Abstract
Purpose
To investigate the clinical significance of the changes in the macular microvasculature in patients with diabetes mellitus type 2 without diabetic retinopathy.
Methods
Fifty-five patients with diabetes mellitus type 2 without diabetic retinopathy and 48 healthy individuals were enrolled in a prospective cross-sectional study. We identified the changes of optical coherence tomography angiography parameters (foveal avascular zone [FAZ] area and circularity, vessel density, and perfusion index) of the 6 × 6-mm macular scan. Correlation and multiple regression analyses were performed between optical coherence tomography angiography parameters and previously known diabetes mellitus type 2-related demographic and systemic characteristics, and serum biochemical markers.
Results
FAZ parameters and perfusion index of the superficial and deep vascular plexus showed significant correlation with serum insulin level, and homeostasis model assessment indices. In multiple linear regression analysis, low insulin levels predicted increased FAZ areas in both the superficial (β = –0.007; P = 0.030) and deep layers (β = –0.010; P = 0.018) and a decreased perfusion index in the deep layer (β = 0.003; P = 0.001).
Conclusions
The expansion and loss of circularity of the FAZ and the decrease in the perfusion index may be affected by insulin resistance and secretory capacity in patients with diabetes mellitus type 2 with no diabetic retinopathy.
Keywords: optical coherence tomography angiography, diabetes mellitus, diabetic retinopathy, foveal avascular zone, vessel density, perfusion index, insulin, homeostasis model assessment
Optical coherence tomography angiography (OCT-A) noninvasively allows quantitative and qualitative evaluation of both the structure and flow of the stratified retinal vasculature in human participants. A number of OCT-A studies have been performed in patients with diabetes with diabetic retinopathy (DR), revealing decreased parafoveal vascular density and flow, especially in the deep capillary layer,1,2 and enlargement and impaired integrity of the foveal avascular zone (FAZ).3,4 Alterations in the FAZ area and circularity are important indicators of microcirculatory disturbance involving the macula as well as vessel-based metrics.5 Recent studies have shown contradicting results for changes in microvascular perfusion by using OCT-A in patients with diabetes without DR. Most reported a decrease in perifoveal perfusion parameters in the deep retinal layer,6,7 or in the superficial layer.8,9 However, Goudot et al.10 found no significant differences, and Rosen et al.11 described greater capillary perfusion in diabetics with no DR compared with controls.
In addition, these early microvascular changes detected using OCT-A in diabetic eyes without DR are not very specific findings. For example, patients with other retinal diseases (e.g., AMD12 and retinal vascular occlusion13) and patients with neurodegenerative disease (e.g., Alzheimer's disease14 and Parkinson's disease15) also demonstrate nonspecific microvascular alterations in their macula, similar to those observed with DR. Therefore, the clinical relevance of microvascular changes preceding retinopathy should be established before these changes can be used as novel retinal biomarkers for DR in practice. However, to our knowledge, there is no well-known DR biomarker that shows a significant correlation with the OCT-A parameters.6,16
Among various glycemic indices such as glucose levels, glycated albumin, glycated hemoglobin (HbA1c), HbA1c is the most well-known DR biomarkers.17 In diabetes mellitus, pancreatic β-cell function and insulin sensitivity are assessed by using postprandial C-peptide-to-glucose ratio, C-peptide increment, insulin increment, homeostasis model assessment of β-cell function (HOMA-B) and insulin resistance (HOMA-IR).18,19 The role of insulin indices as DR biomarker is of increasing interest.20 We expected that subclinical microvascular abnormalities of the macula appearing on OCT-A assessments might serve as a noninvasive and useful biomarker showing a significant correlation with some of the most representative and common existing biomarkers of DR.
Therefore, we designed a study to address two major objectives. First, to accurately identify the microvascular alterations in patients who did not develop retinopathy even with a long DM history, by using an OCT-A device and an image processing program. Second, to investigate the clinical relevance and the possibility of using OCT-A findings as a novel retinal biomarker. We compared these findings with the easily obtainable and common monitoring parameters (demographic characteristics, glycemic data, and insulin secretory/resistant factors) for diabetes and DM-related vascular complications.
Methods
We prospectively assessed a cross-sectional series of participants, consisting of patients with type 2 diabetes mellitus without clinical signs of DR (DM group) and age- and sex-matched normal participants (control group). All patients with type 2 diabetes mellitus whose diagnosis was confirmed by the diabetologist at the Department of Endocrinology at Gangnam Severance Hospital, Yonsei University College of Medicine (Seoul, Korea), were recruited from the Department of Ophthalmology at the same institute between February 2017 and January 2018. We only included patients older than 18 years with type 2 diabetes mellitus (use of insulin or oral hypoglycemic agents and HbA1c ≥ 6.5%) for more than 10 years without clinical signs of DR confirmed on fundus examination and color fundus photographs. When the fundus of the DM patients showed any stage of DR (dot-and-blot hemorrhages, cotton wool spots, microaneurysms, and nonperfusion areas) or diabetic macular edema with or without hard exudates, the patients were excluded. Control group was recruited among patients who underwent cataract surgery in the Department of Ophthalmology at the same institute. We only included normal patients who had no history of diabetes mellitus with normal range of fasting glucose in the preoperative evaluation and with no medications known to affect glucose tolerance.
The other exclusion criteria were as follows: presence of retinal diseases (e.g., dry and wet AMD, central serous chorioretinopathy, retinal vascular occlusion, epiretinal membrane, and macular hole), optic nerve diseases (e.g., optic neuritis, glaucoma, and ischemic optic neuropathy), significant media opacity or uveitis; high refractive error of greater than ±4 diopters; and a previous history of intraocular procedures within 6 months or any anti-inflammatory topical therapy within 3 months. Institutional review board approval was obtained, and the study complied with the guidelines of the Declaration of Helsinki. All study participants provided informed consent.
Clinical Assessments
Age, sex, duration of diabetes, and antidiabetic drugs administered were recorded for each participant. We measured resting systolic and diastolic blood pressure, and body mass index (BMI, kg/m2) was calculated on the basis of the weight and height measurements.
After an overnight fast, blood samples were obtained before and 120 minutes after ingestion of a standardized mixed meal, and laboratory assessments of the following biomarkers were performed: glucose, insulin, C-peptide, HbA1c, and glycated albumin. Pancreatic β-cell function and insulin sensitivity were evaluated using the following indices21: HOMA-B = ([basal insulin (pM) x 3.33]/[basal glucose (mM) – 3.5]), HOMA-IR = ([basal insulin (pM) x basal glucose (mM)]/135), and postprandial C-peptide-to-glucose ratio, which was defined as follows22: (stimulated C-peptide [ng/mL]/stimulated glucose [mg/dL]) x 100.
All participants underwent measurements of the best corrected visual acuity (logMAR) and IOP (mm Hg) with a noncontact tonometer and spherical equivalent (diopter) measurement using an auto-refractor (KR-1; Topcon Medical Systems, Inc., Tokyo, Japan). Detailed anterior segment and fundus examination was performed by a retinal specialist (MK. Wide-field (180°–200°) color fundus imaging was performed using a laser scanning ophthalmoscopy device (Optomap; Optos Plc., Dunfermline, UK).
OCT-A Scanning and Quantitative Measurement
The macula was scanned using a Zeiss Cirrus 5000 HD-OCT (Zeiss Meditec. Inc., Jena, Germany). This instrument uses a wavelength of 840 nm with a scanning rate of 68,000 A-scans per second. For all acquisitions, the FastTrac retinal-tracking technology was used to reduce motion artifacts. The resulting 6 × 6-mm angiography cube contains 245 B-scan slices. Each B-scan consists of 245 A-scans, and each A-scan is 1024 pixels deep. The 6 × 6-mm angiography images were obtained. Scanned images with a signal strength of less than 7 (from a maximum of 10) were not used in subsequent analysis. Moreover, the images that were decentered, poorly focused, or horizontally misaligned were excluded from the analysis.
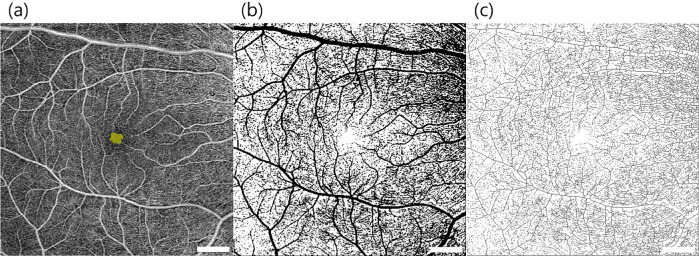
We acquired segment slabs of the superficial (Fig. 1a) and the deep (Fig. 1b) vascular plexuses of the retina from the 6 × 6-mm angiography images. The outer border of the inner plexiform layer was used to produce each slab in the customized settings of OCT-A (Figs. 1c–d). In cases with inaccurate segmentation, we manually adjusted the boundary between the superficial and deep vascular layers. The projection artifacts of the superficial layer were removed in the deep layer images by using a software built-in Zeiss OCT-A viewer. All images were exported into the ImageJ 1.50 software (National Institutes of Health, Bethesda, MD). FAZ was manually bordered by using the polygon selection tool (Fig. 2a), after which its area and circularity (formula= 4 × π × area/[perimeter]2) were automatically measured using ImageJ software.23 A circularity value closer to 1 indicates a circular shape, and closer to 0 indicates more irregular shape.24 We analyzed the vessel density and perfusion index of the 6 × 6-mm macula except the FAZ (central foveal 0.5-mm radius area). Vessel density (mm−1) was calculated after skeletonization (Fig. 2b) of the binarized image according to the methods described in previous studies.25 The perfusion index was defined as spatial averaging output after image binarization (Fig. 2c) by using autothresholding at 3 SDs above the mean noise level.25 Only one eye of each patient was selected for the analysis (the eye with the better OCT-A image quality). Two independent readers (EYC and SEP) obtained and evaluated the OCT-A findings, and the average values were used for the statistical analysis.
Figure 1.
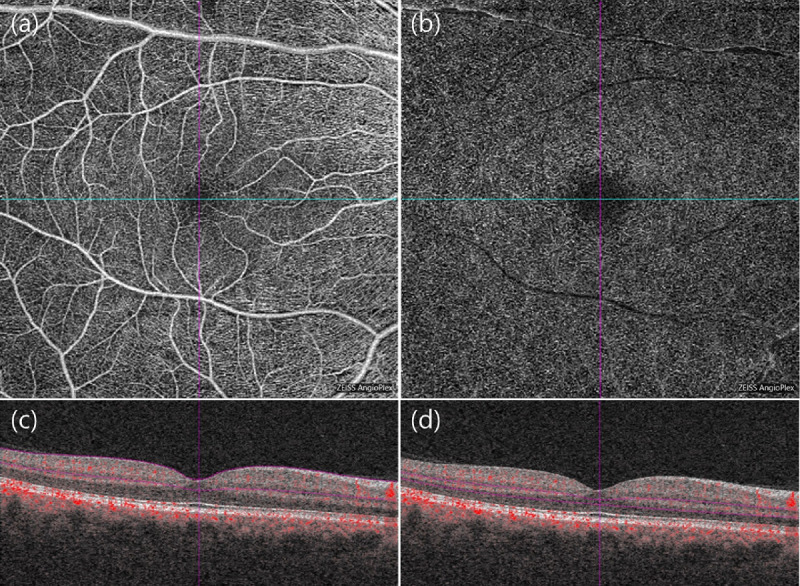
Optical coherence tomographic angiography (OCT-A) images of the right eye of a 57-year-old man with type 2 diabetes with no DR on clinical examination. En-face OCT-A images (a, b) and corresponding cross-sectional B-scan with flow (c, d) within the 6 × 6-mm macular cubes were captured at the level of the superficial capillary plexus (SCP; a, c) and the deep capillary plexus (DCP; b, d), respectively. The dotted purple lines on the B-scan image represent the boundaries of the segmentation slabs with each vascular network from which the OCT-A image was generated. The projection artifacts of the SCP layer were removed in the DCP during en-face OCT-A.
Figure 2.
Image processing steps for microvascular analysis of an en face optical coherence tomographic angiography projection image within the 6 × 6-mm macular cubes at the level of the superficial capillary plexus. (a) FAZ area is shown in yellow. (b) A binarized image was used to measure the perfusion index, and (c) skeletonization was performed to calculate the vessel density using the tools provided in the ImageJ software. The scan-logo in the bottom right of the images was masked for analysis.
Statistical Analysis
Statistical analysis was performed using SPSS software 21 (SPSS, Inc., Chicago, IL). All quantitative data were expressed as mean ± SD. P values of less than 0.05 were considered to be statistically significant. Basic characteristics were compared between DM patients with no DR and controls by performing the independent t-test for continuous variables and chi-square tests for categorical variables. We used the Pearson's correlation test to assess the relationship between OCT-A parameters and serum parameters. Stepwise multiple linear regression analysis was performed using OCT-A values for the macular microvasculature to model the relationship between these values and demographic, systemic, glycemic, and insulin secretory/resistant parameters.
Results
Of the 117 examined participants, 14 were excluded (6 eyes with epiretinal membrane or inner retinal atrophy, 5 eyes with drusen or RPE change, 3 eyes with low quality of image) and the data for the remaining 103 eyes (103 participants) were included in the analysis. Fifty-five patients had been diagnosed with type 2 diabetes mellitus but showed no signs of DR, and the remaining 48 individuals were healthy control participants. Basic systemic and ocular characteristics of the participants were summarized in Table 1, and all comparable variables were similar between the groups. In the DM without DR group, the median duration of diabetes was 17.9 years (range, 10.2–27.5 years).
Table 1.
Basic Characteristics of Healthy Individuals (Control Group) and Patients with Diabetes with No DR (DM Group)
| Control Group (n = 48) | DM Group (n = 55) | P Value | |
|---|---|---|---|
| Eyes (right/left) | 24/24 | 33/22 | 0.41* |
| Age (years) | 62.9 ± 12.9 | 62.5 ± 12.1 | 0.91† |
| Sex (M/F) | 20/ 28 | 31/ 24 | 0.63* |
| BMI (kg/m2) | 24.4 ± 3.4 | 24.2 ± 3.5 | 0.82† |
| Systolic blood pressure (mm Hg) | 131.8 ± 19.6 | 133.9 ± 15.6 | 0.68† |
| Diastolic blood pressure (mm Hg) | 77.7 ± 9.3 | 78.4 ± 9.3 | 0.80† |
| DM duration (years) | — | 17.9 ± 7.7 | — |
| BCVA (logMAR) | 0.03 ± 0.04 | 0.04 ± 0.06 | 0.41† |
| IOP (mm Hg) | 12.9 ± 1.3 | 14.6 ± 1.0 | 0.76† |
| Spherical equivalent (diopter) | –0.02 ± 1.80 | –0.04 ± 1.60 | 0.67† |
BCVA, best-corrected visual acuity.
Values are expressed as mean ± SD, except where indicated as the number of participants
Chi square test.
Independent t-test.
Table 2 shows a comparison of the FAZ and microvascular measurements in the superficial and deep vascular plexuses. Significant differences were more remarkable in the superficial vascular plexus. The FAZ area was larger in the DM without DR group (0.37 ± 0.13 mm2) than in the control group (0.29 ± 0.11 mm2; P = 0.012), whereas the FAZ circularity showed a decrease in patients with diabetes (0.77 ± 0.07) compared with controls (0.81 ± 0.05; P = 0.028). The vessel density (19.46 ± 3.03 mm−1 vs. 21.46 ± 3.08 mm−1; P = 0.014) and perfusion index (0.29 ± 0.03 vs. 0.33 ± 0.06; P = 0.001) of the macula were significantly lower in patients with diabetes than in healthy participants. In the deep vascular plexus, only the perfusion index showed a moderate decrease in patients with diabetes (0.38 ± 0.04 for control vs. 0.36 ± 0.06 for DM without DR; P = 0.042). Increased area and decreased circularity of the FAZ and a decrease in vessel density were also noted in the deep vascular plexus, although these results did not reach statistical significance.
Table 2.
Comparison of the FAZ and Microvascular Measurements in the Superficial and Deep Capillary Plexuses between Healthy Individuals (Control Group) and Patients With Diabetes With No DR (DM Group)
| Control Group | DM Group | P Value* | |
|---|---|---|---|
| SCP | |||
| FAZ area (mm2) | 0.29 ± 0.11 | 0.37 ± 0.13 | 0.012 |
| FAZ circularity | 0.81 ± 0.05 | 0.77 ± 0.07 | 0.028 |
| Vessel density (mm−1) | 21.46 ± 3.08 | 19.46 ± 3.03 | 0.014 |
| Perfusion index | 0.33 ± 0.06 | 0.29 ± 0.03 | 0.001 |
| DCP | |||
| FAZ area (mm2) | 0.71 ± 0.23 | 0.75 ± 0.19 | 0.44 |
| FAZ circularity | 0.77 ± 0.07 | 0.76 ± 0.06 | 0.87 |
| Vessel density (mm−1) | 25.43 ± 1.96 | 25.32 ± 3.38 | 0.87 |
| Perfusion index | 0.38 ± 0.04 | 0.36 ± 0.06 | 0.042 |
DCP, deep capillary plexus; SCP, superficial capillary plexus.
P values in bold indicate statistical significance (P < 0.05).
Independent t-test.
Table 3 summarizes the results of correlation analyses for microvascular parameters and demographic and systemic characteristics (age, BMI, systolic blood pressure, diastolic blood pressure, and DM duration) of the participants. Age showed a significant correlation with all OCT-A parameters for the deep vascular plexus (r = 0.294 and P = 0.027 for the FAZ area; r = −0.284 and P = 0.032 for FAZ circularity; r = −0.274 and P = 0.033 for vessel density; and r = −0.407 and P = 0.001 for perfusion index). BMI was positively correlated with the perfusion index of the deep vascular plexus (r = 0.267; P = 0.045). An increased of systolic blood pressure was related with a decrease of vessel density of SCP (r = –0.303; P = 0.041).
Table 3.
Correlation Analysis for the Macular Microvascular Parameters and Systemic Markers in Patients with Diabetes with No DR
| Systemic Markers, r (P Value)* | |||||
|---|---|---|---|---|---|
| Age | BMI | SBP | DBP | DM Duration | |
| SCP parameters | |||||
| FAZ area (mm2) | –0.033 | 0.031 | 0.079 | 0.114 | 0.009 |
| (0.802) | (0.826) | (0.603) | (0.455) | (0.963) | |
| FAZ circularity | –0.133 | 0.114 | 0.017 | –0.004 | –0.007 |
| (0.312) | (0.401) | (0.908) | (0.981) | (0.971) | |
| Vessel density (mm−1) | –0.166 | –0.037 | – 0.303 | –0.035 | 0.081 |
| (0.202) | (0.786) | (0.041) | (0.815) | (0.655) | |
| Perfusion index | –0.013 | 0.095 | 0.006 | –0.079 | –0.148 |
| (0.919) | (0.483) | (0.969) | (0.600) | (0.412) | |
| DCP parameters | |||||
| FAZ area (mm2) | 0.294 | 0.031 | 0.284 | 0.266 | 0.018 |
| (0.027) | (0.826) | (0.058) | (0.074) | (0.926) | |
| FAZ circularity | –0.284 | 0.019 | 0.027 | –0.157 | 0.097 |
| (0.032) | (0.893) | (0.859) | (0.296) | (0.618) | |
| Vessel density (mm−1) | –0.274 | 0.147 | 0.014 | –0.224 | 0.053 |
| (0.033) | (0.274) | (0.926) | (0.135) | (0.769) | |
| Perfusion index | –0.407 | 0.267 | –0.278 | –0.072 | –0.191 |
| (0.001) | (0.045) | (0.062) | (0.636) | (0.287) | |
DBP, diastolic blood pressure; DCP, deep capillary plexus; DM, diabetes mellitus; SCP, superficial capillary plexus; SBP, systolic blood pressure.
P values in bold indicate statistical significance (P < 0.05).
Pearson's correlation test.
Table 4 summarizes the results of correlation analyses for microvascular parameters and serum DM-related biomarkers in the DM group. Among the various glycemic and insulin secretory/resistant parameters, insulin-related values showed significant relationships with macular microvascular changes in Pearson's correlation analyses. Low insulin levels showed a moderate correlation with an increase in the FAZ area of the superficial vascular plexus (r = –0.584; P = 0.022) and deep vascular plexus (r = –0.545; P = 0.036). HOMA-IR values were more strongly correlated with the FAZ area of the superficial vascular plexus (r = –0.454; P = 0.034) and deep vascular plexus (r = –0.568; P = 0.009). HOMA-B values also showed a significant negative correlation with the FAZ area of the superficial vascular plexus (r = –0.430; P = 0.046) and deep vascular plexus (r = –0.551; P = 0.012). Interestingly, a decrease in the perfusion index of the deep vascular plexus was significantly related to low insulin levels (r = 0.734; P = 0.002), HOMA-IR scores (r = 0.616; P = 0.002), and HOMA-B scores (r = 0.648; P = 0.001). Additionally, the perfusion index of the superficial vascular plexus and HOMA-B scores demonstrated moderate positive correlation (r = 0.458; P = 0.032). There was no significant association between the microvascular parameters of the superficial and deep retina and the HbA1c, glycated albumin, and glucose levels and postprandial C-peptide-to-glucose ratio.
Table 4.
Correlation Analysis for the Microvascular Parameters and Serum Biochemical Markers of Diabetes in Patients with Diabetes with No DR
| Serum Biochemical Markers, r (P Value)* | |||||||
|---|---|---|---|---|---|---|---|
| HbA1c | GA | Glc | Ins | HOMA-IR | HOMA-B | PCGR | |
| SCP parameters | |||||||
| FAZ area (mm2) | –0.149 | –0.035 | –0.035 | –0.584 | –0.454 | –0.430 | 0.298 |
| (0.42) | (0.84) | (0.84) | (0.022) | (0.034) | (0.046) | (0.30) | |
| FAZ circularity | –0.257 | –0.118 | –0.119 | 0.006 | 0.024 | 0.089 | 0.006 |
| (0.16) | (0.52) | (0.51) | (0.98) | (0.91) | (0.69) | (0.98) | |
| Vessel density (mm−1) | 0.211 | –0.089 | –0.091 | 0.139 | 0.033 | 0.217 | –0.038 |
| (0.24) | (0.62) | (0.61) | (0.62) | (0.69) | (0.33) | (0.89) | |
| Perfusion index | 0.041 | –0.193 | –0.194 | 0.386 | 0.249 | 0.458 | –0.097 |
| (0.82) | (0.28) | (0.27) | (0.15) | (0.26) | (0.032) | (0.74) | |
| DCP parameters | |||||||
| FAZ area (mm2) | 0.052 | 0.003 | 0.004 | –0.545 | –0.568 | –0.551 | 0.317 |
| (0.79) | (0.98) | (0.98) | (0.036) | (0.009) | (0.012) | (0.29) | |
| FAZ circularity | –0.040 | 0.099 | 0.098 | 0.360 | 0.228 | 0.244 | –0.406 |
| (0.84) | (0.60) | (0.61) | (0.18) | (0.33) | (0.30) | (0.16) | |
| Vessel density (mm−1) | 0.206 | 0.247 | 0.247 | 0.190 | 0.118 | 0.095 | –0.197 |
| (0.25) | (0.16) | (0.16) | (0.49) | (0.60) | (0.66) | (0.50) | |
| Perfusion index | 0.159 | 0.101 | 0.099 | 0.734 | 0.616 | 0.648 | –0.266 |
| (0.38) | (0.57) | (0.58) | (0.002) | (0.002) | (0.001) | (0.35) | |
DCP, deep capillary plexus; GA, glycated albumin; Glc, glucose; Ins, insulin; PCGR, postprandial C-peptide-to-glucose ratio; SCP, superficial capillary plexus.
P values in bold indicate statistical significance (P < 0.05).
Pearson's correlation test.
The determinants of important OCT-A parameters (FAZ areas of both superficial and deep vascular plexuses and the perfusion index of the deep vascular plexus) in diabetic participants obtained by multiple linear regression analysis are presented in Table 5. We performed multiple linear regression analysis by entering only factors such as demographic factors, including age and BMI, and the insulin secretory/resistant parameters that were found to be significant in the Pearson's correlation test. Of the demographic factors included, age was significantly associated with the perfusion index of the deep vascular plexus (standardized beta [STD β] = –0.002; P = 0.002). Among the insulin-related parameters we entered, the insulin level was significantly associated with the FAZ area of the superficial vascular plexus (STD β = –0.007; P = 0.030), the FAZ area of the deep vascular plexus (STD β = –0.010; P = 0.018), and the perfusion index of the deep vascular plexus (STD β = 0.003; P = 0.001).
Table 5.
Multiple Linear Regression Models for Significant Macular Microvascular Parameters in Patients with Diabetes with No DR
| FAZ Area of SCP | FAZ Area of DCP | Perfusion Index of DCP | ||||
|---|---|---|---|---|---|---|
| STD β | P Value | STD β | P Value | STD β | P Value | |
| Age | 0.150 | 0.53 | 0.210 | 0.37 | –0.002 | 0.002 |
| BMI | 0.207 | 0.41 | –0.135 | 0.58 | 0.212 | 0.19 |
| Insulin | –0.007 | 0.030 | –0.010 | 0.018 | 0.003 | 0.001 |
| HOMA-IR | 0.214 | 0.20 | 0.119 | 0.91 | –0.145 | 0.84 |
| HOMA-B | 0.141 | 0.16 | 0.257 | 0.77 | 0.065 | 0.91 |
DCP, deep capillary plexus; SCP, superficial capillary plexus.
P values in bold indicate statistical significance (P < 0.05).
Discussion
In this study, we demonstrated two main findings. First, in patients with a long history of type 2 diabetes but with no DR, a decrease in vessel density and perfusion and impairment of the FAZ border occur earlier in the superficial vascular plexus than in the deep vascular plexus. Second, serum insulin level and HOMA values show a moderate inverse correlation with the levels of macular microvascular impairment.
Previous studies using fluorescein angiography have demonstrated reduction of capillary density and enlargement of FAZ in patients with diabetes with no retinopathy.26,27 Simonett et al.6 reported a lower vessel density in the deep capillary plexus in patients with type 1 diabetes with no and mild signs of nonproliferative DR. Subsequent reports noted that in patients with diabetes without signs of DR: the capillary nonperfusion initiates in the deep capillary plexus rather than the superficial capillary plexus.7,23 In contrast, Vujosevic et al.8 reported perifoveal capillary loss in the superficial capillary plexus before the clinical signs of DR in patients with type 1 and type 2 diabetes. In the early stage of nonproliferative DR, capillary dropout was also identified in the superficial capillary plexus.9 A decreased vessel density has also been reported in both the superficial and deep capillary plexuses in type 2 diabetes mellitus without DR.28 In contrast to the 6 × 6-mm2 scans used in our study, previous studies cited herein have used the central 3 × 3-mm2 scan for their analysis.
Interstudy differences in the retinal segmentation presets among OCT-A imaging systems and the lack of correction for the differences in segmentation boundaries can cause significant errors in OCT-A measurements.29 In addition, OCT-A scanning has artifact-related limitations caused by the projection of superficial vasculature on the deep retinal and choroidal plexuses.30 Therefore, unlike the superficial retinal layer, accurate information related to the deep retinal layer is difficult to obtain on screening or diagnosis of DR.16 In this study, we adjusted the anatomic slab segmentation, applied a projection-removal process on every deep vascular plexus image, and included the entire 6 × 6-mm2 macular area in the microvasculature analysis. Through this process, we confirmed impaired macular perfusion in clinically normal diabetic eyes, which was more prominent in the superficial vascular plexus than in the deep vascular plexus. This finding indicates that perfusion defects may occur initially in the superficial layer and then progress to the deep layer. However, our results may have some limitations because we did not adjust the image magnification errors induced by axial length variation.31,32
Although some investigators have reported FAZ enlargement and loss of integrity in DR,3,28 others did not find significant changes.6,23 We believe that these discrepancies could be attributed to differences in patient characteristics, OCT-A devices, and the methods used for quantitative analysis. In our study, patients with diabetes with no signs of DR showed FAZ area enlargements with loss of circularity in the superficial vascular plexus. The FAZ area also showed a significant relationship with the insulin-associated biochemical parameters in both superficial and deep vascular plexuses. Therefore, we could conclude that the very early microvascular changes before DR also include damage to the foveal center of the superficial vascular plexus and the deep vascular plexus.
The insulin secretory/resistant parameters, including the serum insulin level and HOMA-IR and HOMA-B scores, showed significant correlation with the FAZ and the perfusion index of the macula. The results of Pearson's correlation and multiple linear regression analyses revealed that the FAZ area tended to increase with a decrease in insulin level, and the perfusion index of the deep vascular plexus tended to decrease with older age and a decrease in insulin level. HbA1c is the most well-known biomarker in DR33; however, our results first revealed that it does not show a significant relationship with the initiating microvascular changes. HbA1c, which measures the time-averaged mean levels of glycemia, can only represent the glycemic control of the previous weeks and is insufficient to predict the overall glycemic control. In addition, it has recently been discovered that diabetic complications may arise from a single, hyperglycemia-induced superoxide production in the cellular level.34,35
With respect to the levels of insulin secretion in conjunction with the stages of type 2 diabetes mellitus, it is well-known that serum levels of insulin decrease in patients with progressive diabetes because of a reduction in β-cell mass and sequential disturbances in β-cell function.36 The results of our study suggest that low insulin levels may also reflect the development of DR in patients with long-standing diabetes with no signs of DR. If insulin can be confirmed as a significant biomarker, it will allow more cost-effective screening to identify asymptomatic DM individuals at the risk of developing DR.
In conclusion, we evaluated the clinical relevance of macular microvascular parameters quantitatively measured by OCT-A on demographic and glucometabolic parameters, and revealed a novel finding that the fasting insulin level and HOMA-IR and HOMA-B scores in patients with type 2 diabetes mellitus without DR reflect the very early microvascular changes leading to DR. Pancreatic β-cell function and insulin sensitivity might be a more sensitive serum biomarker than HbA1c levels, glucose fluctuation, and DM duration for early detection of diabetic vascular complications. This value can be a useful marker for determination of the need for more aggressive antidiabetic therapy. Further longitudinal prospective studies are needed to investigate a causal relationship between insulin secretory capacity and resistance, macular vascular damage, and other vascular complications of diabetes.
Acknowledgments
Disclosure: E.Y. Choi, None; S.E. Park, None; S.C. Lee, None; H.J. Koh, None; S.S. Kim, None; S.H. Byeon, None; M. Kim, None
References
- 1. Chen Q, Ma Q, Wu C, et al.. Macular vascular fractal dimension in the deep capillary layer as an early indicator of microvascular loss for retinopathy in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2017; 58: 3785–3794. [DOI] [PubMed] [Google Scholar]
- 2. Nesper PL, Roberts PK, Onishi AC, et al.. Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017; 58: 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016; 254: 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang F, Cheung CY. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017; 58: 1766. [DOI] [PubMed] [Google Scholar]
- 5. Kwon J, Choi J, Shin JW, Lee J, Kook MS. Alterations of the foveal avascular zone measured by optical coherence tomography angiography in glaucoma patients with central visual field defects. Invest Ophthalmol Vis Sci. 2017; 58: 1637–1645. [DOI] [PubMed] [Google Scholar]
- 6. Simonett JM, Scarinci F, Picconi F, et al.. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017; 95: e751–e755. [DOI] [PubMed] [Google Scholar]
- 7. Scarinci F, Picconi F, Giorno P, et al.. Deep capillary plexus impairment in patients with type 1 diabetes mellitus with no signs of diabetic retinopathy revealed using optical coherence tomography angiography. Acta Ophthalmol. 2018; 96: e264–e265. [DOI] [PubMed] [Google Scholar]
- 8. Vujosevic S, Muraca A, Alkabes M, et al.. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina (Philadelphia, Pa). 2017; 39: 435–445. [DOI] [PubMed] [Google Scholar]
- 9. Shen C, Yan S, Du M, Zhao H, Shao L, Hu Y. Assessment of capillary dropout in the superficial retinal capillary plexus by optical coherence tomography angiography in the early stage of diabetic retinopathy. BMC Ophthalmol. 2018; 18: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goudot MM, Sikorav A, Semoun O, et al.. Parafoveal OCT angiography features in diabetic patients without clinical diabetic retinopathy: a qualitative and quantitative analysis. J Ophthalmol. 2017; 2017: 8676091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosen RB, Andrade Romo JS, Krawitz BD, et al.. Earliest evidence of preclinical diabetic retinopathy revealed using OCT angiography (OCTA) perfused capillary density. Am J Ophthalmol. 2019; 203: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toto L, Borrelli E, Mastropasqua R, et al.. Association between outer retinal alterations and microvascular changes in intermediate stage age-related macular degeneration: an optical coherence tomography angiography study. Br J Ophthalmol. 2017; 101: 774–779. [DOI] [PubMed] [Google Scholar]
- 13. Seknazi D, Coscas F, Sellam A, et al.. Optical coherence tomography angiography in retinal vein occlusion: correlations between macular vascular density, visual acuity, and peripheral nonperfusions area on fluorescein angiography. Retina (Philadelphia, Pa). 2017; 38: 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bulut M, Kurtulus F, Gozkaya O, et al.. Evaluation of optical coherence tomography angiographic findings in Alzheimer's type dementia. Br J Ophthalmol. 2018; 102: 233–237. [DOI] [PubMed] [Google Scholar]
- 15. Miri S, Shrier EM, Glazman S, et al.. The avascular zone and neuronal remodeling of the fovea in Parkinson disease. Ann Clin Transl Neurol. 2015; 2: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Durbin MK, An L, Shemonski ND, et al.. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017; 135: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O'Neal DN, Januszewski AS. Biomarkers in diabetic retinopathy. Rev Diabet Stud. 2015; 12: 159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ludvigsson J. C-peptide in diabetes diagnosis and therapy. Front Biosci (Elite Ed). 2013; 5: 214–223. [DOI] [PubMed] [Google Scholar]
- 19. Hirata T, Higashiyama A, Kubota Y, et al.. HOMA-IR values are associated with glycemic control in Japanese subjects without diabetes or obesity: the KOBE study. J Epidemiol. 2015; 25: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SR, Lee YH, Lee SG, et al.. Urinary N-acetyl-beta-D-glucosaminidase, an early marker of diabetic kidney disease, might reflect glucose excursion in patients with type 2 diabetes. Medicine (Baltimore). 2016; 95: e4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 22. Lee EY, Hwang S, Lee SH, et al.. Postprandial C-peptide to glucose ratio as a predictor of beta-cell function and its usefulness for staged management of type 2 diabetes. J Diabetes Investig. 2014; 5: 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carnevali A, Sacconi R, Corbelli E, et al.. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetologica. 2017; 54: 695–702. [DOI] [PubMed] [Google Scholar]
- 24. Okada M, Hersh D, Paul E, van der Straaten D. Effect of centration and circularity of manual capsulorrhexis on cataract surgery refractive outcomes. Ophthalmology. 2014; 121: 763–770. [DOI] [PubMed] [Google Scholar]
- 25. Alibhai AY, Moult EM, Shahzad R, et al.. Quantifying microvascular changes using OCT angiography in diabetic eyes without clinical evidence of retinopathy. Ophthalmol Retina. 2018; 2: 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arend O, Wolf S, Jung F, et al.. Retinal microcirculation in patients with diabetes mellitus: dynamic and morphological analysis of perifoveal capillary network. Br J Ophthalmol. 1991; 75: 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arend O, Wolf S, Harris A, Reim M. The relationship of macular microcirculation to visual acuity in diabetic patients. Arch Ophthalmol. 1995; 113: 610–614. [DOI] [PubMed] [Google Scholar]
- 28. Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017; 58: 190–196. [DOI] [PubMed] [Google Scholar]
- 29. Rommel F, Siegfried F, Kurz M, et al.. Impact of correct anatomical slab segmentation on foveal avascular zone measurements by optical coherence tomography angiography in healthy adults. J Curr Ophthalmol. 2018; 30: 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang FY, Ng DS, Lam A, et al.. Determinants of quantitative optical coherence tomography angiography metrics in patients with diabetes. Sci Rep. 2017; 7: 2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sampson DM, Gong P, An D, et al.. Axial length variation impacts on superficial retinal vessel density and foveal avascular zone area measurements using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017; 58: 3065–3072. [DOI] [PubMed] [Google Scholar]
- 32. Linderman R, Salmon AE, Strampe M, Russillo M, Khan J, Carroll J. Assessing the accuracy of foveal avascular zone measurements using optical coherence tomography angiography: segmentation and scaling. Transl Vis Sci Technol. 2017; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ting DS, Tan KA, Phua V, Tan GS, Wong CW, Wong TY. Biomarkers of diabetic retinopathy. Curr Diab Rep. 2016; 16: 125. [DOI] [PubMed] [Google Scholar]
- 34. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 35. Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006; 116: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meier JJ, Bonadonna RC. Role of reduced beta-cell mass versus impaired beta-cell function in the pathogenesis of type 2 diabetes. Diabetes Care. 2013; 36: S113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]