Abstract
This review reflects a collaboration between the American Registry of Pathology (the publisher of the Armed Forces Institute of Pathology Fascicles) and Annals of Diagnostic Pathology. It is part of a series of expert recommendations on topics encountered in daily practice. The authors, 4 pathologists with expertise in breast pathology and a breast surgeon with a clinical and research interest in lobular carcinoma in situ (LCIS), met by conference call in September 2019 to develop recommendations for evaluating and reporting LCIS. Herein, we summarize the diagnostic criteria of classic LCIS and LCIS subtypes according to the most recent WHO criteria, discuss how best to distinguish LCIS from ductal carcinoma in situ in problematic cases (including the uses and limitations of E-cadherin immunohistochemistry), and review outcome and management issues for patients with LCIS.
Most pathologists readily recognize and diagnose the classic type of lobular carcinoma in situ (LCIS) as first described in detail by Foote and Stewart almost 80 years ago [1]. However, more recently recognized subtypes of LCIS often create diagnostic problems because of the presence of histologic features that overlap with those of ductal carcinoma in situ (DCIS), including nuclear pleomorphism, necrosis, and marked distension of involved spaces. Although E-cadherin immunohistochemisty is commonly used by pathologists to help distinguish LCIS from DCIS in problematic cases, the limitations and pitfalls of this approach are not widely appreciated [2,3].
Over the last several decades, epidemiologic and clinical follow-up studies, pathologic studies, and molecular and genetic studies have led to a greater understanding of classic LCIS, its clinical significance, and its management implications. However, lack of standardized terminology and the relative infrequency of LCIS subtypes have made it very difficult to determine the clinical significance and optimal management of these lesions. Thus, in practice, pathologists are often uncertain as to how to best classify LCIS lesions with features that deviate from those of the classic type and clinicians are uncertain as to how best to manage these patients.
The purpose of this article is to review the current diagnostic criteria for classic LCIS and its subtypes based on the most recent WHO recommendations, discuss the uses and limitations of E-cadherin immunohistochemistry in distinguishing LCIS from DCIS in problematic cases, and present the available data on outcome and management of patients with classic LCIS and LCIS subtypes identified in surgical specimens and in core needle biopsies.
1. Classic LCIS
In the most recent WHO classification, classic LCIS is the term used to describe lesions of the terminal duct lobular unit (TDLU) characterized by a dyscohesive proliferation of type A and/or B epithelial cells [4]. Type A cells are small cells with uniform hyperchromatic nuclei while type B cells have slightly larger vesicular nuclei with mild variability in size and shape and with small nucleoli (Fig. 1). More than 50% of the acini in a TDLU must be filled and expanded by the neoplastic cells to qualify as LCIS; lesser degrees of involvement warrant a diagnosis of atypical lobular hyperplasia (ALH). Type A and type B cells may be mixed in individual proliferations and even within a single involved space. The cytoplasm of classic LCIS cells is typically pale to lightly eosinophilic. In almost all cases, at least some cells contain intracytoplasmic vacuoles (also termed lumina) that may contain an eosinophilic globule. These vacuoles may be so subtle that histochemical stains for mucin are required for their demonstration or they may be large enough to produce signet ring cell forms with peripheral displacement of the nuclei. The cells of classic LCIS typically show strong expression of estrogen receptor (ER) and progesterone receptor (PR) and lack HER2 protein overexpression and gene amplification. Classic LCIS cells also characteristically demonstrate loss of membrane expression of the adhesion molecule E-cadherin [2,3] as well as alterations in the expression of other proteins in the cadherin-catenin complex including loss of membrane expression of β-catenin and cytoplasmic (rather than membrane) expression of p120 catenin [3] (Fig. 1) (see below).
Fig. 1.
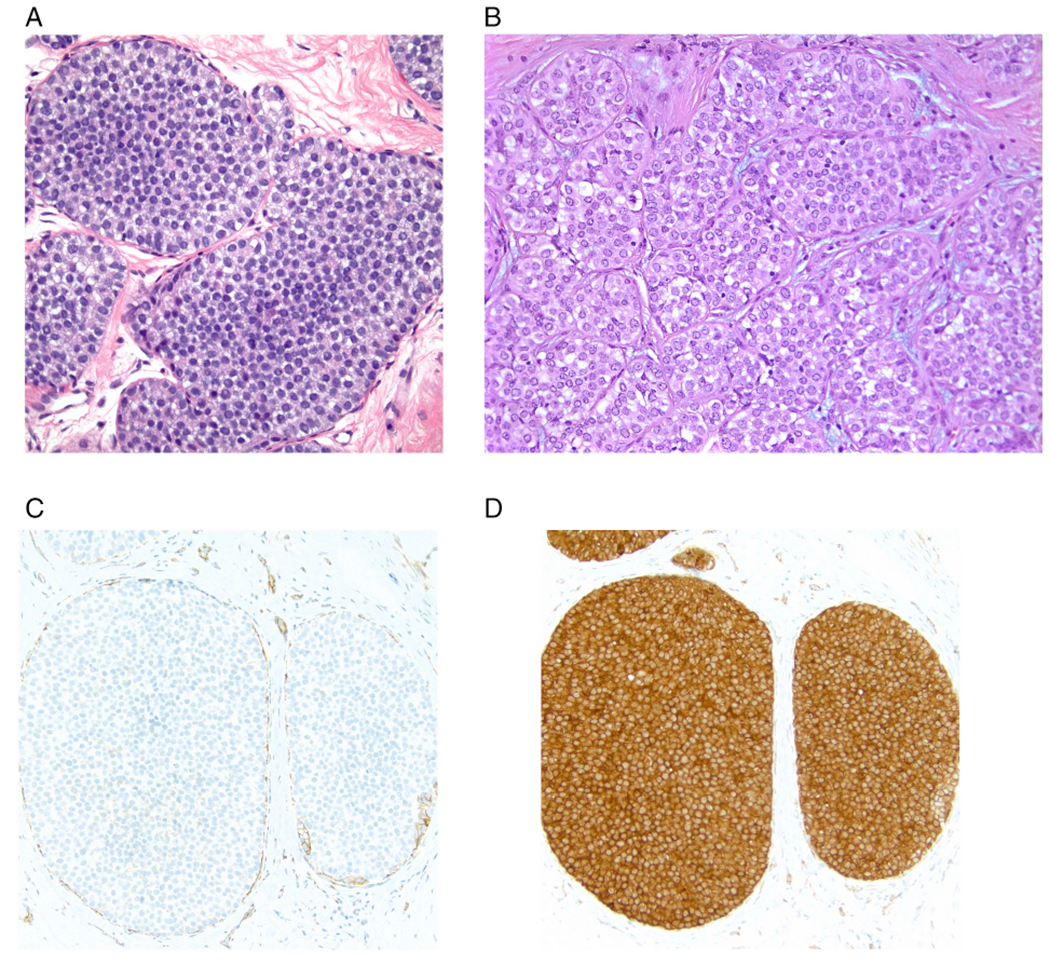
Classic lobular carcinoma in situ (LCIS). A. Type A cells. Small cells with uniform nuclei. B. Type B cells. The nuclei are larger and slightly more variable in size and shape than those seen in Type A cells. The chromatin is vesicular and small nucleoli are evident in some of the nuclei. C. E-cadherin immunostain demonstrating loss of membrane expression in the LCIS cells (note staining of surrounding myoepithelial cells). D. p120 catenin immunostain showing cytoplasmic staining of the LCIS cells.
2. LCIS subtypes
In addition to classic LCIS there are two subtypes currently recognized by WHO: pleomorphic LCIS and florid LCIS [4]. Several other terms have been used in clinical practice for lesions with features that deviate from those of classic LCIS, including “non-classic LCIS” and “variant LCIS” without further clarification. WHO does not recommend the use of these terms due to lack of sufficient specificity to guide patient management [4], and we concur with this viewpoint.
Pleomorphic LCIS and florid LCIS share with classic LCIS the solid proliferation of dyscohesive neoplastic cells within TDLUs but differ from classic LCIS with regard to the degree of nuclear atypia and/or lobular acinar expansion. Pleomorphic LCIS is defined by its cytologic features, in particular the presence of marked nuclear pleomorphism with at least some nuclei > 4 times size of a lymphocyte nucleus and/or nuclei that are equivalent to those seen in high-grade DCIS [4,5]. Occasional cases show cells with multinucleation. Apocrine features (defined as the presence of abundant eosinophilic, granular cytoplasm and large rounded nuclei with prominent nucleoli) may be observed in a subset of pleomorphic LCIS, lesions that have been variously termed apocrine pleomorphic LCIS, pleomorphic apocrine LCIS, or pleomorphic LCIS with apocrine features [6] (Fig. 2). Lobular acini involved by pleomorphic lobular cells are often substantially expanded but may be only mildly distended or show no distension; the latter still qualify as pleomorphic LCIS since there is no recognized category of pleomorphic ALH (analogous to not having a high grade atypical ductal hyperplasia).
Fig. 2.
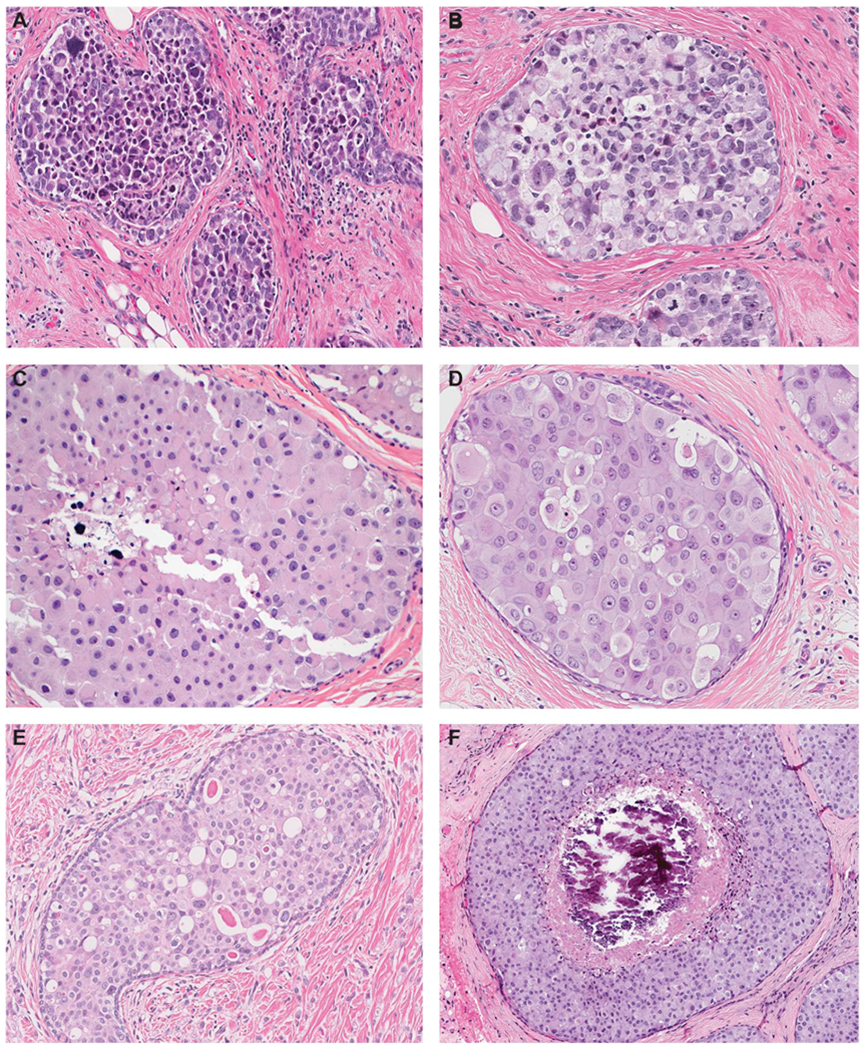
Pleomorphic lobular carcinoma in situ (LCIS). A. Pleomorphic LCIS is characterized by a solid proliferation of dyscohesive cells, similar to classic LCIS, but with marked nuclear pleomorphism equivalent to high-grade DCIS. B. In this pleomorphic LCIS, many neoplastic cells have signet-ring cell morphology with large intracytoplasmic mucin-filled vacuoles that displace the nuclei to the periphery. Also noted are multinucleated cells, which may be present in pleomorphic LCIS but are not observed in classic LCIS. C and D. A subset of pleomorphic LCIS have apocrine features and are characterized by large cells with abundant eosinophilic, granular cytoplasm and large rounded nuclei with prominent nucleoli. E. Many neoplastic cells in this example of pleomorphic LCIS demonstrate prominent intracytoplasmic vacuoles, some of which contain a large eosinophilic globule. The finding of prominent intracytoplasmic vacuoles in an in situ lesion favors LCIS over DCIS, but may also create the false impression of glandular structures mimicking DCIS. Note the presence of associated invasive lobular carcinoma in the background. F. Pleomorphic LCIS is often, but not always, associated with comedo necrosis and calcifications.
Florid LCIS is defined by its architectural features. In these lesions the LCIS cells exhibit the cytologic features of classic LCIS (type A, type B, or a mixture of type A and B cells), but there is marked distention of TDLUs or ducts, creating a confluent mass-like architecture. Florid LCIS should have at least one of two architectural features: 1) little to no intervening stroma between markedly distended acini of involved TDLUs; 2) a minimum size cut-off of an expanded acinus or duct filling at least one high-power field (an area equivalent to ~40–50 cells in diameter) [7–10] (Fig. 3).
Fig. 3.
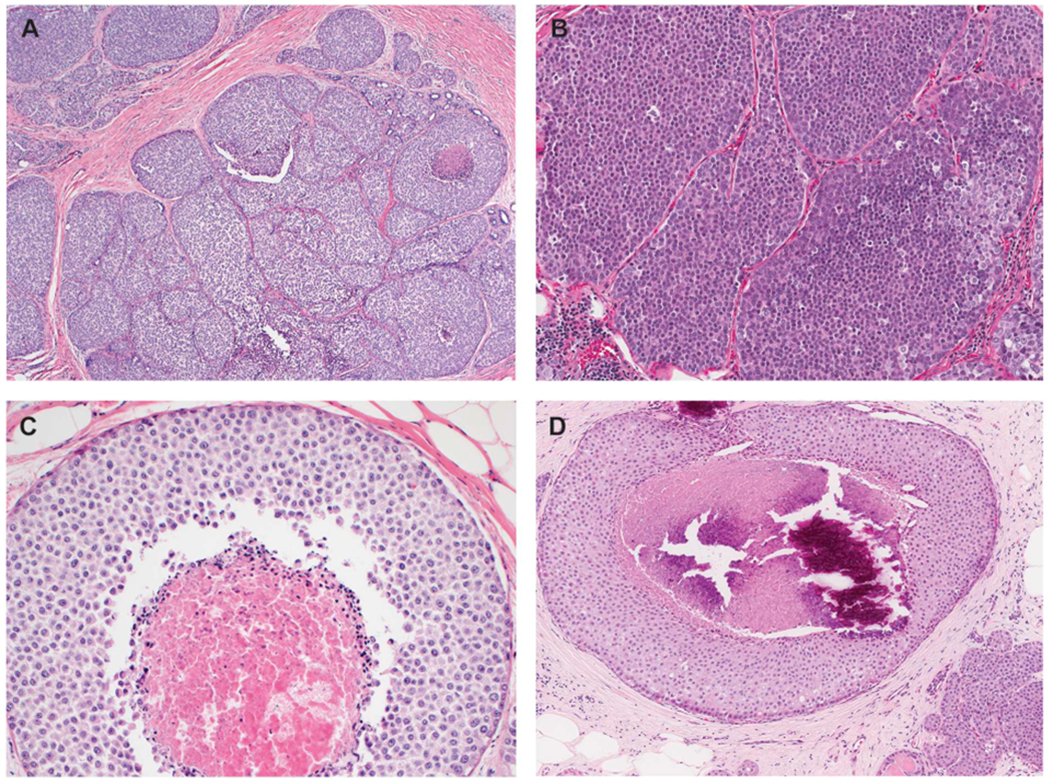
Florid lobular carcinoma in situ (LCIS). A. Florid LCIS has cytologic features identical to those of classic LCIS but is distinguished by marked distention of TDLUs or ducts, creating a confluent mass-like appearance at low power view. To qualify for florid subtype, an LCIS lesion should demonstrate at least one of the two architectural features depicted in B and C: (B) the spaces are expanded to a point that there is little to no intervening stroma between the markedly distended acini and ducts; (C) the expanded duct fills at least one high power field (an area equivalent to ~40–50 cells in diameter). Similar to pleomorphic LCIS, these lesions often demonstrate comedo necrosis. D. Florid LCIS with comedo necrosis and calcifications. Note the presence of classic LCIS with similar cytologic features at right lower corner, the presence of which should alert the pathologist the possibility of a LCIS subtype and not solid pattern DCIS.
Both pleomorphic LCIS and florid LCIS frequently demonstrate comedo necrosis and calcifications, the latter leading to detection by mammography. However, the presence of comedo necrosis is not required for the diagnosis of either of these morphologic subtypes.
Some pleomorphic LCIS cases may also exhibit a florid growth pattern and these lesions are best classified as pleomorphic LCIS. Other LCIS cases may display a florid growth pattern but demonstrate a spectrum of cytologic atypia ranging from classic to pleomorphic, and a designation of mixed pleomorphic and florid LCIS can be applied to these lesions.
The cytologic and/or architectural features of pleomorphic LCIS and florid LCIS can mimic solid-pattern DCIS. In the authors’ experience, pleomorphic LCIS and florid LCIS are more likely to be erroneously categorized as DCIS than vice versa. Awareness of these LCIS subtypes is important for correct classification of in situ lesions and proper patient management. Distinction between pleomorphic LCIS and florid LCIS from DCIS can usually be established by careful morphologic evaluation, as summarized in Table 1.
Table 1.
Morphologic features useful for the distinction between lobular carcinoma in situ (LCIS) and ductal carcinoma in situ (DCIS).
| Morphologic feature | Favor LCIS | Favor DCIS |
|---|---|---|
| Loss of cell-to-cell adhesion | + | |
| Prominent intracytoplasmic lumina and/or signet-ring cell morphology | + | |
| Solid pattern only | + | |
| Classic LCIS and/or ALH in adjacent breast | + | |
| Sharply-defined cell membranes | + | |
| Polarization of cells around extracellular lumina and/or at periphery of involved spaces | + |
However, these morphologic features are not entirely specific and show overlap between LCIS and DCIS. For example, loss of cellular cohesion, a helpful morphologic clue for a lobular phenotype, may not be apparent in LCIS, especially in florid LCIS. Conversely, DCIS with necrosis/degeneration or suboptimal fixation may appear dyscohesive. Intracytoplasmic lumina and signet-ring cells are often observed in LCIS (as well as in invasive lobular carcinoma[ILC]) and may be seen in any of the morphologic subtypes of LCIS; however, these cytologic features are not specific for a lobular phenotype as they may also be seen in some examples of DCIS. Polarization of cells around extracellular lumina to form glandular structures, micropapillae, and papillary architecture are not expected in LCIS, but can occasionally be observed when the neoplastic lobular cells involve other lesions such as collagenous spherulosis and papillomas. The majority of DCIS lesions have mixed architectural patterns; when an in situ lesion is entirely of solid pattern, the possibility of pleomorphic or florid LCIS should be considered and further investigated. Lastly, the vast majority of pleomorphic LCIS and florid LCIS are seen in association with classic LCIS and/or ALH. Although classic LCIS and ALH can be seen in association with DCIS, their presence should alert the pathologist to these morphologic subtypes of LCIS.
Given the overlapping morphological features of pleomorphic/ florid LCIS and DCIS, immunostains for E-cadherin and other components of the cadherin-catenin complex can be useful to assist in correct classification. However, the distinction between LCIS and DCIS cannot rely solely on immunohistochemical markers, which must be interpreted in the context of the histologic features (see below).
At the present time there are no biomarkers that are helpful in distinguishing classic LCIS from florid LCIS. The cells of both are typically strongly and diffusely positive for ER and rarely show HER2 overexpression or gene amplification. Most pleomorphic LCIS are also ER positive and HER2 negative, but the apocrine variant is often ER negative (and androgen receptor positive) and may show HER2 overexpression or gene amplification [6]. Thus, while the presence of HER2 overexpression by immunohistochemistry could possibly be a marker to distinguish pleomorphic from classic LCIS in problematic cases, the sensitivity and specificity of HER2 immunostaining in this context is unknown.
Emerging molecular and clinical outcome studies have suggested that pleomorphic LCIS and florid LCIS are genetically and, likely, biologically more advanced lesions than classic LCIS [11,12] and that these LCIS subtypes represent direct precursor lesions to ILC. In fact, most (59 to 87%) pleomorphic LCIS and florid LCIS are ultimately found to have associated invasive carcinoma either on core needle biopsy or excision, with the majority (84–100%) being ILC [7,13–16]. Therefore, the presence of these lesions should prompt a careful search for subtle invasion in the adjacent breast tissue, which may be associated with a slightly cellular stroma or be masked by inflammatory cells. Cytokeratin immunostains are a very helpful diagnostic adjunct to highlight the presence of these subtle invasive foci (Fig. 4).
Fig. 4.
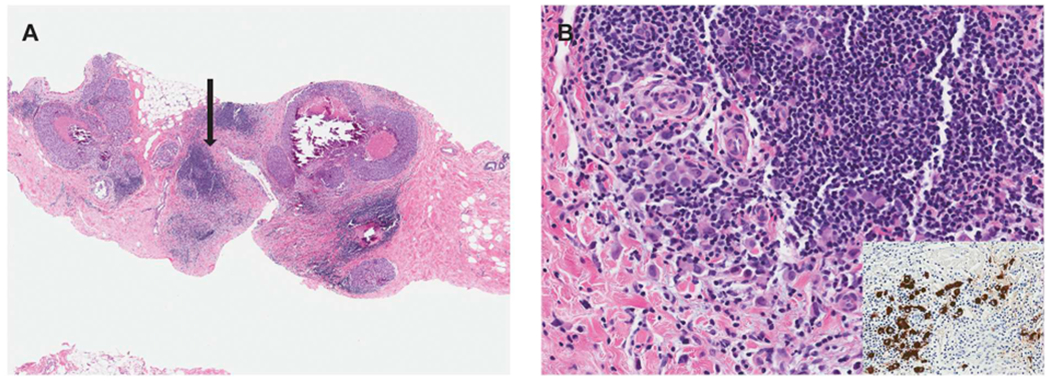
Pleomorphic LCIS associated with subtle invasive lobular carcinoma. A. This core biopsy shows pleomorphic LCIS with comedo necrosis and calcifications and foci of chronic inflammation. B. High power evaluation of one of the inflammatory foci (highlighted by the arrow in panel A) reveals subtle invasive lobular carcinoma admixed with and masked by the lymphocytic infiltrate. A keratin immunostain (inset) is very useful in highlighting the invasive tumor cells.
In some cases of LCIS the combination of cytologic and architectural features do not fit neatly into any of the three WHO accepted categories (Fig. 5). Since evidence-based data on how best to categorize such cases is lacking, pathologists must depend on pragmatic guidelines. In some cases, it may be difficult to distinguish classic LCIS from pleomorphic or florid LCIS. For example, some LCIS lesions have nuclear features that are borderline between classic LCIS and pleomorphic LCIS or architectural features that are borderline between classic LCIS and florid LCIS since in situ lobular lesions, like intraductal proliferative lesions, demonstrate a continuous spectrum of cytologic and architectural features. For these borderline lesions, we recommend an approach similar to that recommended by Page and Rogers for ductal lesions, i.e., “when in doubt, the more benign diagnosis (i.e. classic LCIS) is appropriate” [17]. Thus, LCIS lesions that are on the borderline between classic LCIS composed of type B cells and pleomorphic LCIS should be categorized as classic LCIS composed of type B cells. Similarly, lesions with cytologic features of classic LCIS that show distension of the acini borderline between classic LCIS and florid LCIS should be designated as classic LCIS. It is important to emphasize that lesions with extensive classic LCIS but lacking marked acinar expansion should not be categorized as florid LCIS (i.e., “florid” should not be used as an adjective to describe the extent of the LCIS since this term is now used by WHO to describe a specific subtype of LCIS [4]).
Fig. 5.
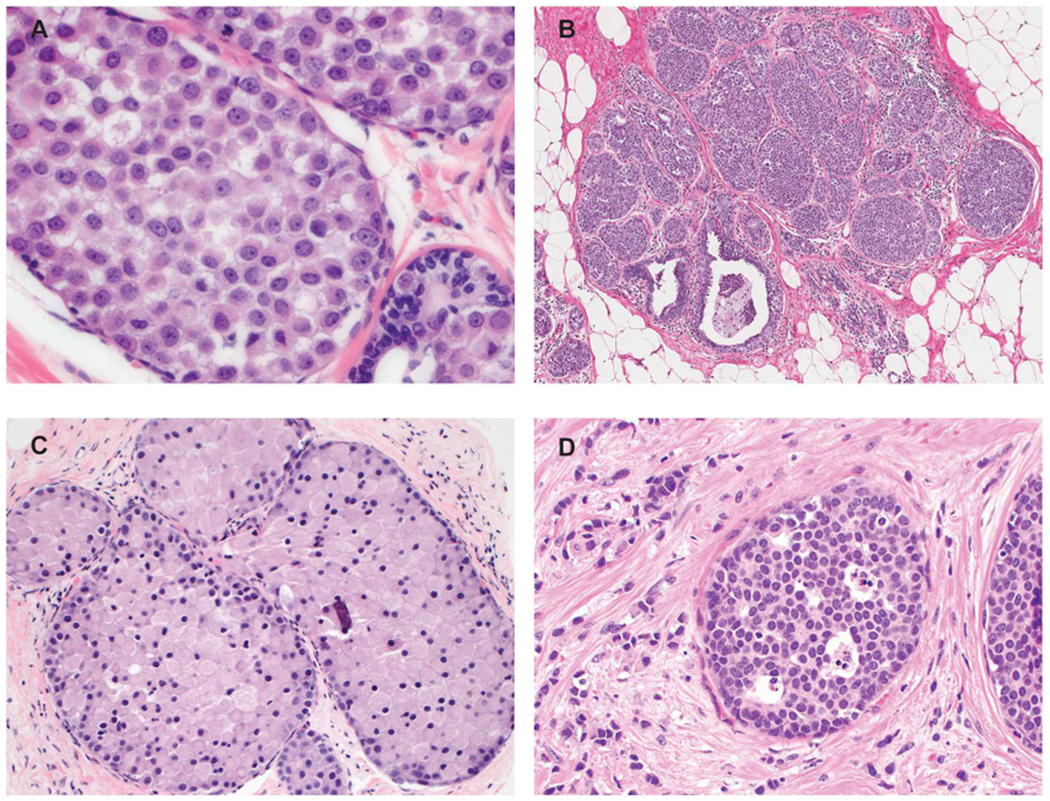
Borderline and unusual LCIS lesions. A. In this LCIS case, the nuclei are larger than those of typical type B cells but fall short of the marked atypia required for pleomorphic LCIS. The lesion should be categorized as classic LCIS composed of type B cells. B. This LCIS lesion shows abundant proliferation with significantly expanded acini. However, the acini remain separate from one another with persistence of intervening interlobular stroma and none of the distended acini fill one high power field. The lesion does not meet the criteria for florid LCIS and should be classified as classic LCIS. C. This example of LCIS with apocrine features but lacking marked nuclear pleomorphism is challenging to classify. It should not be diagnosed as apocrine pleomorphic LCIS or simply “non-classic LCIS”. One suggested approach is to give the descriptive diagnosis “LCIS with apocrine features” with a comment in the report. D. This LCIS lesion is composed of classic lobular cells (type A) but shows single-cell apoptosis and minute foci of necrosis. In the absence of marked nuclear atypia or duct-lobular expansion, the lesion is best classified as classic LCIS.
Another problematic area is LCIS cases with apocrine cytologic features that do not have sufficient nuclear atypia for designation as apocrine pleomorphic LCIS (Fig. 5). These lesions typically lack ER and PR expression (as in apocrine lesions of the breast in general), a biomarker profile that is different from classic LCIS, which is consistently ER positive. There are currently no clinical or molecular data to provide guidelines regarding how to best categorize these lesions. Given this, we recommend reporting such cases as “LCIS with apocrine features” and indicating that the lesion does not meet the criteria for pleomorphic or florid LCIS, but that the features are unusual for classic LCIS and that its clinical significance is uncertain.
3. E-cadherin biology, dysfunction, and diagnostic utility
E-cadherin is a member of the calcium-dependent cadherin family of transmembrane proteins that regulate epithelial cell adhesion and maintenance of cell polarity by forming adherens junctions between neighboring cells and the actin cytoskeleton. The intracytoplasmic domain of E-cadherin associates with actin by binding alpha-, beta- and gamma-catenins and the cadherin-catenin complex is strengthened by membranous p120 catenin [18–20]. When E-cadherin is lost or functionally impaired, intercellular adhesion is disrupted leading to programmed cell death in the detached cells. The downregulation or dysfunction of membranous E-cadherin in ALH, LCIS and ILC is associated with the loss of membranous alpha, beta and gamma-catenins as well as the cytoplasmic accumulation of p120 catenin [21]. The upregulation of cytoplasmic p120 catenin mediates resistance to programmed cell death despite cellular dyscohesion and promotes cell motility in lobular lesions [22,23].
The E-cadherin protein is the product of the CDH1 gene located on the long arm of chromosome 16 (16q22.1). Functional inactivation or downregulation of E-cadherin can occur through genetic or epigenetic mechanisms [2,24]. The loss of E-cadherin expression is most commonly caused by loss of heterozygosity at 16q with subsequent bi-allelic inactivation of CDH1 due to inactivating somatic mutations, promoter hypermethylation or other forms of transcriptional repression. Somatic mutations of CDH1 are frequently truncating, however, in-frame deletions and missense mutations have also been identified [25], which result in nonfunctional protein expression. Rarely, germline mutations in the CDH1 gene can be associated with the development of lobular neoplasms [26].
Immunohistochemistry for E-cadherin is commonly used to differentiate between ductal and lobular carcinomas (both in situ and invasive). The cells of ductal proliferations, like normal breast epithelium, typically show complete, circumferential membranous expression of E-cadherin [2]. In ALH, LCIS, and ILC, there is loss of E-cadherin protein expression in most cases, resulting in absence of membrane staining by immunohistochemistry (Fig. 1). It is important to note, however, that in up to 23% of ILC, there is retained membranous expression of E-cadherin, but the protein is nonfunctional and the staining pattern is aberrant [25,27–32]. The frequency of E-cadherin expression in LCIS lesions is less clear since few studies have addressed this issue; however, the reported frequency ranges from 0 to 9%.
Aberrant patterns of E-cadherin staining most often appear as incomplete, fragmented and beaded membranous staining of variable intensity. However, complete, circumferential membranous staining with an intensity ranging from weak to strong may also be seen. In addition, diffuse cytoplasmic staining may be observed. A dot-like, perinuclear Golgi-type pattern has also been described in cases where the mutated protein remains in the Golgi apparatus (Fig. 6). It should be noted that normal or proliferating ductal epithelial cells as well as myoepithelial cells adjacent to or admixed with LCIS cells show membranous expression of E-cadherin which may be mistakenly interpreted as E-cadherin staining of the LCIS cells themselves (Fig. 7). Therefore, careful correlation with the histologic findings on the corresponding hematoxylin and eosin-stained sections is essential to avoid erroneous interpretation of E-cadherin immunostains.
Fig. 6.
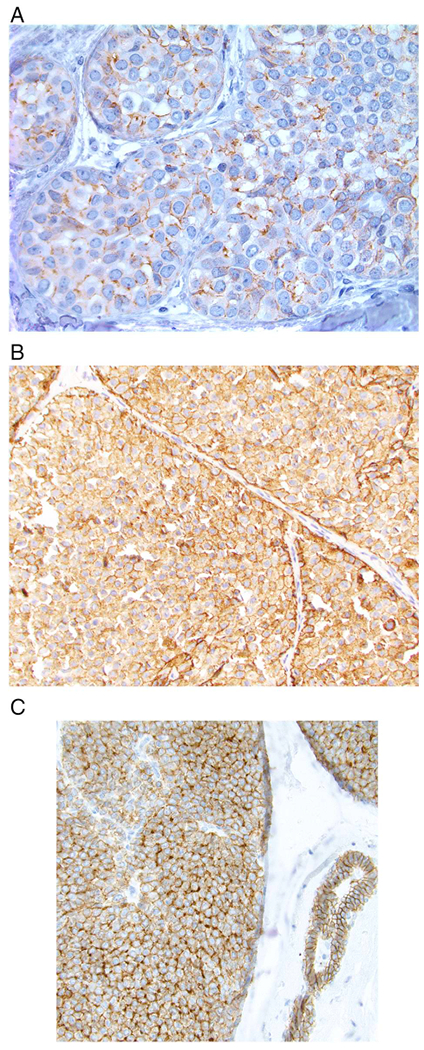
Examples of aberrant E-cadherin expression in lobular carcinoma in situ. A. Most often, aberrant E-cadherin expression is characterized by weak, partial, fragmented, or beaded membrane staining. B. In some cases there is more extensive membranous staining as well as diffuse cytoplasmic staining. C. An example of aberrant E-cadherin expression in which some cells show a perinuclear, dot-like pattern of staining (note normal membranous expression of E-cadherin in the duct on the right side of the image).
Fig. 7.
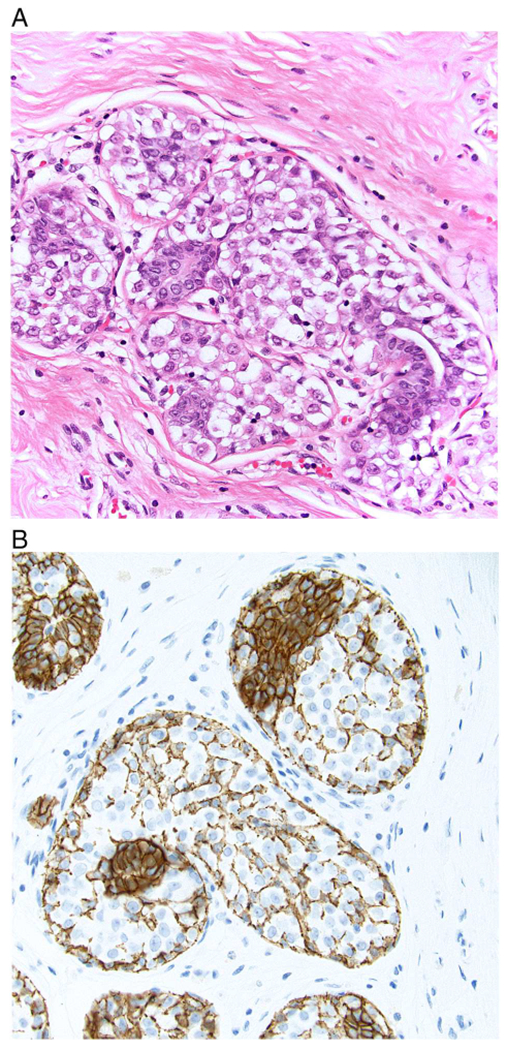
A. In this example of lobular carcinoma in situ (LCIS), residual normal ductal epithelial cells are evident on the H and E-stained section. B. E-cadherin immunostain shows strong membranous staining of residual ductal epithelial cells as well as staining of myoepithelial cells and their processes that are present between the LCIS cells. The E-cadherin staining of these normal elements should not be mistaken for staining of the LCIS cells.
When cases demonstrate classic lobular morphology, the diagnoses of ALH, LCIS and ILC can be made without E-cadherin immunohistochemistry. However, in diagnostically challenging cases, especially when E-cadherin immunostaining is difficult to interpret, additional immunohistochemical stains for other members of the cadherin-catenin complex may be helpful. In particular, demonstrating the loss of membranous beta-catenin and/or aberrant cytoplasmic p120 staining is supportive of a lobular phenotype [2]. Importantly, the interpretation of E-cadherin immunohistochemistry is further complicated by discrepant staining patterns observed using different E-cadherin antibodies [33] and false positive staining when the antibody staining protocol used has not been adequately optimized and validated [34].
It is important to emphasize that since aberrant membranous E-cadherin staining can be seen in bona fide cases of LCIS and ILC, the presence of membranous staining for E-cadherin in and of itself should not be used to classify an in situ or invasive carcinoma as “ductal”, as having “ductal features”, or as showing “ductal differentiation”. Further, given that the distinction between a lobular and ductal phenotype has more substantial clinical and management implications for in situ lesions than for invasive lesions, we recommend that staining for E-cadherin be used primarily for problematic in situ lesions rather than for invasive lesions. In addition, terms such as “in situ carcinoma with ductal and lobular features” or “invasive carcinoma with ductal and lobular features” should be reserved for those lesions that have hybrid or indeterminate features on hematoxylin and eosin-stained sections and in which immunostains for E-cadherin and the other members of the cadherin-catenin complex are ambiguous and do not permit definitive categorization of a lesion as lobular or ductal.
4. Outcome and management issues
4.1. Classic LCIS
Long-term follow-up studies have indicated that classic LCIS is associated with a 7- to 10-fold increase in breast cancer risk compared to that in women in the reference population. The absolute risk of developing breast cancer for a woman with LCIS is approximately 1–2% per year and this risk persists for at least 25 years (depending on age at diagnosis). Most studies with long follow-up (> 15 years) have indicated that the risk of subsequent cancer is conferred equally to both breasts, whereas some studies suggest ipsilateral breast cancers predominate the first 5 to 10 years [35–38]. Clinical and histologic features that might identify which patients with LCIS are more likely to develop invasive breast cancer have not been consistently identified. A family history of breast cancer in women <40 years of age, maximal distension of involved spaces, a mixture of type A and B cells, >10 involved spaces, a higher proportion of slides involved, and focal E-cadherin staining have all been reported to be associated with a greater risk of cancer development [35,38,39]. However, the association between these features and the development of invasive breast cancer is not sufficiently strong or reproducible to be of value in clinical practice. A recent study demonstrated that LCIS cases cluster into two distinct groups based on their gene expression signatures and identified candidate genes that may be involved in tumor progression [40]. Whether these signatures will be of clinical value in predicting the risk of subsequent breast cancer among patients with LCIS remains to be determined.
The observation that ILC is much more prevalent among the cancers that develop in women with a prior or concurrent history of LCIS (accounting for 23% to 75% of cancers) and that LCIS and coexistent ILC frequently share genetic alterations and are clonally related is evidence that LCIS is a non-obligate precursor of invasive breast cancer in addition to a marker of a generalized increase in breast cancer risk, in at least some cases [41–43]. At the present time, however, it is not possible to determine which LCIS lesions are more likely to behave as risk factors and which are more likely to behave as true precursors of invasive disease. Therefore, the most prudent management for patients with classic LCIS remains active surveillance, with or without chemoprevention with selective estrogen receptor modulators (tamoxifen or raloxifene) or an aromatase inhibitor (exemestane) [44]. It is not necessary to assess or report upon the status of the microscopic margins of excision in cases of classic LCIS since the presence of classic LCIS at the margin does not require additional surgery. Further, for patients with mixed classic and pleomorphic or florid LCIS assessment and reporting of margins is not required for the classic LCIS component.
When classic LCIS is diagnosed on core needle biopsy, careful radiologic-pathologic correlation is required. If multidisciplinary review demonstrates concordant imaging findings and there are no other lesions in the core biopsy which themselves require excision (e.g., atypical ductal hyperplasia), recent data suggest that the upgrade rates to DCIS or invasive cancer are sufficiently low that routine surgical excision of classic LCIS is not required [45,46].
4.2. LCIS subtypes
The clinical implications and appropriate management of pleomorphic LCIS and florid LCIS are more problematic for a number of reasons: 1) these lesions are rare; 2) the terminology and diagnostic criteria for these LCIS subtypes have been inconsistent; and 3) there are no long-term follow-up studies akin to those available for classic LCIS to provide information on the natural history of these lesions [36,47–54]. As a result, there is no consensus regarding their optimal management.
Data regarding the risk of local recurrence after simple excision and future development of invasive breast cancer after a diagnosis of these LCIS subtypes are limited to a few small, retrospective series of patients with pleomorphic lobular carcinoma in situ [5,53–56] (Table 2). Given the retrospective nature of these reports, the variable management strategies, and the limited number of cases in all reported series, it is not possible to draw any definitive conclusions regarding the long-term risk associated with these lesions. Patients with pleomorphic and florid LCIS were not specifically evaluated in the breast cancer prevention trials. While the majority of these lesions, like classic LCIS, are ER positive and HER2 negative suggesting that there is the potential to consider chemoprevention as an option for risk reduction, 5 of 10 patients reported in the literature who developed DCIS or invasive cancer following a diagnosis of pleomorphic LCIS had been treated with chemoprevention (Table 2).
Table 2.
Clinical outcomes among patients with pleomorphic lobular carcinoma in situ.
| Number of eases and treatment | Follow-up | Recurrent P-LCIS | Ipsilateral DCIS or IDC/ILC | |
|---|---|---|---|---|
| Sneige 2002 [5] | 5 excision only | 17 mos (mean) | 1 (12 mos) | – |
| Downs-Kelly 2011 [55] | 20 excision +/− CP or RT | 46 mos (mean) | 1a (19 mos) | – |
| Khoury 2014 [53] | 29 excision +/− CP or RT | 55.5 mos (median) | 2 | 4b (3 ILC, 1 IDC) |
| Flanagan 2015 [54] | 7 excision +/− CP or RT | 4.1 yrs. (mean) | – | – |
| DeBrot 2017 [56] | 7 excision +/− CP | 67 mos (median) | 3 (56 mos) | 4c (1 DCIS, 2 ILC, 1 IDC) |
| Savage 2018 [64] | 10 excision only +/− CP 2 excision + RT |
3.4 yrs. (median) | 0 | – |
| Desai 2018 [59] | 11 excision + / − CP or RT | 47 mos (median) | 1 | 1 (ILC) |
| Nakhlis 2019 [57] | 25 excision only | 58 mos (median) | 0 | 1 (DCIS) |
Abbreviations: P-LCIS, pleomorphic lobular carcinoma in situ; DCIS, ductal carcinoma in situ IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; CP, chemoprevention; RT, radiation therapy.
This patient was treated by CP.
3 of these 4 patients were treated by CP (2 patients who developed ILC, 1 who developed IDC).
1 of these 4 patients was treated by CP (the patient who developed DCIS).
The significance of these LCIS subtypes at surgical margins is also unclear. Retrospective data with relatively few events suggest that there may be a higher rate of local recurrence in patients with positive margins than in those with negative margins, but these data are insufficient to draw firm conclusions about the clinical significance of positive margins [53–59]. The paucity of data on the significance of margin involvement has resulted in disparate opinions among surgeons regarding the need for re-excision for positive margins in such cases. In one survey of 351 breast surgeons 24% said they always re-excise, 53% said they never re-excise, and 23% said they sometimes re-excise for pleomorphic LCIS at a margin of excision [60]. Until more data are available, the decision about how to approach these LCIS subtypes at the margin is best made in a multidisciplinary fashion with discussion among the surgeon, radiologist, and pathologist. Given that the limited available data do not convincingly demonstrate an increased risk of subsequent invasive cancer based on the margin status for these LCIS subtypes, the approach to re-excision and the importance of achieving a negative margin should be made pragmatically based on an individual patient’s overall risk profile and personal preferences. In addition, there are no data to support the use of radiation therapy following excision of pleomorphic or florid LCIS.
In contrast, the management of patients with these LCIS subtypes detected on core needle biopsy is more straightforward. Although most series are limited by small numbers of cases, the reported upgrade rates at surgical excision of pleomorphic LCIS, florid LCIS and lesions variously termed “non-classical” and “variant” LCIS average almost 40% (Table 3) [7,54,57,58,61–63]. As such, routine excision following a core needle biopsy diagnosis of pleomorphic or florid LCIS is warranted to rule out an associated carcinoma. Whether or not surgical excision is necessary for LCIS lesions that have features that deviate from classic LCIS but that do not fit neatly into the categories of pleomorphic or florid LCIS (e.g., LCIS with apocrine features but lacking high-grade nuclear atypia or florid architecture) is uncertain due to lack of data. In such cases, radiologic-pathologic correlation and global risk assessment is recommended to determine the need for excision.
Table 3.
Upgrade rate at excision for lobular carcinoma in situ (LCIS) subtypes diagnosed on core needle biopsy.
| LCIS subtype | Number with excisions | # (%) with upgrade | |
|---|---|---|---|
| Georgian-Smith & Lawton, 2001 [65] | P-LCIS | 5 | 2 (40%) |
| Lavoue et al., 2007 [66] | P-LCIS | 10 | 3 (33%) |
| Chivukula et al., 2008 [61] | PLCIS | 12 | 3 (25%) |
| Carder et al., 2010 [63] | P-LCIS | 8 | 2 (25%) |
| Sullivan et al., 2010 [67] | V-LCIS | 11 | 4 (36%) |
| P-LCIS | 17 | 3 (18%) | |
| Niell et al., 2012 [68] | P-LCIS | 4 | 4 (100%) |
| Meroni et al., 2014 [69] | P-LCIS | 12 | 6 (50%) |
| Flanagan et al., 2015 [54] | P-LCIS | 21 | 11 (52%) |
| Susnik et al., 2016 [62] | V-LCIS | 15 | 4 (27%) |
| Guo et al., 2018 [70] | P-LCIS | 23 | 14 (60%) |
| Savage et al., 2018 [64] | P-LCIS | 15 | 4 (27%) |
| Massanat et al., 2018 [16] | P-LCIS | 22 | 8 (36.3%) |
| Shamir et al., 2019 [7] | P-LCIS, F-LCIS | 14 | 5 (36%) |
| Foschini et al., 2019 [15] | P-LCIS, F-LCIS | 70 | 31 (44%) |
| Nakhlis et al., 2019 [57] | V-LCIS | 76 | 27 (36%) |
| Total | 335 | 131 (39%) |
Abbreviations: P-LCIS, pleomorphic lobular carcinoma in situ; F-LCIS, florid lobular carcinoma in situ; V-LCIS, variant lobular carcinoma in situ (including either a combination of pleomorphic and florid LCIS, or cases in which the variants were not clearly defined according to current criteria).
5. Conclusions
Although the diagnosis of classic forms of LCIS is usually straightforward, the recognition of LCIS subtypes (i.e., pleomorphic and florid LCIS) and their distinction from DCIS remains problematic. Familiarity with the most recent WHO criteria for the diagnosis of LCIS and its subtypes and an understanding of the uses and pitfalls of E-cadherin immunohistochemistry are important in arriving at the correct diagnosis in difficult cases. There is general consensus regarding the clinical significance and management of patients with classic LCIS. In contrast, the management of patients with pleomorphic and florid LCIS is more problematic due to the rarity of these lesions and paucity of outcome data. Given these limitations, a pragmatic approach is to individualize management of these patients based on multidisciplinary evaluation and global risk assessment.
Acknowledgments
This publication is supported by an unrestricted educational grant from NeoGenomics.
References
- [1].Foote FW Jr, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol 1941;17:419–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dabbs DJ, Schnitt SJ, Geyer FC, Weigelt B, Baehner FL, Decker T, et al. Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. Am J Surg Pathol 2013;37(7):e1–11. [DOI] [PubMed] [Google Scholar]
- [3].Canas-Marques R, Schnitt SJ. E-cadherin immunohistochemistry in breast pathology: uses and pitfalls. Histopathology. 2016;68(1):57–69. [DOI] [PubMed] [Google Scholar]
- [4].Chen YYDT, King TA, Palacios J, Shin SJ, Simpson PT. Lobular carcinoma in situ In: Board TWCE, editor. Breast Tumours. Lyon: International Agency for Research on Cancer; 2019. p. 71–4. [Google Scholar]
- [5].Sneige N, Wang J, Baker BA, Krishnamurthy S, Middleton LP. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductal-lobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol 2002;15(10):1044–50. [DOI] [PubMed] [Google Scholar]
- [6].Chen YY, Hwang ES, Roy R, DeVries S, Anderson J, Wa C, et al. Genetic and phenotypic characteristics of pleomorphic lobular carcinoma in situ of the breast. Am J Surg Pathol 2009;33(11):1683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shamir ER, Chen YY, Chu T, Pekmezci M, Rabban JT, Krings G. Pleomorphic and florid lobular carcinoma in situ variants of the breast: a clinicopathologic study of 85 cases with and without invasive carcinoma from a single academic center. Am J Surg Pathol 2019;43(3):399–408. [DOI] [PubMed] [Google Scholar]
- [8].Alvarado-Cabrero I, Picon Coronel G, Valencia Cedillo R, Canedo N, Tavassoli FA. Florid lobular intraepithelial neoplasia with signet ring cells, central necrosis and calcifications: a clinicopathological and immunohistochemical analysis of ten cases associated with invasive lobular carcinoma. Arch Med Res 2010;41(6):436–41. [DOI] [PubMed] [Google Scholar]
- [9].Shin SJ, Lai A, De Vries S, Suzuki J, Roy R, Hwang ES, et al. Florid lobular carcinoma in situ: molecular profiling and comparison to classic lobular carcinoma in situ and pleomorphic lobular carcinoma in situ. Hum Pathol 2013;44(10): 1998–2009. [DOI] [PubMed] [Google Scholar]
- [10].Wen HY, Brogi E. Lobular carcinoma in situ. Surg Pathol Clin 2018;11 (1):123–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shamir ER, Chen YY, Krings G. Genetic analysis of pleomorphic and florid lobular carcinoma in situ variants: frequent ERBB2/ERBB3 alterations and clonal relationship to classic lobular carcinoma in situ and invasive lobular carcinoma. Mod Pathol 2020. January 9, 2020. [DOI] [PubMed] [Google Scholar]
- [12].Harrison BT, Nakhlis F, Dillon DA, Soong TR, Garcia EP, Schnitt SJ, et al. Genomic profiling of pleomorphic and florid lobular carcinoma in situ reveals highly recurrent ERBB2 and ERRB3 alterations. Mod Pathol. 2020 January 13, 2020. [DOI] [PubMed] [Google Scholar]
- [13].Bagaria SP, Shamonki J, Kinnaird M, Ray PS, Giuliano AE. The florid subtype of lobular carcinoma in situ: marker or precursor for invasive lobular carcinoma? Ann Surg Oncol 2011;18(7):1845–51. [DOI] [PubMed] [Google Scholar]
- [14].Fadare O, Dadmanesh F, Alvarado-Cabrero I, Snyder R, Stephen Mitchell J, Tot T, et al. Lobular intraepithelial neoplasia [lobular carcinoma in situ] with comedo-type necrosis: a clinicopathologic study of 18 cases. Am J Surg Pathol 2006;30(11):1445–53. [DOI] [PubMed] [Google Scholar]
- [15].Foschini MP, Miglio R, Fiore R, Baldovini C, Castellano I, Callagy G, et al. Preoperative management of Pleomorphic and florid lobular carcinoma in situ of the breast: report of a large multi-institutional series and review of the literature. Eur J Surg Oncol. 2019;45:2279–86. [DOI] [PubMed] [Google Scholar]
- [16].Masannat YA, Husain E, Roylance R, Heys SD, Carder PJ, Ali H, et al. Pleomorphic LCIS what do we know? A UK multicenter audit of pleomorphic lobular carcinoma in situ. Breast. 2018;38:120–4. [DOI] [PubMed] [Google Scholar]
- [17].Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol 1992;23(10): 1095–7. [DOI] [PubMed] [Google Scholar]
- [18].Hatzfeld M The p120 family of cell adhesion molecules. Eur J Cell Biol 2005;84(2–3):205–14. [DOI] [PubMed] [Google Scholar]
- [19].Noren NK, Liu BP, Burridge K. Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 2000;150(3):567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci 2008;65(23):3756–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sarrio D, Perez-Mies B, Hardisson D, Moreno-Bueno G, Suarez A, Cano A, et al. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene. 2004;23(19):3272–83. [DOI] [PubMed] [Google Scholar]
- [22].Schackmann RC, van Amersfoort M, Haarhuis JH, Vlug EJ, Halim VA, Roodhart JM, et al. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. J Clin Invest 2011;121(8):3176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shibata T, Kokubu A, Sekine S, Kanai Y, Hirohashi S. Cytoplasmic p120ctn regulates the invasive phenotypes of E-cadherin-deficient breast cancer. Am J Pathol 2004; 164 (6): 2269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and ’omics. Breast Cancer Res 2015;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Da Silva L, Parry S, Reid L, Keith P, Waddell N, Kossai M, et al. Aberrant expression of E-cadherin in lobular carcinomas of the breast. Am J Surg Pathol 2008;32(5):773–83. [DOI] [PubMed] [Google Scholar]
- [26].Corso G, Intra M, Trentin C, Veronesi P, Galimberti V. CDH1 germline mutations and hereditary lobular breast cancer. Fam Cancer 2016;15(2):215–9. [DOI] [PubMed] [Google Scholar]
- [27].Rakha EA, Patel A, Powe DG, Benhasouna A, Green AR, Lambros MB, et al. Clinical and biological significance of E-cadherin protein expression in invasive lobular carcinoma of the breast. Am J Surg Pathol 2010;34(10):1472–9. [DOI] [PubMed] [Google Scholar]
- [28].Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol 1996;105(4):394–402. [DOI] [PubMed] [Google Scholar]
- [29].Acs G, Lawton TJ, Rebbeck TR, LiVolsi VA, Zhang PJ. Differential expression of E-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol 2001. ;115(1):85–98. [DOI] [PubMed] [Google Scholar]
- [30].Goldstein NS. Does the level of E-cadherin expression correlate with the primary breast carcinoma infiltration pattern and type of systemic metastases? Am J Clin Pathol 2002;118(3):425–34. [DOI] [PubMed] [Google Scholar]
- [31].Qureshi HS, Linden MD, Divine G, Raju UB. E-cadherin status in breast cancer correlates with histologic type but does not correlate with established prognostic parameters. Am J Clin Pathol 2006;125(3):377–85. [PubMed] [Google Scholar]
- [32].Sarrio D, Moreno-Bueno G, Hardisson D, Sanchez-Estevez C, Guo M, Herman JG, et al. Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int J Cancer 2003;106(2):208–15. [DOI] [PubMed] [Google Scholar]
- [33].Choi YJ, Pinto MM, Hao L, Riba AK. Interobserver variability and aberrant E-cadherin immunostaining of lobular neoplasia and infiltrating lobular carcinoma. Mod Pathol 2008;21 (10): 1224–37. [DOI] [PubMed] [Google Scholar]
- [34].Wells JM, Pipa J, Shin SJ. Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. Am J Surg Pathol 2014;38(3):434–5. [DOI] [PubMed] [Google Scholar]
- [35].Schnitt SJ, Morrow M. Lobular carcinoma in situ: current concepts and controversies. Semin Diagn Pathol 1999;16(3): 209–23. [PubMed] [Google Scholar]
- [36].Lakhani SR, Schnitt S, O’Malley F, van de Vijver M, Simpson PT, Palacios J, et al. Lobular neoplasia In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, editors. WHO classification of tumours of the breast. Lyon: IARC Press; 2012. p. 78–80. [Google Scholar]
- [37].Chuba PJ, Hamre MR, Yap J, Severson RK, Lucas D, Shamsa F, et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol 2005;23(24):5534–41. [DOI] [PubMed] [Google Scholar]
- [38].King TA, Pilewskie M, Muhsen S, Patil S, Mautner SK, Park A, et al. Lobular carcinoma in situ: a 29-year longitudinal experience evaluating clinicopathologic features and breast cancer risk. J Clin Oncol 2015;33(33):3945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goldstein NS, Bassi D, Watts JC, Layfield U, Yaziji H, Gown AM. E-cadherin reactivity of 95 noninvasive ductal and lobular lesions of the breast. Implications for the interpretation of problematic lesions. Am J Clin Pathol 2001;115(4):534–42. [DOI] [PubMed] [Google Scholar]
- [40].Andrade VP, Morrogh M, Qin LX, Olvera N, Giri D, Muhsen S, et al. Gene expression profiling of lobular carcinoma in situ reveals candidate precursor genes for invasion. Mol Oncol 2015;9(4):772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hwang ES, Nyante SJ, Yi Chen Y, Moore D, DeVries S, Korkola JE, et al. Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer. 2004;100(12):2562–72. [DOI] [PubMed] [Google Scholar]
- [42].Begg CB, Ostrovnaya I, Carniello JV, Sakr RA, Giri D, Towers R, et al. Clonal relationships between lobular carcinoma in situ and other breast malignancies. Breast Cancer Res 2016;18(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sakr RA, Schizas M, Carniello JV, Ng CK, Piscuoglio S, Giri D, et al. Targeted capture massively parallel sequencing analysis of LCIS and invasive lobular cancer: repertoire of somatic genetic alterations and clonal relationships. Mol Oncol 2016; 10(2):360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Morrow M, Schnitt SJ, Norton L. Current management of lesions associated with an increased risk of breast cancer. Nat Rev Clin Oncol 2015;12(4):227–38. [DOI] [PubMed] [Google Scholar]
- [45].Nakhlis F, Gilmore L, Gelman R, Bedrosian I, Ludwig K, Hwang ES, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in-situ in patients with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016;23(3):722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Murray MP, Luedtke C, Liberman L, Nehhozina T, Akram M, Brogi E. Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision. Cancer. 2013;119(5):1073–9. [DOI] [PubMed] [Google Scholar]
- [47].Jacobs TW. Recently recognized variants of lobular carcinoma in situ (LCIS) with an emphasis on management of LCIS on core needle biopsy. Pathol Case Rev 2003;8:211–9. [Google Scholar]
- [48].Murray M, Brogi E. Lobular carcinoma in situ, classical type and unusual variants In: Collins LC, editor. Current Concepts in Breast Pathology. Surgical Pathology Clinics. 2. Philadelphia: W.B. Saunders; 2009. p. 273–99. [DOI] [PubMed] [Google Scholar]
- [49].Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol 2010;27(1):49–61. [DOI] [PubMed] [Google Scholar]
- [50].Jorns J, Sabel MS, Pang JC. Lobular neoplasia: morphology and management. Arch Pathol Lab Med 2014;138(10): 1344–9. [DOI] [PubMed] [Google Scholar]
- [51].Masannat YA, Bains SK, Pinder SE, Purushotham AD. Challenges in the management of pleomorphic lobular carcinoma in situ of the breast. Breast. 2013;22(2):194–6. [DOI] [PubMed] [Google Scholar]
- [52].Murray L, Reintgen M, Akman K, Cox C, Cox J, Reintgen D, et al. Pleomorphic lobular carcinoma in situ: treatment options for a new pathologic entity. Clin Breast Cancer 2012;12(1):76–9. [DOI] [PubMed] [Google Scholar]
- [53].Khoury T, Karabakhtsian RG, Mattson D, Yan L, Syriac S, Habib F, et al. Pleomorphic lobular carcinoma in situ of the breast: clinicopathological review of 47 cases. Histopathology. 2014;64(7):981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Flanagan MR, Rendi MH, Calhoun KE, Anderson BO, Javid SH. Pleomorphic lobular carcinoma in situ: radiologic-pathologic features and clinical management. Ann Surg Oncol 2015;22(13):4263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Downs-Kelly E, Bell D, Perkins GH, Sneige N, Middleton LP. Clinical implications of margin involvement by pleomorphic lobular carcinoma in situ. Arch Pathol Lab Med 2011;135(6):737–43. [DOI] [PubMed] [Google Scholar]
- [56].De Brot M, Koslow Mautner S, Muhsen S, Andrade VP, Mamtani A, Murray M, et al. Pleomorphic lobular carcinoma in situ of the breast: a single institution experience with clinical follow-up and centralized pathology review. Breast Cancer Res Treat 2017;165(2):411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nakhlis F, Harrison BT, Giess CS, Lester SC, Hughes KS, Coopey SB, et al. Evaluating the rate of upgrade to invasive breast cancer and/or ductal carcinoma in situ following a core biopsy diagnosis of non-classic lobular carcinoma in situ. Ann Surg Oncol 2019;26(1):55–61. [DOI] [PubMed] [Google Scholar]
- [58].Fasola CE, Chen JJ, Jensen KC, Allison KH, Horst KC. Characteristics and clinical outcomes of pleomorphic lobular carcinoma in situ of the breast. Breast J 2018;24(1):66–9. [DOI] [PubMed] [Google Scholar]
- [59].Desai AA, Jimenez RE, Hoskin TL, Day CN, Boughey JC, Hieken TJ. Treatment outcomes for pleomorphic lobular carcinoma in situ of the breast. Ann Surg Oncol 2018;25(10): 3064–8. [DOI] [PubMed] [Google Scholar]
- [60].Blair SL, Emerson DK, Kulkarni S, Hwang ES, Malcarne V, Ollila DW. Breast surgeon’s survey: no consensus for surgical treatment of pleomorphic lobular carcinoma in situ. Breast J 2013;19(1): 116–8. [DOI] [PubMed] [Google Scholar]
- [61].Chivukula M, Haynik DM, Brufsky A, Carter G, Dabbs DJ. Pleomorphic lobular carcinoma in situ (PLCIS) on breast core needle biopsies: clinical significance and immunoprofile. Am J Surg Pathol 2008;32(11):1721–6. [DOI] [PubMed] [Google Scholar]
- [62].Susnik B, Day D, Abeln E, Bowman T, Krueger J, Swenson KK, et al. Surgical outcomes of lobular neoplasia diagnosed in core biopsy: prospective study of 316 cases. Clin Breast Cancer 2016;16(6):507–13. [DOI] [PubMed] [Google Scholar]
- [63].Carder PJ, Shaaban A, Alizadeh Y, Kumarasuwamy V, Liston JC, Sharma N. Screen-detected pleomorphic lobular carcinoma in situ (PLCIS): risk of concurrent invasive malignancy following a core biopsy diagnosis. Histopathology. 2010;57(3):472–8. [DOI] [PubMed] [Google Scholar]
- [64].Savage JL, Jeffries DO, Noroozian M, Sabel MS, Jorns JM, Helvie MA. Pleomorphic lobular carcinoma in situ: imaging features, upgrade rate, and clinical outcomes. AJR Am J Roentgenol 2018;211(2):462–7. [DOI] [PubMed] [Google Scholar]
- [65].Georgian-Smith D, Lawton TJ. Calcifications of lobular carcinoma in situ of the breast: radiologic-pathologic correlation. AJR Am J Roentgenol 2001. ;176(5):1255–9. [DOI] [PubMed] [Google Scholar]
- [66].Lavoue V, Graesslin O, Classe JM, Fondrinier E, Angibeau H, Leveque J. Management of lobular neoplasia diagnosed by core needle biopsy: study of 52 biopsies with follow-up surgical excision. Breast. 2007;16(5):533–9. [DOI] [PubMed] [Google Scholar]
- [67].Sullivan ME, Khan SA, Sullu Y, Schiller C, Susnik B. Lobular carcinoma in situ variants in breast cores: potential for misdiagnosis, upgrade rates at surgical excision, and practical implications. Arch Pathol Lab Med 2010;134(7):1024–8. [DOI] [PubMed] [Google Scholar]
- [68].Niell B, Specht M, Gerade B, Rafferty E. Is excisional biopsy required after a breast core biopsy yields lobular neoplasia? AJR Am J Roentgenol 2012;199(4):929–35. [DOI] [PubMed] [Google Scholar]
- [69].Meroni S, Bozzini AC, Pruneri G, Moscovici OC, Maisonneuve P, Menna S, et al. Underestimation rate of lobular intraepithelial neoplasia in vacuum-assisted breast biopsy. Eur Radiol 2014;24(7):1651–8. [DOI] [PubMed] [Google Scholar]
- [70].Guo T, Wang Y, Shapiro N, Fineberg S. Pleomorphic lobular carcinoma in situ diagnosed by breast Core biopsy: clinicopathologic features and correlation with subsequent excision. Clin Breast Cancer 2018;18(4). e449–e54. [DOI] [PubMed] [Google Scholar]


