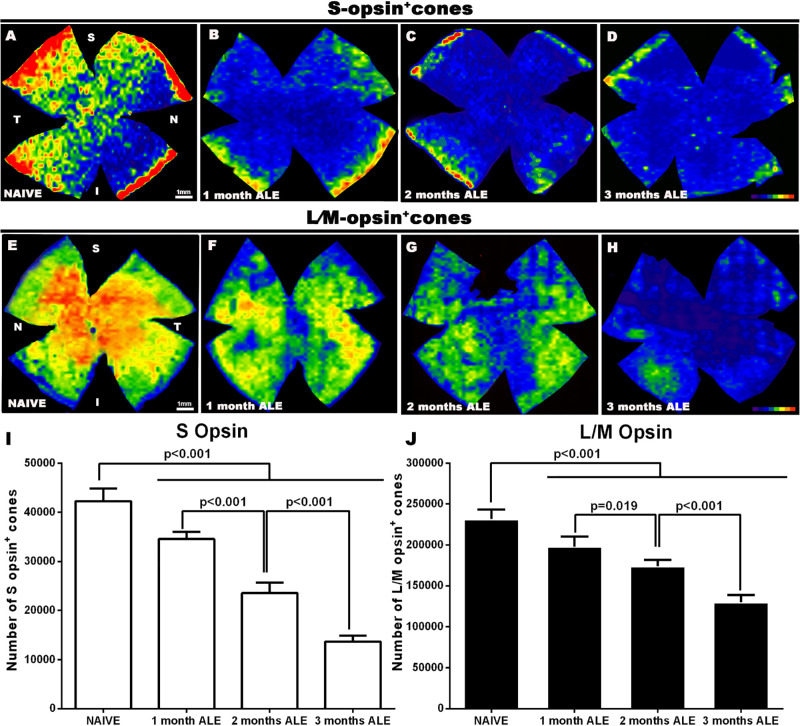
In the control group (naïve), the mean numbers ± standard deviations of S- and L/M- opsin+ cones were 41,998 ± 2,151 (n = 8; Fig. 1) and 228,314 ± 10,957 (n = 8; Fig. 1), respectively. One month ALE, the population of S- and L/M- opsin+ cones had decreased significantly by 18% and 15%, respectively, when compared with naïve retinas (Fig. 1; n = 8 each group; P < 0.001; one-way ANOVA, Tukey test). Cone degeneration progressed further with time, and by 2 months ALE there was a significant loss of 44% and 24% of S- and L/M-opsin+ cones, respectively, and by 3 months ALE this loss increased even further to 68% and 44%, respectively (Fig. 1; n = 8 each group; P < 0.001; one-way ANOVA, Tukey test). Thus, our data show that light exposure causes a significant progressive loss of cones during at least 3 months.
Figure 1.
Survival of S and L/M- opsin+ cones. Representative isodensity maps of (A–D) S- and (E–H) L/M- opsin+ cones in (A, E) naïve animals and in experimental animals at (B, F) 1, (C, G) 2, and (D ,H) 3 months ALE. Progressive death of both cone populations can be observed in experimental animals throughout the retina. Color scale of S-opsin+ cones/mm2: 0 (purple) to ≥1300 (red). Color scale of L/M-opsin+ cones/mm2: 0 (purple) to ≥6500 (red). (I, J) Graphs showing the mean number of (I; white bars) S-opsin+ cones and (J; black bars) L/M-opsin+ cones in naïve and experimental animals at increasing times ALE. Light exposure causes a significant progressive death of both cone populations (One-way ANOVA, Tukey test).
Topography of Cone Loss After Light Exposure
Although S and L/M cone loss started at 1 month ALE in the superior retina (named the “arciform photosensitive area”, see next paragraph) and later spread to the central retina (Figs. 1 and 2; see next paragraph), at 1 month, the cone isodensity maps showed a larger decrease of L/M-cones in the superior retina, and of S-cones in the central and equatorial retina (Figs. 1B,F). Two and 3 months ALE, only cold colors (i.e., lower densities) could be seen in the cone isodensity maps (Figs. 1D,H), showing that both cone populations were greatly diminished all throughout the retina (Fig. 1).
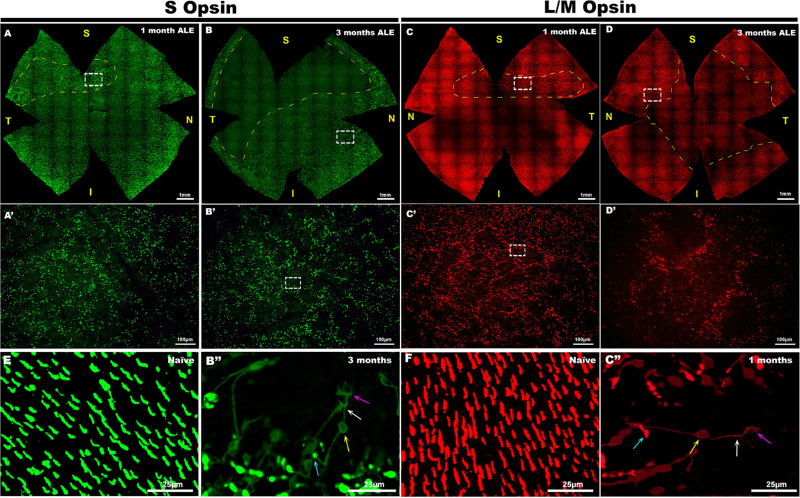
Figure 2.
Light exposure causes a disruption of the normal photoreceptor mosaic and morphological changes in the surviving photoreceptors. S- (left two columns, green) and L/M- (right two columns, red) cone immunodetection in whole mounts of the (A, B) left and (C, D) right retinas of four representative experimental animals, (A, C) 1 and (B, D) 3 months ALE. In the whole-mounted retinas, the dashed yellow lines surround, at 1 month ALE, the area of the superotemporal retina where the rings of (A) S- and (C) L/M-cone degeneration first appear and, at 3 months ALE (B, D), the areas devoid of S- and L/M cones. (A–D) Insets are shown at a higher power in A′–D′, respectively, to show the characteristic rings of photoreceptor degeneration. The insets in B′ and C′ are shown at higher power in B″ and C″ to illustrate the degenerating (B″) S and (C″) L/M cones at the periphery of the rings. Degenerating cones show opsin expression in the outer segments (blue arrow), somas (yellow arrow), inner segments (white arrow), axons and terminals (purple arrow), and their outer segments are located in the periphery of the rings, whereas their axons are directed towards the center of the rings.
In addition, when we analyzed qualitatively cone distribution in these retinas, we observed that the normal, more or less homogeneous, photoreceptor mosaic was altered by the appearance of rings devoid of cones, henceforward rings of cone degeneration (Fig. 2). One month ALE, these rings of cone degeneration appeared in a distinctive area of the superior or sometimes superotemporal retina (Figs. 2 A, A′). The area in the superior retina in which the rings first appeared coincided with the “arciform photosensitive area” of the rat retina13,15,16,20,67,68 (Figs. 2A,C). However, with time, the rings of the superior retina spreaded through the central and inferior retina and at the same time increased in size and merged (Figs. 2–4). Two months ALE, the size of area occupied by the rings of cone degeneration as well as the size of the rings increased (Figs. 3 A-C; 4A,C) and by 3 months ALE, there were rings also in the inferior retina up to the retinal periphery (Figs. 2B,D). The coalescence of the rings of cone degeneration caused with time the disappearance of the rings and thus of both cone populations in large retinal areas. By 2 months, the superior retina was largely devoid of both cone populations (Figs. 2,4). By 3 months, there was a large area devoid of both types of cones that varied between animals and occupied the superior and sometimes the temporal and the central peripapillary retina (Figs. 2B,D; 4A).
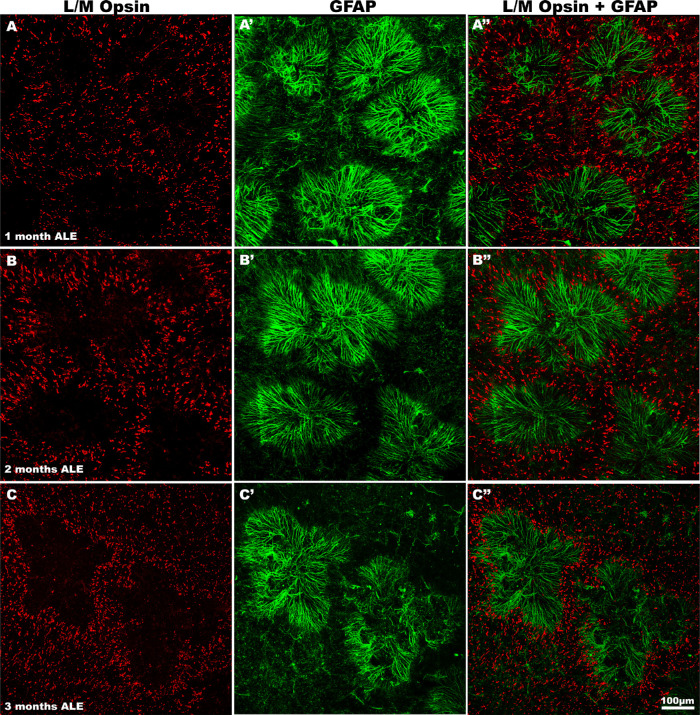
Figure 3.
The rings of cone degeneration are occupied by rosettes of Müller cells. Magnifications of the same regions of representative retinas immunodetected for L/M opsin (left column), GFAP (center column), or a merged image (right column) to show the rings of L/M-cone degeneration at 1 (first row), 2 (second row), and 3 (third row) months ALE. The rings devoid of L/M-cones are occupied by radial processes of Müller cells in the form of rosettes.
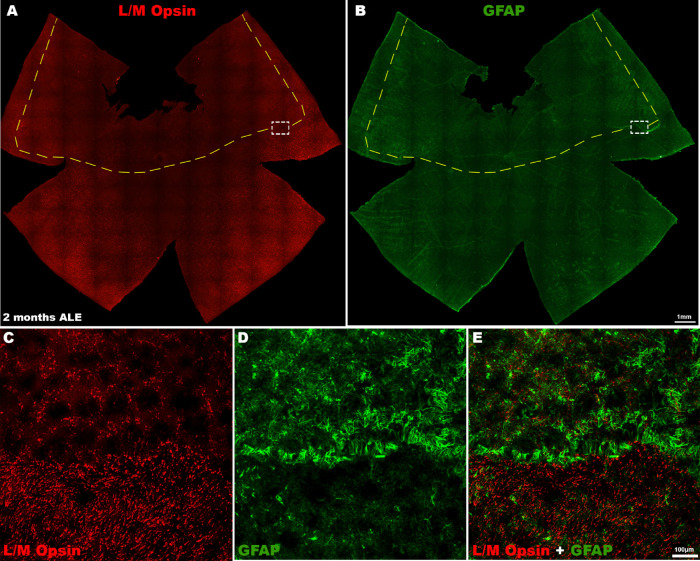
Figure 4.
L/M-cone loss and GFAP expression 2 months ALE. Photomontages of the same representative retinal whole mount of an animal processed two months ALE and immunoreacted for (A, red) L/M opsin and (B, green) GFAP showing that the superior “photosensitive area” of the rat retina is at this time point devoid of (A) L/M-cones, whereas there is an increased expression of (B) GFAP in this area and specially in its boundaries. (A, B) Insets are shown at higher power in (C) and (D), respectively, and (E) is a merged image of (C) and (D) to show the cone loss and increased GFAP immunoreactivity at the limits of the photosensitive area.
Within the rings of cone degeneration, the surviving cones displayed morphological changes that included shortened outer segments and redistribution of both S and L/M opsins to the inner segments, somas and axons (Figs. 2 B″, C″). The surviving cones showed polarity, their inner segments being directed toward the center of the rings and their outer segments toward the periphery. These changes were more evident in the central part of the rings probably because in this region only a few cones were present and their processes could be better visualized.
Macro- and Microglial Cell Implication in Photoreceptor Degeneration After Light Exposure
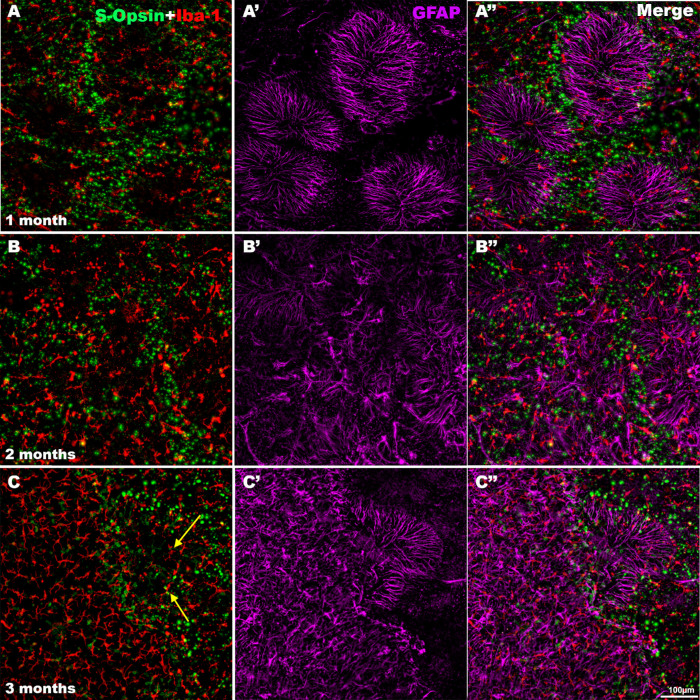
In control animals, GFAP expression was only found in astrocytes, in the innermost retinal layers. One month ALE, we found Müller cell gliosis: intense GFAP immunoreactivity in the processes of the Müller cells that were at this time point radially distributed, forming rosettes that occupied the center of the rings of cone degeneration, thus presumably filling the space left by the dead cones (Figs. 3 and 5). Two months ALE, when the rings began to increase in size and coalesce with other rings, the immunoreactive processes of the Müller cells lost in part their radial distribution and their intermediate filament expression was lost at the center of the rings (Figs. 4 D, E and 5 B-B″, see the following section). When the rings coalesced and formed large areas devoid of cones in the superior retina (the “arciform photosensitive area”), the increased GFAP expression in the Müller cells processes was thus more marked in the boundaries of this area (Fig. 4E).
Figure 5.
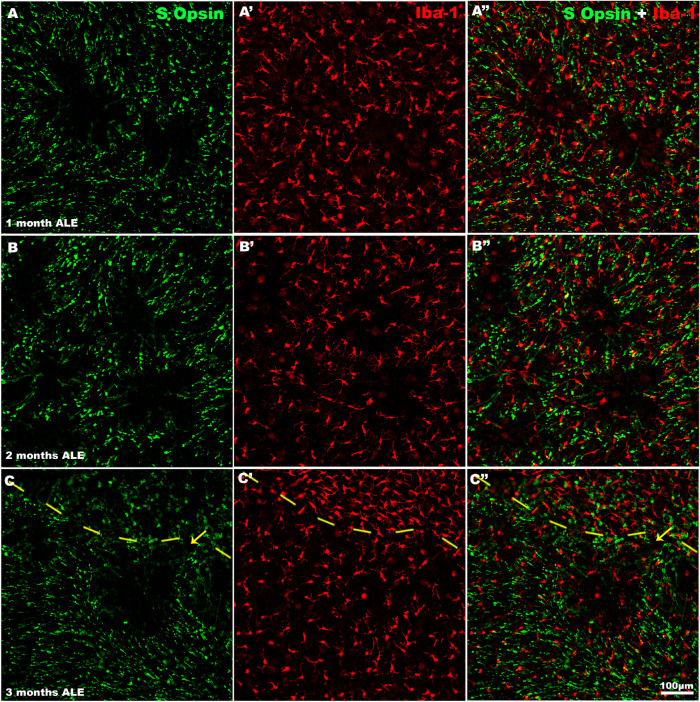
Microglial and Müller cell involvement during cone S degeneration. Magnifications of the same regions from the superior retina of representative animals processed (A-A″) 1, (B-B″) 2, and (C-C″) 3 months ALE and immunoreacted for S-opsin and Iba-1 (left column), GFAP (purple, central column), or merged images (right column). One month ALE, GFAP-labeled processes of Müller cells with a radial distribution were seen inside the (A-A″) rings and activated microglial cells were usually seen in the borders of the rings, but there were also some in the center of some rings. Two months ALE, the rings merged, and GFAP immunoreactivity decreased at the center of the rings, whereas at their periphery, the GFAP-positive processes lost in part their radial distribution (B′). Three months ALE, the areas completely devoid of cones (left part of C-C″) were filled by activated microglia that had resumed a mosaic distribution (C, C″) and GFAP-positive processes that were radially oriented only at the boundaries of the cone degenerated area (C′, C″). Arrows point to activated microglial cells inside of a ring of cone degeneration.
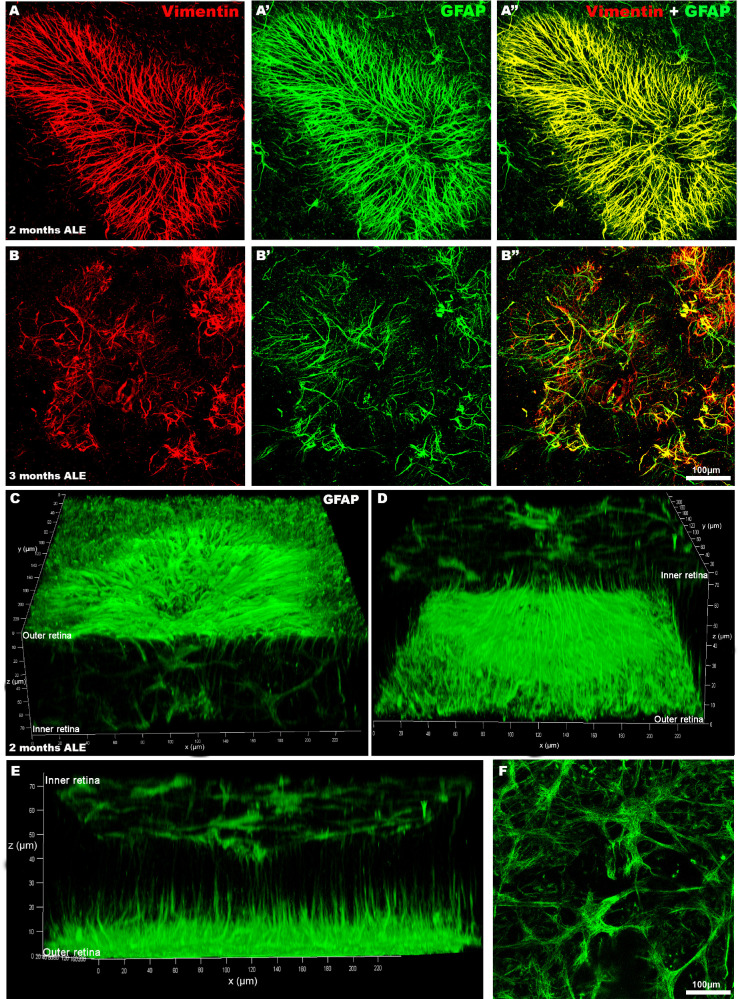
The radial GFAP immunoreactive processes found within the rings of cone degeneration were also Vimentin immunoreactive and therefore unequivocally correspond to processes of Müller cells (Figs. 5 C-C″ and 6 A-B″). Moreover, confocal microscopy revealed that these radial GFAP immunoreactive processes were located in the outer retina at the level of the photoreceptor outer segment layer (Figs. 6 C and D), but that they originated in more internal retinal layers where they ran vertically and became more horizontal in the most outer layers. All of these features indicate that these radial processes correspond to the outer processes of the Müller cells that form the outer glial seal.
Figure 6.
GFAP-positive radial processes within the rings of cone degeneration correspond to the external processes of Müller cells. Confocal photomicrophotographs of representative rings of cone degeneration in retinal whole mounts 2 (A-A″) or 3 (B-B″) months ALE immunoreacted for Vimentin (A, B, A″, B″) and GFAP (A′, B′, A″, B″, C–F). The GFAP radial immunoreactive processes observed in the center of the rings, 2 (first row) or 3 (second row) months ALE are also Vimentin (A″, B″) positive, and thus correspond to the outer processes of Müller cells. Confocal three-dimensional reconstructions of the GFAP immunoreactivity in a ring of cone degeneration using different tilting degrees (C–E), to show GFAP immunoreactivity through the retina. The inner and outer retina have been indicated in the z axis. There are GFAP-labeled Müller cell processes in the inner retina that run vertically and become horizontal and radially oriented in the most external retinal layers (C–E) and GFAP-positive processes in the most inner retinal layers that correspond to the Müller cell end feet and the astrocytes of the nerve fiber layer (see a representative microphotograph of the inner GFAP immunoreactive layer, shown also in the upper part of D, in F).
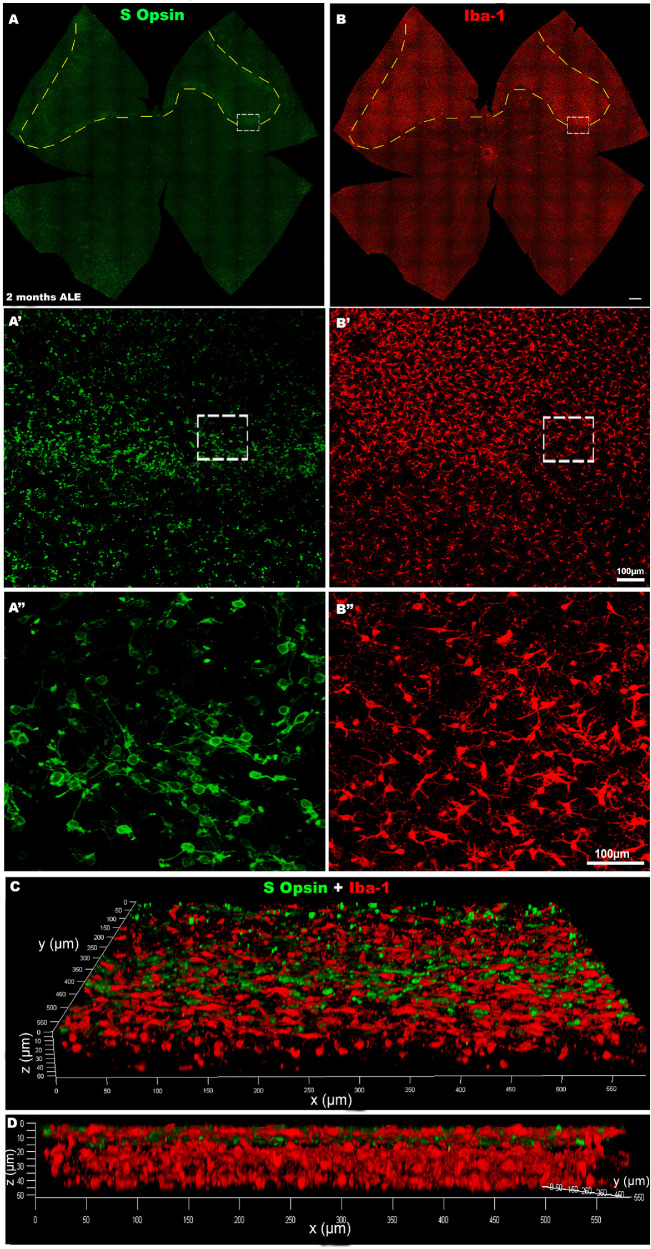
In control animals, microglial cells were inactive and homogeneously distributed in the different retinal layers, as described.54,69,70 One month ALE, we found many activated microglial cells at the level of the photoreceptor outer segment layer. These cells were mainly located in the periphery of the rings of cone degeneration although there were also some at the center of the rings (Figs. 5 A-A″ and 7 A-A″). These cells showed an enlarged soma, and thickening and retraction of their processes, the typical characteristics of their activated state. Confocal microscopy confirmed that these activated microglial cells of the outer retinal layers were at the level of the photoreceptor layers (Figs. 8 C, D). Two or more months ALE, most of the activated microglial cells had migrated toward the periphery of the rings of cone degeneration (Figs. 5 and 7). However, when the rings of cone degeneration of the superior retina coalesced, the activated microglial cells of the outer retinal layers were most evident in the superior retina, in the named “arciform photosensitive area” (Fig. 8B,) where they showed again an homogeneous distribution and in this region there were increased densities of activated microglial cells (Figs. 5 and 8B).
Figure 7.
Microglial cells and the rings of cone degeneration. Magnifications of the same representative retinal regions immunolabeled for S-opsin (left column), Iba-1 (center column), or merged images (right column) of animals processed at 1 (first row), 2 (second row), and 3 (third row) months ALE to show the rings of S-cone degeneration. Iba-1 immunoreactive microglial cells were observed surrounding the rings (A′, B′), but also inside the rings A′–C′). The interrupted line in C-C″ separates the photosensitive area devoid of cones (upper part) from the less degenerated retina (lower part) where a ring devoid of S-cones but containing microglial cells is close to it.
Figure 8.
S-cone loss and microglial cell reactivity 2 months ALE. Photomontages of the same representative retinal whole mounts of an animal processed 2 months ALE and immunoreacted for S-cones (A, green) and for Iba-1 (B, red) showing an area of the superior retina (dashed line) devoid of S-cones (A) and filled by activated microglial cells (B). Magnifications (A′-B″) of the border of the cone death area. The insets in A′ and B′ are shown at higher power in A″ and B″, to show that at the limit the S-cone morphological changes and the microglial cell activation are more marked. Three-dimensional confocal reconstruction of the same area shown in A″ and B″ to document the location within the retina of the activated microglial cells (0 µm in the z axis corresponds to the photoreceptor outer segment layer).
At the same time as microglial cells were migrating from the center to the periphery of the rings, (from 1 to 2 months ALE in the superior retina, but later in the inferior retina) we observed a change in Müller cell immunoreactivity. At one month ALE, the outer processes were of Müller cells showed increased GFAP and vimentin expression and an orderly radial distribution in the center of the rings. Two and 3 months ALE when the rings fused, the outer processes of Müller cells of the rings lost GFAP and vimentin immunoreactivity especially at the center of the rings, whereas the processes at the periphery of the rings were still immunoreactive and became intermingled with the processes of the Müller cells in the neighboring rings (Figs. 5 B′-C″).
Discussion
In the present study, we have quantified for the first time the progression of S- and L/M-cone loss and the behavior of macro and microglial cells in a model of light-induced photoreceptor degeneration. We suggest a plausible coordination between microglial and Müller cells in the course of photoreceptor degeneration.
Cone Loss in Light-induced Retinal Degeneration
Our results show that light exposure causes a progressive loss of S- and L/M-cones that is presumably influenced by the primary loss of rods, that occurs before cone loss in this rat model, as it happens in other nocturnal animals with rod-dominated retinas15,71 (see next paragraphs). Interestingly, although the densities of L/M-cones are higher in control retinas than the densities of S-cones, the loss of S-cones ALE is more extensive, suggesting that this cone population may be more sensitive to white light, which is in accordance with previous studies of light induced retinal degeneration.13,15,17 We and others have previously proposed that this fact could be due to S-cone sensitivity to the blue component of light,17,71–74 which is the most phototoxic or to differential sensitivity of the S cones to oxidative stress.13,17,74,75
Previous studies from our laboratory have shown that photoreceptor loss ALE follows a spatiotemporal pattern that begins in the superior or superotemporal retina and spreads with time to the other retinal regions.2,13,15,16 We have observed some variability in the location of the area were photoreceptor loss starts, because sometimes sits in the superior retina and sometimes extends also to the temporal retina.2,13,15,16 Here we show that in the superior retina, where the phototoxic injury commences, the loss of S- and L/M-cones proceeds with the appearance of rings of cone degeneration that appear delayed to rod loss in this area.15 These rings of cone degeneration have been previously described both for rods and cones in this model of light exposure13,15 but also in several rat models of inherited retinal degeneration in which cone loss is secondary to rod degeneration.23–30,32,76 Although in this study we did not perform a double immunostaining of both cone populations in the same retinas, we know from previous works in our laboratory that these rings of cone degeneration lack both types of cones.13,23 In this model, these rings devoid of cones follow a similar, but delayed, spatiotemporal pattern than that observed for rods.15 One month ALE, rings devoid of cones are mainly distributed in the superior or superotemporal retina, whereas rods have been lost all throughout the retina.15 Interestingly, in the previously mentioned rat models of inherited photoreceptor degeneration, the rings of cone degeneration are found in the equatorial retina of the four retinal quadrants, although with a tendency to be larger and more abundant in the superior retina.13,15,23,25,31 Thus, it seems that there are spatiotemporal differences in the appearance of rings of cone degeneration related to the cause of the degeneration, because when the primary cause of the degeneration is light exposure they appear first in the superior (superotemporal) retina,13,15 the light-sensitive area of the rat retina.13,15,16,20,67,68
Our group has documented previously that light exposure causes a blood-retinal breakdown and maximal photoreceptor death in an “arciform” area situated in the superotemporal area of the rat retina.2,13,15,16 This is the area where the rings of cone degeneration first appear ALE. Other authors have found also that in normal rats67,68,71,77–81 and in rats with inherited retinal degeneration80,82,83 light exposure causes maximal photoreceptor loss in the superior retina and therefore have named this area the “photosensitive area” of the rat retina. Furthermore, photoreceptor death is also maximal in this area in various rat models of retinitis pigmentosa.31 Our group has also documented that the superior retina, where the rings of cone degeneration first appear, shows the highest densities of L/M-cones64 and retinal ganglion cells,84 and other authors have also documented increased densities of retinal ganglion cells in the superotemporal retina85 and longer rod outer segments in the superior retina.86 Thus, the characteristics of the superior or superotemporal retina suggest that it could be the most specialized area of the rat retina for vision and maybe the equivalent to the visual streak in the rat retina.64
The exact cause of the reorganization of the cone mosaic in empty rings in various models of photoreceptor degeneration is still a matter of debate. Initially, it was not clear whether the rings of cone degeneration were due to cone migration25,32 or cone loss.28 Because the rings become larger with time ALE and because the numbers of cones decrease also with time ALE, it is clear from this and other studies that they are the result of cone loss13,15–17 that seems to propagate from the center to the periphery of the rings and at the same time the ring size increases.32 Interestingly, we have not been able to find rings devoid of photoreceptors in an animal model of retinal degeneration induced by taurine depletion that causes primary loss of cones,13 and it is tempting to suggest that these rings may be related to a rod-cone dependent survival mechanism.10,19–21,25,32,42,87,88–91
The rings in our model and in other animal models23 contain degenerating cones whose inner segments are directed towards the center of the rings. These degenerating cones show a redistribution of the opsin expression to their inner segment, soma, axons, and synaptic terminals.23,25,27 Redistribution of opsin to the cone soma and inner segment has been also observed in other animal models of inherited retinal degeneration,23,25,32 in human diseases such as retinitis pigmentosa92 and age-related macular degeneration,93 and in experimental retinal detachment.94 Therefore, opsin redistribution may be a feature of degenerating cones.
Involvement of Retinal Glia in Light-induced Photoreceptor Degeneration
Both Müller and microglial cells respond to retinal cell damage and are known to phagocytose under pathological conditions. Although the exact role of gliosis in retinal degenerations remains still unknown,34,35,46,57,69,70,95 it is known to contribute to early retinal remodeling45,48 and disrupts the normal retinal morphology. Müller cells are the major contributors of gliosis46,48 and the first glial cells that respond to photoreceptor stress,45,48,96 showing a hypertrophy45,81,97 and increased GFAP expression.24,32,35,40,41,44–46,98 Thus, Müller cell reaction could have an important role in the appearance of rings during photoreceptor degeneration.32
In this study, we show that when photoreceptor loss begins ALE, Müller cells hypertrophy, show increased GFAP and vimentin expression and extend their external processes horizontally and radially within the rings of cone degeneration, probably in an attempt to form the glial seal (see next paragraph). A previous work has indicated that these clusters appear “firework-like structures”24 and in addition, it has been proposed that cones may migrate through the processes of Müller cells from the center to the periphery of the rings.99 With time ALE, the rings increase in size and merge, and the increased expression of the intermediate filaments in the processes of Müller cells ceases at the center of the rings while it continues in the periphery of the rings and, when the rings merge, at the boundaries of the “photosensitive area.” This is in accordance with previous works that have shown Müller cell hyperreactivity ALE in the superior retina.100,101 Also when the rings merge the radial distribution of the outer processes of Müller cells at the periphery of the rings is somehow lost because they intermingle with the processes of the cells in the neighboring rings.
Although Müller cells may participate in inherited and light-induced retinal degenerations through photoreceptor phagocytosis,48,49 it seems likely that these cells have other functions in cone ring formation, because in a model of inherited retinal degeneration the disruption of Müller cell metabolism through the intravitreal injection of alpha aminoadipic acid eliminates the rings of cone degeneration.32 In inherited retinal degenerations, it has also been shown that retinal remodeling courses with hypertrophy of the processes of Müller cells in the outer retina that form a glial sealing to isolate the remnant of the neural retina from the RPE and choroid.32,87,102 Indeed, as photoreceptor degeneration progresses, the glial seal is more evident,103 covering the entire outer retina at late stages.43,45,46 This sequence of events suggests that Müller cells hypertrophy in the early phases of photoreceptor degeneration and participate in the phagocytosis of the dead rods and cones. Later, the external processes of Müller cells form the glial seal, and the surviving cones may migrate through them radially24 to produce the rings of cone degeneration.32,99 The exact function of the glial seal is not known but it may have a mechanic function that confers the retina some stability in the course of the degeneration,34 but also a protective function, because in the late stages of retinal remodeling, the focal gaps in the glial seal may allow the migration of retinal pigment epithelium cells to the inner retina,3,42,102,104–106 which triggers a series of progressive events that, ultimately, cause retinal ganglion cell death.2,3,16,22,42,63,102,104–107
The retinal microglial cells may also have an important role in the inherited retinal degenerations, because these cells become activated and migrate to the outer retinal layers where they phagocytose the dead photoreceptors.81,108,109 We have recently shown, using two different rat models of inherited retinal degeneration with different etiologies, that microglial cell activation begins at the same time as photoreceptor death, but that the Müller cell reaction is somewhat delayed,19,35 and that microglial cell inhibition reduces photoreceptor loss.19,57 In this study, we show that 1 month ALE, there are high numbers of activated microglial cells and/or macrophages in the photoreceptor layers at the center and periphery of the rings of cone degeneration, suggesting that these cells may be involved in the phagocytosis of the dead photoreceptors. Other authors have also found migration of microglia and macrophages to the outer retina in other models of light-induced retinal degeneration59,110 and activated microglial cells at the center of the rings of photoreceptor degeneration in a rat model of inherited retinal degeneration, before the occupation of the rings by processes of Müller cells.29 Microglial cells and Müller cells might act coordinately to phagocytose photoreceptor debris during retinal degeneration48,49,60,111,112; Müller cells may release factors that induce microglial activation and migration,96,100,103 and may also form a scaffold to guide microglial migration,49,60 whereas activated microglia may also release factors that influence Müller cells behavior.111,113 Specifically, microglial activation may influence the morphology and function of Müller cells, stimulating Müller cell gliosis but a decrease of their phagocytic activity.49,60,114 We also show in this study that 2 or 3 months ALE, the microglial cells disappear from the center of the rings and concentrate in their periphery and, when all the cones disappear, the microglial cells resume their normal homogeneous distribution within the areas devoid of cones. Other authors have suggested that microglial cells may migrate in close contact with Müller cells.49,60 In this work, we also document that long-term ALE when the rings of the cone degeneration fuse, the microglial cells resume their homogeneous distribution at the same time as the Müller cells at the center of the rings lose the increased expression of intermediate filaments and the Müller cell processes at the periphery of the rings lose their radial disposition because they interlace with processes of cells in the neighboring rings. This sequence of events suggests that Müller cell gliosis at the center of the rings may impede the normal mosaic distribution of microglial cells or that microglial cells could influence Müller cells gliosis. In any case, the changes observed in glial cell morphology and behavior ALE are the result of cone degeneration but also of the microglial-Müller cells crosstalk.48,60,111,114
In conclusion, our results document, for the first time, the relationship between cone degeneration, and microglial and Müller cell reactivity in the initial phases of retinal remodeling in an animal model of light-induced retinal degeneration. Because in this model we observe rings of cone degeneration that we and other authors have observed before in animal models of inherited retinal degeneration with primary degeneration of rods but not of cones,13 we suggest that they occur secondarily to light-induced rod degeneration in combination with other factors such as microglial cell activation and proliferation and Müller cell gliosis.24,32,40,41,99 The sequence of events suggests a coordinated intervention of microglial and Müller cells. Activated microglial cells in the center of the rings could be the responsible for the phagocytosis of dead cones, whereas Müller cells may play several roles at different stages of the degeneration, such as cone phagocytosis, cone and microglial cell migration, and formation of the glial seal. Microglial cells may also influence the behavior of Müller cells. A better understanding of the role of the retinal micro- and macroglia in photoreceptor degeneration may allow us to intervene in the disease.
Acknowledgments
Supported by Fundación Séneca, Agencia de Ciencia y Tecnología Región de Murcia (19881/GERM/15), and the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional “Una Manera de Hacer Europa” (SAF2015-67643-P, PI16/00380, RD16/0008/0026, PI16/00031, PI19/00203, PI19/00071).
Disclosure: J. Di Pierdomenico, None; A. Martínez-Vacas, None; D. Hernández-Muñoz, None; A.M. Gómez-Ramírez, None; F.J. Valiente-Soriano, None; M. Agudo-Barriuso, None; M. Vidal-Sanz, None; M.P. Villegas-Pérez, None; D. García-Ayuso, None
References
- 1. Glickman RD. Phototoxicity to the retina: mechanism of damage. Int J Toxicol. 2002; 21: 473–490. [DOI] [PubMed] [Google Scholar]
- 2. García-Ayuso D, Salinas-Navarro M, Agudo-Barriuso M, Alarcón- Martínez L, Vidal-Sanz M, Villegas-Pérez MP. Retinal ganglion cell axonal compression by retinal vessels in light-induced retinal degeneration. Mol Vis. 2011; 17: 1716–1733 [PMC free article] [PubMed] [Google Scholar]
- 3. García-Ayuso D, Di Pierdomenico J, Vidal-Sanz M, Villegas-Pérez MP. Retinal ganglion cell death as a late remodeling effect of photoreceptor degeneration. Int J Mol Sci. 2019; 20: E4649. doi: 10.3390/ijms20184649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krigel A, Berdugo M, Picard E, et al.. Light-induced retinal damage using different light sources, protocols and rat strains reveals LED phototoxicity. Neuroscience. 2016; 339: 296–307. [DOI] [PubMed] [Google Scholar]
- 5. Hafezi F, Marti A, Munz K, Remé CE. Light-induced apoptosis: differential timing in the retina and pigment epithelium. Exp Eye Res. 1997; 64: 963–970. [DOI] [PubMed] [Google Scholar]
- 6. Wenzel A, Grimm C, Samardzija M, Remé CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005; 24: 275–306. [DOI] [PubMed] [Google Scholar]
- 7. Behar-Cohen F, Martinsons C, Vienot F, et al.. Light-emitting diodes (LED) for domestic lighting: any risks for the eye? Prog Retin Eye Res. 2011; 30: 239–257. [DOI] [PubMed] [Google Scholar]
- 8. Arnault E, Barrau C, Nanteau C, et al.. Phototoxic action spectrum on a retinal pigment epithelium model of age related macular degeneration exposed to sunlight normalized conditions. PLoS One. 2013; 8: e71398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010; 11: 273–284. [DOI] [PubMed] [Google Scholar]
- 10. Chrysostomou V, Valter K, Stone J. Cone-rod dependence in the rat retina: variation with the rate of rod damage. Invest Ophthalmol Vis Sci. 2009; 50: 3017–3023. [DOI] [PubMed] [Google Scholar]
- 11. Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Susceptibility to retinal light damage in transgenic rats with rhodopsin mutations. Invest Ophthalmol Vis Sci. 2003; 44: 486–492. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Zhang Y, Chen S, Vernon G, Wong WT, Qian H. Light-dependent OCT structure changes in photoreceptor degenerative rd 10 mouse retina. Invest Ophthalmol Vis Sci. 2018; 59: 1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. García-Ayuso D, Di Pierdomenico J, Hadj-Said W, et al.. Taurine depletion causes ipRGC loss and increases light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2018; 59: 1396–1409. doi: 10.1167/iovs.17-23258. [DOI] [PubMed] [Google Scholar]
- 14. García-Ayuso D, Di Pierdomenico J, Valiente-Soriano FJ, et al.. β-alanine supplementation induces taurine depletion and causes alterations of the retinal nerve fiber layer and axonal transport by retinal ganglion cells. Exp Eye Res. 2019; 188: 107781. doi: 10.1016/j.exer.2019.107781. [DOI] [PubMed] [Google Scholar]
- 15. García-Ayuso D, Galindo-Romero C, Di Pierdomenico J, Vidal-Sanz M, Agudo-Barriuso M, Villegas Pérez MP. Light-induced retinal degeneration causes a transient downregulation of melanopsin in the rat retina. Exp Eye Res. 2017; 161: 10–16. doi: 10.1016/j.exer.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 16. Marco-Gomariz MA, Hurtado-Montalbán N, Vidal-Sanz M, Lund RD, Villegas-Pérez MP. Phototoxic-induced photoreceptor degeneration causes retinal ganglion cell degeneration in pigmented rats. J Comp Neurol. 2006; 498: 163–179 [DOI] [PubMed] [Google Scholar]
- 17. Ortín-Martínez A, Valiente-Soriano FJ, García-Ayuso D, et al.. A novel in vivo model of focal light emitting diode-induced cone-photoreceptor phototoxicity: neuroprotection afforded by brimonidine, BDNF, PEDF or bFGF. PLoS One. 2014; 9: e113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith SO. Structure and activation of the visual pigment rhodopsin. Annu Rev Biophys. 2010; 39: 309–28. [DOI] [PubMed] [Google Scholar]
- 19. Di Pierdomenico J, García-Ayuso D, Agudo-Barriuso M, Vidal-Sanz M, Villegas-Pérez MP. Role of microglial cells in photoreceptor degeneration. Neural Regen Res. 2019; 14: 1186–1190. doi: 10.4103/1673-5374.251204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Narayan DS, Wood JP, Chidlow G, Casson RJ. A review of the mechanisms of cone degeneration in retinitis pigmentosa. Acta Ophthalmol. 2016; 94: 748–754 [DOI] [PubMed] [Google Scholar]
- 21. Sahel JA, Léveillard T. Maintaining Cone Function in Rod-Cone Dystrophies. Adv Exp Med Biol. 2018; 1074: 499–509. [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Ayuso D, Di Pierdomenico J, Agudo-Barriuso M, Vidal-Sanz M, Villegas-Pérez MP. Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death. Neural Regen Res. 2018; 13: 1885–1886. doi: 10.4103/1673-5374.239436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. García-Ayuso D, Ortín-Martínez A, Jiménez-López M, et al.. Changes in the photoreceptor mosaic of P23H-1 rats during retinal degeneration: implications for rod-cone dependent survival. Invest Ophthalmol Vis Sci. 2013; 54: 5888–900. doi: 10.1167/iovs.13-12643. [DOI] [PubMed] [Google Scholar]
- 24. Fernández-Sánchez L, Lax P, Campello L, Pinilla I, Cuenca N. Astrocytes and Müller cell alterations during retinal degeneration in a transgenic rat model of retinitis pigmentosa. Front Cell Neurosci. 2015; 9: 484. doi: 10.3389/fncel.2015.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ji Y, Zhu CL, Grzywacz NM, Lee EJ. Rearrangement of the cone mosaic in the retina of the rat model of retinitis pigmentosa. J Comp Neurol. 2012; 520: 874–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ji Y, Yu WQ, Eom YS, et al.. The effect of TIMP-1 on the cone mosaic in the retina of the rat model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2014; 56: 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hombrebueno JR, Tsai MM, Kim HL, De Juan J, Grzywacz NM, Lee EJ. Morphological changes of short-wavelength cones in the developing S334ter-3 transgenic rat. Brain Res. 2010; 1321: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Tao W, Luo L. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PLoS One. 2010; 5: e9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu CL, Ji Y, Lee EJ, Grzywacz NM. Spatiotemporal pattern of rod degeneration in the S334ter-line-3 rat model of retinitis pigmentosa. Cell Tissue Res. 2013; 351: 29–40. [DOI] [PubMed] [Google Scholar]
- 30. Yu WQ, Eom YS, Shin JA, et al.. Reshaping the cone-mosaic in a rat model of retinitis pigmentosa: modulatory role of ZO-1 expression in DL-alpha-aminoadipic acid reshaping. PLoS One. 2016; 11: e0151668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. LaVail MM, Nishikawaa S, Steinberg RH, et al.. Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration. Exp Eye Res. 2018; 167: 56–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee EJ, Ji Y, Zhu CL, Grzywacz NM. Role of Müller cells in cone mosaic rearrangement in a rat model of retinitis pigmentosa. Glia. 2011; 59: 1107–1117. [DOI] [PubMed] [Google Scholar]
- 33. Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003; 76: 463–471. [DOI] [PubMed] [Google Scholar]
- 34. Cuenca N, Fernández-Sánchez L, Campello L, et al.. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014; 43: 17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 35. Di Pierdomenico J, García-Ayuso D, Pinilla I, et al.. Early events in retinal degeneration caused by rhodopsin mutation or pigment epithelium malfunction: differences and similarities. Front Neuroanat. 2017; 11: 14. doi: 10.3389/fnana.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014; 15: 431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bringmann A, Pannicke T, Grosche J, et al.. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006; 25: 397–424 [DOI] [PubMed] [Google Scholar]
- 38. Bringmann A, Wiedemann P. Müller glial cells in retinal disease. Ophthalmologica. 2012; 227: 1–19. [DOI] [PubMed] [Google Scholar]
- 39. Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013; 61: 651–78. [DOI] [PubMed] [Google Scholar]
- 40. Bringmann A, Iandiev I, Pannicke T, et al.. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009; 28: 423–451. [DOI] [PubMed] [Google Scholar]
- 41. Vogler S, Pannicke T, Hollborn M, et al.. Müller cell reactivity in response to photoreceptor degeneration in rats with defective polycystin-2. PLoS One. 2013; 8: e61631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003; 22: 607–655. [DOI] [PubMed] [Google Scholar]
- 43. Jones BW, Watt CB, Frederick JM, et al.. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol.2003; 464: 1–1. [DOI] [PubMed] [Google Scholar]
- 44. Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016; 150: 149–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfeiffer RL, Marc RE, Jones BW. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog Retin Eye Res. 2019; 19: 30030–30038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hippert C, Graca AB, Barber AC, et al.. Müller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS One. 2015; 10: e0120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Francisco-Morcillo J, Bejarano-Escobar R, Rodríguez-León J, Navascués J, Martín-Partido G. Ontogenetic cell death and phagocytosis in the visual system of vertebrates. Dev Dyn. 2014; 243: 1203–1225. doi: 10.1002/dvdy.24174. [DOI] [PubMed] [Google Scholar]
- 48. Sakami S, Imanishi Y, Palczewski K. Müller glia phagocytose dead photoreceptor cells in a mouse model of retinal degenerative disease. FASEB J. 2019; 33: 3680–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bejarano-Escobar R, Sánchez-Calderón H, Otero-Arenas J, Martín-Partido G, Francisco-Morcillo J. Müller glia and phagocytosis of cell debris in retinal tissue. J Anat. 2017; 231: 471–483. doi: 10.1111/joa.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silverman SM, Wong WT. Microglia in the retina: roles in development, maturity, and disease. Annu Rev Vis Sci. 2018; 4: 45–77. [DOI] [PubMed] [Google Scholar]
- 51. Sominsky L, De Luca S, Spencer SJ. Microglia: key players in neurodevelopment and neuronal plasticity. Int. J. Biochem. Cell Biol. 2018; 94: 56–60. [DOI] [PubMed] [Google Scholar]
- 52. Rashid K, Akhtar-Schaefer I, Langmann T. Microglia in retinal degeneration. Front Immunol. 2019; 10: 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007; 81: 1345–1351 [DOI] [PubMed] [Google Scholar]
- 54. Sobrado-Calvo P, Vidal-Sanz M, Villegas-Pérez MP. Rat retinal microglial cells under normal conditions, after optic nerve section, and after optic nerve section and intravitreal injection of trophic factors or macrophage inhibitory factor. J Comp Neurol. 2007; 501: 866–878. [DOI] [PubMed] [Google Scholar]
- 55. Galindo-Romero C, Valiente-Soriano FJ, Jiménez-López M, et al.. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Invest Ophthalmol Vis Sci. 2013; 54: 974–985 [DOI] [PubMed] [Google Scholar]
- 56. Di Pierdomenico J, García-Ayuso D, Jiménez-López M, et al.. Different ipsi- and contralateral glial responses to anti-VEGF and triamcinolone intravitreal injections in rats. Invest Ophthalmol Vis Sci. 2016; 57: 3533–3544. [DOI] [PubMed] [Google Scholar]
- 57. Di Pierdomenico J, Scholz R, Valiente-Soriano FJ, et al.. Neuroprotective dffects of FGF2 and minocycline in two animal models of inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2018; 59: 4392–4403. doi: 10.1167/iovs.18-24621. [DOI] [PubMed] [Google Scholar]
- 58. Salvador-Silva M, Vidal-Sanz M, Villegas-Pérez MP. Microglial cells in the retina of Carassius auratus: effects of optic nerve crush. J Comp Neurol. 2000. 21: 431–447. [DOI] [PubMed] [Google Scholar]
- 59. Valiente-Soriano FJ, Ortín-Martínez A, Di Pierdomenico J, et al.. Topical brimonidine or intravitreal BDNF, CNTF or bFGF protect cones against phototoxicity. Trans Vis Sci Tech. 2019; 8: 0 (forthcoming) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang M, Wong WT. Microglia-Müller cell interactions in the retina. Adv Exp Med Biol. 2014; 801: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Conedera FM, Pousa AMQ, Mercader N, Tschopp M, Enzmann V. Retinal microglia signaling affects Müller cell behavior in the zebrafish following laser injury induction. Glia. 2019; 67: 1150–1166. doi: 10.1002/glia.23601. [DOI] [PubMed] [Google Scholar]
- 62. Arroba AI1, Alvarez-Lindo N, van Rooijen N, de la Rosa EJ. Microglia-Müller glia crosstalk in the rd10 mouse model of retinitis pigmentosa. Adv Exp Med Biol. 2014; 801: 373–9. doi: 10.1007/978-1-4614-3209-8_47. [DOI] [PubMed] [Google Scholar]
- 63. García-Ayuso D, Salinas-Navarro M, Agudo M, et al.. Retinal ganglion cell numbers and delayed retinal ganglion cell death in the P23H rat retina. Exp Eye Res. 2010; 91: 800–810. [DOI] [PubMed] [Google Scholar]
- 64. Ortín-Martínez A, Jiménez-López M, Nadal-Nicolás FM, et al.. Automated quantification and topographical distribution of the whole population of S- and L-cones in adult albino and pigmented rats. Invest Ophthalmol Vis Sci. 2010; 5: 3171–3183. [DOI] [PubMed] [Google Scholar]
- 65. Vidal-Sanz M, Nadal-Nicolás FM, Valiente-Soriano FJ, Agudo-Barriuso M, Villegas-Pérez MP. Identifying specific RGC types may shed light on their idiosyncratic responses to neuroprotection. Neural Regen Res. 2015; 10: 1228–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ortín-Martínez A, Nadal-Nicolás FM, Jiménez-López M, et al.. Number and distribution of mouse retinal cone photoreceptors: differences between an albino (Swiss) and a pigmented (C57/BL6) strain. PLoS One. 2014; 9: e102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tanito M, Kaidzu S, Ohira A, Anderson RE. Topography of retinal damage in light-exposed albino rats. Exp Eye Res. 2008; 87: 292–295. [DOI] [PubMed] [Google Scholar]
- 68. Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol Vis. 2008; 14: 782–806. [PMC free article] [PubMed] [Google Scholar]
- 69. Jonas RA, Yuan TF, Liang YX, Jonas JB, Tay DK, Ellis-Behnke RG. The spider effect: morphological and orienting classification of microglia in response to stimuli in vivo. PLoS One. 2012; 7: e30763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nadal-Nicolás FM, Jiménez-López M, Salinas-Navarro M, Sobrado-Calvo P, Vidal-Sanz M, Agudo-Barriuso M. Microglial dynamics after axotomy-induced retinal ganglion cell death. J Neuroinflammation. 2017; 14: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010; 29: 113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006; 84: 4–15. [DOI] [PubMed] [Google Scholar]
- 73. Vicente-Tejedor J, Marchena M, Ramírez L, et al.. Removal of the blue component of light significantly decreases retinal damage after high intensity exposure. PLoS One. 2018; 13: e0194218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jaadane I, Boulenguez P, Chahory S, et al.. Retinal damage induced by commercial light emitting diodes (LEDs). Free Radic Biol Med. 2015; 84: 373–384. [DOI] [PubMed] [Google Scholar]
- 75. Hadj-Saïd W, Froger N, Ivkovic I, et al.. Quantitative and topographical analysis of the losses of cone photoreceptors and retinal ganglion cells under taurine depletion. Invest Ophthalmol Vis Sci. 2016; 57: 4692–4703. [DOI] [PubMed] [Google Scholar]
- 76. Xia X, Li Y, Huang D, Oncostatin M. Protects rod and cone photoreceptors and promotes regeneration of cone outer segment in a rat model of retinal degeneration. PLoS One. 2011; 6: e18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rapp LM, Williams TP. The role of ocular pigmentation in protecting against retinal light damage. Vision Res. 1980; 20: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 78. Williams TP, Howell WL. Action spectrum of retinal light damage in albino rats. Invest Ophthalmol Vis Sci. 1983; 24: 285–287. [PubMed] [Google Scholar]
- 79. Borges JM, Edward DP, Tso MO. A comparative study of photic injury in four inbred strains of albino rats. Curr Eye Res. 1990; 9: 799–803. [DOI] [PubMed] [Google Scholar]
- 80. Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM, Organisciak DT. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem Photobiol. 2002; 75: 547–553. [DOI] [PubMed] [Google Scholar]
- 81. Rutar M, Provis JM, Valter K. Brief exposure to damaging light causes focal recruitment of macrophages, and long-term destabilization of photoreceptors in the albino rat retina. Curr Eye Res. 2010; 35: 631–43. [DOI] [PubMed] [Google Scholar]
- 82. Kaitz M, Auerbach E. Effect of early and late light exposure on the inherited retinal degeneration in rats. Experimental Eye Research. 1978,26: 699–704. [DOI] [PubMed] [Google Scholar]
- 83. Organisciak DT, Li M, Darrow RM, Farber DB. Photoreceptor cell damage by light in young Royal College of Surgeons rats. Curr Eye Res. 1999; 19: 188–196. [DOI] [PubMed] [Google Scholar]
- 84. Salinas-Navarro M, Mayor-Torroglosa S, Jiménez-López M, et al.. A computerized analysis of the entire retinal ganglion cell population and its spatial distribution in adult rats. Vision Res. 2009; 49: 115–26. [DOI] [PubMed] [Google Scholar]
- 85. Fukuda Y. A three-group classification of rat retinal ganglion cells: histological and physiological studies. Brain Res. 1977; 119: 327–334. [DOI] [PubMed] [Google Scholar]
- 86. Battelle BA, LaVail MM. Rhodopsin content and rod outer segment length in albino rat eyes: modification by dark adaptation. Exp Eye Res. 1978; 26: 487–497. [DOI] [PubMed] [Google Scholar]
- 87. Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol. 2003; 28: 139–147 [DOI] [PubMed] [Google Scholar]
- 88. Léveillard T, Mohand-Saïd S, Lorentz O, et al.. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004; 36: 755–759. [DOI] [PubMed] [Google Scholar]
- 89. Ripps H. Cell death in retinitis pigmentosa: gap junctions and the ‘bystander’ effect. Exp Eye Res. 2002; 74: 327–336. [DOI] [PubMed] [Google Scholar]
- 90. Cronin T, Raffelsberger W, Lee-Rivera I, et al.. The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Differ. 2010; 17: 1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mei X, Chaffiol A, Kole C, et al.. The thioredoxin encoded by the rod-derived cone viability factor gene protects cone photoreceptors against oxidative stress. Antioxid Redox Signal. 2016; 24: 909–923. [DOI] [PubMed] [Google Scholar]
- 92. Bonilha VL, Rayborn ME, Bell BA, et al.. Histopathological comparison of eyes from patients with autosomal recessive retinitis pigmentosa caused by novel EYS mutations. Graefes Arch Clin Exp Ophthalmol. 2015; 253: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shelley EJ, Madigan MC, Riccardo R, Penfold PL, Provis JM. Cone degeneration in aging and age-related macular degeneration. Arch Ophthalmol. 2009; 127: 483–492. [DOI] [PubMed] [Google Scholar]
- 94. Lewis GP, Erickson PA, Anderson DH, Fisher SK. Opsin distribution and protein incorporation in photoreceptors after experimental retinal detachment. Exp Eye Res. 1991; 53: 629–640. [DOI] [PubMed] [Google Scholar]
- 95. Rathnasamy G, Foulds WS, Ling EA, Kaur C. Retinal microglia – a key player in healthy and diseased retina. Prog Neurobiol. 2019; 173: 18–40. [DOI] [PubMed] [Google Scholar]
- 96. Lu YZ, Natoli R, Madigan M, et al.. Photobiomodulation with 670 nm light ameliorates Müller cell-mediated activation of microglia and macrophages in retinal degeneration. Exp Eye Res. 2017; 165: 78–89. [DOI] [PubMed] [Google Scholar]
- 97. Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016; 51: 1–40 [DOI] [PubMed] [Google Scholar]
- 98. Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Tucker J, Marc RE. Retinal remodeling and metabolic alterations in human AMD. Front. Cell. Neurosci. 2016,10: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lewis GP, Fisher SK. Müller cell outgrowth after retinal detachment: Association with cone photoreceptors. Invest Ophthalmol Vis Sci. 2000; 41: 1542–1545. [PubMed] [Google Scholar]
- 100. Rutar M, Natoli R, Valter K, Provis JM. Early focal expression of the chemokine Ccl2 by Müller cells during exposure to damage-inducing bright continuous light. Invest Ophthalmol Vis Sci. 2011; 52: 2379–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rutar M, Natoli R, Chia RX, Valter K, Provis JM. Chemokine-mediated inflammation in the degenerating retina is coordinated by Müller cells, activated microglia, and retinal pigment epithelium. J Neuroinflammation. 2015; 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jones BW, Watt CB, Marc RE. Retinal remodelling. Clin Exp Optom. 2005; 88: 282–91. [DOI] [PubMed] [Google Scholar]
- 103. Ray A, Sun GJ, Chan L, Grzywacz NM, Weiland J, Lee EJ. Morphological alterations in retinal neurons in the S334ter-line3 transgenic rat. Cell Tissue Res. 2010; 339: 481–491. [DOI] [PubMed] [Google Scholar]
- 104. Villegas-Pérez MP, Lawrence JM, Vidal-Sanz M, et al.. Ganglion cell loss in RCS rat retina: a result of compression of axons by contracting intraretinal vessels linked to the pigment epithelium. J Comp Neurol. 1998; 392: 58–77 [PubMed] [Google Scholar]
- 105. Villegas-Pérez MP, Vidal-Sanz M, Lund RD. Mechanism of retinal ganglion cell loss in inherited retinal dystrophy. Neuroreport. 1996; 7: 1995–1999. [DOI] [PubMed] [Google Scholar]
- 106. Wang S, Villegas-Pérez MP, Holmes T, et al.. Evolving neurovascular relationships in the RCS rat with age. Curr Eye Res. 2003; 27: 183–96 [DOI] [PubMed] [Google Scholar]
- 107. García-Ayuso D, Salinas-Navarro M, Nadal-Nicolás FM, et al.. Sectorial loss of retinal ganglion cells in inherited photoreceptor degeneration is due to RGC death. Br J Ophthalmol. 2014; 98: 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Thanos S. Sick photoreceptors attract activated microglia from the ganglion cell layer: a model to study the inflammatory cascades in rats with inherited retinal dystrophy. Brain Res. 1992; 588: 21–28 [DOI] [PubMed] [Google Scholar]
- 109. Roque RS, Imperial CJ, Caldwell RB. Microglial cells invade the outer retina as photoreceptors degenerate in Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 1996; 37: 196–203 [PubMed] [Google Scholar]
- 110. Natoli R, Jiao H, Barnett NL, et al.. A model of progressive photo-oxidative degeneration and inflammation in the pigmented C57BL/6J mouse retina. Exp Eye Res. 2016; 147: 114–127. [DOI] [PubMed] [Google Scholar]
- 111. Roche SL, Ruiz-Lopez AM, Moloney JN, Byrne AM, Cotter TG. Microglial induced Müller cell gliosis is attenuated by progesterone in a mouse model of retinitis pigmentosa. Glia. 2018; 66: 295–310. [DOI] [PubMed] [Google Scholar]
- 112. Zhang S, Zhang S, Gong W, et al.. Müller cell regulated microglial activation and migration in rats with N-Methyl-N-Nitrosourea-induced retinal degeneration. Front Neurosci. 2018; 12: 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dharmarajan S, Fisk DL, Sorenson CM, Sheibani N, Belecky-Adams TL. Microglia activation is essential for BMP7- mediated retinal reactive gliosis. Journal of Neuroinflammation. 2017; 14: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang M, Ma W, Zhao L, Fariss RN, Wong WT. Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J Neuroinflammation. 2011; 8: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]