Abstract
Mammalian follicles are composed of oocytes, granulosa cells, and theca cells. Theca cells form in the secondary follicles, maintaining follicular structural integrity and secreting steroid hormones. Two main sources of theca cells exist: Wilms tumor 1 positive (Wt1+) cells native to the ovary and Gli1+ mesenchymal cells migrated from the mesonephros. Normal folliculogenesis is a process where oocytes, granulosa cells, and theca cells constantly interact with and support each other through autocrine and paracrine mechanisms. The proliferation and differentiation of theca cells are regulated by oocyte-derived factors, including growth development factor 9 and bone morphogenetic protein 15, and granulosa cell-derived factors, including desert hedgehog, Indian hedgehog, kit ligand, insulin-like growth factor 1, as well as hormones such as insulin and growth hormones. Current research on the origin of theca cells is limited. Identifying the origin of theca cells will help us to systematically elaborate the mechanisms of follicular formation and development.
Keywords: Theca cell, Hedgehog pathway, Growth development factor 9, Bone morphogenetic protein 15, TGF-β superfamily, Kit ligand
Introduction
The ovary is an important female reproductive organ. As the body develops, continuous and dynamic changes occur in the ovary. Follicles are the basic units of ovarian structure and function and are mainly composed of three cell components: oocytes, granulosa cells, and theca cells. The process of folliculogenesis is regulated by signals from these cell components and ultimately manifests as the growth, differentiation, and maturation of the three cell components.
Theca cells are first observed in secondary follicles with two or more layers of granulosa cells even though true theca layers have not been formed.[1] In the antral follicle stage, complete theca layers surrounding granulosa cells are composed of theca cells as the majority, vascular structures and immune cells.[2] The theca interna is composed of theca cells with a steroidogenesis function, vascular endothelial cells, and immune cells, while the theca externa is mainly composed of fibroblast-like cells.[3] In general, the function of theca layers in antral follicles can be summarized as the synthesis of hormones and secretory factors such as androgens and bone morphogenetic proteins (BMPs), the transport of nutrients to granulosa cells, cumulus cells and oocytes through vascular structures, and the provision of structural support for spherical follicles. After rupture of the theca layers and basal lamina during ovulation, theca cells invade into granulosa cell layers as well as vascular and immune cells, resulting in the formation of the corpus luteum.[4] In addition to forming the corpus luteum, the more common fate of theca cells is atresia. Theca cells undergo apoptosis early or late during follicle atresia according to the stage of folliclogenesis.[5]
Though critical, investigations into theca cells have long been neglected. Many questions about theca cells and theca layers remain to be answered. For example, where do the cells in the process of recruitment originate? Do fibroblast-like cells in the theca externa play a role in ovulation and what is the extent of the role? Can theca cell lines be cultured in vitro? How do vascular and immune factors in theca layers affect the follicular ecosystem? Focusing on the question of where theca cells originate, this article discusses the origin of theca cells and the factors involved in their recruitment and differentiation, which will help us comprehensively understand the mystery of theca cells’ life cycle.
Origin and Differentiation of Theca Cells
The formation of theca layers is an important physiological event in the early stage of folliculogenesis. Two main theories address the origin of theca cells. In 2007, several scientists accidentally purified and isolated putative theca cells from the ovaries of newborn mice after luteinizing hormone (LH)-induced differentiation.[6] These cells were similar to fibroblasts in morphology and could be induced to differentiate into steroidogenic cells.[6] These cells are regarded as theca stem cells because of their ability to self-renew and differentiate in vivo and in vitro,[6] reflecting the first theory that theca cells originate from stem cells. However, whether the isolated cells were real theca stem cells was uncertain due to their impurities resulting from the material acquisition method,[6] and no further studies have been found to reinforce this theory.
The other more accepted theory is that theca cells originate from progenitor cells in embryos. This theory is based on a study that found two theca cell progenitors by constructing a Gli+ cell lineage tracing model of the mouse embryogenesis process. These two progenitors are Wilms tumor 1 positive (Wt1+) cells from gonadal primordium and Gli1+ cells migrated from the mesonephros[7] [Figure 1]. Furthermore, by comparing the transcriptomes of these two progenitors, genes associated with steroidogenesis, including steroidogenic acute regulatory steroidogenic acute regulatory (Star), cytochrome P450 17A1 (Cyp17a1), cytochrome P450 11A1 (Cyp11a1), and LH/choriogonadotropin receptor (Lhcgr), were found to be enriched in mesonephros-derived Gli1+ cells, while estrogen receptor 1 (Esr1), Wt1, and genes involved in cell growth and proliferation were more enriched in ovary-derived Gli1+ cells. The significance of the two progenitors may be that they can differentiate into different functional cells and play different but synergetic roles in folliculogenesis and the maintenance of endocrine function.
Figure 1.
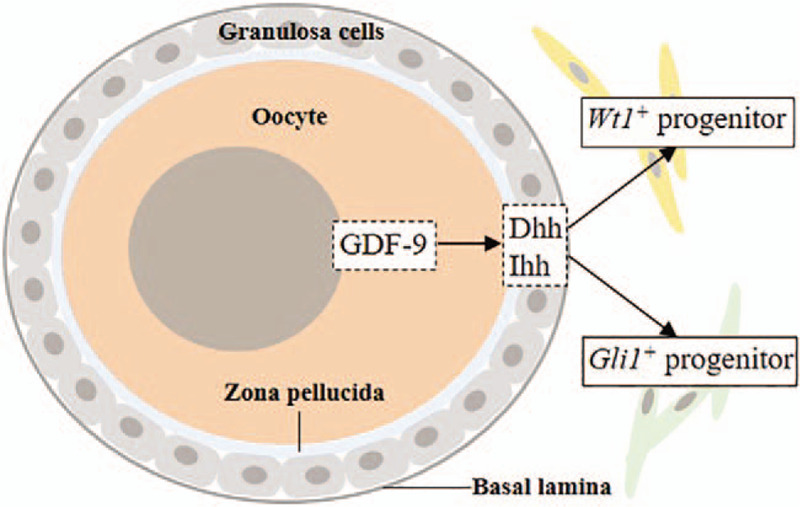
Model for the origin of theca cells derived from two kinds of progenitor cells. GDF-9: Growth differentiation factor 9; Dhh: Desert hedgehog; Ihh: India hedgehog.
This pattern of origination and differentiation through two progenitors is similar to that of Leydig cells in the testes. During the development of rodent embryos from embryonic day (E)12.5 to E15.5, Leydig cells underwent a dramatic increase in number, some of which were derived from steroidogenic factor 1-positive (Sf1+) cells in situ in the gonad, while others were derived from the mesonephros, coelomic epithelium, and neural crest.[8] Based on these findings, we can also identify some similarities in the development of reproductive organs. In the process of embryonic development, some cells can influence the differentiation orientation of adjacent cells. This phenomenon in embryonic development may be caused by in situ signaling by differentiated gonadal cells to induce the differentiation and migration of neighboring mesonephros cells.
Signaling Molecules Involved in the Origin and Differentiation of Theca Cells
Research on the molecular mechanism regulating the origin and differentiation of theca cells is limited and superficial at present, and establishing an accepted model to reveal this mechanism is therefore difficult. According to previous research, we can infer that the origin and differentiation of theca cells must not be regulated by a single factor but rather by multiple factors that form a complex network [Figure 2]. However, whether one or more of these factors play a key regulatory role remains unknown.
Figure 2.
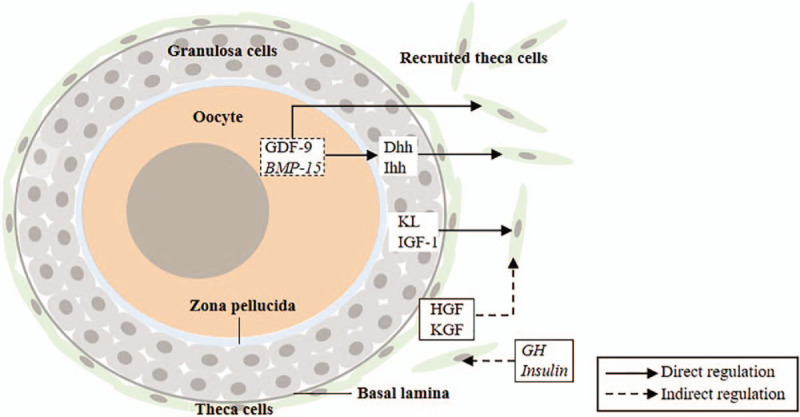
Signaling molecules that regulate the recruitment, differentiation, and proliferation of theca cells. GDF-9 and BMP-15 are oocyte-derived factors. Dhh, Ihh, KL, LIF, and KGF are granulosa cell-derived factors. HGF and KGF are theca cell-derived factors. GH and insulin are factors from the outside of the ovary. Arrows indicate the relationship of positive regulation. The arrows pointing to the recruited theca cells indicate that these factors contribute to the recruitment, differentiation, and proliferation of theca cells. Factors in italics indicate that their regulation on theca is conjectural and uncertain. GDF-9: Growth differentiation factor 9; BMP-15: Bone morphogenetic protein 15; Dhh: Desert hedgehog; Ihh: India hedgehog; KL: Kit ligand; IGF-1: Insulin-like growth factor 1; LIF: Leukemia inhibitory factor; KGF: Keratinocyte growth factor; GH: Growth hormone.
Despite the existing uncertainty, the differentiation of theca cells is known to be regulated by the local follicular environment. A granulosa-theca cell co-culture experiment showed that theca cell proliferation can be stimulated and steroid hormone secretion can be increased by the presence of granulosa cells.[9,10] In addition, granulosa cells are involved in the differentiation and acquisition of LH responsiveness in stromal cells of the ovarian cortex.[11] Moreover, studies have found that the formation of theca layers can be affected by oocytes.[12]
Consistent with the results of co-culture experiments, most of the factors involved in the differentiation of theca cells were synthesized by oocytes and granulosa cells in previous studies. The proliferation, differentiation, and steroidogenesis of theca cells can be modulated by these factors. In addition, hormones from other parts of the body may also be transported to the ovary through the circulatory system to affect theca cell recruitment, but research on this topic is limited. The following section will discuss the functions of oocyte-derived (growth development factor 9 [GDF-9], BMP-15) and granulosa cell-derived factors (desert hedgehog [Dhh], Indian hedgehog [Ihh], kit ligand [KL], and insulin-like growth factor 1 [IGF-1]).
Hedgehog Pathway
The hedgehog (Hh) pathway is currently believed to play an important role in regulating the origin of theca cells. The Hh pathway was first identified in Drosophila for its function in regulating the formation of body segments.[13] Later, this pathway was also found to be crucial for regulating sexual differentiation,[14] normal organ development and pathological processes,[15] especially tumorigenesis.[16] Based on the study in Drosophila, the Hh pathway was found to mainly consist of the ligand Hh, the receptor patched (PTCH),[17] the intermediate protein smoothened (SMO),[18] and the transcription factor cubitus interruptus (Ci).[19] The process of signal transduction can be described as follows.[19] PTCH without Hh binding inhibits the expression and activity of SMO on the membrane. This inhibition results in phosphorylation of Ci and causes its ubiquitination to form a 75-kD fragment (Ci-75). These fragments accumulate in the cytoplasm and diffuse into the nucleus to inhibit the expression of target genes.[20] When Hh is present, SMO is activated via its phosphorylation sites, protein kinase A and casein kinase 1.[21] Then, SMO transmits signals to Ci through the Costal-2 (Cos2)-fused (Fu) complex, causing the dephosphorylation and release of Ci into the cytoplasm.[22] The expression of target genes is the result of both Ci accumulation and Ci-75 fragment decreases.[23]
The ligand Hh has three homologous genes in mammals: Shh, Ihh, and Dhh. The expression of Dhh, Ptch1, Gli1, and Gli2 was absent in fetal mouse ovaries,[24] while in adult mouse ovaries, the Hh pathway was activated. Dhh and Ihh were mainly produced by granulosa cells, while PTCH1/2, huntingtin-interacting protein 1 and Gli1 were mainly located in adjacent theca cells.[7,25] In the same study that found the two theca cell progenitors, the function of the Hh pathway in regulating theca cell formation and differentiation was also identified.[7] In mice with specific knockout of Dhh or Ihh in granulosa cells, the expression of steroidogenic genes, such as Cyp17a1, Star, and hydroxysteroid dehydrogenase 3B1 (Hsd3b1), was significantly decreased, and the formation of theca layers, as well as the growth of antral follicles, was inhibited.[7] Moreover, the expression of Dhh and Ihh in granulosa cells was regulated by oocyte-secreted GDF-9[7] [Figure 1], resulting in the formation of an axis composed of GDF-9 (oocytes), Dhh/Ihh (granulosa cells), and PTCH (theca cells), which plays a crucial role in the formation of theca layers. Sequencing human oocytes and granulosa cells at each stage of folliculogenesis showed that similar to rodents, DHH and IHH were highly expressed in granulosa cells of all stages, especially antral follicles, while SHH expression was low. GDF-9 was highly expressed in oocytes at all stages.[26] Therefore, this axis also exists in human follicles and mediates the formation and differentiation of theca layers.
SMO is a seven-transmembrane signal transduction protein located on the cell membrane and serves as a “bridge” linking PTCH and Gli in the Hh pathway. Amhr2cre/+SmoM2 mice were genetically engineered with a cre/loxP strategy to conditionally express Smo-dominant active allele SmoM2 in the ovaries.[27,28] The expression of Smo in these mutant mice was significantly higher than that in controls.[27] Current studies related to SMO in follicles are mostly based on this transgenic mouse model. These mutant mice were first found to be infertile and to lack smooth muscle in follicles. Specifically, from the perspective of the follicular growth trajectory, oocytes were trapped in follicles after entering the ovulation stage. In mutants, the expression levels of genes related to smooth muscle calponin 1 (Cnn1), desmin (Des), actin, gamma 2, smooth muscle, enteric (Actg2), and transgelin (Tagln) were significantly decreased, and α-smooth muscle actin (α-SMA), a smooth muscle fibroblast marker, showed low expression.[27] This team then explored the causes of anovulation further and found that the density of endothelial tubes labeled by platelet endothelial cell adhesion molecule (PECAM) was increased in the ovarian cortex, and muscle-type vascular support cells were insufficient in theca layers.[28] Vasoconstriction of follicular vessels is an important factor causing follicle rupture and ovulation.[29] Therefore, vascular dysplasia is one of the reasons why excessive activation of the Hh pathway (namely, over-expression of Smo) leads to the occurrence of anovulation.
TGF-β Superfamily (GDF-9 and BMP-15)
The differentiation and proliferation of theca cells are controlled by ovary-derived signals, and the TGF-β superfamily plays an important role. The composition of the TGF-β signaling pathway in ovaries varies across species.[30]
BMP-15 and GDF-9 are the two most noteworthy members involved in oocyte regulation of theca cell function. Several events are affected by the absence of GDF-9, such as oocyte maturation, ovulation, follicular cell proliferation, and steroid hormone synthesis. GDF-9 is thus an essential regulator in the development of many mammalian follicles.[31–35] The effect of GDF-9 on theca cells can be divided into two parts: proliferation and physiological function. Ovarian Gdf9-deficient mice lost the ability to recruit theca progenitors.[12]In vivo experiments in rats and in vitro experiments in cattle showed that GDF-9 can promote the proliferation of theca cells.[36,37] The GDF-9 (oocyte)-Dhh/Ihh (granulosa cell)-PTCH (theca cell) signaling axis mentioned above might be the regulatory mechanism in this process.[7] The effect of GDF-9 on the physiological function of theca cells is mainly reflected in the expression level changes of steroid hormones, such as androgens and progesterone (PRG). When oocytes were injected with GDF-9 anti-sense oligos, CYP17A1 mRNA and androgen production were inhibited in follicles, which could be relieved by exogenous GDF-9.[38] GDF-9 may also promote the growth of follicles indirectly by up-regulating androgen levels as androgen is the main raw material for the synthesis of estrogen by granulosa cells.[38] Theca cells at different stages of folliculogenesis have diverse responses to GDF-9. In small follicles, GDF-9 could reduce the production of PRG and androstenedione (AND) in theca cells in a dose-dependent manner, whereas in large follicles, theca cells did not respond to IGF-1-induced cell proliferation and steroid production.[36] BMP-15, which is secreted by oocytes, plays an important role in regulating follicular cell recruitment and development, ovulation, atresia, and steroid hormone secretion.[30] Some observations in vitro showed that BMP-15 promotes the expression of genes related to theca cell maturation. Due to the strong correlation between oocyte-derived GDF-9 and BMP-15 in various species, their regulatory effects on granulosa cells and cumulus cells are also comparable. However, unlike GDF-9 that had lots of experimental results to support, the regulation of BMP-15 on theca cells’ origin and recruitment was mostly based on speculation and more evidence was needed to clarify this problem.
BMP-4 and BMP-7 are two important factors that regulate ovarian folliculogenesis and steroidogenesis. BMP-7 is mainly expressed in the theca interna, while BMP-4 is expressed in both the theca interna and externa.[39,40] Similar to mothers against decapentaplegic (Smad) 2/3 are the main intracellular signal transduction molecules of these two ligands. Studies on the functions of these two factors have mainly focused on the influence of folliculogenesis, granulosa cell proliferation, and hormone secretion. BMP-7 can positively regulate folliculogenesis progression before the antral follicle stage but significantly inhibited ovulation.[41] Similar to BMP-7, BMP-4 could also promote the transformation from primordial follicles to primary follicles.[42] Both BMP-4 and BMP-7 could affect the process of follicle-stimulating hormone-stimulated steroidogenesis in granulosa cells, where estradiol (E2) was promoted while PRG was inhibited.[40,43] Recently, studies have shown that BMP-4 and BMP-7 can also regulate the function of theca cells through autocrine mechanisms.[44] This regulation was mainly reflected in the inhibition of androgen production in cultured theca cells.[43,44] The mechanism was BMP-4- and BMP-7-induced down-regulation of the expression of enzymes associated with steroidogenesis, such as CYP17A1, StAR, and 3β-hydroxysteroid dehydrogenase, through aggregation of intracellular phosphorylated Smad1.[44] Overall, the main target follicular cells of BMP-4 and BMP-7 are granulosa cell and oocyte, and their main effect on theca cell is regulating the steroidogenesis but not recruitment or proliferation.
The receptors of TGF-β and BMP ligands are both transmembrane serine/threonine kinase receptors, which have similar characteristics, and comprise type I receptors, including activin receptor-like kinase 1 to 7 (ALK 1–7), and type II receptors, including activin type II/IIB receptor (Act RII/IIB), anti-Mullerian hormone receptor type 2 (AMHRII), TGF-β receptor type 2 (TGF-βRII), and BMP receptor type 2 (BMPRII).[45,46] In the presence of ligands, two type I receptors form heterotetramers with two type II receptors, which are then phosphorylated, further activating the downstream Smad pathway.[46] GDF-9 is mainly bound to the ALK5 and BMPRII receptors, while BMP-15 is mainly bound to the ALK6 and BMPRII receptors.[47] ALK5 activates Smad2/3, and ALK6 activates Smad1/5/8.[47] In addition to forming homodimers, GDF-9 and BMP-15 can also function as heterodimers, which activate the downstream Smad2/3 pathway by binding to the receptors formed by ALK4/5/7 and BMPRII.[48,49] Heterodimers are more bioactive than homodimers in promoting cumulus expansion in vivo in mice and humans.[49] The distribution of these receptors on theca cells in different species is also heterogeneous. For example, BMPRIA (also known as ALK3) and BMPRIB (also known as ALK6) were expressed in rat theca cells, but BMPRII was not.[39] Immunohistochemistry showed that BMPRIA, BMPRIB, and BMPRII were all weakly expressed in theca cells of porcine antral follicles.[50] Although the relationship between the TGF-β superfamily and folliculogenesis and follicular function has been extensively studied, evidence for the effect of these factors on theca cells is still limited. The distribution of these receptors on theca cells suggests to some extent that these ligands may directly act on theca cells, which requires further exploration.
Kit Ligand and the c-Kit System
KL is the first granulosa cell-derived factor that has been confirmed to be involved in the regulation of theca cell function.[51] KLs, which are also known as stem cell factor, steel factor, and mast cell growth factor, are mainly secreted by granulosa cells in many mammals.[51–53] According to the alternative splicing forms of mRNA, KL can be divided into the soluble (KL-1) and membrane spanning (KL-2) subtypes. KL-2 mainly mediates anchoring adhesion and direct signal communication between cells, while KL-1 mainly functions through autocrine and paracrine mechanisms.[54] Initially, these two forms are similarly anchored to the plasma membrane. KL-1 has a cleavage site encoded by exon 6, which is absent in KL-2.[55] The expression patterns of KL subtypes are diverse in different mammals. KL-1 and KL-2 are both expressed in antral follicles in goats, whereas only KL-1 can be expressed in the early stage of folliculogenesis.[56] KL-1 is preferentially expressed in the ovarian surface epithelium in rats.[57] KL-2 is highly expressed in the early stage of human folliculogenesis, while KL-1 is mainly expressed in the pre-ovulatory stage.[58] Interestingly, western blot results showed that in addition to 36 kD (KL-1) and 33 kD (KL-2) bands, an unknown 29 kD band emerged in human pre-antral and antral follicles, which disappeared after the addition of an anti-KL antibody, indicating the existence of another KL subtype in humans, which may be highly expressed in all follicle stages.[58]
C-kit, the membrane receptor of KL, belongs to the type III tyrosine kinase receptor family and has five extracellular immunoglobulin-like loops, an α-helix transmembrane domain, and two intracellular tyrosine kinase domains.[54] The presence of KL will dimerize two c-kit monomers, the two inner immunoglobulin-like loops will reorient and move closer to each other, and then further interactions will occur between the two α-helices.[54,59,60] Subsequently, the intracellular tyrosine residues are phosphorylated and then transmit signals via the phosphoinositide 3-kinase (PI3K), phosphatase and tensin homolog, mitogen-activated protein kinase, and Janus kinase/signal transducers and activators of transcription pathways to various parts of the cell.[60] C-kit can be expressed in the theca cells of many vertebrates and is abundant in the secondary and antral theca cells of mice, bovines, hogs, and goats[51,52,55] but is less abundant in the antral theca cells of rabbits.[53] In human premenopausal ovaries, the temporal and spatial expression specificities of KL and c-kit systems are not significant. Immunohistochemistry results showed that these two factors were expressed more or less in various cell types (granulosa cells, theca cells, and stromal cells) of all stages except for atresia follicles (from primordial to antral),[58] suggesting that this signaling system in humans may function in different manners, including in a paracrine and autocrine manner.[58]
The regulatory effects of KL on theca cells include recruitment, proliferation, and steroidogenesis. Parrott and Skinner[61] called KL the “theca cell organizer” because they found that KL could promote the proliferation and recruitment of specific undifferentiated stromal cells to surround primary follicles. Interestingly, KL could increase the production of steroids (mainly AND) in theca cells but had no effect on the production of PRG or AND in stromal cells (high expression of AND and PRG is a significant and vital theca cell feature); that is, KL alone cannot drive stromal cells to differentiate.[61,62] KL is thought to be associated with androgen synthesis in somatic cells of reproductive organs. The peak of c-kit in Sertoli cells is consistent with the time of testicular androgen peaks in various animals’ reproductive cycles. In the reproductive cycle of the seasonal breeder green frog, the time at which c-kit peaked in Sertoli cells coincided with the time at which androgen peaked in the testis.[63] The concentration of soluble c-kit in human follicles was significantly correlated with the concentrations of testosterone (T) and ADH in FF.[64] However, the relationship between KL and steroidogenesis in theca cells and the associated regulatory mechanisms remain to be explored in more detail.
Keratinocyte growth factor (KGF) and hepatocyte growth factor (HGF) are two theca cell-derived factors that can modulate the expression of KL in granulosa cells,[65,66] and KL can modulate the expression of KGF and HGF in response,[65,66] creating a positive feedback loop between granulosa cells and theca cells. Therefore, the recruitment and proliferation of theca cells may be regulated by KGF and HGF indirectly through this feedback loop.
Insulin-Like Growth Factor
IGF, including IGF-1 and IGF-2, is a protein that is typically produced by the liver in response to GH stimulation.[67] IGF-1, which is produced by granulosa cells locally in the ovary,[68] is recognized to stimulate the proliferation and steroidogenesis of theca cells in a paracrine manner.[1,69] In the presence of IGF-1, IGF receptor is activated to transfer signals to organelles and the nucleus through the PLC/PKC and PI3K/Akt pathways.[70] Furthermore, extensive evidence has shown that IGF-1 can promote the differentiation of theca cells in vitro. For example, the expression of LH receptor, which indicated the differentiation of theca cells, could be increased when IGF-1 was added to rat theca cell culture medium.[71] Androgen could be synthesized by cultured human stromal tissue with insulin and IGF addition to the medium.[72] Furthermore, another non-negligible function of IGF-1 is increasing the number of gap junctions between theca-granulosa cells and granulosa cell-oocytes, thus facilitating signal communication between follicular cell components.[73]
Growth hormone (GH), another irreplaceable hormone contributing to the growth and development of the body, is the precursor in the liver for IGF-1 synthesis. GH could positively regulate the secretory activity of IGF-1 through the cAMP/protein kinase A signaling pathway in cultured bovine granulosa cells[74] and thus indirectly stimulate the proliferation and differentiation of theca cells. Previous studies reported the presence of GH receptor (GHR) mRNA in bovine theca cells,[75] implying a direct effect of GH on theca cells. However, evidence for this direct effect in humans was insufficient because GHR mRNA was not detected in the human theca interna and externa.[76] GH can also impact the effect of Gn on follicular somatic cells. The density of follicle-stimulating hormone receptor and LH receptor could be increased in human granulosa cells when treated with GH,[77] but whether GH has a similar effect on theca cells remains questionable.
Other Signals
Many other factors may be related to the differentiation and function of theca cells. Basic fibroblast growth factor, a factor produced by oocytes, granulosa cells, and theca cells,[78,79] could promote folliculogenesis as well as growth and steroidogenesis of theca cells and granulosa cells.[79,80] PCOS is a reproductive endocrine disorder in women of childbearing age characterized by hyper-androgenemia. Ovarian theca cells are thought to be one of the main sources of excess androgen in PCOS patients. Retinoic acid and GATA6 can increase the expression of CYP17A1, thus promoting androgen production.[81,82] By comparing the transcriptomes of normal and PCOS theca cells, the expression of GATA6, retinoic acid synthase, aldehyde dehydrogenase 6 (ALDH6), and retinol dehydrogenase 2 (RDH2) was found to be increased in PCOS patients.[82,83] Another important change in PCOS theca cells was inhibition of the wingless-int (Wnt) signaling pathway as demonstrated by up-regulation of Wnt antagonists (secreted frizzled-related protein 4 [sFRP4]and dickkopf-related protein 1 [DKK1]) and down-regulation of Wnt5A.[83] In addition, Wnt signals may also play an important role in folliculogenesis. A study comparing the transcriptomes of theca layers from normal bovine antral follicles with different diameters (3–5 and 9–12 mm) found that Wnt was the most significant pathway as evidenced by down-regulation of Wnt2B and up-regulation of the Wnt inhibitor frizzle-related protein (FRZB) in large follicles.[2] This change may indicate that the Wnt signaling pathway plays a role in the development of theca layers. However, due to the complex cellular composition of theca layers, whether this change occurs in theca cells or other cell types and whether the cause is a change in cell function or cell type composition remain to be further explored.[2] Hyper-insulinemia is also an important clinical manifestation of PCOS. Excessive insulin can increase CYP17A1 activity in theca cells[62] and promote LH-, IGF-I-, and GDF-9-mediated androgen production.[38,84] PCOS is a complicated reproductive system disease affected by many factors, such as genetic and environmental factors. Understanding the functional status of theca cells in PCOS patients will be helpful for studying the physiological function, regulatory factors, and pathological status of theca cells.
Conclusions
Folliculogenesis is a dynamic process involving oocytes and somatic cells. Any changes or loss in the function of a cell component can affect normal folliculogenesis. Although research on theca cells has been neglected to date, the role of theca cells in folliculogenesis, especially in antral follicles and the later stages, is undeniably essential. The formation of theca layers involves many events, such as the recruitment and growth of theca cells and the development of vascular structures. The complex and orderly regulatory system composed of various factors derived from oocytes, granulosa cells, and signaling pathways in theca cells plays an important role in the recruitment and differentiation of theca cells. At the same time, theca cells can also produce a variety of factors involved in the regulation of follicular function. Studying the derivation, differentiation, and function of theca cells will help us to systematically elaborate the mechanism of follicular formation and development, improve the quality of follicles and the developmental potential of embryos, and treat clinical PCOS and other diseases that may involve theca cell dysfunction.
Funding
This work was supported by grants from the National Key Technology R&D Program of China (Nos. 2017YFC1002002 and 2018YFC1004001), the National Science Foundation of China (No. 81571386), and the Special Research Project of Chinese Capital Health Development (No. 2018-2-4095).
Conflicts of interest
None.
Footnotes
How to cite this article: Liu T, Qin QY, Qu JX, Wang HY, Yan J. Where are the theca cells from: the mechanism of theca cells derivation and differentiation. Chin Med J 2020;133:1711–1718. doi: 10.1097/CM9.0000000000000850
References
- 1.Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction 2010; 140:489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- 2.Hatzirodos N, Hummitzsch K, Irving-Rodgers HF, Rodgers RJ. Transcriptome profiling of the theca interna in transition from small to large antral ovarian follicles. PLoS One 2014; 9:e97489.doi: 10.1371/journal.pone.0097489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto S, Konishi I, Tsuruta Y, Nanbu K, Mandai M, Kuroda H, et al. Expression of vascular endothelial growth factor (VEGF) during folliculogenesis and corpus luteum formation in the human ovary. Gynecol Endocrinol 1997; 11:371–381. doi: 10.3109/09513599709152564. [DOI] [PubMed] [Google Scholar]
- 4.Richards JS, Ren YA, Candelaria N, Adams JE, Rajkovic A. Ovarian follicular theca cell recruitment, differentiation, and impact on fertility: 2017 update. Endocr Rev 2018; 39:1–20. doi: 10.1210/er.2017-00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irving-Rodgers HF, van Wezel IL, Mussard ML, Kinder JE, Rodgers RJ. Atresia revisited: two basic patterns of atresia of bovine antral follicles. Reproduction 2001; 122:761–775. doi: 10.1530/rep.0.1220761. [PubMed] [Google Scholar]
- 6.Honda A, Hirose M, Hara K, Matoba S, Inoue K, Miki H, et al. Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells. Proc Natl Acad Sci U S A 2007; 104:12389–12394. doi: 10.1073/pnas.0703787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Peng J, Matzuk MM, Yao HH. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat Commun 2015; 6:6934.doi: 10.1038/ncomms7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barsoum IB, Yao HH. Fetal Leydig cells: progenitor cell maintenance and differentiation. J Androl 2010; 31:11–15. doi: 10.2164/jandrol.109.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotsuji F, Kamitani N, Goto K, Tominaga T. Bovine theca and granulosa cell interactions modulate their growth, morphology, and function. Biol Reprod 1990; 43:726–732. doi: 10.1095/biolreprod43.5.726. [DOI] [PubMed] [Google Scholar]
- 10.Tajima K, Orisaka M, Yata H, Goto K, Hosokawa K, Kotsuji F. Role of granulosa and theca cell interactions in ovarian follicular maturation. Microsc Res Tech 2006; 69:450–458. doi: 10.1002/jemt.20304. [DOI] [PubMed] [Google Scholar]
- 11.Orisaka M, Tajima K, Mizutani T, Miyamoto K, Tsang BK, Fukuda S, et al. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol Reprod 2006; 75:734–740. doi: 10.1095/biolreprod.105.050344. [DOI] [PubMed] [Google Scholar]
- 12.Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol 1999; 13:1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 13.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 14.Franco HL, Yao HH. Sex and hedgehog: roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosome Res 2012; 20:247–258. doi: 10.1007/s10577-011-9254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finco I, LaPensee CR, Krill KT, Hammer GD. Hedgehog signaling and steroidogenesis. Annu Rev Physiol 2015; 77:105–129. doi: 10.1146/annurev-physiol-061214-111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monkkonen T, Lewis MT. New paradigms for the hedgehog signaling network in mammary gland development and breast cancer. Biochim Biophys Acta Rev Cancer 2017; 1868:315–332. doi: 10.1016/j.bbcan.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingham PW, Taylor AM, Nakano Y. Role of the Drosophila patched gene in positional signalling. Nature 1991; 353:184–187. doi: 10.1038/353184a0. [DOI] [PubMed] [Google Scholar]
- 18.van den Heuvel M, Ingham PW. Smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature 1996; 382:547–551. doi: 10.1038/382547a0. [DOI] [PubMed] [Google Scholar]
- 19.Varjosalo M, Li SP, Taipale J. Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev Cell 2006; 10:177–186. doi: 10.1016/j.devcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Collins RT, Cohen SM. A genetic screen in Drosophila for identifying novel components of the hedgehog signaling pathway. Genetics 2005; 170:173–184. doi: 10.1534/genetics.104.039420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apionishev S, Katanayeva NM, Marks SA, Kalderon D, Tomlinson A. Drosophila smoothened phosphorylation sites essential for hedgehog signal transduction. Nat Cell Biol 2005; 7:86–92. doi: 10.1038/ncb1210. [DOI] [PubMed] [Google Scholar]
- 22.Ho KS, Suyama K, Fish M, Scott MP. Differential regulation of hedgehog target gene transcription by Costal2 and suppressor of fused. Development 2005; 132:1401–1412. doi: 10.1242/dev.01689. [DOI] [PubMed] [Google Scholar]
- 23.Lum L, Beachy PA. The hedgehog response network: sensors, switches, and routers. Science 2004; 304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 24.Yao HH, Whoriskey W, Capel B. Desert hedgehog/patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 2002; 16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijgerde M, Ooms M, Hoogerbrugge JW, Grootegoed JA. Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology 2005; 146:3558–3566. doi: 10.1210/en.2005-0311. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Yan Z, Qin Q, Nisenblat V, Chang HM, Yu Y, et al. Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions. Mol Cell 2018; 72:1021–1034.e4. doi: 10.1016/j.molcel.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Ren Y, Cowan RG, Harman RM, Quirk SM. Dominant activation of the hedgehog signaling pathway in the ovary alters theca development and prevents ovulation. Mol Endocrinol 2009; 23:711–723. doi: 10.1210/me.2008-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren Y, Cowan RG, Migone FF, Quirk SM. Overactivation of hedgehog signaling alters development of the ovarian vasculature in mice. Biol Reprod 2012; 86:174.doi: 10.1095/biolreprod.112.099176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migone FF, Cowan RG, Williams RM, Gorse KJ, Zipfel WR, Quirk SM. In vivo imaging reveals an essential role of vasoconstriction in rupture of the ovarian follicle at ovulation. Proc Natl Acad Sci U S A 2016; 113:2294–2299. doi: 10.1073/pnas.1512304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update 2016; 23:1–18. doi: 10.1093/humupd/dmw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook-Andersen H, Curnow KJ, Su HI, Chang RJ, Shimasaki S. Growth and differentiation factor 9 promotes oocyte growth at the primary but not the early secondary stage in three-dimensional follicle culture. J Assist Reprod Genet 2016; 33:1067–1077. doi: 10.1007/s10815-016-0719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gode F, Gulekli B, Dogan E, Korhan P, Dogan S, Bige O, et al. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil Steril 2011; 95:2274–2278. doi: 10.1016/j.fertnstert.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 33.Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, et al. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod 2004; 70:900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- 34.Juengel JL, McNatty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 2005; 11:143–160. doi: 10.1093/humupd/dmh061. [DOI] [PubMed] [Google Scholar]
- 35.Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 2008; 135:111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 36.Spicer LJ, Aad PY, Allen DT, Mazerbourg S, Payne AH, Hsueh AJ. Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biol Reprod 2008; 78:243–253. doi: 10.1095/biolreprod.107.063446. [DOI] [PubMed] [Google Scholar]
- 37.Vitt UA, McGee EA, Hayashi M, Hsueh AJ. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology 2000; 141:3814–3820. doi: 10.1210/endo.141.10.7732. [DOI] [PubMed] [Google Scholar]
- 38.Orisaka M, Jiang JY, Orisaka S, Kotsuji F, Tsang BK. Growth differentiation factor 9 promotes rat preantral follicle growth by up-regulating follicular androgen biosynthesis. Endocrinology 2009; 150:2740–2748. doi: 10.1210/en.2008-1536. [DOI] [PubMed] [Google Scholar]
- 39.Erickson GF, Shimasaki S. The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrinol 2003; 1:9.doi: 10.1186/1477-7827-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimasaki S, Zachow RJ, Li D, Kim H, Iemura S, Ueno N, et al. A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci U S A 1999; 96:7282–7287. doi: 10.1073/pnas.96.13.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod 2001; 65:994–999. doi: 10.1095/biolreprod65.4.994. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 2003; 69:1265–1272. doi: 10.1095/biolreprod.103.018671. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Du SY, Ding M, Dou X, Zhang FF, Wu ZY, et al. The BMP4-Smad signaling pathway regulates hyperandrogenism development in a female mouse model. J Biol Chem 2017; 292:11740–11750. doi: 10.1074/jbc.M117.781369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology 2005; 146:1883–1892. doi: 10.1210/en.2004-1303. [DOI] [PubMed] [Google Scholar]
- 45.de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev 2004; 15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Massague J. TGF-beta signal transduction. Annu Rev Biochem 1998; 67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 47.Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet 2018; 35:1741–1750. doi: 10.1007/s10815-018-1268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mottershead DG, Ritter LJ, Gilchrist RB. Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15. Mol Hum Reprod 2012; 18:121–128. doi: 10.1093/molehr/gar056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, et al. Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A 2013; 110:E776–E785. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quinn RL, Shuttleworth G, Hunter MG. Immunohistochemical localization of the bone morphogenetic protein receptors in the porcine ovary. J Anat 2004; 205:15–23. doi: 10.1111/j.0021-8782.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parrott JA, Skinner MK. Direct actions of kit-ligand on theca cell growth and differentiation during follicle development. Endocrinology 1997; 138:3819–3827. doi: 10.1210/endo.138.9.5368. [DOI] [PubMed] [Google Scholar]
- 52.Brankin V, Hunter MG, Horan TL, Armstrong DG, Webb R. The expression patterns of mRNA-encoding stem cell factor, internal stem cell factor and c-kit in the prepubertal and adult porcine ovary. J Anat 2004; 205:393–403. doi: 10.1111/j.0021-8782.2004.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutt KJ, McLaughlin EA, Holland MK. Kit/kit ligand in mammalian oogenesis and folliculogenesis: roles in rabbit and murine ovarian follicle activation and oocyte growth. Biol Reprod 2006; 75:421–433. doi: 10.1095/biolreprod.106.051516. [DOI] [PubMed] [Google Scholar]
- 54.Merkwitz C, Lochhead P, Tsikolia N, Koch D, Sygnecka K, Sakurai M, et al. Expression of KIT in the ovary, and the role of somatic precursor cells. Prog Histochem Cytochem 2011; 46:131–184. doi: 10.1016/j.proghi.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod 2006; 12:61–69. doi: 10.1093/molehr/gal010. [DOI] [PubMed] [Google Scholar]
- 56.Silva JR, van den Hurk R, van Tol HT, Roelen BA, Figueiredo JR. The kit ligand/c-kit receptor system in goat ovaries: gene expression and protein localization. Zygote 2006; 14:317–328. doi: 10.1017/S0967199406003832. [DOI] [PubMed] [Google Scholar]
- 57.Ismail RS, Cada M, Vanderhyden BC. Transforming growth factor-beta regulates Kit ligand expression in rat ovarian surface epithelial cells. Oncogene 1999; 18:4734–4741. doi: 10.1038/sj.onc.1202865. [DOI] [PubMed] [Google Scholar]
- 58.Tuck AR, Robker RL, Norman RJ, Tilley WD, Hickey TE. Expression and localisation of c-kit and KITL in the adult human ovary. J Ovarian Res 2015; 8:31.doi: 10.1186/s13048-015-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemmon MA, Ferguson KM. A new twist in the transmembrane signaling tool-kit. Cell 2007; 130:213–215. doi: 10.1016/j.cell.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Liu H, Chen X, Focia PJ, He X. Structural basis for stem cell factor-KIT signaling and activation of class III receptor tyrosine kinases. EMBO J 2007; 26:891–901. doi: 10.1038/sj.emboj.7601545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parrott JA, Skinner MK. Kit ligand actions on ovarian stromal cells: effects on theca cell recruitment and steroid production. Mol Reprod Dev 2000; 55:55–64. doi: 10.1002/(SICI)1098-2795(200001)55:1<55::AID-MRD8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 62.Huang CT, Weitsman SR, Dykes BN, Magoffin DA. Stem cell factor and insulin-like growth factor-I stimulate luteinizing hormone-independent differentiation of rat ovarian theca cells. Biol Reprod 2001; 64:451–456. doi: 10.1095/biolreprod64.2.451. [DOI] [PubMed] [Google Scholar]
- 63.Raucci F, Di Fiore MM. The c-kit receptor protein in the testis of green frog Rana esculenta: seasonal changes in relationship to testosterone titres and spermatogonial proliferation. Reproduction 2007; 133:51–60. doi: 10.1530/rep.1.01009. [DOI] [PubMed] [Google Scholar]
- 64.Tanikawa M, Harada T, Mitsunari M, Onohara Y, Iwabe T, Terakawa N. Expression of c-kit messenger ribonucleic acid in human oocyte and presence of soluble c-kit in follicular fluid. J Clin Endocrinol Metab 1998; 83:1239–1242. doi: 10.1210/jcem.83.4.4746. [DOI] [PubMed] [Google Scholar]
- 65.Parrott JA, Skinner MK. Thecal cell-granulosa cell interactions involve a positive feedback loop among keratinocyte growth factor, hepatocyte growth factor, and kit ligand during ovarian follicular development. Endocrinology 1998; 139:2240–2245. doi: 10.1210/endo.139.5.6018. [DOI] [PubMed] [Google Scholar]
- 66.Parrott JA, Mosher R, Kim G, Skinner MK. Autocrine interactions of keratinocyte growth factor, hepatocyte growth factor, and kit-ligand in the regulation of normal ovarian surface epithelial cells. Endocrinology 2000; 141:2532–2539. doi: 10.1210/endo.141.7.7581. [DOI] [PubMed] [Google Scholar]
- 67.Silva JR, Figueiredo JR, van den Hurk R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009; 71:1193–1208. doi: 10.1016/j.theriogenology.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 68.Schams D, Berisha B, Kosmann M, Amselgruber WM. Expression and localization of IGF family members in bovine antral follicles during final growth and in luteal tissue during different stages of estrous cycle and pregnancy. Domest Anim Endocrinol 2002; 22:51–72. doi: 10.1016/s0739-7240(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 69.Duleba AJ, Spaczynski RZ, Olive DL. Insulin and insulin-like growth factor I stimulate the proliferation of human ovarian theca-interstitial cells. Fertil Steril 1998; 69:335–340. doi: 10.1016/s0015-0282(97)00473-1. [DOI] [PubMed] [Google Scholar]
- 70.Ipsa E, Cruzat VF, Kagize JN, Yovich JL, Keane KN. Growth hormone and insulin-like growth factor action in reproductive tissues. Front Endocrinol (Lausanne) 2019; 10:777.doi: 10.3389/fendo.2019.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magoffin DA, Weitsman SR. Insulin-like growth factor-I regulation of luteinizing hormone (LH) receptor messenger ribonucleic acid expression and LH-stimulated signal transduction in rat ovarian theca-interstitial cells. Biol Reprod 1994; 51:766–775. doi: 10.1095/biolreprod51.4.766. [DOI] [PubMed] [Google Scholar]
- 72.Barbieri RL, Makris A, Ryan KJ. Insulin stimulates androgen accumulation in incubations of human ovarian stroma and theca. Obstet Gynecol 1984; 64:73S–80S. doi: 10.1097/00006250-198409001-00019. [DOI] [PubMed] [Google Scholar]
- 73.Zhao J, Taverne MA, Van Der Weijden GC, Bevers MM, Van Den Hurk R. Insulin-like growth factor-I (IGF-I) stimulates the development of cultured rat pre-antral follicles. Mol Reprod Dev 2001; 58:287–296. doi: 10.1002/1098-2795(200103)58:3<287::AID-MRD7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 74.Sirotkin AV, Makarevich AV. GH regulates secretory activity and apoptosis in cultured bovine granulosa cells through the activation of the cAMP/protein kinase A system. J Endocrinol 1999; 163:317–327. doi: 10.1677/joe.0.1630317. [DOI] [PubMed] [Google Scholar]
- 75.Shimizu T, Murayama C, Sudo N, Kawashima C, Tetsuka M, Miyamoto A. Involvement of insulin and growth hormone (GH) during follicular development in the bovine ovary. Anim Reprod Sci 2008; 106:143–152. doi: 10.1016/j.anireprosci.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Sharara FI, Nieman LK. Identification and cellular localization of growth hormone receptor gene expression in the human ovary. J Clin Endocrinol Metab 1994; 79:670–672. doi: 10.1210/jcem.79.2.7519196. [DOI] [PubMed] [Google Scholar]
- 77.Regan SLP, Knight PG, Yovich JL, Arfuso F, Dharmarajan A. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells, with improved pregnancy outcomes. Fertil Steril 2018; 110:1298–1310. doi: 10.1016/j.fertnstert.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 78.Yamamoto S, Konishi I, Nanbu K, Komatsu T, Mandai M, Kuroda H, et al. Immunohistochemical localization of basic fibroblast growth factor (bFGF) during folliculogenesis in the human ovary. Gynecol Endocrinol 1997; 11:223–230. doi: 10.3109/09513599709152538. [DOI] [PubMed] [Google Scholar]
- 79.van Wezel IL, Umapathysivam K, Tilley WD, Rodgers RJ. Immunohistochemical localization of basic fibroblast growth factor in bovine ovarian follicles. Mol Cell Endocrinol 1995; 115:133–140. doi: 10.1016/0303-7207(95)03678-4. [DOI] [PubMed] [Google Scholar]
- 80.Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol 2001; 175:123–130. doi: 10.1016/s0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- 81.Wickenheisser JK, Nelson-DeGrave VL, Hendricks KL, Legro RS, Strauss JF, 3rd, McAllister JM. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90:4858–4865. doi: 10.1210/jc.2005-0330. [DOI] [PubMed] [Google Scholar]
- 82.Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, Urbanek M, et al. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem 2003; 278:26380–26390. doi: 10.1074/jbc.M300688200. [DOI] [PubMed] [Google Scholar]
- 83.Wood JR, Ho CK, Nelson-Degrave VL, McAllister JM, Strauss JF., 3rd The molecular signature of polycystic ovary syndrome (PCOS) theca cells defined by gene expression profiling. J Reprod Immunol 2004; 63:51–60. doi: 10.1016/j.jri.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril 1993; 59:323–331. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]


