Abstract
Background
Spinal metastases can cause metastatic epidural spinal cord compression (MESCC), which can result in neurological dysfunction and impaired quality of life. This study investigated the safety and effectiveness of posterior decompression surgery and radiofrequency ablation followed by vertebroplasty in spinal metastasis from lung cancer.
Material/Methods
From June 2008 to September 2015, a retrospective analysis was conducted in 15 patients with spinal metastasis from lung cancer. All cases suffered MESCC and underwent posterior decompression surgery to relieve the compression of spinal cord, and had radiofrequency ablation followed by vertebroplasty. All patients received postoperative multidisciplinary therapy. The operative time, blood loss, complications, pain, neurologic deficit, quality of life, and survival were assessed preoperatively and postoperatively.
Results
Patients were followed from 6 to 56 months. The mean time of operation was 154±50 minutes and the mean blood loss was 210±90 mL. In the pre-operation analysis found the mean visual analogue scale (VAS) was 7.86±0.86. In the post-operation analysis at 3 months, the mean VAS score was 3.51±1.32. The VAS improved significantly (t=7.95, P<0.01). The Frankel grade was improved 1 grade or 2 grades in 14 patients when pre-operation was compared to post-operation. Only 1 patient kept Frankel grade D after surgery. Eight patients with sphincteric dysfunction preoperatively were improved after surgery. The EORTC QLQ-C30 score was 86.13±8.51 preoperatively and 52.21±13.28 postoperatively. The quality of life was improved significantly (t=11.8, P<0.01). The median survival time was 11 months.
Conclusions
Through posterior decompression surgery and radiofrequency ablation followed by vertebroplasty, the quality of life was improved significantly. This palliative treatment was effective and safe in spinal metastasis from lung cancer.
MeSH Keywords: Lung Neoplasms, Pulsed Radiofrequency Treatment, Spinal Cord Compression, Vertebroplasty
Background
There are 1.3 million people who have died annually worldwide because of lung cancer [1]. About 65% of patients with lung cancer will have bone metastasis. The most common site of bone metastases is the spine. Metastatic epidural spinal cord compression (MESCC) has been found in 5% to 10% of all cancer patients, and it is a very common complication of spinal metastasis [2]. MESCC is associated with mobility, sphincteric function, and so on. The median survival of patients with bone metastases from lung cancer is from 2.1 to 9.9 months [3–5].
In several studies, some patients with spinal metastasis from lung cancer underwent total en bloc spondylectomy (TES), with the median survival 26 to 46.3 months [6,7]. However, many studies have shown that TES may caused excessive morbidity, blood loss, and long surgery time. Murakami et al. suggested that TES should be performed in selected patients with controlled primary lung cancer, localized spinal metastases, and no visceral metastases [7]. In these patients, surgery can improve prognosis through TES. But the complication of TES remains high even in selected patients. So, risks and benefits must be weighed before TES is performed. Only a few selected cases to receive TES; the majority of cases undergo the palliative surgery.
In 1981, Harrington was the first to use cement to strengthen vertebral stability in treating 14 patients with spinal metastasis through an open procedure [8]. In 1987, Deramond was the first to inject bone cement percutaneously in treating spinal hemangioma [9]. Today, percutaneous vertebroplasty is widely used in treatment of spinal metastases, but percutaneous vertebroplasty is associated with higher complication rates especially related to cement leakage [10–13].
Radiofrequency ablation device generates 460 kHz radio frequency current, with high-speed ion vibration and friction generated in the surrounding tissues through the electrodes, which is then transformed into heat energy. When the temperature reaches a certain target temperature, the local tissues and cells will undergo thermal coagulation. Radiofrequency ablation was initially used in the treatment of liver cancer and achieved good therapeutic effect. In the 21st century, radiofrequency ablation combined with vertebroplasty for treatment of spinal metastasis was introduced. Several studies have shown that the combination of radiofrequency ablation with percutaneous vertebroplasty is safe and effective in spinal metastases [14–16]. Radiofrequency ablation performed in spinal metastases can cause coagulation necrosis of tumor cells and reduce the complications of cement leakage.
Considering that the life expectancy of lung cancer patients with bone metastasis is short, the main purpose of surgical treatment is to relieve pain, improve neurological function, and enhance the quality of life. We studied the safety and effectiveness of posterior decompression surgery and radiofrequency ablation followed by vertebroplasty in spinal metastasis from lung cancer.
Material and Methods
There were 15 patients with spinal metastasis from lung cancer enrolled from June 2008 to September 2015. Patients include in the study meet the following criteria: 1) patients had metastatic epidural spinal cord compression (MESCC) which caused neurological dysfunction or pain or paraplegic for shorter than 48 hours. 2) Life expectancy was at least 3 months. 3) The patients have a general status good enough to undergo surgery. The contraindications to surgery were the following: 1) suitable status for total en bloc spondylectomy; 2) contraindications to general anesthesia; 3) intolerable to potential surgical complications.
In pre-operation assessment, pain, neurologic deficit, and quality of life were evaluated by visual analogue scale (VAS), Frankel grade, and European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire, respectively. The biomechanical situation was described using the spinal instability neoplastic score (SINS).
All patients were given routine examinations such as cardiopulmonary function tests, ultrasound, blood routine, blood coagulation routine, blood group, biochemical routine, etc. Patients had magnetic resonance imaging (MRI) and x-ray studies. MRI was used because it can identify spinal cord compression clearly.
Surgery
General anesthesia was used in all patients, and the operation position was the prone position. Under C-arm guidance, we identified the lesion segment and made a median longitudinal incision. We stripped bilateral erector spine muscles to expose lamina, severed the supraspinal ligament and interspinal ligament, and removed spinous process, bilateral lamina, and ligamentum flavum. The spinal cord and nerve roots were fully exposed, the compression of the spinal cord was fully relieved, and the fluctuation of the spinal cord was restored.
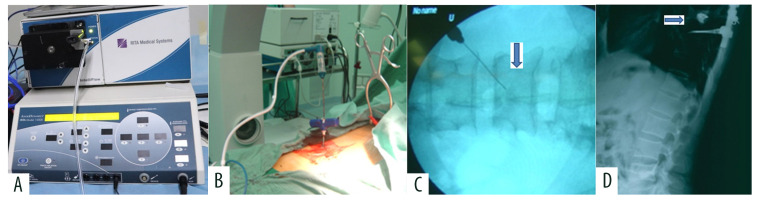
After the posterior decompression was finished, radiofrequency ablation was performed in the lesion vertebral body. The radiofrequency ablation device was RITA® 1500 RF electrosurgical generator (RITA Medical Systems, Inc., Mountain View, CA, USA) and UniblateTM Rita RF electrodes (RITA Medical Systems, Inc., Mountain View, CA, USA). The 13-gauge bone puncture needle established the channel through a transpedicular approach under C-arm guidance. We used monopolar electrodes with 1 cm or 2 cm active tip in the vertebral lesion (Figure 1). The power setting was 75 W. The maximum temperature of the electrode active tip reached 85°C. Radiofrequency ablation lasted for 10 minutes. The monopolar electrode was removed after radiofrequency ablation. Vertebroplasty was done slowly under C-arm guidance. The amount of bone cement was about 3–5 mL. Heart rate, blood pressure, and electrocardiogram were closely monitored during the process. At last, under C-arm guidance, we reconstructed spinal stability with screw-rod internal fixation (Figure 2).
Figure 1.
Spinal metastases with Tomita type 7. (A) Radiofrequency ablation device. (B, C) Monopolar electrodes with 2 cm active tip was in L4 vertebral lesion (↓). (D) Posterior surgery indirectly removes spinal cord compression from T8 vertebral lesion (→). After radiofrequency ablation, vertebroplasty was performed, and finally screw rod internal fixation was performed.
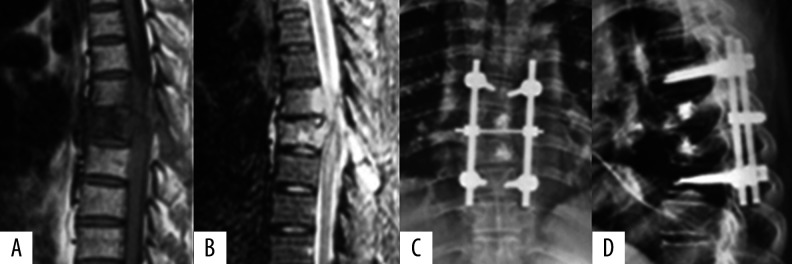
Figure 2.
A 52-year-old male with spinal metastases from lung cancer. (A, B) Preoperative magnetic resonance imaging showed spinal metastases in T6 and T7 vertebral body. Spinal cord was compressed. (C, D) Postoperative x-ray photographs showed nodular high-density bone cement images in vertebral body of T6, T7, and L1, posterior screw fixation in T5 and T8 vertebral body. Vertebral collapse and the adjacent intervertebral space narrowed were not seen.
Postoperative adjuvant therapy
All patients received postoperative multidisciplinary therapy including radiotherapy, chemotherapy, targeted therapy, and zoledronic acid. In all, 14 patients received radiotherapy; the total dose was 30–45 Gy given in 10–15 fractions; 8 patients received chemotherapy (4–6 cycles); 7 patients underwent gene detection found epidermal growth factor receptor (EGFR) mutation, received targeted drug treatment; 10 patients received zoledronic acid intravenously every 4 weeks.
Follow-up
All the patients received follow-up. In pre-operation evaluation and postoperative 3-month follow-up, pain, neurologic deficit, and quality of life were evaluated by visual analogue scale (VAS), Frankel grade, and EORTC QLQ-C30 questionnaire, respectively. Urinary sphincter function was observed as well.
Statistical analysis
VAS and EORTC QLQ-C30 scores were analyzed with paired-samples t-test. Survival analysis used Kaplan-Meier method. P-value <0.05 was considered statistically significant.
Results
From June 2008 to September 2015, 15 cases who met the inclusion criteria were enrolled, including 9 males and 6 females. The average age was 50.1 years (range 40–68 years). All patients were assessed by Tomita score and Tomita classification. Tomita score was 6–7. The spinal instability neoplastic score (SINS) was 12.1. Tomita classification [10] was as follows: Tomita type 4 (3 patients), Tomita type 5 (4 patients), Tomita type 6 (4 patients), Tomita type7 (4 patients). Patients were followed from 6 to 56 months. No patients were lost during follow-up.
Operative time, blood loss, and bone cement leakage
There were 15 patients who received posterior decompression surgery to relieve compression of the spinal cord, and radiofrequency ablation followed by vertebroplasty. At the same time, the spinal stability was reconstructed with screw-rod internal fixation. The mean time of operation was 154±50 minutes and mean blood loss was 210±90 mL. Cement leakage was observed in 2 patients. The rate of polymethylmethacrylate (PMMA) leakage was 13.3%. One patient had peri-vertebral soft tissue leakage and the other patient had adjacent disc leakage. There were no clinical symptoms caused by leakage.
Visual analogue scale (VAS)
All patients reported that pain was markedly relieved through surgery. In the pre-operation evaluation, the mean visual analogue scale (VAS) was 7.86±0.86. In postoperative 3-month evaluation, the mean VAS score was 3.51±1.32. The VAS improved significantly (t=7.95, P<0.01) (Table 1). One patient progressed in 8 months and had pain that was severe again; he received radiotherapy and the pain was controlled.
Table 1.
The comparison of clinical data between pre-surgery and post-surgery.
| Symptom/feature | Pre op (%) | Post op (%) |
|---|---|---|
| VAS | 7.86±0.86 | 3.51±1.32 |
| Sphincteric dysfunction | 86.7% (13/15) | 26.7% (4/15) |
| EORTC QLQ-C30 | 86.13±8.51 | 52.21±13.28 |
| Frankel C | 60.0% (9/15) | 0 (0/15) |
| Frankel D | 40.0% (6/15) | 60.0% (9/15) |
| Frankel E | 0 (0/15) | 40.0% (6/15) |
VAS – visual analogue scale; EORTC – European Organization for Research and Treatment of Cancer (EORTC).
Frankel grade and KPS
Neurologic deficits were evaluated by Frankel grade (Table 1). In the pre-operation assessment, 9 patients had Frankel grade C and 6 patients had Frankel grade D. In the post-operative assessment at 3 months, no patient had Frankel grade C, 9 patients had Frankel grade D, and 6 patients had Frankel grade E. Frankel grade was improved 1 grade or 2 grades in 14 patients. Only 1 patient kept grade D after surgery. The neurologic deficit of the spinal cord was improved significantly.
Sphincteric dysfunction
In all, 13 patients had sphincteric dysfunction. The urinary sphincter function of 2 patients was continent. At 3-months postoperatively, 8 out of 13 patients (61.5%) who were incontinent before surgery recovered by this treatment, but still 5 patients suffered from sphincteric dysfunction. Two patients who was normal before the operation were continent after the operation (Table 1).
EORTC QLQ-C30 score
All the patients completed the EORTC QLQ-C30 questionnaire. The EORTC QLQ-C30 score was 86.13±8.51 preoperatively and 52.21±13.28 postoperatively. The quality of life was reported to be improved significantly (t=11.8, P<0.01).
Survival
One patient was lost to follow-up and the other patients died. The mean survival was 19.6 months. The median survival was 11.0 months; the 1-year survival rate was 53.3%; the 2-year survival rate was 26.7%; the 3-year survival rate was 20.0% (Figure 3).
Figure 3.
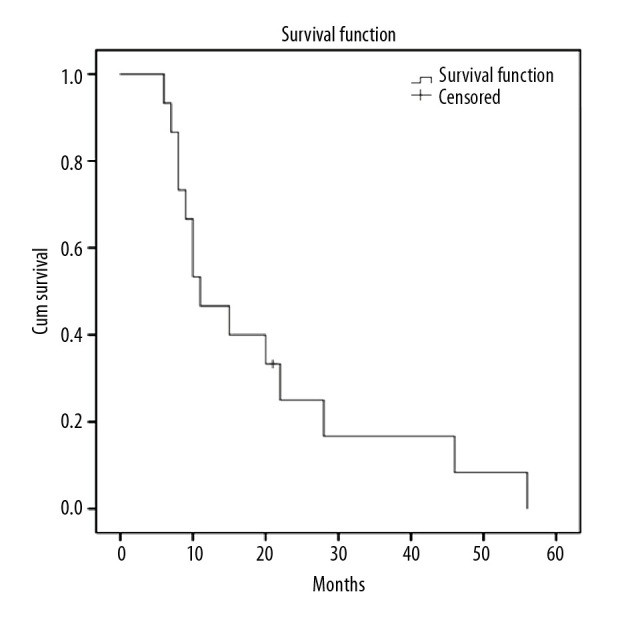
The follow-up period of the patients ranged from 6 to 56 months. The mean survival was 19.6 months. The median survival time was 11.0 months.
Complications
No patient died during the treatment. Three patients felt pain around the injection site, which subsides after 48 hours. The cement leakage percentage was 13.3%, but there were not clinical symptoms caused by leakage. One patient had an incision infection and one patient had a urinary tract infection.
Discussion
Expected survival for patients with bone metastasis from lung cancer is short. It is controversial whether such patients should undergo surgical treatment. First of all, we must estimate the life expectancy accurately. At the same time, we must fully weigh the risks and benefits of surgery. The aim of treatment is to relieve symptoms rapidly and improve the quality of life. In this study, 15 patients had lung cancer with spinal metastasis. The life expectancy of these patients was more than 3 months. All patients underwent posterior decompression surgery and radiofrequency ablation, followed by vertebroplasty. The quality of life of patients was improved significantly. We found that this palliative operation was effective and safe for patients with spinal metastases from lung cancer, and the procedure had acceptable postoperative complications and mortality rates.
It is well accepted that surgical treatment can only be considered when the patient’s expected survival time is more than 3 months [17]. In our study, 15 cases underwent palliative surgical intervention to manage axial pain and spinal instability. First of all, in our patient cases, the compression of spinal cord was indirectly decompressed. Then spinal stability was restored by vertebroplasty and instrumental spinal fixation. All patients reported that pain was rapidly relieved through this palliative surgery. Frankel grade was improved by 1 grade or 2 grades in 14 patients. Only 1 patient kept a grade D after surgery. The neurological deficit of the spinal cord was improved significantly as well in our patient cases. At 3-months postoperatively, 8 out of 13 patients (61.5%) who were incontinent before surgery recovered. The EORTC QLQ-C30 score was 86.13±8.51 preoperatively and 52.21±13.28 postoperatively. The quality of life was enhanced markedly (t=11.8, P<0.01). These findings were consistent with several previous studies [18,19].
As we know, total en bloc spondylectomy (TES) is associated with excessive morbidity, blood loss, and long surgery time; TES is appropriate in patients with controlled primary lung cancer, localized spinal metastases, and no visceral metastases [7]. Even if patients with surgery indications can undergo TES, the incidence of complications remains high. However, the survival time of spinal metastasis from lung cancer is limited. The treatment should be palliative rather than invasive [20]. Laufer et al. [21] suggested that treatment of spinal metastases must emphasize durable tumor control while minimizing treatment-related complications and morbidity, while giving consideration to effective chemotherapy, radiotherapy, and surgical treatment options to achieve this goal. Wu et al. [22] proposed that posterior decompression and occipitocervical fixation followed by intraoperative vertebroplasty was a safe and valuable method with less invasion to treat spinal metastasis. In our study, the Tomita sore of most patients was 6–7 and life expectancy was limited. TES is known to be highly invasive in nature. TES was not considered suitable in these patients because of the potential high operative-related complications and morbidity with short predicted survival. Thus, we performed palliative surgery to relieve compression of the spinal cord and reconstruct spinal stability. The mean time of the palliative operation was 154±50 minutes and the mean blood loss was 210±90 mL. At the same time, the pain, function, and quality of life of patients were improved with less complication, less blood loss, and lower morbidity.
Currently, percutaneous vertebroplasty is mainly used in treatment of spinal metastases, but percutaneous vertebroplasty is associated with higher complication rates such as cement leakage [10–13]. The most serious cement leakage complication associated with percutaneous vertebral augmentation is cement extravasation into the epidural space and neural foramina leading to spinal cord compression and radicular pain. Malignant vertebral body lesions are often associated with epidural extensions or posterior cortical disruption, which may increase the risk of cement leakage. Percutaneous vertebroplasty also can cause physical displacement of tumors, which may lead to tumor cells advancing through physical holes in the vertebral body or via vertebral veins.
Combining radiofrequency ablation with percutaneous vertebroplasty in spine metastases has been described previously in several studies [23,24]. These studies showed that if radiofrequency ablation was performed before percutaneous vertebroplasty, the risk of cement leakage was reduced [25,26]. Georgy [27] found that combining radiofrequency ablation with percutaneous cement augmentation seemed to be effective in treating painful metastatic vertebral pathological fractures. Moreover, radiofrequency ablation can destroy sensory nerve fibers, reduce the lesion volume, and kill tumor cells. In our study, cement leakage was observed in 2 patients. The cement leakage percentage was 13.3%. One case of leakage was perivertebral soft tissue leakage and the other case was adjacent disc leakage. There were no clinical symptoms caused by either leakage. We found that radiofrequency ablation could reduce the complication of vertebroplasty-associated bone cement leakage.
Patients with spinal metastases from lung cancer have short survival expectancy. Spinal instability may lead to pathological fractures and further paraplegia. Fortunately, palliative posterior decompression surgery with stabilization and postoperative multidisciplinary treatment have been shown to be effective in such patients [28,29]. With multidisciplinary therapy in lung cancer, the patient’s survival time can be significantly prolonged [30,31]. In our study, 14 patients received radiotherapy, 8 patients received chemotherapy (4–6 cycles), 7 patients received targeted therapy, and 10 patients received zoledronic acid. The median survival time was 11.0 months. The 1-year survival rate was 53.3%. The 2-year survival rate was 26.7%. The 3-year survival rate was 20.0%. Survival of patients was longer than the preoperative life expectancy in some patients. Prolonged survival may be associated with improved quality of life by treatment with palliative surgery and multidisciplinary therapy. Perhaps, the existing scoring system should be improved in the future. We suggested that postoperative adjuvant therapy including medication and radiation should be routinely administered in patients who receive palliative posterior decompression and stabilization surgery.
There were several limitations to this study. According to the criteria for enrollment, only 15 patients were enrolled in 7 years. In this study, the sample was small, which precluded valid analysis of these results. However, some patients received different treatments including radiotherapy, chemotherapy, targeted-therapy, and bisphosphonates before or after surgery. It was difficult to analysis prognosis for a limited sample and there were other interference factors. This study was a retrospective analysis without a control group. A randomized controlled trial is required to verify the results of our study.
Conclusions
In the present study, we found that posterior decompression surgery and radiofrequency ablation followed by vertebroplasty was effective and safe in relieving pain and neurological deficit, and improved quality of life. We believe that survival of patients will be significantly prolonged with the introduction of new targeted drugs for lung cancer. Patients with spinal metastasis from lung cancer should be evaluated for multidisciplinary therapy. Posterior decompression surgery and radiofrequency ablation followed by vertebroplasty may be a suitable option for patients with MESCC.
Abbreviations
- MESCC
metastatic epidural spinal cord compression
- RFA
radiofrequency ablation
- VP
vertebroplasty
- PVP
percutaneous vertebroplasty
- VAS
visual analogue scale
- KPS
Karnofsky Score
- TES
total en bloc spondylectomy
- SINS
spinal instability neoplastic score
- L
lumbar vertebra
- T
thoracic vertebra
Footnotes
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The present study was approved by Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) and it conforms to the provisions of the Declaration of Helsinki. Written informed consent was obtained from all individuals in the present study.
Conflict of interests
None.
Source of support: This work was supported by National Natural Science Foundation of China grant number 81872184
References
- 1.WHO Cancer. World Health Organization; 2006. Retrieved on 2007-06-25. [Google Scholar]
- 2.Moussazadeh N, Laufer I, Yamada Y, Bilsky MH. Separation surgery for spinal metastases: Effect of spinal radiosurgery on surgical treatment goals. Cancer Contr. 2014;21:168–74. doi: 10.1177/107327481402100210. [DOI] [PubMed] [Google Scholar]
- 3.Hessler C, Vettorazzi E, Madert J, et al. Actual and predicted survival time of patients with spinal metastases of lung cancer: Evaluation of the robustness of the Tokuhashi score. Spine (Phila Pa 1976) 2011;36:983–89. doi: 10.1097/BRS.0b013e3181e8f7f8. [DOI] [PubMed] [Google Scholar]
- 4.Hosono N, Ueda T, Tamura D, et al. Prognostic relevance of clinical symptoms in patients with spinal metastases. Clin Orthop Relat Res. 2005;(436):196–201. doi: 10.1097/01.blo.0000160003.70673.2a. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. National cancer institute of Canada clinical trials group. Erlotinib in previously treated non-small-cell cancer. N Engl J Med. 2005;353(2):123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 6.Grunenwald DH, Mazel C, Girard P, et al. Radical en bloc resection for lung cancer invading the spine. J Thorac Cardiovasc Surg. 2002;123:271–79. doi: 10.1067/mtc.2002.119333. [DOI] [PubMed] [Google Scholar]
- 7.Murakami H, Kawahara N, Demura S, et al. Total en bloc spondylectomy for lung cancer metastasis to the spine. J Neurosurg Spine. 2010;13:414–17. doi: 10.3171/2010.4.SPINE09365. [DOI] [PubMed] [Google Scholar]
- 8.Harrington KD. The use of methylmethacrylate for vertebral-body replacement and anterior stabilization of pathological fracture-dislocations of the spine due to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(1):36–46. [PubMed] [Google Scholar]
- 9.Galibert P, Deramond H, Rosat P, Le Gars D. [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty]. Neurochirurgie. 1987;33(2):166–68. [in French] [PubMed] [Google Scholar]
- 10.Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: A systematic review of 69 clinical studies. Spine. 2006;23(17):1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia GH, Barzilay PH, Manoj EN, et al. Cement leakage in percutaneous vertebroplasty: Effect of preinjection gelfoam embolization: Clinical case series. Spine. 2006;31(8):915–19. doi: 10.1097/01.brs.0000209307.03930.38. [DOI] [PubMed] [Google Scholar]
- 12.Duran C, Sirvanci M, Aydogan M, et al. Pulmonary cement embolism: A complication of percutaneous vertebroplasty. Acta Radiologica. 2007;48(8):854–59. doi: 10.1080/02841850701422153. [DOI] [PubMed] [Google Scholar]
- 13.Luetmer MT, Bartholmai BJ, Rad AE, et al. Asymptomatic and unrecognized cement pulmonary embolism commonly occurs with vertebroplasty. Am J Neuroradiol. 2011;32(4):654–57. doi: 10.3174/ajnr.A2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer O, Lohrmann C, Markmiller M, et al. Technical innovation. Combined treatment of a spinal metastasis with radiofrequency heat ablation and vertebroplasty. Am J Roentgenol. 2003;180:1075–77. doi: 10.2214/ajr.180.4.1801075. [DOI] [PubMed] [Google Scholar]
- 15.van der Linden E, Kroft LJ, Dijkstra PD. Treatment of vertebral tumor with posterior wall defect using image-guided radiofrequency ablation combined with vertebroplasty: Preliminary results in 12 patients. J Vasc Interv Radiol. 2007;18:741–47. doi: 10.1016/j.jvir.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann RT, Jakobs TF, Trumm C, et al. Radiofrequency ablation in combination with osteoplasty in the treatment of painful metastatic bone disease. J Vasc Interv Radiol. 2008;19:419–25. doi: 10.1016/j.jvir.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 17.White BD, Stirling AJ, Paterson E, et al. Diagnosis and management of patients at risk of or with metastatic spinal cord compression: Summary of NICE guidance. BMJ. 2008;337:a2538. doi: 10.1136/bmj.a2538. [DOI] [PubMed] [Google Scholar]
- 18.Quan GM, Vital JM, Aurouer N, et al. Surgery improves pain, function and quality of life in patients with spinal metastases: A prospective study on 118 patients. Eur Spine J. 2011;20:1970–78. doi: 10.1007/s00586-011-1867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. J Neurosurg Spine. 2008;8:271–78. doi: 10.3171/SPI/2008/8/3/271. [DOI] [PubMed] [Google Scholar]
- 20.Yang SZ, Tang Y, Zhang Y, et al. Prognostic factors and comparison of conservative treatment, percutaneous vertebroplasty, and open surgery in the treatment of spinal metastases from lung cancer. World Neurosurg. 2017;108:163–75. doi: 10.1016/j.wneu.2017.08.130. [DOI] [PubMed] [Google Scholar]
- 21.Laufer I, Rubin DG, Lis E, et al. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18:744–51. doi: 10.1634/theoncologist.2012-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Tan M, Qi Y, et al. Posterior decompression and occipitocervical fixation followed by intraoperative vertebroplasty for metastatic involvement of the axis. BMC Musculoskelet Disord. 2018;19(1):11. doi: 10.1186/s12891-018-1928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronemeyer DH, Schirp S, Gevargez A. Image-guided radiofrequency ablation of spinal tumors: Preliminary experience with an expandable array electrode. Cancer J. 2002;8:33–39. doi: 10.1097/00130404-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsuka A, Yamakado K, Maeda M, et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004;15:707–12. doi: 10.1097/01.rvi.0000133507.40193.e4. [DOI] [PubMed] [Google Scholar]
- 25.Chi JH, Gokaslan ZL. Vertebroplasty and kyphoplasty for spinal metastases. Curr Opin Support Palliat Care. 2008;2(1):9–13. doi: 10.1097/SPC.0b013e3282f5d907. [DOI] [PubMed] [Google Scholar]
- 26.Kam NM, Maingard J, Kok HK, et al. Combined augmentation and radiofrequency ablation in the management of spinal metastases: An update. Curr Treat Options Oncol. 2017;18(12):74. doi: 10.1007/s11864-017-0516-7. [DOI] [PubMed] [Google Scholar]
- 27.Georgy BA, Wong W. Plasma-mediated radiofrequency ablation assisted percutaneous cement injection for treating advanced malignant vertebral compression fractures. Am J Neuroradiol. 2007;28(4):700–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Uei H, Tokuhashi Y, Maseda M, et al. clinical results of multidisciplinary therapy including palliative posterior spinal stabilization surgery and postoperative adjuvant therapy for metastatic spinal tumor. J Orthop Surg Res. 2018;13(1):30. doi: 10.1186/s13018-018-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, Wang G, Han X, et al. Comparison of the therapeutic effects of surgery combined with postoperative radiotherapy and standalone radiotherapy in treating spinal metastases of lung cancer. Clin Neurol Neurosurg. 2016;141:38–42. doi: 10.1016/j.clineuro.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Schilsky RL. Personalizing cancer care: American Society of Clinical Oncology presidential address 2009. J Clin Oncol. 2009;27:3725–30. doi: 10.1200/JCO.2009.24.6827. [DOI] [PubMed] [Google Scholar]
- 31.Zhao W, Wang H, Hu JH, et al. Palliative pain relief and safety of percutaneous radiofrequency ablation combined with cement injection for bone metastasis. Jpn J Clin Oncol. 2018;48(8):753–59. doi: 10.1093/jjco/hyy090. [DOI] [PubMed] [Google Scholar]