Abstract
The suprachiasmatic nucleus (SCN) of the hypothalamus consists of a highly heterogeneous neuronal population networked together to allow precise and robust circadian timekeeping in mammals. While the critical importance of SCN neurons in regulating circadian rhythms has been extensively studied, the roles of SCN astrocytes in circadian system function are not well understood. Recent experiments have demonstrated that SCN astrocytes are circadian oscillators with the same functional clock genes as SCN neurons. Astrocytes generate rhythmic outputs that are thought to modulate neuronal activity through pre- and post-synaptic interactions. In this study, we developed an in silico multicellular model of the SCN clock to investigate the impact of astrocytes in modulating neuronal activity and affecting key clock properties such as circadian rhythmicity, period and synchronization. The model predicted that astrocytes could alter the rhythmic activity of neurons via bidirectional interactions at tripartite synapses. Specifically, astrocyte-regulated extracellular glutamate was predicted to increase neuropeptide signaling from neurons. Consistent with experimental results, we found the astrocytes could increase the circadian period and enhance neural synchronization according to their endogenous circadian period. The impact of astrocytic modulation of circadian rhythm amplitude, period, and synchronization was predicted to be strongest when astrocytes had periods between 0–2 h longer than neurons. Increasing the number of neurons coupled to the astrocyte also increased its impact on period modulation and synchrony. These computational results suggest that signals which modulate astrocytic rhythms or signaling (e.g., as a function of season, age or treatment) could cause circadian rhythm disruptions or serve as putative therapeutic targets.
Keywords: Astrocytes, Neuronal Coupling, Suprachiasmatic Nucleus, Circadian Rhythms, Mathematical Modeling
INTRODUCTION
The suprachiasmatic nucleus (SCN) in mammals consists of approximately 20,000 neurons that act as circadian oscillators and about one third as many astrocytes that have a poorly understood role in circadian system function (Silver, 2018). Neurons generating rhythms in gene expression and firing frequency are coupled together for robustness and synchronized to establish 24-hour circadian rhythms for regulating various physiological and behavioral processes (Belle and Diekman, 2018; Hastings et al., 2014; Welsh et al., 2010). Impairment of the circadian clock has been implicated in numerous disorders including sleep problems, mental illness, and metabolic diseases (Foster and Kreitzman, 2014; Liu et al., 2007). Neuronal coupling is mediated by neurotransmitters triggered by action potentials and released from presynaptic neurons (Allen et al., 2017; Colwell, 2011; Herzog et al., 2017). The primary coupling mechanism involves the neurotransmitter vasoactive intestinal polypeptide (VIP), which is necessary and sufficient for synchronization of the heterogeneous neural population (Aton et al., 2005; Herzog et al., 2017; Vosko et al., 2007) despite its wide range of endogenous periods (Aton et al., 2005; Honma, 2018). The role of other SCN neurotransmitters including γ-aminobutyric acid (GABA) and glutamate in circadian system function is less well understood (Albers et al., 2017; Chi-Castañeda and Ortega, 2018; Evans, 2016; Herzog et al., 2017; Ono et al., 2018).
Astrocytes are ubiquitous throughout the mammalian brain and have been shown to interact with neurons via spatial proximity and gliotransmission at synapses to regulate brain network function, behavior, and plasticity (Araque et al., 1999; Halassa et al., 2007; Volterra and Meldolesi, 2005). SCN astrocytes are rhythmically associated with neurons through intercellular coupling agents including VIP and arginine-vasopressin (AVP) (Becquet et al., 2008; Marpegan et al., 2009). Several recent studies have shown the crucial role of SCN astrocytes in tuning the SCN neuronal clock, contributing to circadian rhythms, and influencing neuronal physiology (Belle and Allen, 2018; Ruben and Hogenesch, 2017). The loss of astrocytic Bmal1 in the SCN lengthened circadian period and locomotor behavior (Tso et al., 2017), while deletion of astrocytic Bmal1 throughout the brain altered daily behavioral rhythms and GABA levels in the brain (Barca-Mayo et al., 2017). When SCN neurons expressed one copy of the tau mutation of CK1ε (which shortens their circadian period) and astrocytes did not express CK1ε (which lengthen their circadian period) (Tso et al., 2017), the circadian period of the SCN and locomotor activity was lengthened.
Inhibition of GABA transporters (GATs) expressed in SCN astrocytes resulted in an elevated tonic GABAA receptor-mediated current and affected the periodicity of Per1 expression in SCN neurons (Moldavan et al., 2017). Astrocytes also have been shown to synaptically communicate with and tune SCN neurons through glutamatergic transmission (Brancaccio et al., 2017; Scofield, 2018). Under constant darkness, the rhythmic release of glutamate via excitatory amino acid transporters (EAATs) enabled astrocytes to maintain higher intracellular calcium levels in presynaptic neurons through glutamate receptor activation, which in turn produced elevated inhibitory GABAergic tone in the dorsal SCN (Brancaccio et al., 2017, 2019). Although these experimental studies demonstrated that astrocytes affect circadian behavior, the individual contributions associated with GABA (Barca-Mayo et al., 2017), glutamate (Brancaccio et al., 2017, 2019) and VIP (Marpegan et al., 2009) are poorly understood.
Mathematical modeling has proven to be a powerful complementary tool to experimentation for understanding the roles of the molecular clock, electrophysiology, cell-to-cell coupling and neural network topology on circadian behavior (Bernard et al., 2007; DeWoskin et al., 2015; Hafner et al., 2012; Kingsbury et al., 2016; Vasalou et al., 2009, 2011; Vasalou and Henson, 2011). To our knowledge, existing models are restricted to neuronal behavior and do not directly include the possible modulatory effects of SCN astrocytes, which are also self-sustained oscillators. Although there was a proposed model of distinct multi-oscillatory units in the circadian system, the model indirectly introduced the concept of neutral elements that did not exhibit self-sustained oscillation and were presumably possible glial cells in such a system (Diez-Noguera, 1994). Also, some astrocyte models have been developed for other brain regions such as the cortex and hippocampus (De Pittà et al., 2012; Manninen et al., 2018; Oschmann et al., 2018). These models span single astrocytes, the neuron-astrocyte tripartite synapse, and larger neuron-astrocyte networks but are not directly applicable to the SCN. The development of SCN-specific neuron-astrocyte network models has the potential to provide mechanistic underpinnings for observed experimental behavior, expand our understanding of circadian timekeeping, and provide experimentally testable hypotheses about astrocytic modulation of neuronal networks.
The goal of this in silico study was to develop a simplified but biophysically-based multicellular model of neuronal-astrocytic SCN networks and to utilize the model to gain better understanding of the synaptic communication mechanisms that may have driven circadian behavior observed in several recent experimental studies (Barca-Mayo et al., 2017; Brancaccio et al., 2017, 2019; Ruben and Hogenesch, 2017; Tso et al., 2017). Our computational model was based on complex bidirectional communication between glial and neuronal cells reported in other brain regions (De Pittà et al., 2012; Manninen et al., 2018; Oschmann et al., 2018) specialized to the SCN to probe potential influences of astrocytes on the neuronal clock (Barca-Mayo et al., 2017; Brancaccio et al., 2017, 2019; Moldavan et al., 2015; Tso et al., 2017). By developing a novel model of an SCN astrocyte, we applied the concept of a tripartite synapse consisting of one astrocyte and two (pre-and post-synaptic) neurons (Vasalou et al., 2011; Vasalou and Henson, 2010) to construct an integrated SCN network model. The model accounted for VIP, GABAergic, and glutamatergic signaling between a single astrocyte and a heterogeneous population of 100 neurons. We focused our computational studies on unraveling the effects of neuronal and astrocytic endogenous periods, bidirectional neuronal-astrocytic signaling, intercellular coupling strength, and neural network topology on circadian rhythmicity, synchronization, and period of the neural population.
MATERIAL AND METHODS
1. SCN neuronal-astrocytic network model
The SCN network model was based on the hypothesis that astrocytes alter neuronal dynamics via bidirectional interactions mediated through the actions of VIP, GABA, and glutamate at tripartite synapses. The integrated model consisted of a neuronal model based on our previous studies (Vasalou et al., 2011; Vasalou and Henson, 2010) and an astrocytic model specifically developed for this study (Fig. 1A). The single-neuron model consisted of three interconnected modules (gene regulation, electrophysiology, and neurotransmitter signaling) following our previous modeling studies.
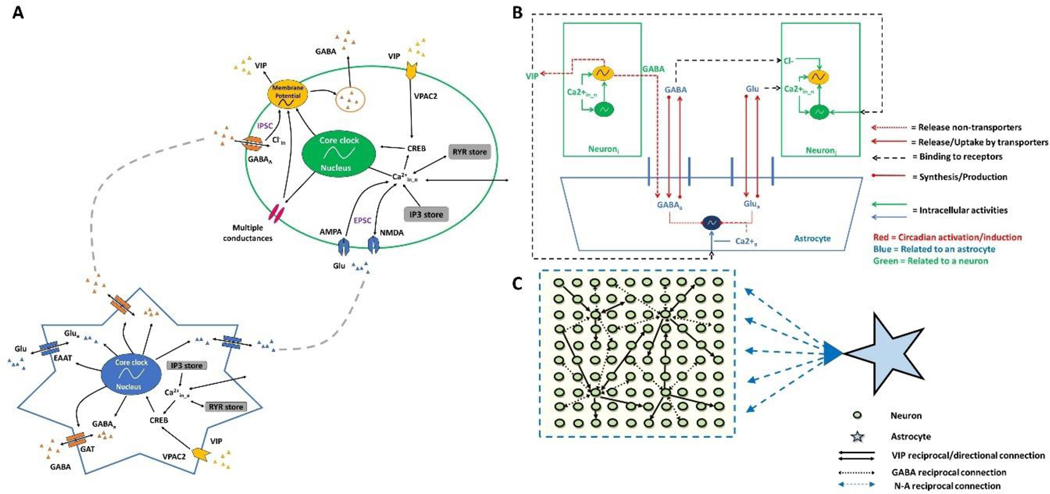
Figure 1. In silico SCN model architecture and neuron-astrocyte interactions.
(A) Schematic representation of the coupled neural and astrocytic oscillators. The neuron model (oval) was modified from (Vasalou and Henson, 2010), whereas we developed the astrocyte model (star) in this study. The astrocyte model included core clock gene regulation, intracellular pathways (e.g., calcium and CREB), GABA (GATs), glutamate (EAATs) transporters, and VIP receptors. (B) Schematic representation of the tripartite synapse model with cell-to-cell communication of both neurons and the astrocyte at synapses mediated through multiple intercellular signaling pathways (VIP, GABA, and glutamate). (C) Schematic representation of the SCN network model with 100 heterogeneous neurons synaptically interacting with a single astrocyte. Neuron-to-neuron connectivity followed a small-world network topology (Vasalou et al., 2009), while astrocyte-to-neuron connectivity followed a mean-field network topology. Note: Inositol 1,4,5-trisphosphate (IP3) and Ryanodine (RYR) are different calcium stores; Glu is glutamate; CREB is a cellular transcription factor; AMPA and NMDA are glutamate receptors; IPSC and EPSC are inhibitory and excitatory postsynaptic currents, respectively.
The astrocyte model retained the same gene regulation module since core clock genes identified in SCN neurons are preserved in SCN astrocytes (Brancaccio et al., 2017; Tso et al., 2017), while the neurotransmitter signaling module was modified as detailed below and the electrophysiology module was eliminated since astrocytes do not exhibit firing behavior. Instead, intracellular calcium represented a proxy for astrocytic excitability (Dallérac et al., 2013). We modeled calcium dynamics and internal calcium pools (i.e., IP3 and ryanodine stores) (Mesiti et al., 2015; Vasalou and Henson, 2010) and included VPAC2 receptors (Masmoudi-Kouki et al., 2007) for binding VIP released by SCN neurons (Marpegan et al., 2009). The astrocyte model also included GABA transporters which potentially regulate GABA concentrations in synaptic and extra-synaptic regions (Moldavan et al., 2015, 2017) as well as glutamate transporters for glutamate uptake and release (Brancaccio et al., 2017, 2019). The astrocyte was treated as a GABAergic and glutamatergic cell (Brancaccio et al., 2017; Schousboe et al., 2013; Yoon et al., 2012) that produced glutamate and stored GABA with circadian variations. Hence, our single astrocyte model included core clock gene regulation, intracellular calcium signaling dynamics, glutamate/GABA synthesis, release and uptake, and VIP binding.
To construct the SCN network model, we applied the concept of the tripartite synapse (Perea et al., 2009; Pérez-Alvarez and Araque, 2013) where an astrocyte can modulate communication between pre-and post-synaptic neurons (Fig. 1B). The tripartite synapse was scaled up to the network level by allowing a heterogeneous population of 100 neurons to be modulated by a single astrocyte (Fig. 1C). The assumption of a single astrocyte in the network was based on knowledge that astrocytes are highly interconnected through gap junctions, essentially sharing their intracellular content (Shinohara et al., 2000), that a single astrocyte can modulate as many as 100,000 synapses (Halassa et al., 2007), and that a neuron is unlikely to be contacted by more than one astrocyte as astrocytes typically form non-overlapping domains (Sofroniew and Vinters, 2010). The astrocyte was assumed to modulate all neuron-to-neuron connections in the simulated network. Following our previous work (Vasalou et al., 2009, 2011), the neuronal network was constructed by placing an ensemble of 100 heterogeneous neurons on an equally-spaced, two-dimensional grid. Approximately 20% of the neurons were randomly selected to synthesize VIP, while all neurons were GABAergic (Vasalou et al., 2009, 2011). Because GABA is commonly recognized as the major inhibitory neurotransmitter in the SCN (Albers et al., 2017), we assumed that GABA signaling was strictly inhibitory following previous modeling studies (e.g., (DeWoskin et al., 2015; Vasalou et al., 2011)). Hence, our study did not explore the effects of excitatory GABA (Ono et al., 2018) as a function of intracellular chloride levels (Klett and Allen, 2017; Myung et al., 2015), time-of-day (Alamilla et al., 2014; Albus et al., 2005; Choi et al., 2008; De Jeu and Pennartz, 2002; Wagner et al., 1997), SCN region (Alamilla et al., 2014; Myung et al., 2015) or light stimulation (Farajnia et al., 2014; Myung et al., 2015). Individual neurons were coupled according to small-world network connectivity (Vasalou et al., 2009, 2011). In this work, 100 heterogeneous neurons were used to construct the small-world network that required a low density of long-range shortcut connections added to the local connections. We did not attempt to differentiate between the dorsal and ventral SCN or account for the effects of light entrainment on circadian behavior. A more complete description of the combined neuronal-astrocytic network including the model equations and parameters is contained in the Supplementary Material.
2. Computational studies and simulation analysis
All simulations were performed within MATLAB by adapting code developed in our previous modeling studies (Vasalou et al., 2009, 2011). To account for heterogeneity among the SCN neurons, we assigned each neuron model randomly perturbed values of the Bmal1 mRNA transcription and degradation rates and the Per mRNA transcription rate as described elsewhere (Vasalou et al., 2011). These random perturbations yielded an uncoupled network with approximately 25% sustained rhythmic neurons with endogenous oscillation periods ranging between 22 and 26 hours as experimentally observed (Aton et al., 2005). The single astrocyte was connected to all 100 neurons, while the neurons were interconnected according to a small-world topology following our previous approach (Vasalou et al., 2009, 2011) by randomly assigning 5% of possible long-range connections. Because each simulation contained a different neural population and neural network topology, we performed five independent simulations for each scenario (see below) and reported mean values along with the standard error of the mean (SEM).
In addition to performing simulations with wild-type SCN cells, we studied the effects of neuronal and astrocytic mutations on circadian behavior to mimic experimental studies (Brancaccio et al., 2017; Tso et al., 2017) (Fig. 2A). These mutations either varied the endogenous cell period or eliminated cell rhythmicity completely through Bmal1 knockout. The endogenous period was changed experimentally by genetic manipulations targeting posttranslational feedback loops. The tau mutation in casein kinase I (the kinase that phosphorylates PER proteins) accelerates the dynamics of circadian timekeeping; therefore the CKI tau mutation was used experimentally to shorten the endogenous cell period (Maywood et al., 2014; Meng et al., 2008; Takahashi, 2016). By contrast, the loss of F-box/leucine-rich-repeat protein or FBXL3 (the ubiquitin ligase for the CRY proteins) (Godinho et al., 2007; Siepka et al., 2007) stabilizes CRY 1 and 2 and increases the endogenous cell period (Maywood et al., 2014). These mutations were implemented in silico by tuning parameters associated with the CKI-PER (k1 and vdPC) and FBXL3-CRY (v3PC and vdCC) pathways (Leloup and Goldbeter, 2003; St John et al., 2014) to achieve shorter or longer endogenous periods (Table S2). To implement Bmal1 knockout (Bmal1−/− ) in astrocytes, we set the Bmal1 mRNA synthesis rate (vsB) to zero and removed the Michaelis-Menten terms governing the maximum rates of GABA/glutamate synthesis and transportation driven by the astrocytic clock genes (Equations 1–4 in the Supplementary Material) such that those rates were purely constitutive (Leloup and Goldbeter, 2003; Mirsky et al., 2009).
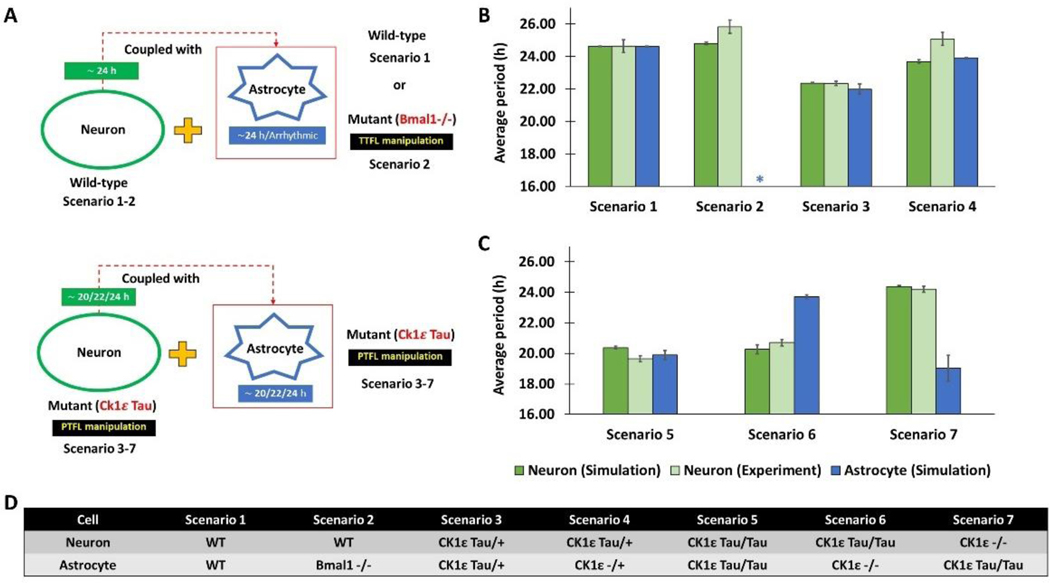
Figure 2. Comparison of neuronal periods from in vitro experiments and in silico simulations for seven different cell combinations.
(A) Schematic diagram showing seven simulation scenarios that mimic recent experiments on SCN astrocytes We compared the average period (Mean±SEM) of neuronal Per mRNA from the model simulations with the neuronal PER2 from experimental observations reported in (Tso et al., 2017) (B) and (Brancaccio et al., 2017) (C). We also presented the simulated periods of the astrocyte. The seven simulation scenarios involve different genetic manipulations that altered the endogenous periods of the astrocyte and the neuronal population as presented in (D). Notes: TTFL = Transcription-Translation Feedback Loop; PTFL= Posttranslational feedback loop; WT=Wild-type (about 24-h period). Asterisk (*) = arrhythmic (no period). Remark: CK1ε Tau/Tau or CK1ε Tau/+ cells have about 20-h and 22-h endogenous periods, respectively, while CK1ε−/− or CK1ε−/+ cells have an about 24-h endogenous period (Meng et al., 2008).
Simulations were performed by initiating cell-to-cell coupling after about seven cycles (t >= 150 h) and running for 25 total cycles (600 h) to assess circadian behavior. The oscillatory behavior of the Per mRNA rhythms was assessed by period, phase and amplitude following the methods presented elsewhere (To et al., 2007; Vasalou et al., 2011; Vasalou and Henson, 2010). We also calculated measures of phase synchrony and amplitude coherence of the Per mRNA signal over a specified period using the synchronization index (SI) (Strogatz, 2000) and order parameter (R) (Gonze et al., 2005), respectively. Both measures yielded values between zero (no coordination) and one (perfect coordination).
RESULTS
1. The astrocyte-neuron network model predicts genotype-dependent circadian periods
We performed simulations to mimic recent experiments aimed at deciphering the role of SCN astrocytes in circadian timekeeping (Brancaccio et al., 2017; Tso et al., 2017). These experiments have shown, counterintuitively, that SCN astrocytes with mutations in the Bmal1 or CK1ε genes (mutations that are presumed to abolish or lengthen circadian rhythms, respectively), produced a similar increase in period of the SCN and locomotor behavior (Tso et al., 2017). When we simulated circadian networks with different endogenous neuronal and astrocytic periods, we found that the circadian period of the astrocytes affects rhythms in the neurons.
We performed simulations for seven distinct scenarios which differed according to the endogenous periods of the astrocyte and the neuronal population (Fig. 2A and 2D). Wild-type neurons were connected to a wild-type astrocyte (Scenario 1) or an astrocyte rendered arrhythmic through Bmal1 knockout (Scenario 2). As observed experimentally for Scenario 1, the model predicted a small increase in the average period of the coupled neurons compared to their endogenous 24-h period (Fig. 2B). When the astrocyte was arrhythmic, the model correctly predicted a small neuronal period increase compared to Scenario 1 (Fig. 2B). Next, we coupled mutant CK1ε Tau/+ neurons with a 22-h period to mutant CK1ε Tau/+ astrocytes with a 22-h period (Scenario 3) or CK1ε −/+ astrocytes with a 24-h period (Scenario 4). The neuronal period was correctly predicted to be 22-h for Scenario 3 and was predicted to be close to the 24-h period of the astrocyte for Scenario 4, while the experimentally determined period for this scenario was ~25-h (Fig. 2B).
To test the effects of even shorter circadian periods in astrocytes and neurons (Fig. 2C), we combined CK1ε Tau/Tau neurons with a 20-h period with either a CK1ε Tau/Tau astrocyte with a 20-h period (Scenario 5) or a CK1ε −/− astrocyte with a 24-h period (Scenario 6). As observed experimentally, both these two scenarios were predicted to generate neuronal periods of ~20-h, although the actual period was slightly higher for Scenario 6 when the Tau mutation was removed from the astrocyte. Finally, CK1ε −/− neurons with a 24-h period were coupled with CK1ε Tau/Tau astrocytes with a 20-h period (Scenario 7), which produced a predicted neuronal period of 24-h in agreement with experiment. Collectively these model predictions suggested that astrocytic rhythmicity was not necessary for neuronal rhythmicity but that the coupled neuronal period was dependent on the endogenous periods of both neurons and astrocytes.
2. Astrocytes can entrain neurons when their endogenous periods are sufficiently close
We sought to determine if differences between the endogenous periods of neurons and astrocytes could be used to predict how astrocytes affect daily rhythms in the SCN. We performed 16 distinct simulations containing cells with endogenous periods ranging from 20-h to 26-h by modifying the parameters associated with PER/CRY stability (Leloup and Goldbeter, 2008; Maywood et al., 2011; St John et al., 2014) (Fig. 3A; see Materials and Method section and Table S2).
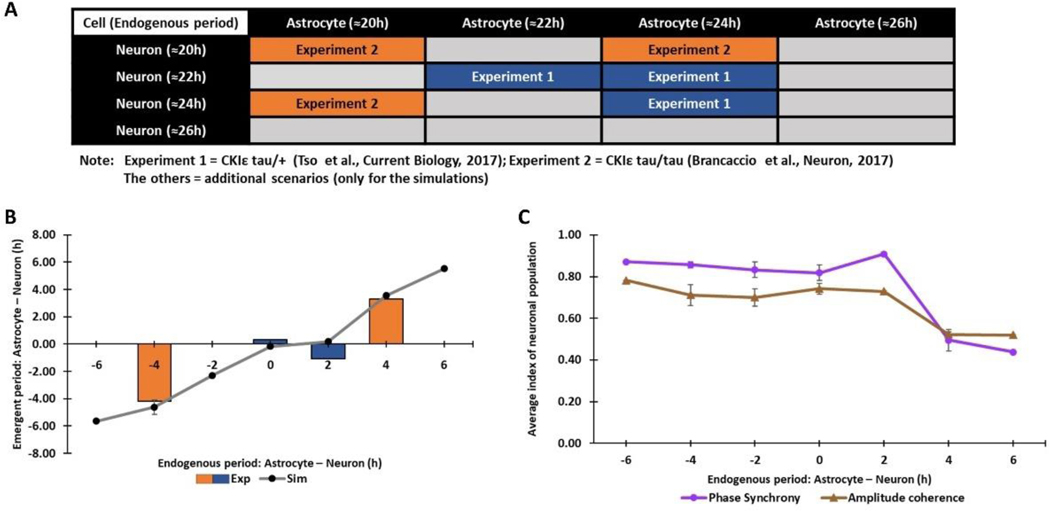
Figure 3. Astrocytic control of the neuronal period depends on the difference between the endogenous neuronal and astrocytic periods.
(A) 16 simulation scenarios performed to investigate the effect of endogenous periods on the coupled neuronal period. Six scenarios could be compared to available data (Brancaccio et al., 2017; Tso et al., 2017), while the other ten scenarios were constructed using endogenous periods reported in other studies (Maywood et al., 2011; Patton et al., 2016; St John et al., 2014). (B) The difference between the endogenous astrocytic and neuronal periods (x-axis) plotted against the difference between the coupled neuronal and astrocytic periods (y-axis) for all 16 simulation scenarios and the six scenarios for which comparable data was available (Brancaccio et al., 2017; Tso et al., 2017). (C) Neuronal phase synchrony and amplitude coherence measures (Mean±SEM) predicted for the 16 simulation scenarios plotted versus the difference between the endogenous astrocytic and neuronal periods.
Our model predicted that differences between the endogenous periods and not the individual periods themselves were the key determinant as to whether the coupled neurons were entrained by the astrocyte (Fig. 3B). We found that the neurons produced an average period close to the astrocytic period when (1) the endogenous astrocytic period was greater than or equal to the endogenous neuronal period; and (2) the difference between the two endogenous periods was approximately 2-h or less. These two conditions were satisfied by seven of the 16 simulations that produced endogenous period differences of 0-h or +2-h in (Fig. 3A and 3B). For the other nine scenarios, the astrocyte was not able to entrain the neurons, and the coupled period of the neuronal population was close to its endogenous period. Thus, our model accurately simulated experimental results implicating astrocytes in modulating the circadian period of the SCN. Furthermore, the model generated experimentally testable predictions that, when both SCN neurons and astrocytes are circadian, their phase synchrony and amplitude coherence will substantially decrease when (1) the endogenous astrocytic period is greater than the endogenous neuronal period; and (2) the difference between the two endogenous periods is greater than two hours (Fig. 3C).
3. Astrocytic modulation of the neuronal population depends on the density of astrocyte-neuron connections
To investigate the putative impact of network structure on astrocytic modulation of neuronal rhythms, we performed additional simulations with wild-type cells in which neuron-to-neuron and astrocyte-to-neuron connectivities were varied. Two types of neuronal networks were considered: small-world (SW) networks as used in Fig. 2 and a nearest-neighbor network formulated as in our previous study (Vasalou et al., 2009). Six types of astrocyte-to-neuron networks were constructed by randomly allowing different percentages (0%, 5%, 25%, 50%, 75%, 100%) of all possible connections, with a mean-field connectivity of 100% used in Fig. 2. Finally, two different period distributions of the uncoupled neuronal population were constructed by varying random perturbation of the Bmal1 transcription rate (vsB) and degradation rate (vmB) (Vasalou et al., 2011). A 2% standard deviation produced relatively narrow distributions (PD1; also used in Fig. 2) with uncoupled periods of 22–26 h, while a larger 3% standard deviation generated a broader distribution (PD2) with periods of 21–29 h. The average number of neuron-to-neuron connections and the total number of neuron-astrocyte connections for all 24 network structures are shown in Fig. S1.
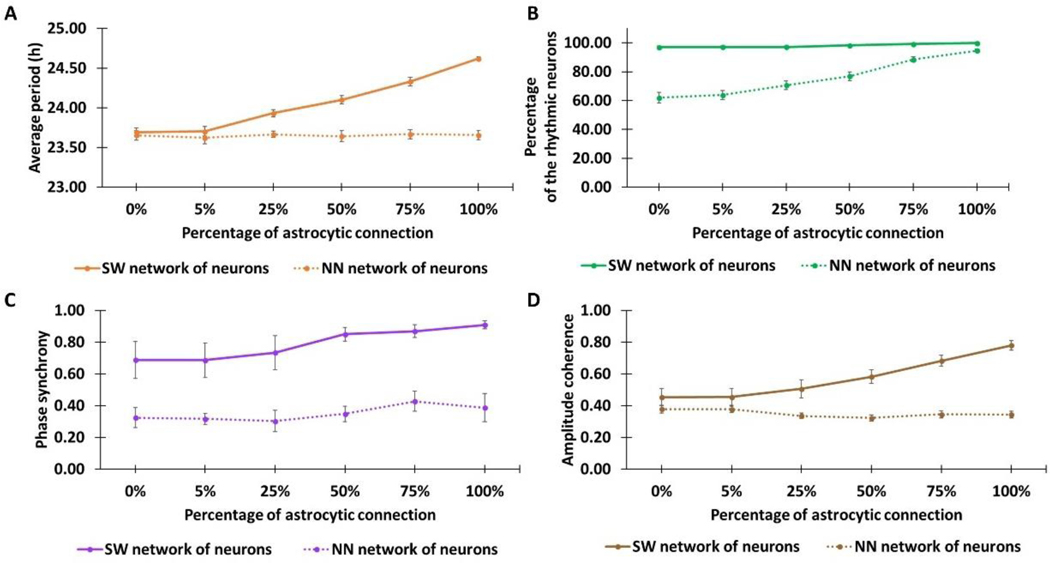
Our model predicted that the extent of astrocytic modulation depended on the density of neuron-to-neurons and astrocyte-to-neuron connections as well as the degree of neuronal heterogeneity. As the percentage of astrocyte-to-neuron connections was increased from 0% to 100% with the narrow period distribution PD1, the neuronal period was predicted to increase ~1-h for the SW network topologies (Fig. 4A). The NN topologies did not exhibit a similar period increase, but the percentage of rhythmic neurons increased substantially as the percentage of astrocyte-to-neuron connections was increased (Fig. 4B). By contrast, astrocytic modulation was not required to achieve high rhythmicity in the SW networks. Phase synchrony (Fig. 4C) and amplitude coherence (Fig. 4D) of the neuronal population was increased in the SW networks as astrocyte-to-neuron connectivity was increased, while the NN networks exhibited low synchrony and coherence independent of astrocytic modulation. While similar predictions were obtained when simulations were performed with the broader period distribution PD2 (Fig. S2), astrocytic modulation generally had less impact on cell-to-cell coordination in terms of phase and amplitude. Unlike the increased period for the SW network, the neuronal period was predicted to decrease ~0.5-h for the NN network topologies as the astrocyte-to-neuron connectivity was increased. Uncoupled period distributions that are either narrow (PD1) or broad (PD2) and coupled period distributions for the 24 network structures are shown in Fig. S3. Collectively, our predictions suggested that astrocytic modulation of the neuronal population within a small-world network depended strongly on the density of astrocyte-neuron connections but only weakly on the heterogeneity of endogenous neuronal periods.
Figure 4. Astrocytic modulation of the neuronal population for different network topologies.
SCN models with two types of neuronal networks (small-world, SW and nearest neighbor, NN (Vasalou et al., 2009)) and six types of astrocyte-to-neuron networks containing different percentages of all possible connections (0%, 5%, 25%, 50%, 75%, and 100%) were constructed and simulated. Four emergent system properties were calculated for each model (Mean±SEM): (A) average period of the neuronal population; (B) percentage of rhythmic neurons; (C) neuronal phase synchrony; and (D) neuronal amplitude coherence. Notes: The simulations were based on narrow neuronal period distribution before coupling (≈ 22–26 h). Simulation results for a broader neuronal period distribution are shown in Fig. S2.
4. Astrocytic modulation of neuronal populations depends on mutual contributions of the multiple intercellular signaling pathways
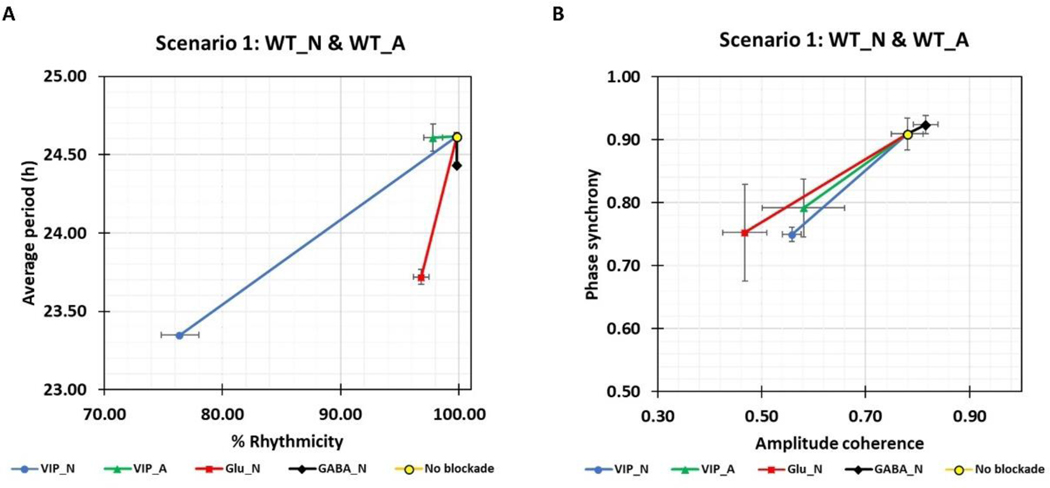
To explore the importance of intercellular signaling pathways on astrocytic modulation, we performed simulations with individual VIP, glutamate and GABA pathways blocked. More specifically, we generated five types of models containing either wild-type cells (no blockade), neurons unable to bind VIP (VIP_N), astrocytes unable to bind VIP (VIP_A), neurons unable to bind glutamate (Glu_N) or neurons unable to bind GABA (GABA_N). For each model type, simulations were performed with cells having no mutations and the six experimentally-realized neuron/astrocyte mutations explored in Fig. 2, generating 35 distinct scenarios.
Simulation results for the five models containing neurons and astrocytes without mutations are shown in Fig. 5. The loss of VIP coupling between neurons was predicted to cause relatively large decreases in the average period and rhythmicity of the neuronal population (Fig. 5A) consistent with experimental results (Hastings et al., 2014). Interestingly, the loss of astrocyte-released glutamate that only binds to the respective receptors in neurons resulted in a decrease of ~1-h in the average period but had little effect on rhythmicity. The blockade of VIP signaling to the astrocyte or GABA signaling to the neurons had little effect on period or rhythmicity. Phase synchrony and amplitude coherence of the neuronal populations were affected similarly by loss of VIP or glutamate signaling to neurons or VIP signaling to the astrocyte (Fig. 5B). Neither phase synchrony nor amplitude coherence was affected by the loss of GABA signaling in neurons. For WT cells (Scenario 1), the model predicted that neuronal VIP and glutamate receptors play dominant roles for SCN neurons-astrocyte interaction at a synapse.
Figure 5. Astrocytic modulation of the neuronal population with different intercellular signaling pathways removed.
SCN models containing wild-type cells (no blockade), neurons unable to bind VIP (VIP_N), astrocytes unable to bind VIP (VIP_A), neurons unable to bind glutamate (Glu_N) or neurons unable to bind GABA (GABA_N) were constructed and simulated. (A) Average neuronal period (Mean±SEM) and percentage of rhythmic neurons (Mean±SEM). (B) Phase synchrony (Mean±SEM) and amplitude coherence (Mean±SEM) of the neuronal population. Note: simulation results for six experimentally-realized neuron/astrocyte mutations (Scenario 2–7 presented in Fig 2.) are shown in Fig. S4.
Corresponding simulation results for the six experimentally-realized neuron/astrocyte mutations (Fig. S4) revealed several trends regarding the mutual effect of endogenous periods and intercellular coupling on astrocytic modulation. An astrocyte with shorter endogenous periods (e.g., about 20 or 22 h) was predicted to generate lower percentages of rhythmic VIP-blockade neurons (Fig. S4B, S4G, and S4I) than the WT astrocyte with a 24-h endogenous period (Fig. 5A). Interestingly, the absence of VIP that binds to neuronal receptors allowed neuronal period modulation by an astrocyte with 4h higher endogenous period. To illustrate, when VIP-blockade neurons with relatively short endogenous periods ~20-h were completely coupled with a wild-type astrocyte with endogenous period ~24-h, the neuronal period was reset towards the astrocytic period (Fig. S4H), and the neuronal population exhibited higher phase-amplitude synchrony (Fig. S4K). By contrast, the blockade of neuron-released VIP that binds to the astrocytic receptors had little impact on neuronal period and rhythmicity (Fig. 5A, S4A-C and S4G-I) but strongly affected phase synchrony and amplitude coherence of the neuronal population, especially when the endogenous period of the astrocyte was ~24-h (Fig. 5B, S4F and S4K).
Glutamate and GABA signaling were also predicted to affect the ability of the astrocyte to entrain the neuronal population. Glutamate blockade consistently resulted in period shortening, reduced rhythmicity, and less phase and amplitude coordination in the neuronal population despite the presence of the astrocyte. GABA blockade of neurons caused the neuronal population to exhibit more variable period resetting that depended on the endogenous neuronal and astrocytic periods. Such GABA blockade slightly rescued desynchronization of the neuronal population when the endogenous neuronal and astrocytic periods were similar (Fig. 5B, S4E, and S4J). Taken together, these model predictions suggest that astrocytic modulation of neuronal behavior is dependent on both endogenous period and intercellular signaling; however, the activation of neuronal VIP and glutamate receptors likely has more impact on the overall neuronal rhythms and astrocytic modulation than GABA receptors.
5. Astrocytes have tunable mechanisms primarily mediated by VIP and glutamate for modulating SCN neuronal period
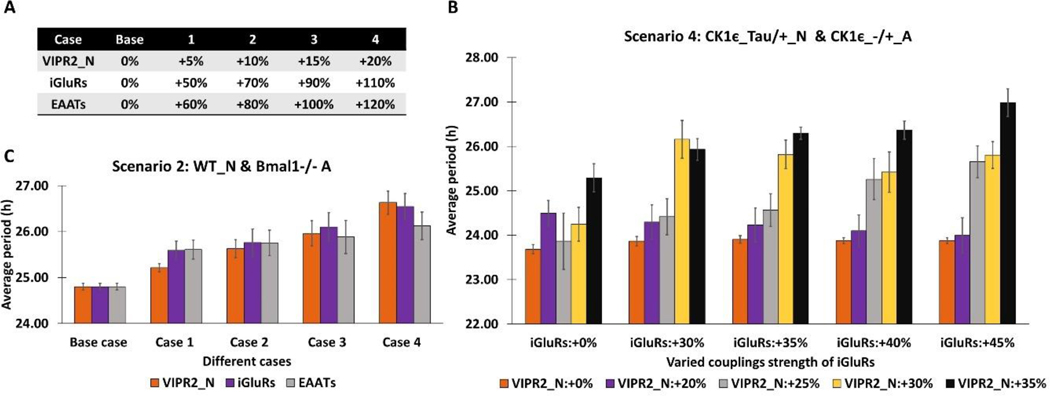
We investigated the possible roles of signaling to and from astrocytes on SCN circadian rhythms beyond the effect of genetically-manipulated neurons and astrocytes (especially for Scenario 2 and 4 in Fig. 2) to better understand neuronal period modulation (Tso et al., 2017). According to our previous computational findings related to the dominant signaling agents (Fig, 5 and S4), we selectively varied coupling strengths of neuronal VIP or glutamate receptors as well as mechanistic rates related to astrocytic glutamate signaling to resimulate Scenario 2 and 4 in addition to modifying parameters associated with astrocytic Bmal1 for arrhythmicity or PER/CRY stability for different endogenous periods. For example, we increased the degree of coupling strengths (the extent of receptor saturation modeled in To et al., 2007; Vasalou and Henson, 2010, 2011) associated with either neuronal VIP (VIPR2_N) or neuronal glutamate (iGluRs) (Fig. 6A). We also specifically enhanced the glutamate synthesis/transport rate regulated by the astrocyte (EAATs) from its nominal values used in this work (Fig. 6A). In the absence of astrocytic rhythmicity (Scenario 2, Fig, 6C), our model predicted that these three model parameters could be independently tuned to yield similar neuronal period increases including the ~1-h increase observed experimentally (Tso et al., 2017) when Bmal1−/− astrocytes were combined with WT neurons (Fig. 6A). Simultaneous increase of VIP and glutamate receptor strengths in networks with CK1ε−/+ astrocytes and CK1ε Tau/+ neurons (Scenario 4, Fig. 6B) could also produce large neuronal period increases, including the ~2-h increase observed experimentally (Tso et al., 2017). Thus, it is possible that loss of Bmal1 or heterozygous CK1ε Tau mutation of astrocytes may indirectly contribute to enhanced VIP and glutamate signaling activity towards the lengthened neuronal period. Collectively, these model predictions suggest that the astrocytes may have tunable mechanisms potentially mediated by VIP (Marpegan et al., 2009) and glutamate (Brancaccio et al., 2017) for modulating SCN neuronal period.
Figure 6. Effect of increased network coupling strengths on the average period of the neuronal population.
Model parameters for network coupling strengths associated with neuronal VIP (VIPR2_N) and glutamate (iGluRs) receptors and the glutamate synthesis/transport rate regulated by the astrocyte (EAATs) were increased from their nominal values. (A) Percentage increases in each parameter used to perform simulations of Scenario 2 (Wild-type neurons and Bmal1−/− astrocytes). (B) Period increases (Mean±SEM) of the simulated neuronal populations for Scenario 2. (C) Period increases (Mean±SEM) of the simulated neuronal populations for Scenario 4 (CK1ε Tau/+ neurons and CK1ε−/+ astrocytes) and simultaneous increases of VIP and glutamate receptor strengths.
DISCUSSION
Our multicellular model was able to qualitatively recapitulate recent experimental findings (Brancaccio et al., 2017; Tso et al., 2017) demonstrating that astrocytic rhythmicity can alter daily rhythms in the SCN and behavior (Fig. 2). Model simulations with wild-type and mutant cells helped rationalize the contexts under which astrocytes were dominant and reset the average period of the coupled neurons away from their endogenous periods and specifically towards the endogenous astrocytic period. Based on these simulations, we concluded that astrocytes were able to entrain neuronal populations when the endogenous astrocytic period was greater than or equal to the endogenous neuronal period and the difference between the two endogenous periods was approximately 2-h or less (Fig. 3). Our model predictions were generated over a wider range of neuronal and astrocytic endogenous periods than experimentally studied to date (Brancaccio et al., 2017; Tso et al., 2017) and could be additionally evaluated using established methods to generate short-period and long-period mutant cells (Godinho et al., 2007; Maywood et al., 2014; Meng et al., 2008; Siepka et al., 2007; Takahashi, 2016) for the future experiments.
Our simulations suggested that the density of astrocyte-to-neurons connections plays a functional role in tuning neuronal dynamics and may augment neuron-to-neuron coupling in circadian timekeeping. More specifically, denser astrocyte-to-neurons connectivity was predicted to result in increased rhythmicity, lengthened period, and enhanced coordination of the neuronal population (Figs. 4 and S1-S3). Our model predicted that denser connectivity also allowed the astrocyte to entrain neuronal populations with broader endogenous period distributions (Fig. S3), although entrainment capability remained limited by the endogenous periods of the two cell types. Additionally, our simulations indicated that a well-coordinated neuronal network achieved through a combination of neuron-to-neuron coupling and astrocyte-to-neuron connections allowed greater astrocytic modulation of neuronal outputs. Since SCN neurons are known to display a wide range of endogenous periods (Gu et al., 2009), astrocytic modulation through adjustable numbers of astrocytes and connections with the neuron population provides a putative mechanism for enhancing neural coordination and enhancing network plasticity. The predicted effect of astrocytic modulation of neuronal circadian rhythms depending upon the network density is also consistent with the experimental finding that a daily synaptic rearrangement of the astrocytic network contributes to a rhythmic change in astrocytes-to-neuron connection degree and facilitates functional alteration of neuronal activity in the SCN (Bosler et al., 2015). When combined with an expanding experimental literature (Barca-Mayo et al., 2017; Brancaccio et al., 2017, 2019; Ruben and Hogenesch, 2017; Tso et al., 2017), our model predictions support the hypothesis that astrocytes provide a complementary means via their synaptic coupling for adjusting SCN neuronal outputs to the extensively studied mechanisms associated with interneuronal coupling (Bernard et al., 2007; Vasalou et al., 2009) and external cues (Komin et al., 2011; Tsumoto et al., 2011; Xu et al., 2012).
Our model predicted that the astrocytic modulation of SCN neurons depended on mutual contributions from multiple intercellular signals (VIP (Marpegan et al., 2009), GABA (Barca-Mayo et al., 2017; Moldavan et al., 2017) and glutamate (Brancaccio et al., 2017; Scofield, 2018)) regulated by not only neurons but also by astrocytes. Our signaling-blockade simulations suggested that the astrocytic binding of neuronally-released VIP is an essential mechanism for establishing bidirectional communication between the two cell types. For instance, when a network was formed with an astrocyte that did not sense VIP but had endogenous period 2-h larger than that of mutant neurons, the astrocyte was not able to drive the neuronal population to higher periods and synchrony (Scenario 4 in Fig. S4.) These results suggest that astrocytic sensing and response to rhythmically-release VIP by neurons might modulate the neuronal population. Furthermore, our model predicted that loss of rhythmicity in the VIP-blockade neuronal populations cannot be compensated by bidirectional coupling with an arrhythmic astrocyte due to Bmal1 knockout (Scenario 2 in Fig. S4.), supporting the hypothesis that astrocytic Bmal1 may be essential for astrocytic-modulated neuronal rhythms (Barca-Mayo et al., 2017).
We hypothesized that astrocytes are capable of tuning the circuit-level dynamics of SCN neurons via complex synaptic interactions mediated mainly through the excitatory signaling (i.e., VIP and glutamate; Brancaccio et al., 2017, 2019) with inhibitory signaling (i.e., GABA; Semyanov et al., 2004; Yoon et al., 2012) playing a less significant role. Analogous to the functional contribution of VIP, glutamate was predicted to play a dominant role in the astrocytic modulation of SCN neurons (Brancaccio et al., 2017, 2019). For example, an astrocyte having an endogenous period 2-h larger than that of glutamate-blockade neurons was not able to drive the neuronal population to higher periods and synchrony (Scenario 4 in Fig. S4.). Inhibitory GABAergic activity that can be mediated by astrocytes through transformed glutamatergic excitation (Héja et al., 2009, 2012) was predicted to have a less direct role in astrocytic tuning of neuronal outputs. Together, our computations suggested that multiple intercellular signals functionally coordinate to mediate astrocyte-to-neuron communication at synapses and generate circadian rhythms. Nevertheless, VIP (Marpegan et al., 2009) and glutamate (Brancaccio et al., 2017, 2019; Scofield, 2018) signaling tend to be the primary coupling mechanisms contributing to astrocyte-modulated neuronal rhythms. Our model omits other intercellular coupling mechanisms that may influence astrocytic-neuronal interactions, including excitatory GABA signaling (Albers et al., 2017; Ono et al., 2018) and arginine vasopressin (AVP) signaling in the shell region of the SCN (Hastings et al., 2018; Honma, 2018).
Our simulations then further demonstrated that increased coupling strengths of neuronal VIP or glutamate receptors or increased glutamate-related activity of genetically modified astrocytes can be possible mechanisms leading to the increased period of neuronal populations modulated by the astrocyte. While arrhythmic or ~24h period astrocytes did not produce a considerable change in neuronal period when nominal values of coupling strengths were used in our model (Fig. 2), we found that loss of Bmal1 or CK1ε Tau mutation in astrocytes could indirectly enhance coupling strength associated with neuronal receptors and mechanistic rates related to astrocytic signaling activity (Fig. 6). These results possibly rationalized the finding of the lengthened neuronal period observed in recent experiments (Tso et al., 2017). We hypothesized that astrocytic Bmal1 might be functionally associated with changes in glutamate synthesis and transport in astrocytes or the coupling strength of VIP (Barca-Mayo et al., 2017) or glutamate receptors in WT neurons. Furthermore, our model suggested that removal of CK1ε tau/+ mutation in astrocytes may increase cellular activities by promoting stronger VIP or glutamate-mediated coupling in mutant neurons. Collectively, we hypothesized that loss of tau mutation or Bmal1 knockout in astrocytes may result in the lengthened neuronal period as experimentally observed (Tso et al., 2017) because such genetic manipulations may be associated with enhanced coupling activities mediated by neuronal VIP and astrocytic glutamate and therefore alter daily rhythms of the neuronal population.
In summary, our computational model supports the emerging view that astrocytes encode circadian information via bidirectional synaptic interactions based on the entrainment of VIP released by neurons and then influence daily neuronal rhythms through release and uptake of GABA and glutamate to adjust the neuronal core clock. We have shown that SCN astrocytes can modulate neuronal populations through multiple mechanisms depending on distinct factors, including intercellular coupling, network topology, and endogenous periods. We believe that such mechanistic models are essential for generating testable predictions that can guide experimental design for investigating interactions between SCN neuronal and astrocytic populations and the functional role of these interactions in circadian timekeeping. For example, astrocytes may broadly interact with neurons across the SCN or specifically communicate with SCN subpopulation (e.g., VIPergic neurons). Furthermore, whether gliotransmitters such as GABA and glutamate affect SCN neuronal activity at the synapse-, cell-, and circuit-level remains obscure. Larger simulated networks containing multiple, heterogeneous astrocytes may be worth considering in future studies. Finally, the effects of external stimuli such as light on astrocytic modulation of neuronal populations and SCN plasticity requires further study (Leone et al., 2015). It would also be intriguing to know if astrocytes vary their number or neuronal contacts as a function of, for example age or season, to alter their modulation of neurons.
Supplementary Material
ACKNOWLEDGMENTS
The research was supported by the National Institutes of Health [grant number U01-EB021956–01].
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
REFERENCES
- Alamilla J, Perez-Burgos A, Quinto D, and Aguilar-Roblero R (2014) Circadian modulation of the Cl- equilibrium potential in the rat suprachiasmatic nuclei. Biomed Res Int 2014:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Walton JC, Gamble KL, McNeill JK, and Hummer DL (2017) The Dynamics of GABA Signaling: Revelations from the Circadian Pacemaker in the Suprachiasmatic Nucleus. Front Neuroendocrinol 44:35–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus H, Vansteensel MJ, Michel S, Block GD, and Meijer JH (2005) A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol 15:886–893. [DOI] [PubMed] [Google Scholar]
- Allen CN, Nitabach MN, and Colwell CS (2017) Membrane Currents, Gene Expression, and Circadian Clocks. Cold Spring Harb Perspect Biol 9:a027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, and Haydon PG (1999) Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci 22:208–215. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, and Herzog ED (2005) Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L, and De Pietri Tonelli D (2017) Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun 8:14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becquet D, Girardet C, Guillaumond F, François-Bellan AM, and Bosler O (2008) Ultrastructural plasticity in the rat suprachiasmatic nucleus. Possible involvement in clock entrainment. Glia 56:294–305. [DOI] [PubMed] [Google Scholar]
- Belle MD and Allen CN (2018) The circadian clock: a tale of genetic–electrical interplay and synaptic integration. Curr Opin Physiol 5:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle MDC and Diekman CO (2018) Neuronal oscillations on an ultra-slow timescale: Daily rhythms in electrical activity and gene expression in the mammalian master circadian clockwork. Eur J Neurosci 48:2696–2717. [DOI] [PubMed] [Google Scholar]
- Bernard S, Gonze D, Čajavec B, Herzel H, and Kramer A (2007) Synchronization-induced rhythmicity of circadian oscillators in the suprachiasmatic nucleus. PLoS Comput Biol 3:667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosler O, Girardet C, Franc J-L, Becquet D, and François-Bellan A-M (2015) Structural plasticity of the circadian timing system. An overview from flies to mammals. Front Neuroendocrinol 38:50–64. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Patton AP, Chesham JE, Maywood ES, and Hastings MH (2017) Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron 93:1420–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio M, Edwards MD, Patton AP, Smyllie NJ, Chesham JE, Maywood ES, and Hastings MH (2019) Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 363:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi-Castañeda D and Ortega A (2018) Circadian Regulation of Glutamate Transporters. Front Endocrinol 9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YIYS, Jung SH, Kim JS, Kim DY, Son EJ, Han HC, Hong SK, Colwell CS, and Kim YIYS (2008) Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci 28:5450–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS (2011) Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci 12:553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallérac G, Chever O, and Rouach N (2013) How do astrocytes shape synaptic transmission? Insights from electrophysiology. Front Cell Neurosci 7:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jeu M and Pennartz C (2002) Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J Neurophysiol 87:834–844. [DOI] [PubMed] [Google Scholar]
- De Pittà M, Volman V, Berry H, Parpura V, Volterra A, and Ben-Jacob E (2012) Computational quest for understanding the role of astrocyte signaling in synaptic transmission and plasticity. Front Comput Neurosci 6:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWoskin D, Myung J, Belle MDC, Piggins HD, Takumi T, and Forger DB (2015) Distinct roles for GABA across multiple timescales in mammalian circadian timekeeping. Proc Natl Acad Sci U S A 112:E3911–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Noguera A (1994) A functional model of the circadian system based on the degree of intercommunication in a complex system. Am J Physiol Integr Comp Physiol 267:R1118–R1135. [DOI] [PubMed] [Google Scholar]
- Evans JA (2016) Collective timekeeping among cells of the master circadian clock. J Endocrinol 230:R27–R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, van Westering TLE, Meijer JH, and Michel S (2014) Seasonal induction of GABAergic excitation in the central mammalian clock. Proc Natl Acad Sci U S A 111:9627–9632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG and Kreitzman L (2014) The rhythms of life: what your body clock means to you! Exp Physiol 99:599–606. [DOI] [PubMed] [Google Scholar]
- Godinho SIH, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, and Romero MR (2007) The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316:897–900. [DOI] [PubMed] [Google Scholar]
- Gonze D, Bernard S, Waltermann C, Kramer A, and Herzel H (2005) Spontaneous synchronization of coupled circadian oscillators. Biophys J 89:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Wang J, and Liu Z (2009) Free-running period of neurons in the suprachiasmatic nucleus: Its dependence on the distribution of neuronal coupling strengths. Phys Rev E 80 80:5–8. [DOI] [PubMed] [Google Scholar]
- Hafner M, Koeppl H, and Gonze D (2012) Effect of network architecture on synchronization and entrainment properties of the circadian oscillations in the suprachiasmatic nucleus. PLoS Comput Biol 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, and Haydon PG (2007) The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13:54–63. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Brancaccio M, and Maywood ES (2014) Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus. J Neuroendocrinol 26:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, and Brancaccio M (2018) Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci 19:453–469. [DOI] [PubMed] [Google Scholar]
- Héja L, Barabás P, Nyitrai G, Kékesi KA, Lasztóczi B, Töke O, Tárkányi G, Madsen K, Schousboe A, Dobolyi Á, Palkovits M, and Kardos J (2009) Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS One 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héja L, Nyitrai G, Kékesi O, Dobolyi Á, Szabó P, Fiáth R, Ulbert I, Pál-Szenthe B, Palkovits M, and Kardos J (2012) Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol 10:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Hermanstyne T, Smyllie NJ, and Hastings MH (2017) Regulating the Suprachiasmatic Nucleus (SCN) Circadian Clockwork: Interplay between Cell-Autonomous and Circuit-Level Mechanisms. Cold Spring Harb Perspect Biol 9:a027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S (2018) The mammalian circadian system: a hierarchical multi-oscillator structure for generating circadian rhythm. J Physiol Sci 68:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury NJ, Taylor SR, and Henson MA (2016) Inhibitory and excitatory networks balance cell coupling in the suprachiasmatic nucleus: A modeling approach. J Theor Biol 397:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett NJ and Allen CN (2017) Intracellular Chloride Regulation in AVP+ and VIP+ Neurons of the Suprachiasmatic Nucleus. Sci Rep 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komin N, Murza a. C, Hernández-García E, and Toral R (2011) Synchronization and entrainment of coupled circadian oscillators. Interface Focus 1:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup JC and Goldbeter A (2003) Toward a detailed computational model for the mammalian circadian clock. Proc Natl Acad Sci U S A 100:7051–7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup JC and Goldbeter A (2008) Modeling the circadian clock: From molecular mechanism to physiological disorders. BioEssays 30:590–600. [DOI] [PubMed] [Google Scholar]
- Leone MJ, Beaule C, Marpegan L, Simon T, Herzog ED, and Golombek DA (2015) Glial and light-dependent glutamate metabolism in the suprachiasmatic nuclei. Chronobiol Int 32:573–578. [DOI] [PubMed] [Google Scholar]
- Liu AC, Lewis WG, and Kay SA (2007) Mammalian circadian signaling networks and therapeutic targets. Nat Chem Biol 3:630–9. [DOI] [PubMed] [Google Scholar]
- Manninen T, Havela R, and Linne ML (2018) Computational models for calcium-mediated astrocyte functions. Front Comput Neurosci 12:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Krall TJ, and Herzog ED (2009) Vasoactive intestinal polypeptide entrains circadian rhythms in astrocytes. J Biol Rhythms 24:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masmoudi-Kouki O, Gandolfo P, Castel H, Leprince J, Fournier A, Dejda A, Vaudry H, and Tonon MC (2007) Role of PACAP and VIP in astroglial functions. Peptides 28:1753–1760. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, Meng Q-J, Nolan PM, Loudon ASI, and Hastings MH (2011) Tuning the Period of the Mammalian Circadian Clock: Additive and Independent Effects of CK1 Tau and Fbxl3Afh Mutations on Mouse Circadian Behavior and Molecular Pacemaking. J Neurosci 31:1539–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, Smyllie NJ, and Hastings MH (2014) The Tau Mutation of Casein Kinase 1ϵ Sets the Period of the Mammalian Pacemaker via Regulation of Period1 or Period2 Clock Proteins. J Biol Rhythms 29:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sládek M, Semikhodskii AS, Glossop NRJ, Piggins HD, Chesham JE, Bechtold DA, Yoo SH, Takahashi JS, Virshup DM, Boot-Handford RP, Hastings MH, and Loudon ASI (2008) Setting Clock Speed in Mammals: The CK1ε tau Mutation in Mice Accelerates Circadian Pacemakers by Selectively Destabilizing PERIOD Proteins. Neuron 58:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiti F, Floor PA, and Balasingham I (2015) Astrocyte to Neuron Communication Channels With Applications. IEEE Trans Mol Biol Multi-Scale Commun 1:164–175. [Google Scholar]
- Mirsky HP, Liu AC, Welsh DK, Kay SA, and Doyle FJ (2009) A model of the cell-autonomous mammalian circadian clock. Proc Natl Acad Sci U S A 106:11107–11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldavan M, Cravetchi O, Williams M, Irwin RP, Aicher SA, and Allen CN (2015) Localization and expression of GABA transporters in the suprachiasmatic nucleus. Eur J Neurosci 42:3018–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldavan MG, Cravetchi O, and Allen CN (2017) GABA transporters regulate tonic and synaptic GABA A receptor-mediated currents in the Suprachiasmatic Nucleus Neurons. J Neurophysiol 118:3092–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung J, Hong S, DeWoskin D, De Schutter E, Forger DB, and Takumi T (2015) GABA-mediated repulsive coupling between circadian clock neurons in the SCN encodes seasonal time. Proc Natl Acad Sci U S A 112:E3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono D, Honma K, Yanagawa Y, Yamanaka A, and Honma S (2018) Role of GABA in the regulation of the central circadian clock of the suprachiasmatic nucleus. J Physiol Sci:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oschmann F, Berry H, Obermayer K, and Lenk K (2018) From in silico astrocyte cell models to neuron-astrocyte network models: A review. Brain Res Bull 136:76–84. [DOI] [PubMed] [Google Scholar]
- Patton AP, Chesham JE, and Hastings MH (2016) Combined Pharmacological and Genetic Manipulations Unlock Unprecedented Temporal Elasticity and Reveal Phase-Specific Modulation of the Molecular Circadian Clock of the Mouse Suprachiasmatic Nucleus. J Neurosci 36:9326–9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, and Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431. [DOI] [PubMed] [Google Scholar]
- Pérez-Alvarez A and Araque A (2013) Astrocyte-neuron interaction at tripartite synapses. Curr Drug Targets 14:1220–1224. [DOI] [PubMed] [Google Scholar]
- Ruben MD and Hogenesch JB (2017) Circadian Rhythms: Move Over Neurons — Astrocytes Mediate SCN Clock Function. Curr Biol 27:R350–R352. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Bak LK, and Waagepetersen HS (2013) Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD (2018) Exploring the Role of Astroglial Glutamate Release and Association With Synapses in Neuronal Function and Behavior. Biol Psychiatry 84:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, and Silver RA (2004) Tonically active GABAA receptors: Modulating gain and maintaining the tone. Trends Neurosci 27:262–269. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Mitushima D, and Kimura F (2000) Effects of gap junction blocker on vasopressin and vasoactive intestinal polypeptide rhythms in the rat suprachiasmatic nucleus in vitro. Neurosci Res 38:43–47. [DOI] [PubMed] [Google Scholar]
- Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, and Takahashi JS (2007) Circadian Mutant Overtime Reveals F-box Protein FBXL3 Regulation of Cryptochrome and Period Gene Expression. Cell 129:1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R (2018) Suprachiasmatic Nucleus Anatomy, Physiology, and Neurochemistry. Oxford Research Encyclopedia of Neuroscience. [Google Scholar]
- Sofroniew M V. and Vinters H V. (2010) Astrocytes: Biology and pathology. Acta Neuropathol 119:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John PC, Hirota T, Kay SA, and Doyle FJ (2014) Spatiotemporal separation of PER and CRY posttranslational regulation in the mammalian circadian clock. Proc Natl Acad Sci U S A 111:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogatz SH (2000) From Kuramoto to Crawford: exploring the onset of synchronization in populations of coupled oscillators. Phys D Nonlinear Phenom 143:1–20. [Google Scholar]
- Takahashi JS (2016) Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18:164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To T-L, Henson MA, Herzog ED, and Doyle FJ (2007) A molecular model for intercellular synchronization in the mammalian circadian clock. Biophys J 92:3792–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M, and Herzog ED (2017) Astrocytes Regulate Daily Rhythms in the Suprachiasmatic Nucleus and Behavior. Curr Biol 27:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto K, Kurosawa G, Yoshinaga T, and Aihara K (2011) Modeling light adaptation in circadian clock: Prediction of the response that stabilizes entrainment. PLoS One 6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasalou C and Henson MA (2010) A multiscale model to investigate circadian rhythmicity of pacemaker neurons in the suprachiasmatic nucleus. PLoS Comput Biol 6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasalou C and Henson MA (2011) A multicellular model for differential regulation of circadian signals in the core and shell regions of the suprachiasmatic nucleus. J Theor Biol 288:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasalou C, Herzog ED, and Henson MA (2009) Small-World Network Models of Intercellular Coupling Predict Enhanced Synchronization in the Suprachiasmatic Nucleus. J Biol Rhythms 24:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasalou C, Herzog ED, and Henson MA (2011) Multicellular model for intercellular synchronization in circadian neural networks. Biophys J 101:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A and Meldolesi J (2005) Astrocytes, from brain glue to communication elements: The revolution continues. Nat Rev Neurosci 6:626–640. [DOI] [PubMed] [Google Scholar]
- Vosko AM, Schroeder A, Loh DH, and Colwell CS (2007) Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol 152:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, and Yarom Y (1997) GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 387:598–603. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, and Kay SA (2010) Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72:551–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Gu C, Pumir A, Garnier N, and Liu Z (2012) Entrainment of the suprachiasmatic nucleus network by a light-dark cycle. Phys Rev E 86:2–6. [DOI] [PubMed] [Google Scholar]
- Yoon BE, Woo J, and Lee CJ (2012) Astrocytes as GABA-ergic and GABA-ceptive cells. Neurochem Res 37:2474–2479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.