Abstract
Purpose
To compare the macroscopic and microscopic histologic changes in eyes treated with micropulse transscleral cyclophotocoagulation (MP-TCP) versus continuous wave transscleral cyclophotocoagulation (CW-TCP).
Methods
Twelve halves of globes from three pairs of adult cadaveric eyes were randomly assigned to nontreated control, CW-TCP, single MP-TCP treatment, or double MP-TCP treatments, and then sectioned for histologic analysis. Presence or absence of the following four unique histologic changes was recorded: splitting within the ciliary process epithelium (splitting), separation of the pigmented ciliary process epithelium from the stroma (separation), coagulation of collagen and destruction of ciliary process stroma (coagulation), and full-thickness destruction of ciliary process epithelium (destruction).
Results
A total of 498 slides were analyzed, and laser scars in all treated specimens were located in the pars plana. Logistic regression analysis showed that compared with controls, CW-TCP-treated specimens were significantly more likely to experience separation (odds ratio [OR] = 11.1, P = 0.02), coagulation (OR = 24.3, P = 0.002), and destruction (OR = 11.1, P = 0.03). Destruction of the ciliary process epithelium was observed exclusively in CW-TCP-treated sections. No significant differences in histologic features were observed between controls and MP-TCP.
Conclusions
MP-TCP does not produce significant histologic changes in cadaveric eyes, whereas CW-TCP treatment does.
Translational Relevance
These findings improve understanding of the mechanism of MP-TCP, help explain the increased rates of adverse effects following CW-TCP treatment compared with MP-TCP, and describe effects of MP-TCP at various doses.
Keywords: cyclophotocoagulation, CW-TCP, MP-TCP, histology
Introduction
Glaucoma is the leading cause of irreversible blindness in the world and its prevalence is expected to increase over the coming years.1 Lowering of intraocular pressure (IOP) has been shown to prevent or delay the onset of glaucomatous vision loss.2,3 Currently, lowering of IOP is achieved by medical treatment with topical or systemic medications, laser therapy, or surgical interventions.
Conventional continuous wave transscleral cyclophotocoagulation (CW-TCP) is a cyclodestructive procedure that lowers IOP presumably by directly damaging the ciliary body, the site of aqueous fluid production.4 Adverse effects, such as postoperative pain, intraocular inflammation, hypotony, and scleral thinning, typically limit its use to eyes with limited visual potential.5–9 Recently, a modified application of CW-TCP, known as micropulse transscleral cyclophotocoagulation (MP-TCP), was introduced. MP-TCP applies laser energy over the ciliary processes in shorter pulses compared with CW-TCP. It is hypothesized that these short pulses lead to less damage to the ciliary process, and thus avoid some of the adverse effects of CW-TCP.10 However, the histopathologic effects of treatment with MP-TCP are unknown.
The purpose of this study is to examine the macroscopic and histologic changes with MP-TCP treatment in adult eyes and to compare these changes to CW-TCP-treated eyes. We hypothesize that the anatomic changes following MP-TCP treatment are similar to, but more nuanced than, those following CW-TCP treatment.
Methods
The University of California San Francisco Institutional Review Board granted a HIPAA Authorization Waiver for this study given that the globes used in this study were from anonymous deceased donors. Inclusion and exclusion criteria stipulated that eyes must be free of prior ocular pathology or surgery (aside from cataract or cataract surgery), and that the individual was older than 18 years of age at time of death. This was assessed by donor history and by ocular examination. In total, five pairs eyes were screened and six globes from three adult cadaveric eyes were obtained from the Minnesota Lions Eye Bank. The selected eyes were harvested within 36 hours of death. The axial lengths of the globes were measured, and the globes were orientated based on muscle insertion.
This study employed a randomized comparative experiment design. Four half-globes from each patient were randomized to untreated control, CW-TCP, single MP-TCP treatment, and double MP-TCP treatment based on an online random number generator varying from one to four by the author (JHL). Each number represented each treatment applied to all three pairs of cadaveric eyes. The half-globes were treated independently, but each pair of cadaveric eyes received all four treatments (i.e., one treatment per half-globe). Treatment was applied prior to hemisection. The Iridex Cyclo G6 Glaucoma Laser System (Iridex, Mountain View, CA) was used to perform the treatments. The continuous wave G-probe (Iridex) was used to perform CW-TCP treatment, and the Micropulse P3 probe (Iridex) was used to perform MP-TCP treatment. CW-TCP treatments were performed by positioning the G-probe on the limbus and parallel to the visual axis, as detailed in the instructions for use by the manufacturer. The G-probe was moved circumferentially around the globe, maintaining contact with the limbus, after each laser application. MP-TCP treatments were performed by placing the Micropulse P3 probe at the limbus and perpendicular to the surface of the globe. Sweeping motions were used to treat the globe circumferentially, excluding the 3 and 9 o'clock positions. CW-TCP treatment parameters included power of 1500 to 2000 mW and duration of 4000 ms. Each half-globe randomized to treatment with CW-TCP was treated with a total of five to seven single laser applications. MP-TCP treatment parameters included power of 2000 mW, duration of 80 seconds, and a duty cycle of 31.3%. Half-globes underwent treatment with sweeping motions for 80 seconds if randomized to single MP-TCP treatment, or 160 seconds if randomized to double MP-TCP treatment. After treatment, each globe was fixed in 10% formaldehyde for 24 to 72 hours then cut into two half-globes for macroscopic examination.
Macroscopic and Histologic Features
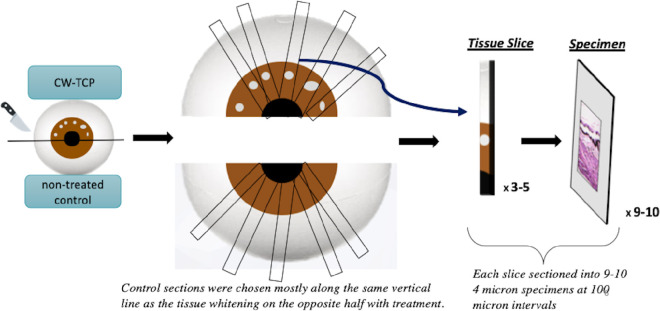
Macroscopic evaluation was performed by two of the authors (KM and MP), who were both masked to treatment condition of the specimens. Superior and inferior halves of each globe were differentially inked to maintain proper orientation after dissection. Each globe was cut posterior to the equator along the coronal plane, the posterior poles were discarded, and anterior poles were examined under a dissecting scope. Variably sized areas of tissue whitening were identified in the pars plana involving the halves randomized to treatment, suggestive of treatment effect (laser burns). The anterior portion of each globe was serially sectioned into three to five tissue slices in the vertical plane perpendicular to the pupil, targeting the area with tissue whitening, and entirely submitted for histologic evaluation. For the control halves, the sections were chosen randomly, and mostly along the same vertical line as the tissue whitening on the opposite half with treatment.
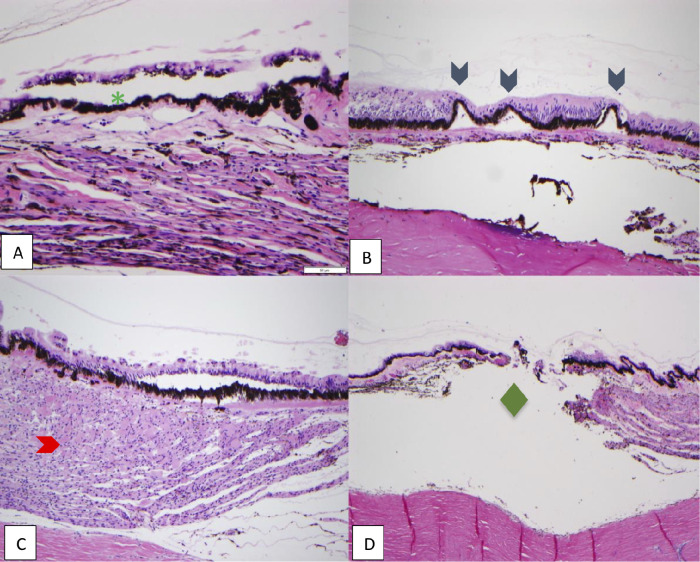
Multiple, 4-µ thick step sections were obtained from formalin-fixed, paraffin-embedded tissues with 100-µ intervals, and were stained with hematoxylin and eosin using an automated stainer (Sakura Tissue-Tek Prisma A1D, Sakura Finetek USA, Inc., Torrence, CA). A cartoon representation of the tissue processing protocol employed in this study is seen in Figure 1. Histologic features were evaluated by three of the authors (KM, MP, and YH). The histologic sections were coded so that the three authors were masked to the section's original eye side, hemisection, tissue slices, and treatment that the tissue received. A consensus among the three authors was achieved for all sections. Histologic changes, which are not typically seen in normal globes, were scored as present or absent in each section, and percent of total sections involved was calculated for each half-globe (frequency). When more than one histologic feature was present, only the most severe histologic change was labeled for the section. Sections missing the ciliary epithelium were excluded from the analysis. Histologic features included in the study are (1) splitting within the ciliary epithelium; (2) separation of the pigmented ciliary epithelium from the stroma; (3) coagulation of collagen and destruction of ciliary body stroma; and (4) full-thickness destruction of ciliary epithelium (Fig. 2).
Figure 1.
Diagrammatic representation of processing of tissue specimens.
Figure 2.
Representative images depicting histologic features included in the study. Hematoxylin and stained sections, 100x magnification. Green asterisk: splitting within the ciliary epithelium, between pigmented and nonpigmented epithelia; blue arrowhead: separation of the pigmented ciliary epithelium from the stroma; red arrowhead: coagulation of collagen and destruction of ciliary body stroma; green diamond: full-thickness destruction of ciliary epithelium. (A) Control, (B) double MP-TCP-treated eye, (C-D) CW-TCP-treated eyes.
Statistical Analyses
Unless otherwise specified, all statistics were performed with IBM SPSS Statistics version 24 (IBM Corporation, Armonk, NY). Presence of histologic features in sections were compared using 4 × 2 Fisher's exact tests, and strength of association between treatment conditions and histologic features was assessed via the Cramér V tests. Stata version 14.0 (StataCorp LLC, College Station, TX) was used to perform a multilevel logistic regression with standard errors adjusted for clustering within section. The number of specimens was selected to provide at least 80% power to detect an association with a Cramer's value of 0.18 or larger between the control and treatment conditions with regards to microscopic changes. P < 0.05 was considered to be statistically significant in all analyses.
Results
All three pairs of eyes were from white adults. The first adult pair was from an 89-year-old man whose cause of death was pneumonia. The second adult pair was from a 62-year-old man whose cause of death was hepatocellular carcinoma. The third adult pair was from a 62-year-old woman whose cause of death was unknown. The mean axial length and standard deviation of the globes was 23.7 ± 0.6 mm.
Macroscopic Changes Following Treatment
Representative images demonstrating the location of the CW-TCP and MP-TCP laser treatments in the cadaveric adult eyes are shown in Figure 3. Both MP-TCP and CW-TCP led to depigmented areas in the cadaveric eyes. CW-TCP caused discrete, well-circumscribed white spots, whereas MP-TCP caused nearly linear, small, irregularly shaped, and streak-like depigmentation of the tissue. Interestingly, all of the depigmented areas were located in the pars plana and not the pars plicata. These observations were consistent in all the cadaveric eye specimens.
Figure 3.
Macroscopic changes following continuous wave and MP-TCP treatment in adult cadaveric eyes. The laser burns (blue arrow) are seen exclusively within the pars plana. Adult eye treated with CW-TCP (A and C). Adult eye treated with MP-TCP (B and D). Note the grossly evident tissue rupture in (C).
Microscopic Changes Following Treatment
In total, 498 ocular histologic sections were analyzed, which were derived from a total of 46 tissue slices containing ciliary epithelium. Four to 18 histologic sections were cut from each tissue slice, with 10.8 ± 3.6 (mean ± standard deviation) sections per slice. The total number of contributing tissue slices and histologic sections per treatment group are listed in Table 1. Representative images demonstrating the histologic changes are shown in Figure 2. The frequencies of each histologic change were calculated across the four treatment groups and compared (Fig. 4 and Table 2). The Fisher's exact tests demonstrated that there were significant differences among treatment groups for full-thickness destruction of ciliary epithelium (P < 0.001), separation of the pigmented ciliary epithelium from the stroma (P < 0.001), and coagulation of collagen and destruction of ciliary stroma (P < 0.001), whereas splitting within the ciliary epithelium occurred at similar rates among the four groups (P = 0.19). With regard to the strength of association between histologic outcome and treatment condition, coagulation of collagen and destruction of ciliary stroma had the strongest association (Cramér's V = 0.44), whereas weaker associations were observed for separation of the pigmented ciliary epithelium from the stroma and full-thickness destruction of ciliary epithelium (Cramér's V = 0.23 and Cramér's V = 0.25, respectively). “Coagulation of collagen and destruction of ciliary body stroma” pairwise comparisons demonstrated that this feature was significantly more common in hemifield treated with CW-TCP compared with MP-TCP1 and MP-TCP2 (Fisher's exact test, P < 0.0001 and P < 0.0001, respectively).
Table 1.
Quantification of Specimens
| Tissue Slices | Histologic Sections | |
|---|---|---|
| Control | 9 | 110 |
| CW-TCP | 15 | 153 |
| Single MP-TCP | 11 | 117 |
| Double MP-TCP | 11 | 118 |
| Total | 46 | 498 |
Figure 4.
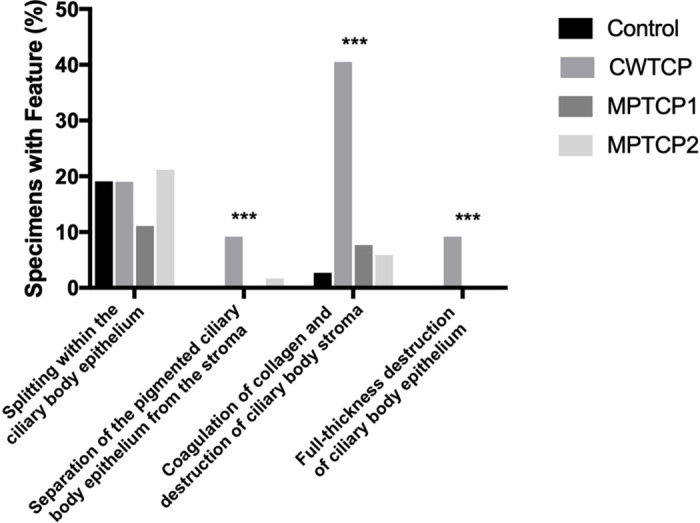
Percentage of sections demonstrating each histologic feature by treatment group. ***Indicates P < 0.001.
Table 2.
Contingency Results for Histologic Outcomes
| Fisher's Exact Test* | Cramér's V Test | |
|---|---|---|
| Splitting within the ciliary epithelium | 0.17 | 0.10 |
| Separation of the pigmented ciliary epithelium from the stroma | <0.001 | 0.23 |
| Coagulation of collagen and destruction of ciliary body stroma | <0.001 | 0.44 |
| Full-thickness destruction of ciliary epithelium | <0.001 | 0.26 |
This table represents a 4 × 2 contingency table comparing the four histologic outcomes among the four different treatment conditions (control, CW-TCP, single MP-TCP, double MP-TCP). *Indicates a P value. A significant P value indicates that at least one treatment group was significantly different from the other treatment groups.
Multilevel logistic regression modeling adjusting for correlation from clustering of multiple sections within a tissue slice was performed for assessing the association between treatment and each histologic feature (Tables 3 and 4). Compared with control specimens without any treatment, the CW-TCP-treated specimens were significantly more likely to demonstrate separation of the pigmented ciliary epithelium from the stroma (odds ratio [OR] = 11.0, P = 0.02), coagulation of collagen and destruction of ciliary stroma (OR = 24.3, P = 0.002), and full-thickness destruction of ciliary epithelium (OR = 11.0, P = 0.03), whereas there was no significant difference in MP-TCP1 and MP-TCP2-treated specimens. None of the treatment groups were significantly different from the control with regard to splitting within the ciliary epithelium between the pigmented and nonpigmented epithelia (P > 0.20).
Table 3.
Likelihood of Presence of Histologic Changes in Each Treatment Group: Control as Reference
| Histologic Outcome | ||||
|---|---|---|---|---|
| Splitting Within the Ciliary Epithelium | Separation of the Pigmented Ciliary Epithelium from the Stroma | Coagulation of Collagen and Destruction of Ciliary Body Stroma | Full-Thickness Destruction of Ciliary Epithelium | |
| Treatment | OR, (95% CI) | OR, (95% CI) | OR, (95% CI) | OR, (95% CI) |
| Control | Reference | Reference | Reference | Reference |
| CW-TCP | 0.99 (0.36–2.78) | 10.98 (1.55–77.84) | 24.3 (3.35–176.2) | 10.98 (1.34–89.85) |
| P = 0.99 | P = 0.02 | P = 0.002 | P = 0.03 | |
| Single MP-TCP | 0.53 (0.19–1.44) | 0.94 (0.07–11.97) | 2.97 (0.30–29.4) | 0.94 (0.07–11.97) |
| P = 0.21 | P = 0.96 | P = 0.35 | P = 0.96 | |
| Double MP-TCP | 1.14 (0.39–3.36) | 1.88 (0.15–24.35) | 2.25 (0.22–23.2) | 0.93 (0.07–11.93) |
| P = 0.81 | P = 0.63 | P = 0.50 | P = 0.96 | |
CI, confidence interval.
Table 4.
Likelihood of Presence of Histologic Changes in each Treatment Group: CW-TCP as Reference
| Histologic Outcome | ||||
|---|---|---|---|---|
| Splitting within the Ciliary Epithelium | Separation of the Pigmented Ciliary ePithelium from the Stroma | Coagulation of Collagen and Destruction of Ciliary Body Stroma | Full-Thickness Destruction of Ciliary Epithelium | |
| Treatment | OR, (95% CI) | OR, (95% CI) | OR, (95% CI) | OR, (95% CI) |
| CW-TCP | Reference | Reference | Reference | Reference |
| Control | 1.01 (0.36–2.81), P = 0.99 | 0.09 (0.01–0.65), P = 0.02 | 0.04 (0.006–0.30), P = 0.002 | 0.09 (0.01–0.75), P = 0.025 |
| Single MP-TCP | 0.53 (0.19–1.44), P = 0.21 | 0.09 (0.01–0.62), P = 0.02 | 0.12 (0.02–0.61), P = 0.01 | 0.09 (0.01–0.72), P = 0.02 |
| Double MP-TCP | 1.15 (0.39–3.41), P = 0.80 | 0.17 (0.02–1.27), P = 0.09 | 0.09 (0.2–0.49), P = 0.005 | 0.08 (0.01–0.72), P = 0.02 |
Analysis was performed using logistic regression model for each treatment condition, and standard errors are adjusted for clustering of histologic features within tissue sections. OR and 95% confidence intervals (CI) are provided. All P values represent the associated treatment group compared with the control.
Discussion
In this article, we studied and compared the macroscopic and microscopic features of MP-TCP-treated, CW-TCP-treated, and untreated control human cadaveric eyes. We found different macroscopic patterns between CW-TCP and MP-TCP treatment, and these treatment effects were isolated to the pars plana. Histologically, CW-TCP induced significant destruction across the ciliary body epithelium and stroma, whereas MP-TCP did not.
Both CW-TCP and MP-TCP treatments result in identifiable macroscopic and histologic changes in treated ocular tissues. Both treatment modalities produced laser burns in the pars plana. This is a notable finding as aqueous humor is mainly produced by ciliary processes in the pars plicata. Previous studies have reported histologic changes in the pars plicata alone, pars plana alone, or both the pars plana and pars plicata following treatment with CW-TCP.4,11–14 In this study, we found that when the laser probe was placed at the limbus as instructed by the manufacturer, the treatment was directed exclusively to the pars plana in both CW-TCP and MP-TCP-treated hemisections. Although IOP in the cadaveric eyes were normalized by injecting balanced solution, it is possible that expansion of the eyeball secondary to increased IOP could have led to laser scar formation closer to the pars plicata. In practice, identification of the pars plicata using transillumination before treatment may improve accuracy of probe location. Nevertheless, application of laser probe with a single size for all patients may lead to variable outcomes due to variation in treatment locations.
This finding also raises questions regarding the mechanism of IOP reduction following treatment. A few studies evaluated the microscopic changes following treatment with CW-TCP, some of which have offered possible explanations for its IOP lowering effect.4,11–16 Postulated hypotheses include laser-induced damage to the ciliary process epithelium leading to decreased aqueous humor production; inflammation leading to decreased aqueous production; release of prostaglandins resulting in increased uveoscleral outflow; and damage to the vascular supply of the ciliary body with resultant decreased aqueous humor production secondary to ciliary body ischemia. These possible mechanisms are congruent with the findings observed in this study.
Microscopically, four unique histologic changes were noted in this study. Splitting within the ciliary epithelium occurred at a similar rate across all four treatment groups, including control tissue, and is probably not a direct effect of the laser treatment but is likely an artifact due to tissue handling and processing. Full-thickness destruction of the ciliary epithelium was seen exclusively in CW-TCP-treated hemisections. Separation of the pigmented epithelium from the stroma was seen only in CW-TCP and double MP-TCP-treated hemisections. Coagulation of collagen and destruction of the ciliary stroma were seen in all four groups but occurred much more commonly in CW-TCP-treated hemisections. Full-thickness destruction of the ciliary epithelium, coagulation of collagen, and destruction of the ciliary stroma likely represent damage of ciliary body tissue, leading to decreased aqueous production. The clinical relevance of separation of pigmented epithelium is not clear and warrants future study.
Logistic regression analysis confirmed that CW-TCP leads to significant histologic changes compared with control hemisections, which is consistent with previous studies.4,13–16 Interestingly, we found that there were no significant histologic changes in MP-TCP-treated section from control section. This may suggest that MP-TCP treatment results in much more nuanced anatomic changes relative to CW-TCP, which may explain its relatively good safety profile.17–19 Moreover, when MP-TCP treatment was doubled, the histologic changes remained minimal compared with control. This provides histologic evidence that extending treatment time up to 160 ms may still be safe with MP-TCP. In addition, the histologic findings imply different IOP lowering mechanism between CW-TCP and MP-TCP. In contrast to CW-TCP, MP-TCP does not appear to destroy the ciliary processes, and thus MP-TCP may lower IOP independent of aqueous production. Further studies should be conducted to explore whether other mechanisms may lead to IOP reduction, such as ciliary body rotation, with subsequent opening of the angle and increased aqueous outflow.
This study has a few limitations. For the MP-TCP treated specimens, burn lesions in the ciliary epithelium were not continuous, and sections were taken at random when processing the specimens. However, an average of 125 slides (SD 19.3) were analyzed per treatment condition, and this large number of slides significantly helped control for confounding results attributed to sampling error. We only used hematoxylin and eosin–stained sections in this study, and other stains, such as Masson trichome, may provide further information about the damaged structures within the ciliary body stroma, which were only interpreted as coagulative changes in collagen in the current study. Furthermore, the laser treatments were applied to and studied in cadaveric eyes with no history of glaucoma. Different tissue responses may occur when diode lasers are applied to living eyes or eyes subjected to chronically elevated IOP given the expected changes associated with tissue injury, inflammation, healing, and scarring.
Conclusions
To our knowledge, this is the first study to assess the histologic effects of MP-TCP treatment applied to cadaveric eyes and to compare MP-TCP treatment with CW-TCP. We found that CW-TCP is associated with variable destruction of the ciliary body across multiple histologic features, whereas MP-TCP did not cause significant damage to the ciliary body. Understanding the histologic effects of MP-TCP treatment will help ophthalmologists and engineers to better understand its mechanism of action and safety profile, as well as to improve its IOP-reduction effects.
Supplementary Material
Acknowledgments
This study was supported in part by grant NIH-NEI EY002162-Core Grant for Vision Research from the National Institutes of Health/National Eye Institute and by an unrestricted departmental grant from Research to Prevent Blindness. Richard K. Lee is supported by the Walter G. Ross Foundation; NEI EY028747-01.
Previous meeting presentations: American Glaucoma Society, Annual Meeting, 2019; American Glaucoma Society, Annual Meeting, 2018; United States and Canadian Academy of Pathology, Annual Meeting, 2018.
Disclosure: K. Moussa, None; M. Feinstein, None; M. Pekmezci, None; J.H. Lee, None; M. Bloomer, None; C. Oldenburg, None; Z. Sun, None; R.K. Lee, None; G.-S. Ying, None; Y. Han, None
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kass MA, Heuer DK, Higginbotham EJ, et al.. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol Chic Ill 1960. 2002; 120: 701–713; discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 3. Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998; 126: 487–497. [DOI] [PubMed] [Google Scholar]
- 4. Blasini M, Simmons R, Shields MB. Early tissue response to transscleral neodymium: YAG cyclophotocoagulation. Invest Ophthalmol Vis Sci. 1990; 31: 1114–1118. [PubMed] [Google Scholar]
- 5. Murphy CC, Burnett CaM, Spry PGD, Broadway DC, Diamond JP. A two centre study of the dose-response relation for transscleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol. 2003; 87: 1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walland MJ. Diode laser cyclophotocoagulation: longer term follow up of a standardized treatment protocol. Clin Experiment Ophthalmol. 2000; 28: 263–267. [DOI] [PubMed] [Google Scholar]
- 7. Mistlberger A, Liebmann JM, Tschiderer H, Ritch R, Ruckhofer J, Grabner G. Diode laser transscleral cyclophotocoagulation for refractory glaucoma. J Glaucoma. 2001; 10: 288–293. [DOI] [PubMed] [Google Scholar]
- 8. Schlote T, Derse M, Rassmann K, Nicaeus T, Dietz K, Thiel HJ. Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma. 2001; 10: 294–301. [DOI] [PubMed] [Google Scholar]
- 9. Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology. 1996; 103: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 10. Amoozgar B, Phan EN, Lin SC, Han Y. Update on ciliary body laser procedures. Curr Opin Ophthalmol. 2017; 28: 181–186. [DOI] [PubMed] [Google Scholar]
- 11. Fankhauser F, van der Zypen E, Kwasniewska S, Rol P, England C. Transscleral cyclophotocoagulation using a neodymium YAG laser. Ophthalmic Surg. 1986; 17: 94–100. [PubMed] [Google Scholar]
- 12. Hampton C, Shields MB. Transscleral neodymium-YAG cyclophotocoagulation. A histologic study of human autopsy eyes. Arch Ophthalmol Chic Ill 1960. 1988; 106: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 13. McKelvie PA, Walland MJ. Pathology of cyclodiode laser: a series of nine enucleated eyes. Br J Ophthalmol. 2002; 86: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walland MJ, McKelvie PA. Diode laser cyclophotocoagulation: histopathology in two cases of clinical failure. Ophthalmic Surg Lasers. 1998; 29: 852–856. [PubMed] [Google Scholar]
- 15. Shields SM, Stevens JL, Kass MA, Smith ME. Histopathologic findings after Nd:YAG transscleral cyclophotocoagulation. Am J Ophthalmol. 1988; 106: 100–101. [DOI] [PubMed] [Google Scholar]
- 16. Belyea DA, Mines MJ, Yao W-J, Dan JA, Bower KS. Telerobotic contact transscleral cyclophotocoagulation of the ciliary body with the diode laser. J Robot Surg. 2014; 8: 49–55. [DOI] [PubMed] [Google Scholar]
- 17. Tan AM, Chockalingam M, Aquino MC, Lim ZI-L, See JL-S, Chew PT. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Experiment Ophthalmol. 2010; 38: 266–272. [DOI] [PubMed] [Google Scholar]
- 18. Aquino MCD, Barton K, Tan AMWT, et al.. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Experiment Ophthalmol. 2015; 43: 40–46. [DOI] [PubMed] [Google Scholar]
- 19. Kuchar S, Moster MR, Reamer CB, Waisbourd M. Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma. Lasers Med Sci. 2016; 31: 393–396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.