Abstract
Efficacy and safety of sodium valproate (SV) and lamotrigine (LTG) in treating refractory epilepsy (RE) in children and the predictive value of serum neuron-specific enolase (NSE) and central nervous system specific S100β protein (S100β) on efficacy assessment were explored. A total of 110 RE children admitted to Xuzhou Children's Hospital, Xuzhou Medical University were enrolled. Patients treated with SV alone served as the control group (n=51), and those treated with SV plus LTG as the study group (n=59). Serum NSE and S100β expression levels were measured by enzyme-linked immunosorbent assay (ELISA). The efficacy, seizure frequency, adverse reactions, concentration of serum brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF), and expression of serum NSE and S100β were observed and compared. The total effective rate in the study group was significantly higher than that in the control group, and the seizure frequency and incidence of adverse reactions were significantly lower than that in the control group. The study group showed remarkably higher BDNF and NGF than the control group after treatment. The expression of serum NSE and S100β in effectively treated children were significantly lower than that in ineffectively treated children. The area under the curve (AUC) of serum NSE and S100β were 0.828 and 0.814 respectively. SV combined with LTG is better and safer than SV alone in the treatment of RE in children. Serum NSE and S100β are of high value in predicting the efficacy.
Keywords: sodium valproate, lamotrigine, refractory epilepsy, NSE, S100β
Introduction
Epilepsy, a neurological disease with seizure susceptibility, has a negative impact on 0.6% of the population in developed countries and 1.6% in developing countries (1,2). Refractory epilepsy (RE) is a kind of drug-resistant epilepsy, which is defined as refractory because it has no successful therapeutic response to a variety of antiepileptic drugs (3). Ten to twenty percent (%) of epileptic children progress to RE, and ~470,000 children suffer from epilepsy, which means there are tens of thousands of RE children (4,5). At present, antiepileptic drugs (AEDs) are the first-line therapy for RE, and the second-line is surgery, diet therapy, and vagus nerve stimulation (6). Although the treatment for RE has been continuously updated, the exploration of high-efficacy AED combinations is still ongoing (7). Our study explored the clinical efficacy and safety of AED regimens for RE children, which is of great value to improve their quality of life.
Sodium valproate (SV) is a first-line anti-epilepsy drug that can be applied to various seizure types in children, but it may also induce teratogenicity, neurocognitive impairment and other side effects (8). Studies have shown that SV plays a neuroprotective role by inhibiting endoplasmic reticulum stress and reducing neuronal apoptosis in epilepsy models induced by experiments (9). SV, an anticonvulsant through regulating neuronal pathways, has a close molecular structure with neurotransmitter γ-aminobutyric acid (GABA), resulting in GABA synergism, which inhibits the occurrence of epileptic onsets and epileptic states (10,11). Lamotrigine (LTG) is a second-generation AED after SV, and also has the function of resisting depression and stabilizing mood (12,13). It is applicable for children and adolescents with various seizure types and syndromes due to its good anticonvulsant, tolerance, broad spectrum activity, and safety (14). SV plus LTG, the most effective AED combination for RE, plays a synergistic role in pharmacodynamics and reduces the seizure frequency of children (15).
At present, there are few studies on the efficacy and safety of SV plus LTG in RE children. Therefore, we evaluated the clinical promotion value of these two AEDs by comparing the efficacy and clinical response.
Patients and methods
General data
A total of 110 RE children admitted to Xuzhou Children's Hospital, Xuzhou Medical University (Xuzhou, China) from February 2018 to March 2019 were enrolled. Patients treated with SV alone served as the control group, and those treated with SV plus LTG as the study group. There were 35 males and 16 females in the control group, aged 3-11 years, with an average age of 6.12±1.05 years, and 34 males and 25 females in the study group, aged 3-12 years, with an average age of 6.18±1.13 years. The study was approved by the Ethics Committee of the Affiliated Xuzhou Children's Hospital of Xuzhou Medical University. The guardians were all informed and signed a fully informed consent form.
Inclusion and exclusion criteria
Inclusion criteria: conforming to the guidelines of RE developed by the International League Against Epilepsy (ILAE) (16); diagnosed by imaging examinations (17); receiving at least two AEDs in the past 6 months to one year; reaching maximum blood drug concentration, and the attacks reduced by at least half; aged 3-12 years. Exclusion criteria: complicated with malignant tumor or severe heart, lung, kidney and liver dysfunction; allergic to the drugs in this study; with incomplete clinicopathological data; pregnant women; not cooperating with this study.
Treatment methods
The patients in the control group were treated with SV alone (J65363, Jinsui Biotechnology Co., Ltd.). If SV was taken and the blood drug concentration range was 50-100 µg/ml, other drugs were gradually discontinued. For patients who had not taken SV, the initial dose was 20 mg/kg/day, which was gradually increased until the blood drug concentration was in the range of 50-100 µg/ml. The patients in the study group were treated with SV plus LTG (Jinsui Biotechnology Co., Ltd., J34775). If SV was taken and the blood drug concentration range was 50-100 µg/ml, LTG at 0.15 mg/kg was taken once a day, with a weekly increase of 0.20 mg/kg/day in the first month, 0.30 mg/kg/day in the second month and 0.50-1.00 mg/kg/day in the third month. If the frequency of RE attacks and related symptoms were controlled or the total dose of LTG reached 10.00 mg/kg/day, LTG had to be stopped. If SV was not used before, the initial dose of SV was first taken, then LTG. The specific administration was as above.
Efficacy assessment
By comparing the average monthly seizure frequency after treatment with the first three months of treatment, the efficacy was quantified. Reduction of seizure frequency by 100%, i.e., no seizures, was considered as control; Reduction of seizure frequency by 75-99% was considered as markedly effective; Reduction of seizure frequency by 50-74% was considered as considered effective; Reduction of seizure frequency by no more than 49% was considered as ineffective; Increase of seizure frequency by at least 25% was considered as deterioration. The total effective rate = (control+markedly effective+effective)/total number of cases x100%.
Outcome measures
The seizure frequency in the two groups in the 3 months before treatment, and 3 and 6 months after treatment was observed and compared to assess the efficacy. The incidence of adverse reactions, serum brain derived neurotrophic factor (BDNF), nerve growth factor (NGF) concentration changes, and the expression of serum neuron-specific enolase (NSE) and central nervous system specific S100β protein (S100β) in effectively and ineffectively treated children were compared.
Detection methods
Elbow venous blood (5 ml) was drawn from the subjects before treatment from 8:00 to 9:00 a.m. 4 weeks after treatment, and placed in a vacuum tube without anticoagulant, then centrifuged at 1,500 x g and 4˚C for 10 min. Sera were collected in an Eppendorf (EP) tube and stored at -60˚C. After taken from the freezer, the sera were dissolved in a refrigerator at 4˚C, and then placed at room temperature for complete dissolution. The expression of BDNF, NGF, NSE, and S100β in serum was detected by enzyme-linked immunosorbent assay (ELISA) (18) in strict accordance with the instructions of the kits (Keshun Biotechnology Co., Ltd., KS017148, KS018187, KS015255, KS13441). Sample, standard and blank wells were set up. Test sample (50 µl) and standard (50 µl) were added to the sample well and standard well, respectively, no treatment for the blank well. The sample and standard wells were each added with 100 µl of horseradish peroxidase labeled antibody, sealed and incubated at 37˚C for 60 min. The liquid was removed, the wells were dried and washed 5 times. Substrates A and B were fully mixed (1:1) and added to all wells (100 µl each well). Afterwards, the plate was sealed, incubation was carried out at 37˚C for 15 min, and 50 µl of termination solution was added to each well. The optical density (OD) value at 450 nm of each well was read by a multifunctional ELISA analyzer (Shanghai Flash Spectrum Biotechnology Co., Ltd., SuPerMax 3000FL), and the concentrations of BDNF, NGF, NSE, and S100β were calculated.
Statistical analysis
This figures were visualized by GraphPad Prism 6 (GraphPad Software). Counting data were expressed by cases/percentage [n (%)], and Chi-square (χ2) test was used for comparison between groups. The measurement data were expressed by mean ± SD, and independent sample t-test was used for the comparison between the two groups. Receiver operating characteristic (ROC) curve was employed to assess the value of serum NSE and S100β in predicting the efficacy in patients. A value of P<0.05 was considered to be statistically significant.
Results
Baseline data
There was no significant difference between the two groups in sex, average age, average course of disease, systolic blood pressure (SBP), diastolic blood pressure (DBP), seizure type, medication history, ADE combination, family history of RE, residence (P>0.05) (Table I).
Table I.
Comparison of baseline data [n (%), mean ± SD].
| Classification | n | Control group (n=51) | Study group (n=59) | χ2/t | P-value |
|---|---|---|---|---|---|
| Sex | 1.416 | 0.234 | |||
| Male | 69 | 35 (68.63) | 34 (57.63) | ||
| Female | 41 | 16 (31.37) | 25 (42.37) | ||
| Average age (years) | 110 | 6.12±1.05 | 6.18±1.13 | 0.287 | 0.775 |
| Average course of disease (years) | 110 | 3.01±0.45 | 3.06±0.52 | 0.535 | 0.594 |
| SBP (mmHg) | 110 | 110.24±4.82 | 109.56±5.06 | 0.718 | 0.474 |
| DBP (mmHg) | 110 | 75.02±4.45 | 74.86±5.10 | 0.174 | 0.862 |
| Seizure type | 0.124 | 0.989 | |||
| Partial seizure | 66 | 31 (60.78) | 35 (59.32) | ||
| Generalized seizure | 19 | 9 (17.65) | 10 (16.95) | ||
| Secondarily generalized seizure | 13 | 6 (11.76) | 7 (11.86) | ||
| Lennox Gastaut syndrome | 12 | 5 (9.81) | 7 (11.87) | ||
| Medication history | - | - | |||
| Carbamazepine | 46 | 21 (-) | 25 (-) | ||
| SV | 56 | 26 (-) | 30 (-) | ||
| Topiramate | 30 | 17 (-) | 13 (-) | ||
| Valnromide | 8 | 5 (-) | 3 (-) | ||
| Phenytoin sodium | 6 | 3 (-) | 3 (-) | ||
| Gabapentin | 6 | 2 (-) | 4 (-) | ||
| AED combination | 2.100 | 0.552 | |||
| 2 | 67 | 30 (58.82) | 37 (62.71) | ||
| 3 | 33 | 18 (35.29) | 15 (25.42) | ||
| 4 | 6 | 2 (3.92) | 4 (6.78) | ||
| 5 | 4 | 1 (1.97) | 3 (5.09) | ||
| Family history of RE | 0.283 | 0.595 | |||
| No | 95 | 45 (88.24) | 50 (84.75) | ||
| Yes | 15 | 6 (11.76) | 9 (15.25) | ||
| Residence | 0.294 | 0.588 | |||
| Rural | 33 | 14 (27.45) | 19 (32.20) | ||
| Urban | 77 | 37 (72.55) | 40 (67.80) |
Comparison of efficacy
Efficacy in the control group: the number of cases of control, markedly effective, effective, ineffective and deterioration were 15, 10, 6, 14 and 6, respectively, with a total effective rate of 60.78%. Efficacy in the study group: the number of cases of control, markedly effective, effective, ineffective and deterioration were 23, 14, 11, 9 and 2, respectively, with a total effective rate of 81.35%. The total effective rate in the study group was significantly higher than that in the control group (P<0.001) (Table II).
Table II.
Comparison of efficacy [n (%)].
| Group | n | Control | Markedly effective | Effective | Ineffective | Deterioration | Total effective rate |
|---|---|---|---|---|---|---|---|
| Control group | 51 | 15 (29.41) | 10 (19.61) | 6 (11.76) | 14 (27.45) | 6 (11.77) | 60.78 |
| Study group | 59 | 23 (38.98) | 14 (23.73) | 11 (18.64) | 9 (15.25) | 2 (3.39) | 81.35 |
| χ2 value | - | - | - | - | - | - | 18.888 |
| P-value | - | - | - | - | - | - | <0.001 |
Comparison of seizure frequency
There was no significant difference in seizure frequency between the study group and the control group 3 months before treatment (P<0.05). At 3 and 6 months after treatment, the frequency in the study group was significantly lower than that in the control group (P<0.05) (Table III).
Table III.
Comparison of seizure frequency (mean ± SD).
| Group | n | 3 months before treatment | 3 months after treatment | 6 months after treatment |
|---|---|---|---|---|
| Control group | 51 | 15.43±2.29 | 10.43±2.29 | 6.97±1.15 |
| Study group | 59 | 15.89±1.04 | 7.89±1.04 | 1.88±0.60 |
| t value | - | 1.387 | 7.659 | 29.660 |
| P-value | - | 0.168 | <0.001 | <0.001 |
Incidence of adverse reactions
After treatment, RE children may present with loss of appetite, hyperactivity, hair loss, lower limb soreness, dizziness, rash and other adverse reactions, and loss of appetite, hyperactivity, lower limb soreness are the main ones. The incidence rate of adverse reactions in the study group was significantly lower than that in the control group (Table IV).
Table IV.
Adverse reactions [n (%)].
| Classification | Control group (n=51) | Study group (n=59) | χ2 value | P-value |
|---|---|---|---|---|
| Loss of appetite | 4 (7.84) | 3 (5.08) | 0.349 | 0.555 |
| Hyperactivity | 4 (7.84) | 2 (3.39) | 1.052 | 0.305 |
| Hair loss | 2 (3.92) | 1 (1.69) | 0.511 | 0.475 |
| Lower limb soreness | 3 (5.88) | 2 (3.39) | 0.531 | 0.392 |
| Dizziness | 2 (3.92) | 1 (1.69) | 0.511 | 0.475 |
| Rash | 1 (1.96) | 0 (0.00) | 1.167 | 0.280 |
| Total | 16 (31.37) | 9 (15.25) | 4.047 | 0.044 |
Comparison of neurotrophic indexes
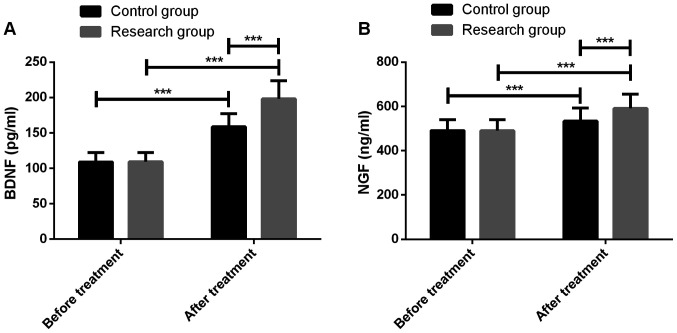
Before treatment, the neurotrophic indexes BDNF and NGF were not significantly different between the two groups (P<0.05), which were significantly increased after treatment (P<0.001), and in the study group they were significantly higher than the control group (P<0.001) (Fig. 1).
Figure 1.
Comparison of neurotrophic indexes. (A) Comparison of BDNF before and after treatment. (B) Comparison of NGF before and after treatment. ***P<0.001. BDNF, brain derived neurotrophic factor; NGF, nerve growth factor.
Expression of serum NSE and S100β
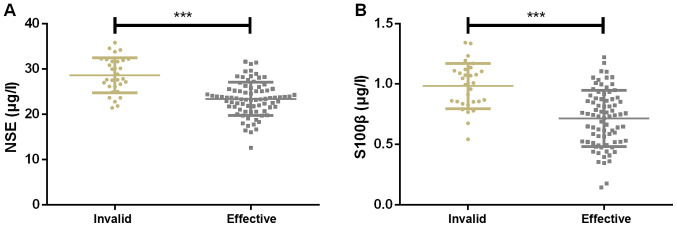
The treatment was effective in 79 children and ineffective in 31 children. The expression of serum NSE was 28.47±3.99 and 21.03±3.18 µg/l in ineffectively treated and effectively treated children, respectively, while that of serum S100β were 0.97±0.23 and 0.65±0.26 µg/l, respectively. Therefore, the expression of serum NSE and S100β in ineffectively treated children were significantly higher than that in effectively treated ones (Fig. 2).
Figure 2.
Expression of serum NSE and S100β. (A) The expression of serum NSE in effectively treated children was significantly lower than that in ineffectively treated children. (B) The expression of serum S100β in effectively treated children was significantly lower than that in ineffectively treated children. ***P<0.001. NSE, neuron-specific enolase. S100β, specific S100β protein.
Serum NSE and S100β in assessing the efficacy
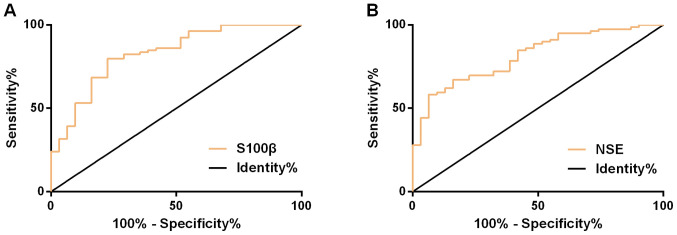
ROC curve demonstrated that the AUC, cut-off, sensitivity, and specificity of serum NSE in assessing the efficacy were 0.828 (95% CI, 0.742-0.914), 26.05, 79.75%, and 77.42%, respectively; while those of serum S100β were 0.814 (95% CI, 0.731-0.896), 0.77, 58.23 and 93.55%, respectively (Fig. 3 and Table V).
Figure 3.
ROC curve of serum NSE and S100β in assessing efficacy. (A) ROC curve of serum NSE in assessing efficacy. (B) ROC curve of serum S100β in assessing efficacy. ROC, receiver operating characteristic; NSE, neuron-specific enolase. S100β, specific S100β protein.
Table V.
Predictive value of serum NSE and S100β on efficacy assessment.
| Group | AUC | 95%CI | S.E | Cut-off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| NSE | 0.828 | 0.742-0.914 | 0.044 | 26.05 | 79.75 | 77.42 |
| S100β | 0.814 | 0.731-0.896 | 0.042 | 0.77 | 58.23 | 93.55 |
NSE, neuron-specific enolase; S100β, specific S100β protein.
Discussion
RE is a chronic and debilitating disease of the nervous system with epileptic seizure caused by accidental discharge of cerebral neurons, which may lead to stigma in patients (19-21). Therefore, appropriate inhibition of neuronal excitability is the key in selection of AEDs in RE children (22). SV has been proved to alleviate neuronal apoptosis in a kainic acid model of epilepsy by enhancing phosphorylation of PKC-dependent GABA A R γ2 Serine 327 (23,24). LTG acts as glutamate antagonist to exert anticonvulsant and sedative functions through its pharmacological mechanism affecting sodium and calcium channels. It can also disturb the pathogenesis of hyperactivity via regulating excitatory neurotransmitters (25). In this study, hair loss, hyperactivity, and lower limb soreness were the main adverse reactions of patients. The seizure frequency in the study group was significantly lower than that in the control group, and the total effective rate and safety of treatment were significantly higher than those in the control group. Therefore, SV plus LTG has high efficacy and safety on RE children and has better inhibitory effect on the seizure frequency compared with SV alone.
We screened two neurotrophic indexes, BDNF and NGF, to compare the improvement of neurotrophic level of RE children treated with drugs that have inhibitory effects on neuronal excitability. BDNF mediates survival, growth, and regeneration of neurons and participates in the regulation of neural plasticity, playing an important role in the healthy brain development, and being of high diagnostic and prognostic value for brain injury (26,27). Tan et al pointed out that BDNF protected neurons by inhibiting the secretion of excitatory amino acids, maintaining calcium homeostasis in neurons, as well as inhibiting the high expression of oxygen free radicals. Moreover, low BDNF level generally indicated the decline of cognitive function in epileptic patients (28). NGF, a typical representative of neurotrophic factors, is responsible for the growth, survival, and differentiation of mature neurons. In addition to being active in a wide array of non-nervous system cells, it is also synthesized by various cell types (29). NGF has a protective effect on basal forebrain cholinergic neurons and may reduce the susceptibility to generalized seizures (30,31). BDNF and NGF were reported to be closely related to epilepsy and involved in the occurrence and progression of focal RE (32). Our findings showed that BDNF and NGF levels in the study group were more significantly increased, indicating that SV combined with LTG was more useful than SV alone in improving neurotrophic levels of RE children.
Finally, we assessed the predictive value of serum NSE and S100β on the efficacy in RE children. NSE and S100β are brain-derived proteins whose high expression is related to the increase of brain injury (33). Shaik et al found that serum NSE of patients with convulsion showed abnormally high expression, suggesting that it was a marker of epilepsy-related neuron injury (34). Another study demonstrated that serum S100β protein level of patients with focal epilepsy was significantly higher than that in healthy controls, which can be a biomarker for neuronal damage in patients with focal RE (35). In this study, the effectively treated children had a significantly lower expression of serum NSE and S100β than those with ineffective treatment, so serum NSE and S100β gradually recovered to normal levels in RE children after treatment. From the ROC curve, we obtained AUC of serum NSE and S100β and the efficacy was 0.828 and 0.814, respectively, which showed that they had better predictive value in efficacy assessment for RE children.
This study confirmed that SV plus LTG has higher efficacy and fewer adverse reactions in the treatment of RE. However, statistics of various attack types and the efficacy of treatment in RE children need to be recorded to know which RE type of children treated by SV plus LTG achieves the highest curative effect.
In conclusion, SV combined with LTG is better and safer than SV alone in the treatment of RE in children, which is more worthy of clinical promotion. Serum NSE and S100β are of high value in predicting the efficacy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DZ wrote the manuscript. DZ and LQ conceived and designed the study. YZ and YS were responsible for the collection and analysis of the experimental data. NZ and XL interpreted the data and drafted the manuscript. LQ and YS revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Xuzhou Children's Hospital, Xuzhou Medical University (Xuzhou, China). Patients who participated in this research, had complete clinical data. Signed informed consents were obtained from the parents or the guardians of the child patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Armeno M, Verini A, Del Pino M, Araujo MB, Mestre G, Reyes G, Caraballo RH. A prospective study on changes in nutritional status and growth following two years of ketogenic diet (KD) therapy in children with refractory epilepsy. Nutrients. 2019;11(1596) doi: 10.3390/nu11071596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGovern RA, Banks GP, McKhann GM. Responsive stimulation in the management of medically refractory epilepsy. In: Epilepsy Surgery and Intrinsic Brain Tumor Surgery. Fountas K and Kapsalaki E (eds). Springer, Cham, pp205-211, 2019. [Google Scholar]
- 3.Sirven JI, Pedley TA, Wilterdink JL. Evaluation and management of drug-resistant epilepsy. UpToDate. https://www.uptodate.com/contents/evaluation-and-management-of-drug-resistant-epilepsy. Accessed August 13, 2018. [Google Scholar]
- 4.Mishra S. Refractory epilepsy in children: A short review. South Asian Res J Med Sci. 2019;1:24–29. [Google Scholar]
- 5.Nigro SE. The efficacy of neurofeedback for pediatric epilepsy. Appl Psychophysiol Biofeedback. 2019;44:285–290. doi: 10.1007/s10484-019-09446-y. [DOI] [PubMed] [Google Scholar]
- 6.Moosa ANV. Antiepileptic drug treatment of epilepsy in children. Continuum (Minneap Minn) 2019;25:381–407. doi: 10.1212/CON.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 7.Golyala A, Kwan P. Drug development for refractory epilepsy: The past 25 years and beyond. Seizure. 2017;44:147–156. doi: 10.1016/j.seizure.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Balagura G, Iapadre G, Verrotti A, Striano P. Moving beyond sodium valproate: Choosing the right anti-epileptic drug in children. Expert Opin Pharmacother. 2019;20:1449–1456. doi: 10.1080/14656566.2019.1617850. [DOI] [PubMed] [Google Scholar]
- 9.Fu J, Peng L, Wang W, He H, Zeng S, Chen TC, Chen Y. Sodium valproate reduces neuronal apoptosis in acute pentylenetetrazole-induced seizures via inhibiting ER stress. Neurochem Res. 2019;44:2517–2526. doi: 10.1007/s11064-019-02870-w. [DOI] [PubMed] [Google Scholar]
- 10.Brown C, Smith C. Sodium valproate. Pract Diabetes. 2018;35:186–187. [Google Scholar]
- 11.Çavuş I, Romanyshyn JC, Kennard JT, Farooque P, Williamson A, Eid T, Spencer SS, Duckrow R, Dziura J, Spencer DD. Elevated basal glutamate and unchanged glutamine and GABA in refractory epilepsy: Microdialysis study of 79 patients at the yale epilepsy surgery program. Ann Neurol. 2016;80:35–45. doi: 10.1002/ana.24673. [DOI] [PubMed] [Google Scholar]
- 12.Johannessen Landmark C, Patsalos PN. Drug interactions involving the new second- and third-generation antiepileptic drugs. Expert Rev Neurother. 2010;10:119–140. doi: 10.1586/ern.09.136. [DOI] [PubMed] [Google Scholar]
- 13.Reimers A. Lamotrigine, bipolar disorder, and the pill-free week. Bipolar Disord. 2019;21:372–373. doi: 10.1111/bdi.12776. [DOI] [PubMed] [Google Scholar]
- 14.Yasam VR, Jakki SL, Senthil V, Eswaramoorthy M, Shanmuganathan S, Arjunan K, Nanjan MJ. A pharmacological overview of lamotrigine for the treatment of epilepsy. Expert Rev Clin Pharmacol. 2016;9:1533–1546. doi: 10.1080/17512433.2016.1254041. [DOI] [PubMed] [Google Scholar]
- 15.Poolos NP, Castagna CE, Williams S, Miller AB, Story TJ. Association between antiepileptic drug dose and long-term response in patients with refractory epilepsy. Epilepsy Behav. 2017;69:59–68. doi: 10.1016/j.yebeh.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Lanteaume L, Guedj E, Bastien-Toniazzo M, Magalahaes A, Mundler O, Bartolomei F. Cognitive and metabolic correlates of emotional vulnerability in patients with temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2012;83:522–528. doi: 10.1136/jnnp-2011-301219. [DOI] [PubMed] [Google Scholar]
- 17.Ollenberger GP, Byrne AJ, Berlangieri SU, Rowe CC, Pathmaraj K, Reutens DC, Berkovic SF, Scheffer IE, Scott AM. Assessment of the role of FDG PET in the diagnosis and management of children with refractory epilepsy. Eur J Nucl Med Mol Imaging. 2005;32:1311–1316. doi: 10.1007/s00259-005-1844-6. [DOI] [PubMed] [Google Scholar]
- 18.Hornbeck PV. Enzyme-linked immunosorbent assays. Curr Protoc Immunol. 2015;110:2.1.1–23. doi: 10.1002/0471142735.im0201s110. [DOI] [PubMed] [Google Scholar]
- 19.Ramaratnam S, Panebianco M, Marson AG. doi: 10.1002/14651858.CD001909.pub2. Lamotrigine add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev: CD001909, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verrotti A, Iapadre G, Di Donato G, Di Francesco L, Zagaroli L, Matricardi S, Belcastro V, Iezzi ML. Pharmacokinetic considerations for anti-epileptic drugs in children. Expert Opin Drug Metab Toxicol. 2019;15:199–211. doi: 10.1080/17425255.2019.1575361. [DOI] [PubMed] [Google Scholar]
- 21.Pagura JR, Alessi R. Epilepsy and seizures. In: The Sports Medicine Physician. Piedade SR, Imhoff AB, Clatworthy M, Moises Cohen M and Espregueira-Mendes J (eds). Springer Cham, pp235-240, 2019. [Google Scholar]
- 22.Hernan AE, Holmes GL. Antiepileptic drug treatment strategies in neonatal epilepsy Elsevier. Prog Brain Res. 2016;226:179–193. doi: 10.1016/bs.pbr.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Li QQ, Jia JN, Cao S, Wang ZB, Wang X, Luo C, Zhou HH, Liu ZQ, Mao XY. Sodium valproate ameliorates neuronal apoptosis in a kainic acid model of epilepsy via enhancing PKC-dependent GABAAR γ2 Serine 327 phosphorylation. Neurochem Res. 2018;43:2343–2352. doi: 10.1007/s11064-018-2659-8. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017;70B:319–327. doi: 10.1016/j.yebeh.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han SA, Yang EJ, Song MK, Kim SJ. Effects of lamotrigine on attention-deficit hyperactivity disorder in pediatric epilepsy patients. Korean J Pediatr. 2017;60:189–195. doi: 10.3345/kjp.2017.60.6.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korley FK, Diaz-Arrastia R, Wu AHB, Yue JK, Manley GT, Sair HI, Van Eyk J, Everett AD, Okonkwo DO, Valadka AB, et al. TRACK-TBI investigators: Circulating brain-derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. J Neurotrauma. 2016;33:215–225. doi: 10.1089/neu.2015.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan XH, Song ZB, Wang H, Wang Q, He JL. Influence of adjuvant levetiracetam therapy on serum nerve cytokines and apoptosis molecules in patients with refractory partial epileptic seizure. Hainan Yixueyuan Xuebao. 2017;23:145–149. (In Chinese) [Google Scholar]
- 29.Skaper SD. Nerve growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology. 2017;151:1–15. doi: 10.1111/imm.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuszynski MH, Yang JH, Barba D, U HS, Bakay RA, Pay MM, Masliah E, Conner JM, Kobalka P, Roy S, et al. Nerve growth factor gene therapy: Activation of neuronal responses in Alzheimer disease. JAMA Neurol. 2015;72:1139–1147. doi: 10.1001/jamaneurol.2015.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silveira DC, Holmes GL, Schachter SC, Geula C, Schomer DL. Increased susceptibility to generalized seizures after immunolesions of the basal forebrain cholinergic neurons in rats. Brain Res. 2000;878:223–227. doi: 10.1016/s0006-8993(00)02703-7. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Lin Y, Kang D, Chen F, Lin K, Su X. Distribution and expression of brain-derived neurotrophic factor, nerve growth factor, and neurotrophic factor-3 in refractory epilepsy-associated focal cortical dysplasia. Clin Neuropathol. 2017;36:233–239. doi: 10.5414/NP301026. [DOI] [PubMed] [Google Scholar]
- 33.Alpdemir M, Özcan O, Alpdemir MF, Şeneş M, Azak A, Duranay M, Yücel M. Serum neuron specific enolase and S-100B levels in hemodialysis and peritoneal dialysis patients. Eur Arch Med Res. 2019;35:83–87. [Google Scholar]
- 34.Shaik AJ, Reddy K, Mohammed N, Tandra SR, Rukmini Mridula Kandadai, Baba Kss S. Neuron specific enolase as a marker of seizure related neuronal injury. Neurochem Int. 2019;131(104509) doi: 10.1016/j.neuint.2019.104509. [DOI] [PubMed] [Google Scholar]
- 35.Calik M, Abuhandan M, Kandemir H, Güzel B, Solmaz A, Celik H, Taskin A, Iscan A. Interictal serum S-100B protein levels in intractable epilepsy: A case-control study. Neurosci Lett. 2014;558:58–61. doi: 10.1016/j.neulet.2013.10.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.