Abstract
Purpose
To determine the relationship between central drusen volume and low-luminance deficit (LLD) in visual acuity (VA) in patients with intermediate age-related macular degeneration (AMD).
Methods
In this cross-sectional study, 42 patients with intermediate AMD underwent testing for VA and low-luminance VA (LLVA), as well as spectral-domain optical coherence tomography. LLD was calculated as the difference between VA and LLVA. Central drusen volume was measured in the central 3 mm of the macula, defined as the volume between the inner border of the retinal pigment epithelium and Bruch's membrane.
Results
Mean ± standard deviation (SD) LLD was 0.32 ± 0.12 logMAR and mean ± SD drusen volume was 0.18 ± 0.09 mm3. No linear relationship was identified between central 3 mm drusen volume and LLD (P = 0.215). R2 for the bivariate linear model was 0.038 (95% confidence interval 0–0.222). Limitation of the analysis to drusen volumes measured in the central 1 mm of the macula did not impact results (R2 = 0.075), nor did incorporation of lens status into the model (R2 = 0.067) or censoring of patients with nonfoveal subretinal drusenoid deposits (R2 = 0.071).
Conclusions
The amount of drusen within the central 3 mm of the macula does not appear to be related to LLD in intermediate AMD. These measures may be manifestations of different underlying pathophysiological mechanisms.
Translational Relevance
Understanding relationships between markers for AMD progression may help guide development of improved clinical grading scales for AMD.
Keywords: retina, age-related macular degeneration, image analysis
Introduction
Age-related macular degeneration (AMD) is a progressive, degenerative disease that is a leading cause of vision loss in the developed world.1 It is estimated that 7% of the US population over the age of 40 years is affected.2 The majority of vision loss in AMD is attributable to late stage disease, which is characterized by geographic atrophy (GA) of the outer retinal layers and/or choroidal neovascularization (neovascular AMD). At present treatment exists for the neovascular form but no therapies are available for individuals with GA.
Treatment for patients with early or intermediate stage “dry” AMD is also not available. This category of AMD patients presents with a wide spectrum of clinical and anatomic disease severities. Visual acuity (VA), the most commonly used measure of visual function, is often unaffected in early and intermediate stage disease.3 To better stratify disease severity and risk of progression to more advanced disease, there is a need to identify additional markers for AMD progression and to understand the relationships between these markers.
Anatomic features have long been used to stage AMD. The presence of large (>125 µm diameter) drusen on color fundus photography is an established risk factor for the development of advanced AMD.4 The exact role of drusen in the progression of AMD is complex, but studies have demonstrated that drusen are associated with photoreceptor thinning,5,6 disruption of the ellipsoid zone,7,8 and the appearance of hyperreflective foci on spectral-domain optical coherence tomography (SD-OCT), a finding that is associated with the development of GA.9,10 Recently, central drusen volume measured on SD-OCT has emerged as a potential marker for AMD severity, with several studies suggesting that central drusen volume is predictive of progression to both neovascular AMD and GA.11–13 Drusen volume is attractive as an endpoint because it is easily measured, reproducible, and can be automated in commercially available SD-OCT software packages.
Functional alternatives to VA have also been explored as potential markers of AMD progression. Such alternatives would therefore be potentially useful as endpoints for treatment trials in intermediate AMD. These tests include low-luminance VA (LLVA), low-luminance deficit (LLD), fundus microperimetry, color contrast sensitivity, and dark adaptation.3,14–16 Among these, LLVA and LLD are the most straightforward and could be easily incorporated into routine clinical trial workup, requiring only the addition of a neutral density filter to standard VA assessments. Difficulty with low-luminance visual tasks is a commonly reported symptom even in earlier stages of AMD17,18 and may portend subsequent disease progression.19 Several studies have shown that LLVA may be depressed in early and intermediate AMD when compared with normal controls.14,20 LLD specifically has been associated with a higher risk of vision loss and lesion enlargement in patients with GA21,22 and worse self-reported night vision symptoms in patients with intermediate AMD.23
Although drusen volume and LLD represent two somewhat divergent approaches to assessing AMD severity, it is plausible that they might be correlated or otherwise somehow related to each other. An understanding of these relationships is necessary if either or both of these parameters are to be utilized within clinical grading scales for AMD or as endpoints in clinical trials for dry AMD. Therefore the objective of the current study was to explore the relationship between LLD and drusen volume in a cohort of patients with intermediate AMD.
Methods
This cross-sectional study was conducted with the approval of the University of Texas Southwestern institutional review board (Dallas, TX). All study protocols were adherent to the tenets set forth in the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
Participants
Following written informed consent, patients with intermediate AMD (defined as the presence of at least one druse ≥125 µm in diameter on color fundus photography)4 underwent examination at the Retina Foundation of the Southwest, Dallas, Texas, USA. All patients had the diagnosis of intermediate AMD confirmed by an experienced retina specialist (KGC). Additional inclusion criteria included age ≥55 years and VA of 20/63 (logarithm of the minimum angle of resolution [logMAR] of 0.50) or better.
Exclusion criteria included any evidence of active choroidal neovascularization or GA (foveal or nonfoveal) on SD-OCT, presence of any other vision-threatening retinal pathology, significant lens changes impairing ability to perform study examinations, lack of measurable soft drusen in the central 3 mm of the macula on SD-OCT, or evidence of subretinal drusenoid deposits (SDD) of >9 disc areas (DA) total or >0.25 DA within 1 mm of the fovea. SDD was assessed by a single grader (KGC) on fundus autofluorescence images or infrared reflectance imaging taken at the baseline visit, or within 6 months of the baseline visit. For patients with intermediate AMD in both eyes, the eye with better VA was designated as the study eye.
Study Examinations
Study examinations included VA testing under both normal and low-luminance conditions, SD-OCT, color fundus photography, and standard ophthalmic examination. VA testing was conducted prior to imaging studies to minimize bleaching of the retina.
VA Testing
VA was measured using an Early Treatment Diabetic Retinopathy Study chart at 4 m, with spectacle correction, at a luminance of 130 candela/m2. Immediately following, LLVA was measured by placing a 2.0-log unit neutral density filter over the study eye and repeating the VA assessment. VA and LLVA were recorded in letters and converted to logMAR for analysis. LLD was calculated as the difference between VA and LLVA.
Imaging
SD-OCT was acquired using the Heidelberg Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). Volume scans were obtained over a field spanning 15° horizontally and 10° vertically centered on the fovea and consisted of 97 horizontal B scans. Retinal layers were automatically segmented by the Heidelberg module. All segmented volume scans were reviewed by a grader (WCO, RAD, KGC), and any errors were manually corrected. Drusen volume was measured in the central 3 mm of the macula using the built-in functionality of the Heidelberg module. Drusen volumes for the central subfield (1 mm) of the macula were also recorded. Specifically, drusen volume was defined as the volume between Bruch's membrane and the inner border of the retinal pigment epithelium (RPE). SDD were not included in the segmentation of the RPE and did not contribute to drusen volume. Images were also assessed for the presence of absence of drusen in the central 1 mm, corresponding to the fovea.
Statistical Analysis
The first available visit per patient was selected for analysis. The relationship between drusen volume and LLD was analyzed using ordinary least squares regression. The confidence interval (CI) for the coefficient of determination (R2) for this comparison was determined using simple quantiles derived from a bootstrap simulation. LLD was compared among phakic and pseudophakic eyes and among eyes with and without foveal drusen using the Student's t-test. Statistical analyses were conducted in R version 3.4.3 (R Project for Statistical Computing; www.r-project.org). A P value <0.05 was considered significant. All reported P values are two-sided.
Results
Forty-two patients with intermediate AMD were included in the study. Baseline demographics are shown in the Table. Mean age was 75 years (range, 55–98 years), mean VA was 0.13 logMAR (approximate Snellen equivalent 20/25; range, 0.48 to –0.18 logMAR), mean ± standard deviation (SD) LLD was 0.32 ± 0.12 logMAR (range, 0.14–0.60 logMAR), mean ± SD 3 mm drusen volume was 0.18 ± 0.09 mm3 (range, 0.11–0.54 mm3), and mean ± SD 1 mm drusen volume was 0.03 ± 0.02 mm3 (range, 0.01–0.10 mm3). Six patients (14%) had nonfoveal SDD ranging from <1 to 9 DA, with LLD ranging from 0.22 to 0.60 logMAR.
Table.
Patient Demographics
| Eye, n (%) OD | 21 (50) |
| Age (years), mean (range) | 75 (55–98) |
| Sex, n (%) female | 28 (67) |
| VA (logMAR), mean (range) | 0.13 (0.48 to –0.18) |
| Approximate Snellen equivalent, mean (range) | 20/25 (20/63–20/12) |
| LLVA (logMAR), mean (range) | 0.44 (0.96–0.10) |
| Approximate Snellen equivalent, mean (range) | 20/50 (20/200–20/25) |
| LLD (letters), mean (range) | 0.32 (0.14–0.60) |
| Central 3 mm drusen volume (mm3), mean (range) | 0.18 (0.11–0.54) |
| Central 1 mm drusen volume (mm3), mean (range) | 0.03 (0.01–0.10) |
| Nonfoveal SDD, n (%) | 6 (14) |
OD, oculus dextrus.
Linear regression (Fig. 1) did not identify a significant relationship (n = 42, P = 0.215) between central 3 mm drusen volume and LLD (β = 0.025 logMAR/0.1 mm3; 95% CI, –0.015 to 0.065 logMAR/0.1 mm3). R2 for the linear model was 0.038, and the 95% CI for R2 as determined by bootstrap simulation was 0 to 0.222. Results were similar when the analysis was repeated using central 1 mm drusen volumes (n = 42, β = 0.016 logMAR/0.01 mm3, P = 0.079). R2 for this model was 0.075. There was no significant difference in LLD between eyes with (n = 36, mean LLD 0.31 logMAR) and without (n = 6, mean LLD 0.33 logMAR) drusen in the central 1 mm of the macula (P = 0.773).
Figure 1.
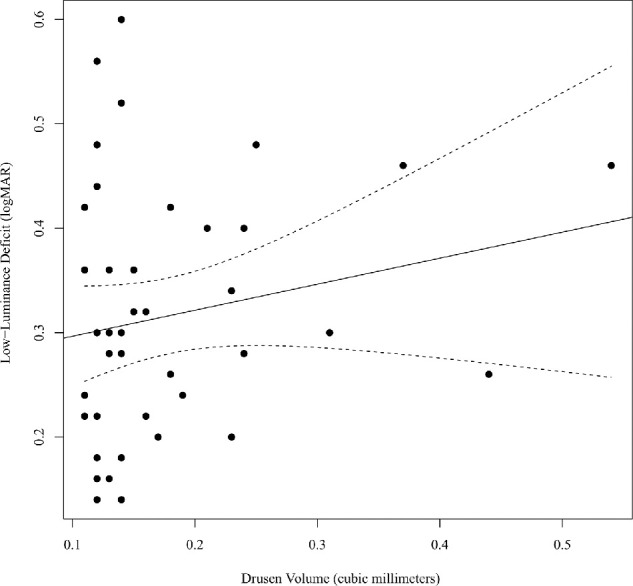
Scatterplot of drusen volume (mm3) versus LLD. The bivariate linear regression line is plotted (solid) with the 95% CI for mean predicted values (dashed).
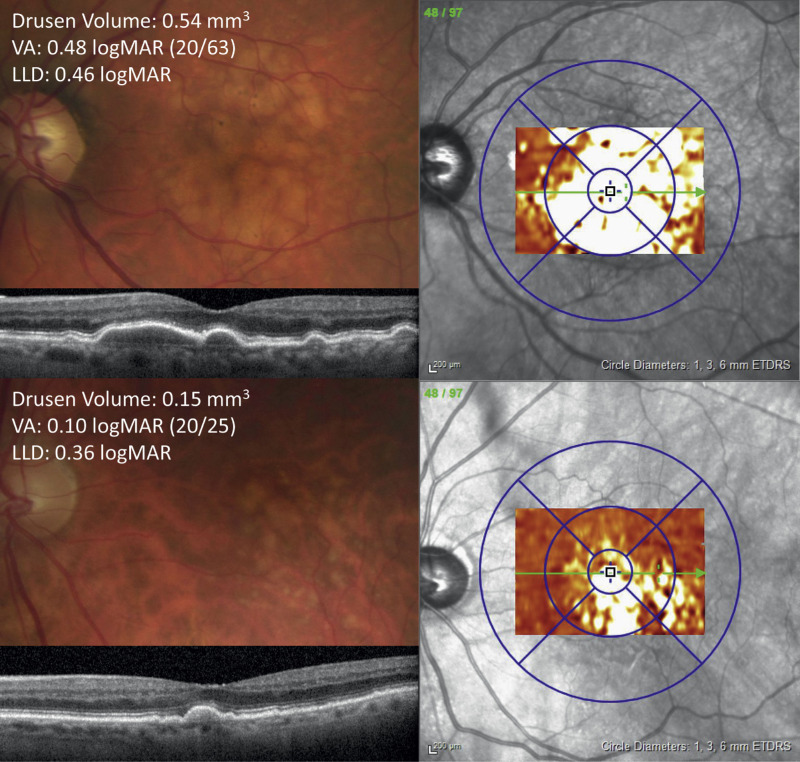
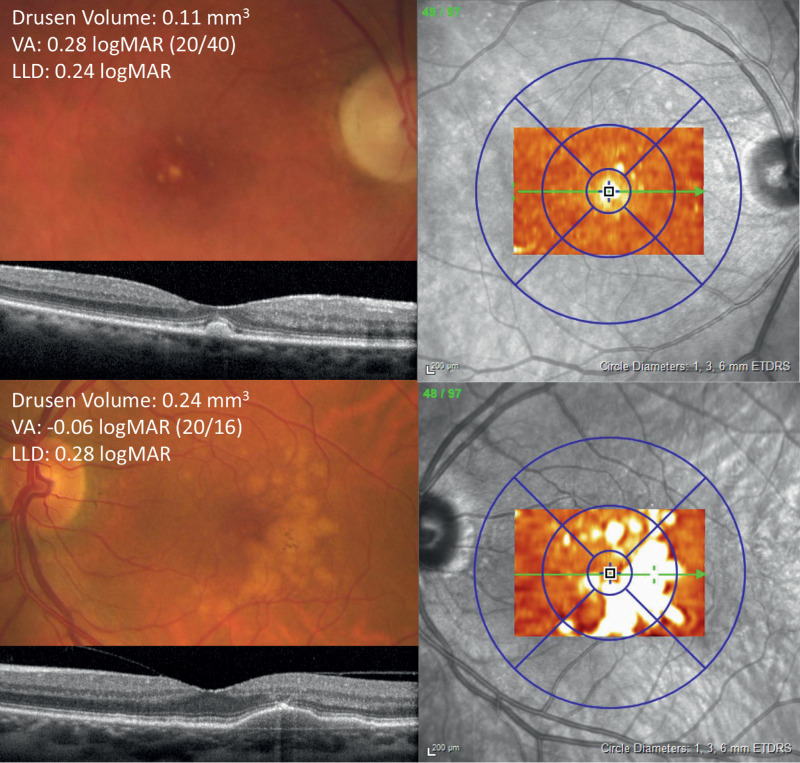
LLD was not significantly different between phakic (n = 20) and pseudophakic (n = 21) eyes (P = 0.241). Inclusion of lens status (phakic vs. pseudophakic) in the linear model for 3 mm drusen volume and LLD did not substantially impact the model (n = 41, βdrusen volume = 0.023 logMAR/0.1 mm3, Pdrusen volume = 0.260), nor did censoring of six cases with nonfoveal SDD (n = 36, β = 0.030 logMAR/0.1 mm3, P = 0.117). R2 was 0.067 for the model including lens status and 0.071 for the model excluding patients with SDD. Representative case examples demonstrating the incongruity between drusen volume and LLD are shown in Figures 2 and 3.
Figure 2.
Color fundus photograph, optical coherence tomography (OCT) line scan, and drusen volume thickness maps for two patients (top and bottom) that both have a relatively high LLD despite a disparity in drusen volumes.
Figure 3.
Color fundus photograph, optical coherence tomography (OCT) line scan, and drusen volume thickness maps for two patients (top and bottom) that both have a relatively small LLD despite a disparity in drusen volumes.
Discussion
In this series, no significant linear relationship was identified between drusen volume and LLD, with central 3 mm drusen volume accounting for only 4% of the variability in LLD (R2). A bootstrap simulation, in which the sample data are resampled over a large number of iterations to generate a distribution for a statistic of interest, was used to generate a 95% CI for R2. This simulation, assuming that subjects in the present sample are representative members of the population of interest (i.e., intermediate AMD patients), revealed a 95% CI of 0 to 0.222 for R2. Thus although both drusen volume and LLD are thought to be markers of AMD severity, the current data suggests that the underlying processes reflected by these measures are not fully congruous.
Low-luminance, or mesopic, conditions represent a transition between rod-dominated scotopic vision and cone-dominated photopic vision during which both rods and cones contribute to visual function. However, their respective contributions are not simply additive, and the precise determinants of mesopic VA are incompletely understood. Modeling of mesopic vision is complicated by a number of factors, including differences in rod and cone sensitivities, interactions between rod and cone signaling, the existence of multiple postreceptoral pathways for signal processing, and different spatial distributions of rods and cones on the retina.24 Rod-cone interactions are particularly complex, involving direct cell–cell coupling through gap junctions, shared neural pathways, and temporal and phase interactions. Furthermore, rods appear to play a role in mediating cone survival.25 Therefore although LLVA is generally considered a measure of cone function owing to the scarcity of rods in the fovea,20,21 the potential contribution of rods to low-luminance dysfunction should not be overlooked, considering that rods are preferentially damaged before cones in AMD.26
Beyond the basic idea that drusen are indicators of an underlying disease process that may also manifest functional impairments such as LLD, there are several plausible links between drusen and retinal function. The presence of drusen has been associated with focal degenerative changes in the overlying photoreceptors, such as loss of photoreceptor density, outer segment thinning, and disruption of the ellipsoid zone as seen on both histological studies and on SD-OCT.5–9,27,28 These changes may explain associations identified between drusen and retinal sensitivity deficits in early and intermediate AMD.7,29–31 It follows that these same morphologic changes may impact LLD,14 although the current study does not provide evidence to support this conclusion. Indeed, even when the analysis was restricted to the central 1 mm (i.e., fovea), in which one might anticipate the greatest degree of correlation with acuity, no significant relationship was identified between drusen volume and LLD. One potential contributor to the absence of an observed correlation in this study is the fact that drusen volume is not strictly unidirectional; that is, although drusen volume tends to increase over time, it is also known that drusen can shrink or disappear altogether, with the latter occurrence sometimes preceding progression to GA or neovascularization.32 The inclusion of some patients who may have been in the midst of drusen regression despite being more advanced in their disease progression might explain the large range of LLD observed at low drusen volumes in this study.
Furthermore, it is likely that more than one mechanism contributes to LLD. Given the importance of rod-cone integration in mesopic vision, one hypothesis is that there may be pathologic postreceptoral changes in the inner retina, secondary to ischemia or photoreceptor damage, that affect visual function.33,34 Another possibility is that thickening of Bruch's membrane in AMD may impair transport of vitamin A and impair photoreceptor kinetics.26,35 It remains possible that other anatomic parameters, such as hyperreflective foci or assessment of ellipsoid zone integrity,31,36 or other known markers of AMD severity, such as pigmentary abnormalities, may correlate more directly with LLD. Further investigation of these potential associations may be revealing.
The authors have observed anecdotally that lens status may impact measurements of LLD, and cataract has previously been associated with poorer subjective low-luminance function.37 In this study, no significant differences in LLD were identified between phakic and pseudophakic eyes, and inclusion of lens status in the linear model did not impact results. However, the amount of lens change in phakic eyes was not specifically assessed in this study.
Similarly, it has been suggested that the presence of SDD may impair low-luminance vision.38 For this reason, patients with fovea-involving SDD or SDD >9 DA were excluded from the present study. However, six patients with SDD not meeting either of these exclusion criteria were included in analysis. Notably, censoring of these patients did not substantially impact the results of the regression analysis, and the risk of bias as a result of the inclusion of this small group of patients is likely low.
It is important to acknowledge that data regarding LLD in early and intermediate AMD have been mixed. In some series, LLD was found to be significantly worse in early and intermediate AMD patients compared with normal patients.16,20 In contrast, Wu et al.14 found that LLD in nonexudative AMD patients was significantly different from normal controls only in patients with nonfoveal GA. The same group later reported that baseline LLD was not correlated with 12-month changes in VA measures or microperimetric sensitivity, although changes in VA were minimal over this period.15 Cocce et al.3 found that LLD was significantly different between early and intermediate AMD patients, but only on computerized assessment. Although the utility of LLD in the classification of AMD remains unclear, it is likely still useful as a practical measure of patient functionality.23
A key consideration in assessing markers for intermediate AMD severity is risk of progression. A number of studies have demonstrated that increased central drusen volume is associated with progression to GA or neovascular AMD, and that above certain thresholds for drusen volume, the risk of progression greatly increases.11–13 Although self-reported night vision symptoms have been associated with progression to GA or neovascularization,19 it is currently unknown whether LLD specifically carries an increased risk of progression from intermediate to advanced AMD. The results of the current study suggest that if LLD does predict disease progression, it may do so independently of drusen volume.
This study has several limitations. First, the sample size is relatively small, and there are a limited number of observations available at higher drusen volumes. These points may therefore have had a disproportionate impact on regression analyses relative to other data points. Second, differences in methodology used to measure drusen volume in the present study may hinder comparison to previous studies on drusen volume. Specifically, the present study utilized Heidelberg SD-OCT images with drusen volume measurements made using the in-built functionality of the Heidelberg module. In contrast, many previous studies utilized Cirrus OCT (Carl Zeiss Meditec, Dublin, CA) with drusen volume measured using Cirrus software or custom software.11–13,39 In the Cirrus module, drusen volume is based on the space between the segmented RPE and an interpolated “virtual” RPE containing no deformations,40 whereas the Heidelberg module measures the space between the inner border of the RPE and Bruch's membrane. Although unlikely to have a substantial impact of the present results, these subtle differences in methodologies may hinder generalizability of the current findings.
Conclusions
There does not appear to be a relationship between drusen volume within the central 3 mm of the macula and LLD in the current series of 42 eyes with intermediate AMD. Instead, these two measures may be indicators of processes that independently contribute to disease progression.
Acknowledgments
The authors thank F. Darell Turner (Ft. Worth, TX) for providing statistical expertise.
Disclosure: W.C. Ou, None; R.A. Denlar, None; K.G. Csaky, Heidelberg Engineering (C)
References
- 1. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012; 96: 614–618. [DOI] [PubMed] [Google Scholar]
- 2. Klein R, Chou C-F, Klein BEK, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011; 129: 75–80. [DOI] [PubMed] [Google Scholar]
- 3. Cocce KJ, Stinnett SS, Luhmann UFO, et al.. Visual function metrics in early and intermediate dry age-related macular degeneration for use as clinical trial endpoints. Am J Ophthalmol. 2018; 189: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferris FL, Wilkinson CP, Bird A, et al.. Clinical classification of age-related macular degeneration. Ophthalmology. 2013; 120: 844–851. [DOI] [PubMed] [Google Scholar]
- 5. Schuman SG, Koreishi AF, Farsiu S, Jung S, Izatt JA, Toth CA. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology. 2009; 116: 488–496.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rogala J, Zangerl B, Assaad N, Fletcher EL, Kalloniatis M, Nivison-Smith L. In vivo quantification of retinal changes associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56: 1689–1700. [DOI] [PubMed] [Google Scholar]
- 7. Iwama D, Tsujikawa A, Ojima Y, et al.. Relationship between retinal sensitivity and morphologic changes in eyes with confluent soft drusen. Clin Experiment Ophthalmol. 2010; 38: 483–488. [DOI] [PubMed] [Google Scholar]
- 8. Hartmann KI, Gomez ML, Bartsch D-UG, Schuster AK, Freeman WR. Effect of change in drusen evolution on photoreceptor inner segment/outer segment junction. Retina. 2012; 32: 1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leuschen JN, Schuman SG, Winter KP, et al.. Spectral-domain optical coherence tomography characteristics of intermediate age-related macular degeneration. Ophthalmology. 2013; 120: 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christenbury JG, Folgar FA, O'Connell RV, Chiu SJ, Farsiu S, Toth CA. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology. 2013; 120: 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nathoo NA, Or C, Young M, et al.. Optical coherence tomography-based measurement of drusen load predicts development of advanced age-related macular degeneration. Am J Ophthalmol. 2014; 158: 757–761.e1. [DOI] [PubMed] [Google Scholar]
- 12. Folgar FA, Yuan EL, Sevilla MB, et al.. Drusen volume and retinal pigment epithelium abnormal thinning volume predict 2-year progression of age-related macular degeneration. Ophthalmology. 2016; 123: 39–50.e1. [DOI] [PubMed] [Google Scholar]
- 13. Abdelfattah NS, Zhang H, Boyer DS, et al.. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Invest Ophthalmol Vis Sci. 2016; 57: 1839–1846. [DOI] [PubMed] [Google Scholar]
- 14. Wu Z, Ayton LN, Guymer RH, Luu CD. Low-luminance visual acuity and microperimetry in age-related macular degeneration. Ophthalmology. 2014; 121: 1612–1619. [DOI] [PubMed] [Google Scholar]
- 15. Wu Z, Ayton LN, Luu CD, Guymer RH. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA Ophthalmol. 2015; 133: 442–448. [DOI] [PubMed] [Google Scholar]
- 16. Chandramohan A, Stinnett SS, Petrowski JT, et al.. Visual function measures in early and intermediate age-related macular degeneration. Retina. 2016; 36: 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scilley K, Jackson GR, Cideciyan AV, Maguire MG, Jacobson SG, Owsley C. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002; 109: 1235–1242. [DOI] [PubMed] [Google Scholar]
- 18. Owsley C, McGwin G, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006; 47: 528–535. [DOI] [PubMed] [Google Scholar]
- 19. Ying G-S, Maguire MG, Liu C, Antoszyk AN. Night vision symptoms and progression of age-related macular degeneration in the Complications of Age-Related Macular Degeneration Prevention Trial. Ophthalmology. 2008; 115: 1876–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puell MC, Barrio AR, Palomo-Alvarez C, Gómez-Sanz FJ, Clement-Corral A, Pérez-Carrasco MJ. Impaired mesopic visual acuity in eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 7310–7314. [DOI] [PubMed] [Google Scholar]
- 21. Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008; 115: 1480–1488, 1488.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al.. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014; 121: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Z, Guymer RH, Finger RP. Low luminance deficit and night vision symptoms in intermediate age-related macular degeneration. Br J Ophthalmol. 2016; 100: 395–398. [DOI] [PubMed] [Google Scholar]
- 24. Stockman A, Sharpe LT. Into the twilight zone: the complexities of mesopic vision and luminous efficiency. Ophthalmic Physiol Opt. 2006; 26: 225–239. [DOI] [PubMed] [Google Scholar]
- 25. Hicks D, Sahel J. The implications of rod-dependent cone survival for basic and clinical research. Invest Ophthalmol Vis Sci. 1999; 40: 3071–3074. [PubMed] [Google Scholar]
- 26. Curcio CA, Owsley C, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000; 41: 2015–2018. [PubMed] [Google Scholar]
- 27. Sadigh S, Cideciyan AV, Sumaroka A, et al.. Abnormal thickening as well as thinning of the photoreceptor layer in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54: 1603–1612. [DOI] [PubMed] [Google Scholar]
- 28. Johnson PT, Brown MN, Pulliam BC, Anderson DH, Johnson LV. Synaptic pathology, altered gene expression, and degeneration in photoreceptors impacted by drusen. Invest Ophthalmol Vis Sci. 2005; 46: 4788–4795. [DOI] [PubMed] [Google Scholar]
- 29. Hartmann KI, Bartsch D-UG, Cheng L, et al.. Scanning laser ophthalmoscope imaging stabilized microperimetry in dry age-related macular degeneration. Retina. 2011; 31: 1323–1331. [DOI] [PubMed] [Google Scholar]
- 30. Acton JH, Smith RT, Hood DC, Greenstein VC. Relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 7618–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu Z, Ayton LN, Luu CD, Guymer RH. Relationship between retinal microstructures on optical coherence tomography and microperimetry in age-related macular degeneration. Ophthalmology. 2014; 121: 1445–1452. [DOI] [PubMed] [Google Scholar]
- 32. Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feuer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011; 118: 2434–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feigl B, Brown B, Lovie-Kitchin J, Swann P. Functional loss in early age-related maculopathy: the ischaemia postreceptoral hypothesis. Eye. 2007; 21: 689–696. [DOI] [PubMed] [Google Scholar]
- 34. Borrelli E, Abdelfattah NS, Uji A, Nittala MG, Boyer DS, Sadda SR. Postreceptor neuronal loss in intermediate age-related macular degeneration. Am J Ophthalmol. 2017; 181: 1–11. [DOI] [PubMed] [Google Scholar]
- 35. Phipps JA, Guymer RH, Vingrys AJ. Loss of cone function in age-related maculopathy. Invest Ophthalmol Vis Sci. 2003; 44: 2277–2283. [DOI] [PubMed] [Google Scholar]
- 36. Wu Z, Cunefare D, Chiu E, et al.. Longitudinal associations between microstructural changes and microperimetry in the early stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016; 57: 3714–3722. [DOI] [PubMed] [Google Scholar]
- 37. Finger RP, Fenwick E, Owsley C, Holz FG, Lamoureux EL. Visual functioning and quality of life under low luminance: evaluation of the German Low Luminance Questionnaire. Invest Ophthalmol Vis Sci. 2011; 52: 8241–8249. [DOI] [PubMed] [Google Scholar]
- 38. Hogg RE, Silva R, Staurenghi G, et al.. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration. Ophthalmology. 2014; 121: 1748–1755. [DOI] [PubMed] [Google Scholar]
- 39. Garcia Filho CA, Yehoshua Z, Gregori G, et al.. Change in drusen volume as a novel clinical trial endpoint for the study of complement inhibition in age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2014; 45: 18–31. [DOI] [PubMed] [Google Scholar]
- 40. Gregori G, Wang F, Rosenfeld PJ, et al.. Spectral domain optical coherence tomography imaging of drusen in nonexudative age-related macular degeneration. Ophthalmology. 2011; 118: 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]