Abstract
Purpose
To evaluate the features of the choroidal structures in the eyes of myopic children obtained by enhanced depth imaging optical coherence tomography (EDI-OCT).
Methods
Ninety-six myopic children with low to moderate myopia (spherical equivalent refractive error [SER], –5.75 to –1.00 diopter) were included in this cross-sectional study. Ocular biometrics were measured using an optical low-coherence reflectometry device. Data of the choroidal structures extracted from a 7500-µm cross-sectional arc of the choroid extending from the temporal optic disc margin, including the total choroidal area, luminal area, stromal area, and choroidal vascularity index, were determined by image binarization of the EDI-OCT. Associations between demographic factors, ocular parameters, and choroidal structures were evaluated using univariate and multiple linear regression analyses.
Results
The study participants (mean age, 11.02 ± 1.70 years) had a mean axial length (AL) of 24.94 ± 0.70 mm. The mean total choroidal area was 2.64 ± 0.49 mm2 (luminal area, 1.68 ± 0.32 mm2; stromal area, 0.95 ± 0.19 mm2), and the choroidal vascularity index was 0.64 ± 0.03. Multiple regression analysis showed that the luminal area was significantly associated with the AL (standard β = –0.24, P = 0.022) after adjusting for sex and corneal radius (CR), whereas the stromal area (standard β = –0.30, P = 0.003) and choroidal vascularity index (standard β = 0.36, P = 0.001) were significantly associated with age after adjusting for sex, CR, and lens thickness (LT). Sex, CR, LT, and SER showed no significant association with choroidal structures after adjusting for age and AL (all P > 0.05).
Conclusions
The luminal area of the choroid tends to decrease with a longer AL, whereas the stromal area tends to decrease with increasing age in myopic children. These findings require further exploration in a longitudinal study.
Keywords: myopia, children, choroidal structure, binarization, EDI-OCT
Myopia is a prevalent public health issue,1 and the mechanism underlying the development and progression of myopia remains unclear. Many studies have suggested that the choroid might play an important role in the visually guided regulation of eye growth.2–5 Nevertheless, these studies have mainly measured the change in choroidal thickness in childhood because the use of spectral domain optical coherence tomography (OCT) and even swept-source OCT, which provide high penetration and resolution, do not allow direct differentiation between the stromal and luminal structures of the choroid. This finding raises the question of whether structural changes in the choroid contribute to myopia development.
Ocular biometrics, such as corneal radius (CR)6 and lens thickness (LT),7 as well as demographic factors, including age and sex,8 have been found to be associated with axial length (AL) elongation in childhood. In addition, numerous animal and human studies have revealed a significant association between AL and choroidal thickness. Eyes with a longer AL are more likely to have thinner choroids,4,9–12 but the components of the choroid that contribute to choroidal thinning during myopic development remain unknown. Therefore determining the association among demographic factors, ocular biometrics, and choroidal components of myopic children might enhance our understanding of the role of the choroid in regulating eye growth in childhood.
The advent of enhanced depth imaging OCT (EDI-OCT) allows more precise, noninvasive assessment of the choroid.13 Previous studies have attempted to assess the choroidal structures in healthy eyes and in eyes with different types of fundus diseases through EDI-OCT scans and the Niblack image binarization technique, and have demonstrated high reproducibility.14–18 Recently, this method was also applied in research on myopia subjects,19,20 but these studies mainly focused on the choroidal structure of myopic adults. Ruiz-Medrano et al.21 studied the structural features of the choroid in healthy children using the binarization method, but this research only reported age-related changes in choroidal components. The potential effect of the AL on the choroidal components of myopic children remains unknown.
The purpose of this study was to assess the choroidal structure of the eyes of myopic children, and analyze the associations among age, sex, CR, LT, AL, spherical equivalent refractive error (SER), and choroidal components by adapting the OCT image segmentation technique proposed by Sonoda et al.18
Methods
This cross-sectional study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the ethical committee of Zhongshan Ophthalmic Center, Sun Yat-sen University. Informed consent was obtained before the start of the study. The inclusion criteria included children between 8 and 15 years of age, low to moderate myopia (SER, –5.75 to –1.00 diopter [D]), with-the-rule astigmatism no greater than –1.50 D, and a corrected distance visual acuity of no worse than 20/25. Subjects with ocular or systemic conditions, use of any myopia-control modalities including rigid contact lenses, multifocal soft contact lenses, and atropine were excluded.
All measurements were conducted between 3 PM and 6 PM to minimize the potential influence of diurnal ocular variations on the results. Ocular biometrics, including CR, LT, and AL, were measured using an optical low-coherence reflectometry device (Lenstar LS 900; Haag Streit AG, Koeniz, Switzerland) after complete mydriasis (0.5% tropicamide plus 0.5% phenylephrine hydrochloride; Xing Qi Ophthalmic Co., Ltd, Shenyang, China). Five consecutive measurements were collected from each subject, and the values were averaged. Measurements, including manifest refraction and OCT scan, were performed twice after complete mydriasis. The OCT scan was performed using a Heidelberg Spectralis instrument (Spectralis HRA + OCT; Heidelberg Engineering, Heidelberg, Germany) with the EDI mode and linear scan pattern. Following the Spectralis user manual guidelines, each participant's keratometry readings and refraction were inputted into the Spectralis software prior to each measurement to account for optical magnification. Each image was obtained using the eye tracking system, which helped orientate and stabilize the OCT scan on the retina. The B-scans of each image included in this study had a quality index of at least 25 dB, and 100 B-scans were averaged to improve the signal-to-noise ratio. The follow-up mode in the Spectralis instrument was utilized to ensure that the same retinal locations were used in all the tests. A single horizontal line scan of 30° (9 mm) through the center of the fovea was used to analyze the subfoveal choroidal thickness and choroidal components. The subfoveal choroidal thickness, which was defined as the distance from the RPE to the chorioscleral interface passing through the center of the fovea, was manually analyzed by two independent observers experienced in analyzing OCT images using the Heidelberg linear measurement tool. The thinnest part of the macula in the image was defined as the location of the center of the fovea.
Image Analysis
When two graders determined that the choroidal structure was distinguishable, the corresponding OCT image was regarded as acceptable and used for subsequent analysis. According to the Sonoda et al.18 study, the sampling of a small area tended to increase the variability in the luminal/stromal area ratio; thus a large 7500-µm wide area of the single horizontal line scan was chosen to determine the binarization of the choroidal area. The nasal margin was determined as the edge of the optic nerve head, and the temporal margin was 7500 µm temporal from the edge of the optic nerve head.
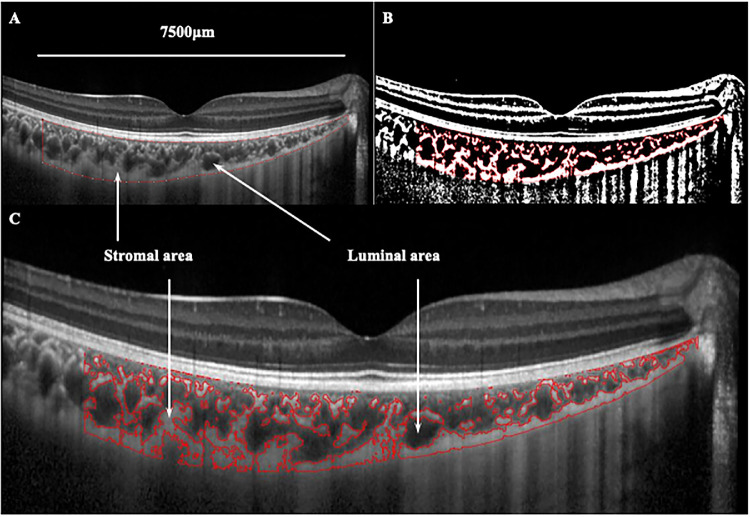
Binarization of the choroidal area in the OCT images was performed by two graders according to a modified Niblack method using ImageJ software version 1.47 (National Institutes of Health, Bethesda, MD, USA). Representative results are shown in Figure 1. Briefly, the portion of the choroidal area that extended vertically from the RPE to the chorioscleral border was determined manually with the ImageJ ROI manager. Three choroidal vessels with lumens larger than 100 µm were then randomly selected using the Oval Selection Tool on the tool bar, and the reflectivity of these areas was determined. The average brightness of the luminal area was set as the minimum value to minimize the noise in the OCT image. The image was then converted to 8 bits and adjusted using the Niblack Auto Local Threshold. The binarized image was converted to an RGB image again, and the luminal area was determined using the Threshold Tool. After adjusting the Set Scale parameters using the information on the conversion factor given by the OCT device individually for each subject, the total choroidal area, luminal area, and stromal area were automatically calculated. The choroidal vascularity index was calculated by dividing the luminal area by the total choroidal area.
Figure 1.
Binarization analysis of choroidal structure from OCT images. (A) Determination of the choroidal region in the original EDI-OCT image with the ImageJ ROI manager. (B) Identification of the choroidal segments using the binarization technique. (C) Overlay of the target choroidal region created by image binarization on the EDI-OCT image.
Data Analyses
Only data from the right eyes were used for the statistical analyses using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). All values were represented as mean ± SD, unless otherwise stated. The normality of the data were first tested using the Kolmogorov-Smirnov test. In the current study, age, AL, CR, LT, SER, subfoveal choroidal thickness, total choroidal area, luminal area, stromal area, and choroidal vascularity index, which showed normal distribution, were used for the statistical analyses. The interobserver agreement of the subfoveal choroidal thickness, cross-sectional total choroidal area, luminal area, and stromal area were assessed by calculating the interclass correlation coefficient and 95% limits of agreement (LOAs). Associations of the choroidal components and subfoveal choroidal thickness with age, sex, AL, CR, LT, and SER were determined through both univariate and multiple linear regression analyses. In this study, age was calculated as the date of examinations minus the date of birth and rounded up as integers. A two-sided P value less than 0.05 was considered statistically significant.
Results
A total of 96 children (57 girls and 39 boys) with a mean age of 11.02 ± 1.70 years were enrolled in the current study. All subjects showed low to moderate myopia with a mean AL of 24.94 ± 0.70 mm. The mean CR was 7.81 ± 0.22 mm, with a small difference between the steep and flat meridians (7.91 ± 0.23 mm vs. 7.71 ± 0.22 mm, respectively; mean difference, 0.20 ± 0.09 mm). The mean LT was 3.31 ± 0.15 mm. The mean subfoveal choroidal thickness was 244 ± 51 µm, and the mean cross-sectional total choroidal area was 2.64 ± 0.49 mm2. The mean luminal and stromal areas were 1.68 ± 0.32 mm2 and 0.95 ± 0.19 mm2, respectively, which yielded a mean choroidal vascularity index of 0.64 ± 0.03. The demographic and choroidal characteristics of the enrolled participants are shown in the Table.
Table.
Demographic and Choroidal Characteristics of all the Participants (n = 96; 57 girls, 39 boys)
| Characteristics | Mean ± SD | Range |
|---|---|---|
| Age (y) | 11.02 ± 1.70 | 8 to 15 |
| Sphere (D) | –2.84 ± 0.99 | –5.25 to –0.75 |
| Cylinder (D) | –0.54 ± 0.46 | –1.50 to 0.00 |
| SER (D) | –3.11 ± 1.05 | –5.75 to –1.00 |
| CR (mm) | 7.81 ± 0.22 | 7.44 to 8.44 |
| LT (mm) | 3.31 ± 0.15 | 3.01 to 3.63 |
| AL (mm) | 24.94 ± 0.70 | 23.19 to 27.06 |
| Choroidal parameters | ||
| Subfoveal choroidal thickness (µm) | 244 ± 51 | 145 to 417 |
| Total choroidal area (mm2) | 2.64 ± 0.49 | 1.53 to 4.02 |
| LA (mm2) | 1.68 ± 0.32 | 0.94 to 2.65 |
| Stromal area (mm2) | 0.95 ± 0.19 | 0.49 to 1.37 |
| Choroidal vascularity index (LA/TCA) | 0.64 ± 0.03 | 0.59 to 0.72 |
LA, luminal area; TCA, total choroidal area.
The interrater agreements of the measurements on the OCT images were reasonably high for all the choroidal parameters. The 95% LOAs of the interrater difference for the subfoveal choroidal thickness, cross-sectional total choroidal area, luminal area, and stromal areas were 0 ± 8 µm, –0.02 ± 0.16 mm2, 0.00 ± 0.12 mm2, and –0.01 ± 0.10 mm2, respectively. The mean interclass correlation coefficients for the subfoveal choroidal thickness, cross-sectional total choroidal area, luminal area, and stromal area were 0.997 (confidence interval [CI], 0.996–0.998), 0.986 (CI, 0.979–0.991), 0.984 (CI, 0.977–0.990), and 0.961 (CI, 0.942–0.974), respectively.
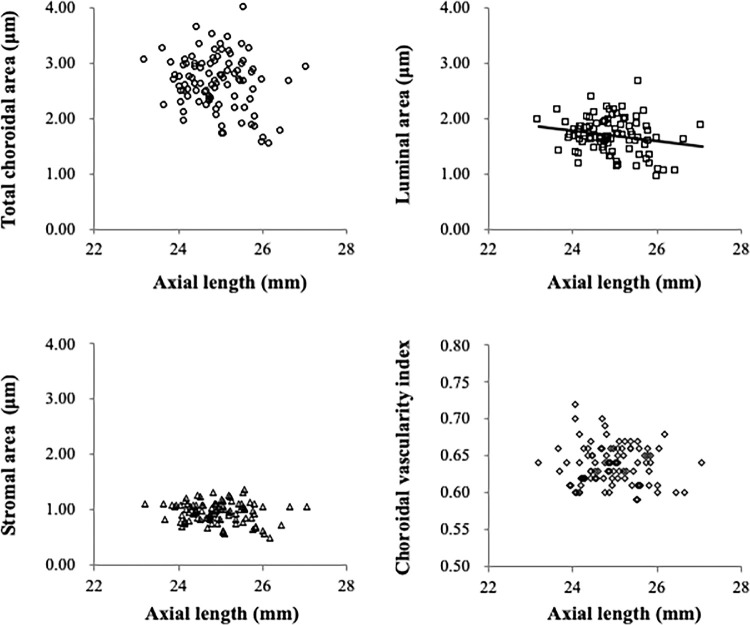
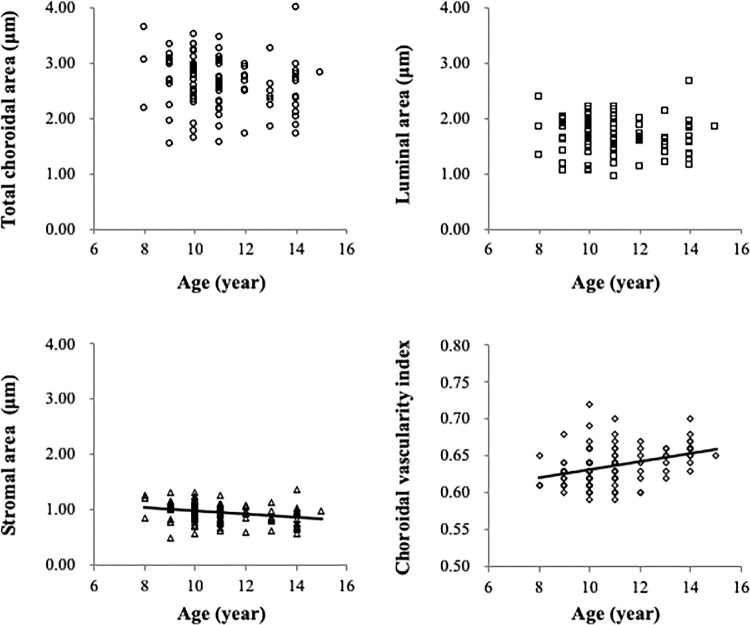
The AL was found to be significantly associated with luminal area (R = –0.20, P = 0.047) but not the total choroidal area (R = –0.19, P = 0.060), stromal area (R = –0.15, P = 0.134), or choroidal vascularity index (R = –0.07, P = 0.480) (Fig. 2). Age was found to be significantly associated with the stromal area (R = –0.30, P = 0.003) and choroidal vascularity index (R = 0.36, P < 0.001), whereas no significant association between age and either total choroidal area (R = –0.18, P = 0.077) or luminal area (R = –0.10, P = 0.319) was found (Fig. 3). Additionally, there was no significant association between SER and any choroidal parameter, and also no sex-related differences (all P > 0.05). Both AL (R = –0.30, P = 0.003) and CR (R = –0.20, P = 0.049) were significantly associated with the subfoveal choroidal thickness.
Figure 2.
Relationship between the AL and components of the choroid determined through univariate analyses. The luminal area was borderline significantly associated with the AL (R = –0.20, P = 0.047) (top right), whereas the total choroidal area (top left), stromal area (bottom left), and choroidal vascularity index (bottom right) exhibited no significant association with the AL (all P > 0.05).
Figure 3.
Relationship between age and components of the choroid determined through univariate analyses. The total choroidal area showed a borderline association with age (R = –0.18, P = 0.077) (top left). Age was significantly associated with stromal area (R = –0.30, P = 0.003) (bottom left) but exhibited no significant association with the luminal area (R = –0.10, P = 0.319) (top right), which revealed a significant association between age and the choroidal vascularity index (R = 0.36, P < 0.001) (bottom right).
The multiple linear regression models included only independent factors because a number of the variables used in the univariate analyses were not independent, for example, LT and AL, SER and AL, and age and AL, which might have artificially inflated the variance in the estimated regression coefficients. Based on the results of univariate analyses, three stepwise linear regression models were established to test the relationship between the luminal area and AL, the stromal area and age, and the choroidal vascularity index and age after adjusting for sex, LT, or CR, respectively. Model 1 showed that the luminal area was significantly correlated to the AL (standard β: –0.20, P = 0.047) after adjusting for sex (P = 0.112) and CR (P = 0.562). In contrast, for the stromal area, age was the main contributing factor (standard β: –0.30, P = 0.003) after adjusting for CR (P = 0.135), LT (P = 0.064), and sex (P = 0.213) in the model 2. The results of the model 3 showed that age exhibited a significant correlation with the choroidal vascularity index (standard β: 0.36, P < 0.001) after adjusting for CR (P = 0.892), LT (P = 0.256), and sex (P = 0.801). Only the AL showed a significant correlation with the subfoveal choroidal thickness (standard β: –0.30, P = 0.003) after adjusting for sex (P = 0.186) and CR (P = 0.758) in the stepwise linear regression analysis.
Discussion
Our knowledge of the choroid in vivo has been significantly improved by adoption of the binarization technique, which allows differentiation of the luminal and stromal components of the choroid in OCT images of the same. Through the acquisition of more detailed information on the choroidal structure using an image binarization technique modified from that developed by Sonoda et al.18, our results demonstrated that an eye with a longer AL, as observed in children with moderate myopia, was more likely to exhibit a thinner subfoveal choroid with a decreased luminal area. Additionally, choroidal stromal area decreased with age, likely underlying the observed increase in the choroidal vascularity index with advancing age in myopic children. The current study, to our knowledge, provides the first description of the structural features of the choroid and the factors influencing them in Chinese children with low to moderate myopia.
Previous studies9–12 have reported a significant association between the AL and choroidal thickness, and association between the choroidal thickness and choroidal components in adults have been described with the help of binarization techniques applied to EDI-OCT images.18–20 Compared with healthy emmetropic adults,19 subfoveal choroidal thinning in myopic adults with longer ALs was found to be associated with a reduction in stromal area, but not of vascular components. However, as reported in the Ruiz-Medrano et al.21 study, the choroidal cross-sectional area, vascular area, and choroidal vascularity index showed significant differences between children and adults. Based on these studies, the structural characteristics of the choroid might show differences between myopic children and myopic adults. In the current study, a thinner subfoveal choroidal thickness was found to be significantly associated with a longer AL but not other factors, such as age, sex, CR, LT and SER, in myopic children. A longer AL also tended to be associated with a smaller choroidal luminal area. Changes in luminal areas may directly influence choroidal thickness, as blood vessels represent the main component of the choroid. Studies using chicks have shown that myopia could result in smaller vessel diameters, lower blood vessel densities,22,23 and a reduced blood flow in the choroid,24 thus the effect of myopia on vascular components might further contribute to the decrease in choroidal thickness observed in the current study. Stromal area, which also contributes to choroidal thickness, showed no significant association with AL in the current study. Taken together, these findings suggest for myopic children that reductions in blood flow and/or blood vessel area may contribute to the subfoveal choroidal thinning observed in eyes with longer ALs.
Interestingly, several effective strategies for inhibiting myopia development, including bright light,25 atropine,26 and orthokeratology lenses,27,28 were found to increase the choroidal thickness. Choroidal thickening under bright light26 and atropine29 were speculated to be related to the increasing release of nitric oxide. Our previous study found that an increase in the large choroidal vascular layer accounted for most of the choroidal thickness thickening in orthokeratology subjects.28 It appears that the increase in the luminal area, which can cause choroidal thickening, might be a helpful signal for inhibiting myopia development.
Age is another important factor for choroidal thickness and choroidal structures. According to previous studies in adults, the choroidal thickness tended to decrease with increasing age.10,11 As reported by Fujiwara et al.,30 choroidal vascular density had negative association with age in healthy subjects aged older than 30 years. Furthermore, Sohn et al.31 found that the amount of fibrillar collagen and cellular components of the choroid decreased with increasing age in eyes from donors aged older than 50 years. It seems that both luminal and stromal components of the choroid have significant association with age in older adults, but the luminal area was reported to exhibit a greater decrease than the stromal area, which explained the reduction in the choroidal vascularity index with increasing age.18 The mean total choroidal area, luminal area, and stromal area of the children examined in the current study were greater than those found in adults in the Sonoda et al.18 study (total choroidal area, 2.45 ± 0.52 mm2 vs. 1.84 ± 0.68 mm2; luminal area, 1.60 ± 0.36 mm2 vs. 1.21 ± 0.48 mm2; stromal area, 0.85 ± 0.18 mm2 vs. 0.63 ± 0.20 mm2). Nevertheless, the association between choroidal thickness and age could be influenced by the speed of axial elongation in children, which differs from that in adults. Read et al.32 investigated longitudinal changes in the choroidal thickness and AL for children and found that choroidal thickness increased in children with normal axial elongation, whereas children undergoing faster AL elongation tended to exhibit less thickening and, in some cases, a thinning of the choroid. This finding might partly explain the lack of significant association between the choroidal thickness and age found in the current study. In contrast to normal adults, the stromal area tended to show a larger decrease than the luminal area in our study, reflected in the increased choroidal vascularity index with increasing age in myopic children. These findings indicate that the choroids of adults and children are structurally different although the underlying mechanism remains unknown. A prospective longitudinal study investigating the changes in choroidal components with increasing age during childhood is warranted.
Previous studies about the effect of sex on choroidal thickness have yielded inconsistent findings. Barteselli et al.33 and Li et al.34 found that the choroidal thickness in men was significantly thicker than that in women, whereas Ruiz-Medrano et al.35 reported no such significant difference. In the current study, no significant differences in either demographic data or choroidal components were found between boys and girls. A previous study indicated that the choroidal thickness significantly increases during puberty even though the eye is undergoing axial elongation.36 The peak age of girls entering puberty has been reported to be approximately 11 to 12 years of age, approximately 2 years earlier than for boys.37,38 As children aged 8 to 15 years were enrolled in the current study, both prepubertal children and children who had already entered puberty would have been included, based on the earlier described criteria. However, as the stage of puberty was not established for individual children in the current study, no specific comments on the effect of puberty can be offered. Therefore studies of children in which both sexes have entered or completed puberty are still necessary to determine the relationship between sex and choroidal components.
There were several other issues in the current study. First, Ruiz-Medrano et al.21 studied the vascular density of the choroid in a healthy population that included normal children through swept-source OCT. In their study, stromal area was not affected by age, whereas in our study, stromal area was found to decrease significantly with increasing age. The reason for this discrepancy in the findings still needs further investigation, but it should be noted that the SER range in the Ruiz-Medrano et al.21 study (±3.00 D) was different from that in Sonoda et al. (from –6.00 to +3.50 D) and our study (from –5.75 to –1.00 D). Furthermore, we did not compare the mean choroid area to that found in the Ruiz-Medrano et al.21 study, which evaluated choroidal components in both normal children and adults, because the B-scan length was different (9 mm in Ruiz-Medrano et al. study vs. 7.5 mm in our study). Second, because the vascular index calculated from a single foveal scan could be considered a good surrogate and a complimentary tool to choroidal thickness for assessment of the choroidal status as suggested by Agrawal et al.,39 we did not further evaluate the choroidal vascular index in different regions. Nevertheless, investigating the choroidal structure in topographical manner is recommended in the future because the choroidal thickness, which has been suggested to be a predictor of the choroidal vascular index in the normal population,40 exhibited an uneven spatial distribution in the macular region.41 Third, the lumens of the choriocapillaris, which appears as dark areas in the binary EDI-OCT images, could not be separately analyzed because of the limitations of the technology. Therefore combining a binarization technique with angio-OCT would allow simultaneous evaluation of both the choriocapillaris density and vascular area. Finally, even though each subject's keratometry readings and refraction were inputted into the Spectralis software before imaging the choroid to estimate the optical magnification, our method did not consider the effect of AL on optical magnification42 because the Spectralis OCT does not allow the use of the AL as an input parameter. This limitation should be considered when interpreting the present data.
Conclusions
The current study characterized structural features of the choroid in myopic children, which was different from the results reported in myopic adults. The luminal area of the choroid tends to decrease with a longer AL that is associated with a thinner subfoveal choroid, whereas the stromal area tends to decrease with increasing age in children with moderate myopia. The technique used to evaluate the choroids of myopic children might be applied in future research into myopia mechanisms and interventions.
Acknowledgments
Supported by a grant from the Science and Technology Program of Guangzhou, China (Grant no. 201803010111).
Disclosure: Z. Li, None; W. Long, None; Y. Hu, None; W. Zhao, None; W. Zhang, None; X. Yang, None
References
- 1. Rudnicka AR, Kapetanakis VV, Wathern AK, et al.. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2016; 100: 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakraborty R, Read SA, Collins MJ.. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res. 2012; 103: 47–54. [DOI] [PubMed] [Google Scholar]
- 3. Chakraborty R, Read SA, Collins MJ.. Hyperopic defocus and diurnal changes in human choroid and axial length. Optom Vis Sci. 2013; 90: 1187–1198. [DOI] [PubMed] [Google Scholar]
- 4. Nickla DL, Wallman J.. The multifunctional choroid. Prog Retin Eye Res. 2010; 29: 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiang ST, Phillips JR, Backhouse S.. Effect of retinal image defocus on the thickness of the human choroid. Ophthalmic Physiol Opt. 2015; 35: 405–413. [DOI] [PubMed] [Google Scholar]
- 6. Scheiman M, Gwiazda J, Zhang Q, et al.. Longitudinal changes in corneal curvature and its relationship to axial length in the Correction of Myopia Evaluation Trial (COMET) cohort. J Optom. 2016; 9: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mutti DO, Mitchell GL, Sinnott LT, et al.. Corneal and crystalline lens dimensions before and after myopia onset. Optom Vis Sci. 2012; 89: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hou W, Norton TT, Hyman L, Gwiazda J.. Axial elongation in myopic children and its association with myopia progression in the Correction of Myopia Evaluation Trial. Eye Contact Lens. 2018; 44: 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Margolis R, Spaide RF.. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147: 811–815. [DOI] [PubMed] [Google Scholar]
- 10. Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y.. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010; 51: 2173–2176. [DOI] [PubMed] [Google Scholar]
- 11. M Kim, Kim SS, Koh HJ, Lee SC. Choroidal thickness, age, and refractive error in healthy Korean subjects. Optom Vis Sci. 2014; 91: 491–496. [DOI] [PubMed] [Google Scholar]
- 12. El-Shazly AA, Farweez YA, ElSebaay ME, WMA E.. Correlation between choroidal thickness and degree of myopia assessed with enhanced depth imaging optical coherence tomography. Eur J Ophthalmol. 2017; 27: 577–584. [DOI] [PubMed] [Google Scholar]
- 13. Ikuno Y, Tano Y.. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009; 50: 3876–3880. [DOI] [PubMed] [Google Scholar]
- 14. Sonoda S, Sakamoto T, Yamashita T, et al.. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. 2014; 55: 3893–3899. [DOI] [PubMed] [Google Scholar]
- 15. Branchini LA, Adhi M, Regatieri CV, et al.. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013; 120: 1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bakthavatsalam M, Ng DS, Lai FH, et al.. Choroidal structures in polypoidal choroidal vasculopathy, neovascular age-related maculopathy, and healthy eyes determined by binarization of swept source optical coherence tomographic images. Graefes Arch Clin Exp Ophthalmol. 2017; 255: 935–943. [DOI] [PubMed] [Google Scholar]
- 17. Okamoto M, Yamashita M, Ogata N.. Effects of intravitreal injection of ranibizumab on choroidal structure and blood flow in eyes with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2018; 256: 885–892. [DOI] [PubMed] [Google Scholar]
- 18. Sonoda S, Sakamoto T, Yamashita T, et al.. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 2015; 159: 1123–1131.e1. [DOI] [PubMed] [Google Scholar]
- 19. Alshareef RA, Khuthaila MK, Goud A, Vupparaboina KK, Jana S, Chhablani J.. Subfoveal choroidal vascularity in myopia: evidence from spectral-domain optical coherence tomography. Ophthalmic Surg Lasers Imaging Retina. 2017; 48: 202–207. [DOI] [PubMed] [Google Scholar]
- 20. Gupta P, Thakku SG, Saw SM, et al.. Characterization of choroidal morphologic and vascular features in young men with high myopia using spectral-domain optical coherence tomography. Am J Ophthalmol. 2017; 177: 27–33. [DOI] [PubMed] [Google Scholar]
- 21. Ruiz-Medrano J, Ruiz-Moreno JM, Goud A, Vupparaboina KK, Jana S, Chhablani J. Age-related changes in choroidal vascular density of healthy subjects based on image binarization of swept-source optical coherence tomography. Retina. 2018; 38: 508–515. [DOI] [PubMed] [Google Scholar]
- 22. Hirata A, Negi A.. Morphological changes of choriocapillaris in experimentally induced chick myopia. Graefes Arch Clin Exp Ophthalmol. 1998; 236: 132–137. [DOI] [PubMed] [Google Scholar]
- 23. Junghans BM, Crewther SG, Liang H, Crewther DP.. A role for choroidal lymphatics during recovery from form deprivation myopia. Optom Vis Sci. 1999; 76: 796–803. [DOI] [PubMed] [Google Scholar]
- 24. Shih YF, Fitzgerald ME, Norton TT, Gamlin PD, Hodos W, Reiner A.. Reduction in choroidal blood flow occurs in chicks wearing goggles that induce eye growth toward myopia. Curr Eye Res. 1993; 12: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lan W, Feldkaemper M, Schaeffel F.. Bright light induces choroidal thickening in chickens. Optom Vis Sci. 2013; 90: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Z, Zhou Y, Xie Z, et al.. The effect of topical atropine on the choroidal thickness of healthy children. Sci Rep. 2016; 6: 34936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Z, Xue F, Zhou J, Qu X, Zhou X.. Effects of orthokeratology on choroidal thickness and axial length. Optom Vis Sci. 2016; 93: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 28. Li Z, Cui D, Hu Y, Ao S, Zeng J, Yang X.. Choroidal thickness and axial length changes in myopic children treated with orthokeratology. Cont Lens Anterior Eye. 2017; 40: 417–423. [DOI] [PubMed] [Google Scholar]
- 29. Ayajiki K, Tanaka T, Okamura T, Toda N.. Evidence for nitroxidergic innervation in monkey ophthalmic arteries in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2000; 279: H2006–H2012. [DOI] [PubMed] [Google Scholar]
- 30. Fujiwara A, Morizane Y, Hosokawa M, et al.. Factors affecting choroidal vascular density in normal eyes: quantification using en face swept-source optical coherence tomography. Am J Ophthalmol. 2016; 170: 1–9. [DOI] [PubMed] [Google Scholar]
- 31. Sohn EH, Khanna A, Tucker BA, Abràmoff MD, Stone EM, Mullins RF.. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci. 2014; 55: 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Read SA, Alonso-Caneiro D, Vincent SJ, Collins MJ.. Longitudinal changes in choroidal thickness and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015; 56: 3103–3112. [DOI] [PubMed] [Google Scholar]
- 33. Barteselli G, Chhablani J, El-Emam S, et al.. Choroidal volume variations with age, axial length, and sex in healthy subjects: a three-dimensional analysis. Ophthalmology. 2012; 119: 2572–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li XQ, Larsen M, Munch IC.. Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Invest Ophthalmol Vis Sci. 2011; 52: 8438–8441. [DOI] [PubMed] [Google Scholar]
- 35. Ruiz-Medrano J, Flores-Moreno I, Peña-García P, Montero JA, Duker JS, Ruiz-Moreno JM.. Macular choroidal thickness profile in a healthy population measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2014; 55: 3532–3542. [DOI] [PubMed] [Google Scholar]
- 36. Li XQ, Jeppesen P, Larsen M, Munch IC.. Subfoveal choroidal thickness in 1323 children aged 11 to 12 years and association with puberty: the Copenhagen Child Cohort 2000 Eye Study. Invest Ophthalmol Vis Sci. 2014; 55: 550–555. [DOI] [PubMed] [Google Scholar]
- 37. Liu YX, Wikland KA, Karlberg J.. New reference for the age at childhood onset of growth and secular trend in the timing of puberty in Swedish. Acta Paediatr. 2000; 89: 637–643. [DOI] [PubMed] [Google Scholar]
- 38. Yip VC, Pan CW, Lin XY, et al.. The relationship between growth spurts and myopia in Singapore children. Invest Ophthalmol Vis Sci. 2012; 53: 7961–7966. [DOI] [PubMed] [Google Scholar]
- 39. Agrawal R, Wei X, Goud A, Vupparaboina KK, Jana S, Chhablani J.. Influence of scanning area on choroidal vascularity index measurement using optical coherence tomography. Acta Ophthalmol. 2017; 95: e770–e775. [DOI] [PubMed] [Google Scholar]
- 40. Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY.. Choroidal vascularity index as a measure of vascular status of the choroid: measurements in healthy eyes from a population-based study. Sci Rep. 2016; 6: 21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ouyang Y, Heussen FM, Mokwa N, et al.. Spatial distribution of posterior pole choroidal thickness by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 7019–7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garway-Heath DF, Rudnicka AR, Lowe T, Foster PJ, Fitzke FW, Hitchings RA.. Measurement of optic disc size: equivalence of methods to correct for ocular magnification. Br J Ophthalmol. 1998; 82: 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]