Abstract
Purpose
To determine the prevalence of a central hyperreflective line in eyes with full-thickness macular holes (FTMH) and lamellar macular holes (LMH) and to elucidate the pathoanatomic importance of this optical coherence tomography (OCT) sign.
Methods
This retrospective analysis evaluated patients with FTMH and LMH at the Stein Eye Institute. Clinical data was collected and SD-OCT volume scans were analyzed for the presence of a central vertical hyperreflective line in 3 separate cohorts: patients with SD-OCT preceding FTMH development, patients with SD-OCT after pars plana vitrectomy (PPVT) treatment for FTMH, and patients with SD-OCT of LMH.
Results
In total, 93 eyes with FTMH and 88 eyes with LMH were identified. Of the 93 FTMH eyes, SD-OCT volume scans were available before development of the FTMH in 12 eyes. Of these, 6 (50%) displayed a vertical hyperreflective line preceding the development of the FTMH. Fifty-one eyes underwent PPVT with resolution of the FTMH, and 26 displayed a hyperreflective line after resolution (51%). Of the 88 eyes with LMH, 22 displayed a hyperreflective line (25%). All hyperreflective lines were noted at the central fovea.
Conclusions
SD-OCT illustrated the presence of a central vertical hyperreflective line preceding FTMH and after resolution of FTMH after PPVT in approximately one-half of cases, and concurrent with LMH in 25% of cases. This vertical hyperreflective line may represent an early SD-OCT marker for the development of FTMH, and may be a sign of central foveal dehiscence owing to disruption of the Muller cell cone.
Keywords: full-thickness macular hole, lamellar macular hole, Muller cell, hyperreflective line, OCT
In a seminal anatomic study, Yamada1 showed that the fovea centralis contains a central cone of Muller cells that extends from the internal limiting membrane (ILM) to the external limiting membrane. Gass2 later described the Muller cell cone and defined this anatomic landmark as a cluster of Muller cells in an inverted cone configuration in the central fovea. Subsequent publications3,4 confirmed the presence of a group of specialized central Muller cells that comprise the floor of the foveola and are referred to as the Muller cell cone. These specialized Muller cells may act like a plug and play a glue-like role to stabilize the central fovea by binding together the foveolar cones.2,5 With disruption, stress may be transmitted through the central fovea predisposing to the development of various disorders, including macular hole formation.5–7
Gaudric et al.8 elegantly described the formation of macular holes using OCT analysis and identified various stages in this progression. Like Gass,2 they proposed that a cleavage plane was present in the central fovea which was stabilized by the Muller cell cone.8 Although the studies from Gaudric et al.8 and other investigators9 have provided detailed OCT analysis of the various stages immediately preceding full-thickness macular hole (FTMH) formation, earlier clinical signs of macular hole development are lacking.
We have identified a vertical hyperreflective line in the central fovea with spectral domain OCT (SD-OCT) that may predate the development of macular hole and may be present well before the stages of macular hole formation described by Gaudric et al.8 While there has been speculation regarding foveal hyperreflectivity preceding macular hole development,9,10 this OCT sign has never been carefully studied. The aim of this study was to determine the prevalence of this OCT marker associated with full thickness and lamellar macular holes (LMHs) and elucidate its pathoanatomic significance in macular disorders such as macular hole formation.
Methods
This study was a retrospective case review series that adhered to the tenets of the Declaration of Helsinki and was conducted in accordance with the regulations of the Health Insurance Portability and Accountability Act. Institutional review board approval was obtained at the UCLA Geffen School of Medicine (D.S.).
Inclusion criteria for the study included eyes with idiopathic FTMH or LMH. Tracked SD-OCT scans before the development of FTMH, in established LMH, or after pars plana vitrectomy (PPVT) were additional requirements to be included in the analysis. Exclusion criteria included poor quality SD-OCT imaging scans and retinal or macular disease (e.g., high myopia, trauma) that confounded the analysis of the macular hole.
A thorough chart review of patients with the diagnosis of FTMH or LMH was performed. Relevant clinical and surgical history information was collected. SD-OCT volume scans (Spectralis; Heidelberg Engineering, Heidelberg, Germany) were reviewed at all visits (before macular hole formation if available, when the macular hole was present, and after vitrectomy when available). The volume scans consisted of either 19 or 25 horizontal B scans centered on the fovea that were tracked with previous volume scans with an automatic retinal tracking threshold set at 25.
SD-OCT definition of a FTMH was a full-thickness defect in the central fovea extending from the ILM to the RPE.11 The FTMH was graded according to the Gaudric OCT classification system8 by two graders (AH and JS). Any discrepancies were resolved by the senior author (DS). A LMH was defined on SD-OCT as an irregular foveal configuration with thinning and a break in the inner fovea in the absence of a full-thickness foveal defect with intact foveal photoreceptors.12 LMH were differentiated into degenerative lamellar holes versus tractional lamellar holes with foveoschisis as originally defined by Govetto et al.13 Patients were then divided into three cohorts: patients with tracked SD-OCT volume scans preceding FTMH, patients with tracked SD-OCT scans after FTMH repair and PPVT, and patients with SD-OCT scans of LMH.
OCT volume scans were carefully studied (AH, CG, IC) in the three cohorts for the presence of a vertical hyperreflective line defined as a hyperreflective linear lesion extending from the ellipsoid zone band (or lower) through the outer nuclear layer to the ILM in the region of the central fovea. If identified, the presence of the hyperreflective line was then confirmed by a second reviewer (JS). Any discrepancies were resolved by the senior author (DS).
All patients who were treated for FTMH with PPVT underwent a three-port PPVT (23 or 25 gauge) under regional anesthesia with peribulbar block. A core and peripheral vitrectomy was performed using a noncontact wide-angle viewing system (Resight, Carl Zeiss Meditec AG, Jena, Germany). In all cases the ILM was peeled around the macular hole, using a flat macular lens and an ILM forceps (Alcon Grieshaber, Fort Worth, TX). Triamcinolone acetonide (Kenalog, Bristol-Myers Squibb, Princeton, NJ) was used to facilitate visualization during macular peeling in some cases. Then, a full air–fluid exchange and postvitrectomy tamponade was performed with a 20% mixture of sulphur hexafluoride.
Statistical analysis was performed with R-software (R Foundation for Statistical Computing, Vienna, Austria). Results are reported as means with range and standard deviation or number with percentage. Independent z-tests were performed for categorical variables (comparison of gender and lamellar hole subtype between patients with and without hyperreflective line). Independent t-tests were performed for interval variables (comparison of age, number of OCTs, mean FTMH stage, and follow-up). These tests were performed separately for each of the three cohorts: patients with tracked SD-OCT volume scans preceding FTMH, patients with tracked SD-OCT scans after FTMH repair and PPVT, and patients with SD-OCT scans of LMH. A P value of less than 0.05 was considered statistically significant. Cohen's Kappa statistics were calculated for intergrader reliability, and are reported as values with a two-sided 95% confidence interval.
Results
A total of 181 eyes were enrolled in the study. From this, 93, 53, and 88 eyes belonged to the pre-FTMH, FTMH treated with PPVT, and LMH cohorts, respectively.
Pre-FTMH Cohort
In total, 93 eyes with FTMH from 89 patients were identified. The average age of patients with a FTMH was 69 (standard deviation, 9.75) and 63% were female.
Of the 93 eyes with FTMH, tracked SD-OCT volume scans were available before the development of the FTMH in 12 (12.9%). Of these 12 eyes, 6 (50%) displayed central hyperreflective lines (Fig. 1) (Table 1).
Figure 1.
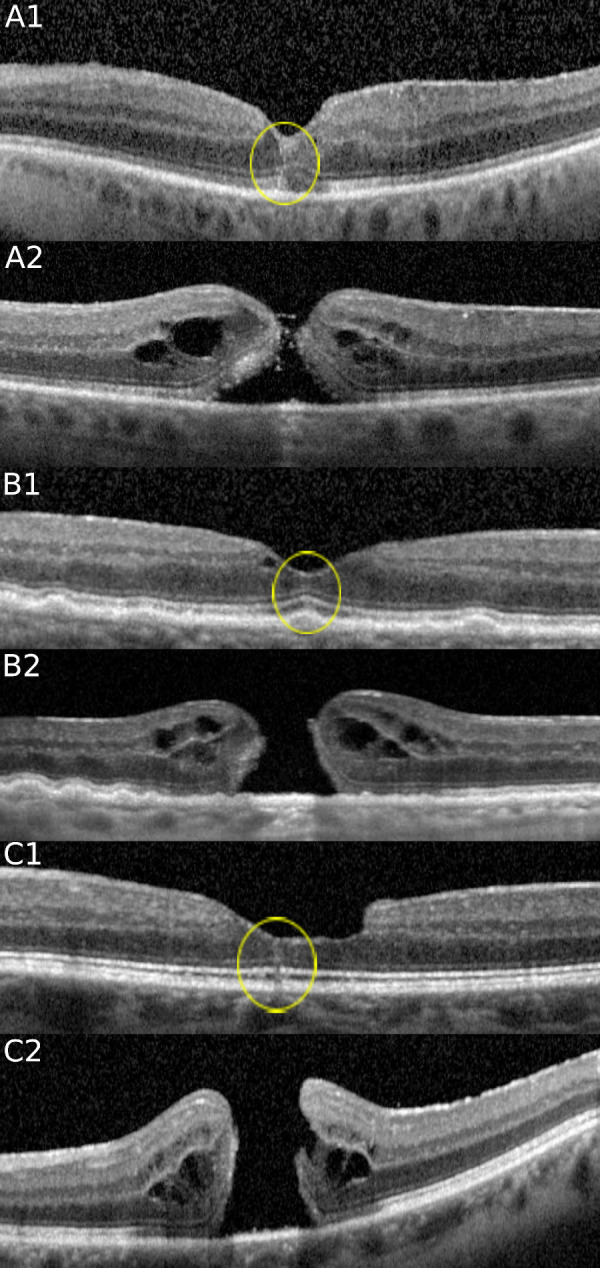
SD-OCT at the fovea of 3 cases before and after FTMH development. Note the presence of a hyperreflective line (yellow circle) preceding FTMH formation in each case. The hyperreflective vertical line extends from the area of the ellipsoid zone band to the ILM in the central fovea in each case. (Case A) In this 69-year-old man, a hyperreflective line at the fovea is noted in the left eye (A1) which progressed to a stage 2 FTMH (A2) just 2 months later. (Case B) In this 88-year-old man, a hyperreflective line at the fovea is noted in the right eye (B1), which evolved into a stage 4 FTMH (B2) 14 months later. (Case C) In this 62-year-old man, a hyperreflective line at the fovea is noted in the left eye (C1), which evolved into a stage 4 FTMH (C2) 4 months later.
Table 1.
Prevalence of Hyperreflective Line
| Group | Total Eyes | No. of Eyes With Adequate Imaging | No. of Eyes With Hyperreflective Lines (%) | No. of Hyperreflective Lines Found in the Central Fovea (%) |
|---|---|---|---|---|
| Before FTMH | 93 | 12 | 6 (50) | 6 (100) |
| After PPVT | 53 | 51 | 26 (51) | 26 (100) |
| LMH | 88 | 88 | 22 (25) | 22 (100) |
A hyperreflective line was seen an average of 616 days before development of the FTMH (50-1606 days; standard deviation, 535 days). The hyperreflective lines were noted on multiple visits for four of six cases. Intergrader analysis of hyperreflective line identification showed strong reliability (K = 0.89 ± 0.073).
The groups of eyes with and without hyperreflective line were compared (1Table 2). There was no significant difference in age or gender (Table 2). Stage of FTMH was also not significantly different (Table 2). Number of OCTs acquired before development of the FTMH and number of days between the first OCT and the first OCT with a FTMH were also not significantly different (Table 2). Intergrader analysis of FTMH staging showed strong reliability (K = 0.86 ± 0.107).
Table 2.
Pre-FTMH Cohort Patient Demographic Characteristics and Hyperreflective Lines Time Course
| Characteristics | Total, 93 | FTMH With Hyperreflective Lines (n = 6) | FTMH Without Hyperreflective Lines (n = 6) | P Values |
|---|---|---|---|---|
| Age y, (standard deviation) | 69.1 (9.75) | 73.2 (10.0) | 69.5 (12.3) | 0.58 |
| Sex (%) | ||||
| Male | 34 (37) | 4 (66.7) | 4 (66.7) | 1.00 |
| Female | 59 (63) | 2 (33.3) | 2 (33.3) | |
| Eye (%) | ||||
| Right | 51 (55) | 2 (33.3) | 5 (83.3) | 0.08 |
| Left | 42 (45) | 4 (66.6) | 1 (16.7) | |
| FTMH stage (standard deviation) | 2.93 (1.00) | 2.33 (0.94) | 2.67 (0.75) | 0.50 |
| Time between hyperreflective line and FTMH, days (standard deviation) | – | 616 (535) | – | – |
| Time between first OCT and OCT with a FTMH, days (standard deviation) | 595.1 (580) | 669.8 (566) | 520.3 (638) | 0.68 |
| No. of OCTS before FTMH | 1.67 (1.52) | 2.4 (0.89) | 2.5 (1.64) | 0.36 |
FTMH: Full thickness macular hole.
FTMH Treated With PPVT Cohort
Of the 93 eyes with FTMH, 53 underwent PPVT, and closure of the hole was noted in 51 eyes (96%) by the time of image analysis. Of these 51, 26 (51%) displayed a hyperreflective line after resolution of the FTMH (Fig. 2) (Table 1). The hyperreflective line occurred an average of 213 days after surgery (8-840 days; standard deviation, 246 days). The hyperreflective lines were noted on multiple visits for 18 of 26 cases. In these 18 cases, the duration of persistence of the hyperreflective line on OCT ranged from 1 week to 20 months before complete resolution. In the other nine cases, the hyperreflective line resolved before the next visit. Comparing the groups of patients with a hyperreflective line after surgery versus those without, there was no significant difference in age, gender, presurgical FTMH stage, number of postsurgical OCTs taken or total follow-up after surgery (Table 3).
Figure 2.
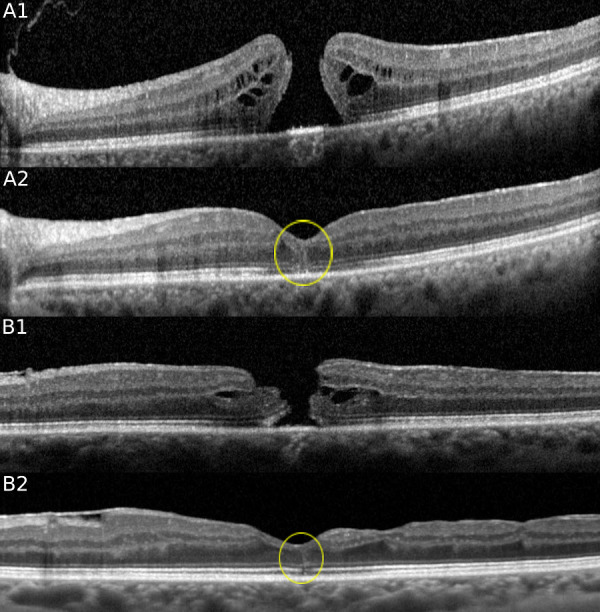
SD-OCT of two cases of FTMH before and after PPVT. Note the presence of a hyperreflective line after resolution of the FTMH after PPVT. (Case A) A 63-year-old woman with a stage 4 FTMH (A1) in the left eye was treated with PPVT 3 weeks after the baseline OCT. A hyperreflective line at the fovea is noted with OCT at the 1-month follow-up (A2), which persisted for 9 months. (Case B) A 75-year old woman with a stage 4 FTMH (B1) in the left eye was treated with PPVT 2 weeks later. A hyperreflective line at the fovea (B2) is noted with OCT 8 months later.
Table 3.
Post Pars Plan Vitrectomy (PPVT) Cohort Patient Demographic Characteristics and Hyperreflective Line Time Course
| Characteristics | Total, 93 | Hyperreflective Lines After PPVT (n = 26) | No Hyperreflective Lines After PPVT (n = 25) | P Values |
|---|---|---|---|---|
| Age, y (standard deviation) | 69.1 (9.75) | 68.3 (8.16) | 73.7 (12.08) | 0.07 |
| Sex (%) | ||||
| Male | 34 (37) | 9 (35) | 11 (44) | 0.52 |
| Female | 59 (63) | 17 (65) | 14 (56) | |
| Eye (%) | ||||
| Right | 51 (55) | 14 (53.8) | 17 (68) | 0.30 |
| Left | 42 (45) | 12 (46.2) | 8 (32) | |
| FTMH stage (standard deviation) | 2.93 (1.00) | 2.91 (0.87) | 2.93 (.1.14) | 0.94 |
| Time between vitrectomy and hyperreflective lines, days (standard deviation) | – | 213 (246) | – | |
| Follow-up, days (standard deviation) | 595.1 (580) | 753 (653) | 1154 (1026) | 0.10 |
| No. of OCTS after vitrectomy (standard deviation) | 1.67 (1.52) | 5.54 (2.7) | 6.77 (3.67) | 0.18 |
LMH Cohort
A total of 88 LMH from 85 patients were screened. The average age in this group was 74.8 (standard deviation 10.55) and 55% of these patients were women. Seven eyes had SD-OCT volume scans before development of the lamellar hole, and none displayed hyperreflective line during this time. Of the 88 eyes with LMH, 22 displayed hyperreflective line associated with the LMH (25%) (Fig. 3) (Table 1). The hyperreflective line was identified on average 247 days after the first OCT with LMH (0–1820 days; standard deviation, 487). The hyperreflective lines were noted on multiple visits for 11 of 22 cases. Comparing groups of patients with hyperreflective line concurrent with the LMH versus those without hyperreflective line, there was no significant difference in age, gender, follow-up, or lamellar hole subtype (Table 4).
Figure 3.
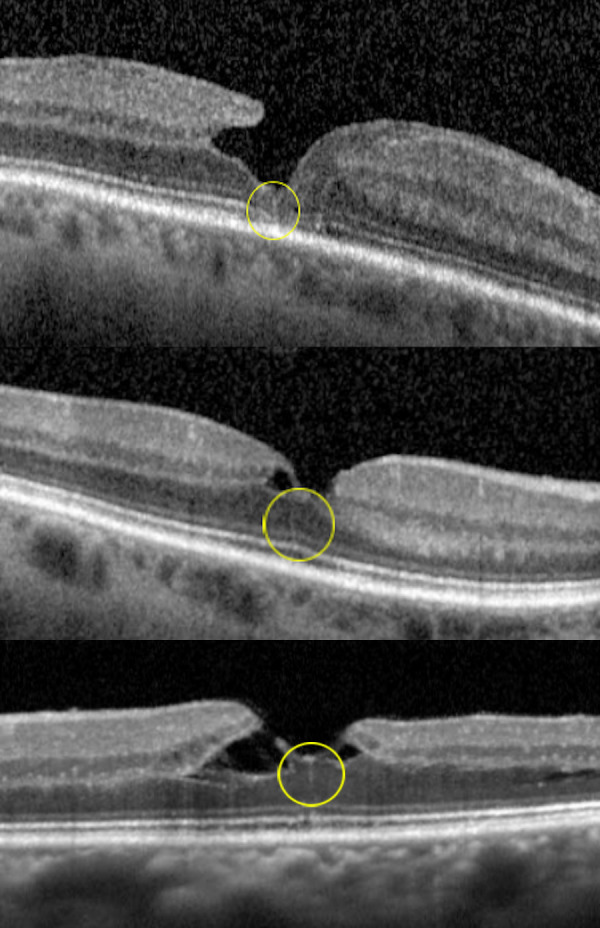
SD-OCT illustrating the presence of a hyperreflective line (yellow circle) associated with a LMH. (Case A) (top) In this 68-year-old man with a lamellar hole in the right eye, a hyperreflective line is noted at the central fovea. (Case B) (middle) In this 61-year-old woman with a lamellar hole in the left eye, a thin hyperreflective line is noted at the central fovea. (Case C) (bottom) In this 71-year-old woman with a tractional lamellar hole and foveoschisis in the left eye, a hyperreflective line is noted at the central fovea.
Table 4.
LMH Cohort Patient Demographic Characteristics and Hyperreflective Line Time Course
| Characteristics | Total, 88 | Hyperreflective Line with LMH (n = 22) | No Hyperreflective Line With LMH (n = 66) | P Values |
|---|---|---|---|---|
| Age, y (standard deviation) | 74.8 (10.55) | 71.2 (10.8) | 76.4 (12.08) | 0.59 |
| Sex (%) | ||||
| Male | 40 (45) | 10 (45) | 30 (45) | 1.00 |
| Female | 48 (55) | 12 (55) | 36 (55) | |
| Eye (%) | ||||
| Right | 43 (45.5) | 7 (31.8) | 36 (55) | 0.06 |
| Left | 45 (54.5) | 15 (68.2) | 30 (45) | |
| Time between first lamellar hole OCT and hyperreflective line, days (standard deviation) | – | 247 (487) | – | |
| Follow-up, days (standard deviation) | 580.9 (628.5) | 610.0 (703.6) | 514 (451.2) | 0.45 |
| Lamellar hole subtype (%) | ||||
| Degenerative lamellar hole | 49 (56) | 14 (64) | 35 (53) | 0.37 |
| Tractional LMH with foveoschisis | 39 (44) | 8 (36) | 31 (47) |
Discussion
We studied the SD-OCT volume scans of eyes with FTMH and LMH and discovered that a hyperreflective line, defined as a vertical linear lesion in the central fovea extending from the ellipsoid zone band to the ILM, can be commonly seen preceding development of a FTMH (50%), after resolution of the macular hole after PPVT (51%), and concurrent with an LMH (25%). The presence of the hyperreflective line preceding FTMH may be an indicator of evolving vitreomacular traction and a very early marker of FTMH or LMH development.
Since the original report by Yamada illustrating the presence of Muller cells in the central fovea, several other reports have been published describing a central cluster of vertical Muller cells in the foveola distinct from the typical z-shaped Muller cells located in the parafoveal region.4,14 Gass2 described the importance of the central Muller cell cone that may act as a glue to hold together and stabilize the central foveola. Bringmann et al.3 described a collection of unique Muller cells that course vertically in the central foveola to the ILM. They later expanded upon these findings, detailing 25 to 35 specialized Muller cells whose processes do not leave the foveola or join the course of the photoreceptor axons in the Henle fiber layer, instead traveling vertically through the “stalk” of the Muller cell cone from the inner layer of the foveola to the external limiting membrane.4 Tschulakow et al.15 used ion beam tomography to elegantly construct a three-dimensional model of the central Muller and photoreceptor cells. This organization was validated with electron microscopy by Syrbe et al.14 who further noted that central Muller cells act to resist tractional forces, and that cysts between central Muller cells may allow for disruption of the integrity of the central fovea if stress is applied. Bringmann et al.4 further supported this finding and noted that disruption of the Muller cell cone, as a result of traction and low resistance to mechanical stretch, leads to destabilization and foveal dehiscence through a central cleavage plane that may arise early in foveal morphogenesis.
During embryologic development, centrifugal displacement of the inner retina combined with centripetal movement of the outer retina may create tangential stress on the central fovea that is stabilized by the central Muller cell cone. These central Muller cells are more weakly bound to photoreceptors and may be prone to destabilization.4 With vitreoretinal traction or other forms of Muller cell disruption, the central fovea destabilizes and the natural forces of the Z-shaped Muller cells override the central Muller cells, leading to dehiscence of the foveola and separation through the central cleavage plane.5,7,14 This process may be captured with SD-OCT as a central hyperreflective line.
Previous studies have outlined the stages of development of a macular hole. Through analysis of FTMH in fellow eyes, Gaudric et al.8 illustrated that the initial stage of macular hole formation is remarkable for anteroposterior vitreomacular traction elicited by evolving posterior vitreous detachment. Cystic formation of the fovea ensues with elevation or flattening of the foveolar pit.8 Coalescence of the cysts into a large central cystic space associated with an incompletely detached flap of vitreoretinal traction is subsequently noted.8 Finally, the operculum detaches completely and the edges of the hole separate.8 Previous studies have identified several systemic risk factors for development of a macular hole including female gender,16–18 age,18 increased plasma fibrinogen levels,19 and previous hysterectomy and oophorectomy.20,21 Morphologic markers such as window defect on fluorescein angiography,22–24 involutional macular thinning,24 and the absence of posterior vitreous detachment24 have also been implicated. Later publications drew attention to minor signs associated with vitreomacular traction before the appearance of intraretinal cysts such as central foveal linear hyperreflectivity or photoreceptor elevation, but this sign did not result in a systematic description.9,10,25 This report, however, uniquely studies the presence of an OCT finding that may represent an earlier stage of macular hole development before the development of an impending macular hole.
Further evidence of the formation of a plane or seam or fault line in the central foveola may be supported by other macular disorders. In various clinical circumstances a central hyperreflective line may be observed. Resolving macular hemorrhage can be observed as a central hyperreflective line with SD-OCT, presumably as the result of residual heme tracking within this central plane. Reorganization of the photoreceptors in multiple evanescent white dot syndrome can also present with a central hyperreflective line (Fig. 4) Vertical hyperreflective lines have also been described after macular grid laser and pan retinal photocoagulation,26 as a result of self-inflicted laser injury,27 and in association with placoid disorders28 but these lesions are parafoveal and are due to migration along the Henle fiber layer.
Figure 4.
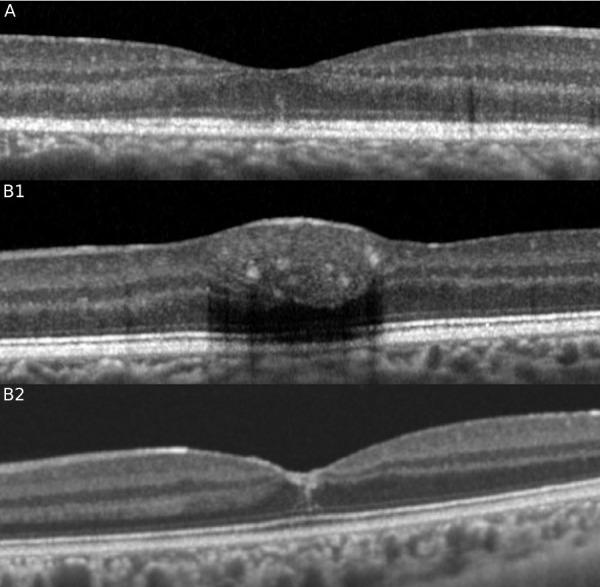
SD-OCT showing the presence of the characteristic hyper reflective line in the central fovea in a case of multiple evanescent white dot syndrome associated with ellipsoid zone disruption (A) and in a separate case of macular hemorrhage (B1) after resolution (B2).
It is important to recognize that the pathogenesis of the linear hyperreflectivity in our three cohorts may be distinct. Although the lines were hyperreflective, vertical and centrally located within the fovea in all three groups, the actual cellular mechanism leading to the hyperreflectivity may be distinctive. However, the common denominator in all cohorts may be the presence of a naturally existing cleavage plane or seam in the central fovea that is either being pulled apart or pulled together in these three clinical situations. The hyperreflective line in eyes after FTMH repair may again relate to the natural seam that is present in the central fovea that is stabilized by the Muller cell cone. Presumably a certain time interval is necessary for this natural seam to resolve as the normal foveal anatomy is restored, and this time course may vary from individual to individual.
Limitations of our study included the small number of SD-OCT volume scans available before FTMH development. Because patients are typically asymptomatic before the development of a macular hole, many patients were not screened before development of the FTMH. The retrospective design of the study did not allow for a consistent imaging protocol or uniform follow-up. The hyperreflective line is admittedly a subtle OCT finding and a previously undefined entity, and was not identified at every visit when repeat OCT datasets were available . However, this OCT feature was noted in multiple visit scans per patient in the majority of cases. Because this finding is one of microscopic dimension, resolution may not be sufficient to capture this abnormality with OCT at every visit. Even with tracked scans, microscopic displacement may still exist between visits and the hyperreflective line may be subsequently missed and not recorded.
This study identified the presence of a central hyperreflective line preceding the development of FTMHs, after resolution of FTMHs after vitrectomy, and concurrent with LMHs. In patients with appropriate imaging, the hyperreflective line was captured in approximately 50% of patients preceding FTMH and after vitrectomy repair, and in 25% of patients with LMH. This linear hyperreflectivity may provide the first visual evidence of the presence of a natural retinal seam at the central fovea, and may serve as the earliest clinical marker of vitreomacular traction and impending macular hole development. Further prospective studies are needed to assess the importance of the hyperreflective line as a biomarker to predict the future development of a macular hole and as a pathoanatomic OCT sign of the presence of a central retinal seam or fault line.
Acknowledgments
Supported by Research To Prevent Blindness Inc (DS), New York, NY and Macula Foundation Inc (DS), New York, New York.
Disclosure: J.M. Scharf, None; A. Hilely, None; R.C. Preti, None; C. Grondin, None; I. Chehaibou, None; G. Greaves, None; K. Tran, None; D. Wang, None; M.S. Ip, Alimera Sciences (C), Allergan (C), Boehringer Ingelheim (C), Genentech, Inc. (C), Omeros (C), Quark (C), ThromboGenics (C); J.P. Hubschman, Alcon (C), Allergan (C, S), Bausch & Lomb (C), Novartis (S); A. Gaudric, Novartis (C), ThromboGenics (C), Topcon (R); D. Sarraf, Amgen (C), Bayer (C), Genentech (C, F), Heidelberg (F), Novartis (S), Optovue (C, F), Regeneron (F), Topcon (F)
References
- 1. Yamada E. Some structural features of the fovea centralis in the human retina. Arch Ophthalmol. 1969; 82: 151–159, doi: 10.1001/archopht.1969.00990020153002. [DOI] [PubMed] [Google Scholar]
- 2. Gass JDM. Müller cell cone, an overlooked part of the anatomy of the fovea centralis. Arch Ophthalmol. 1999; 117: 821, doi: 10.1001/archopht.117.6.821. [DOI] [PubMed] [Google Scholar]
- 3. Bringmann A, Pannicke T, Grosche J, et al.. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006; 25: 397–424, doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4. Bringmann A, Syrbe S, Görner K, et al.. The primate fovea: structure, function and development. Prog Retin Eye Res. 2018; 66: 49–84, doi: 10.1016/j.preteyeres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 5. Chung H, Byeon SH. New insights into the pathoanatomy of macular holes based on features of optical coherence tomography. Surv Ophthalmol. 2017; 62: 506–521, doi: 10.1016/j.survophthal.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 6. Woon WH, Greig D, Savage MD, et al.. Movement of the inner retina complex during the development of primary full-thickness macular holes: implications for hypotheses of pathogenesis. Graefe's Arch Clin Exp Ophthalmol. 2015; 253: 2103–2109, doi: 10.1007/s00417-015-2951-0. [DOI] [PubMed] [Google Scholar]
- 7. Spaide RF. Closure of an outer lamellar macular hole by vitrectomy: Hypothesis for one mechanism of macular hole formation. Retina. 2000; 20: 587–590, doi: 10.1097/00006982-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 8. Gaudric A, Haouchine B, Massin P, Paques M, Blain P, Erginay A. Macular hole formation. Arch Ophthalmol. 1999; 117: 744, doi: 10.1001/archopht.117.6.744. [DOI] [PubMed] [Google Scholar]
- 9. Philippakis E, Astroz P, Tadayoni R, Gaudric A. Incidence of macular holes in the fellow eye without vitreomacular detachment at baseline. Ophthalmologica. 2018; 240: 135–142, doi: 10.1159/000488956. [DOI] [PubMed] [Google Scholar]
- 10. Gaudric A, Tadayoni R.. Macular hole. In: Wilkinson C Schachat A Hinton D, et al., eds. Ryan's Retina. Vol. 3, 6th ed. New York, NY: Elsevier; 2017. [Google Scholar]
- 11. Duker JS, Kaiser PK, Binder S, et al.. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013; 120: 2611–2619, doi: 10.1016/j.ophtha.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 12. Frisina R, Pilotto E, Midena E. Lamellar macular hole: state of the art. Ophthalmic Res. 2019; 61: 73–82, doi: 10.1159/000494687. [DOI] [PubMed] [Google Scholar]
- 13. Govetto A, Dacquay Y, Farajzadeh M, et al.. Lamellar macular hole: two distinct clinical entities? Am J Ophthalmol. 2016; 164: 99–109, doi: 10.1016/j.ajo.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 14. Syrbe S, Kuhrt H, Gärtner U, et al.. Müller glial cells of the primate foveola: an electron microscopical study. Exp Eye Res. 2018; 167: 110–117, doi: 10.1016/j.exer.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 15. Tschulakow A V., Oltrup T, Bende T, Schmelzle S, Schraermeyer U. The anatomy of the foveola reinvestigated. PeerJ. 2018; 6: e4482, doi: 10.7717/peerj.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Witkin AJ, Ko TH, Fujimoto JG, et al.. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006; 113: 388–397, doi: 10.1016/j.ophtha.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCannel CA, Ensminger JL, Diehl NN, Hodge DN. Population-based incidence of macular holes. Ophthalmology. 2009; 116: 1366–1369, doi: 10.1016/j.ophtha.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ali FS, Stein JD, Blachley TS, Ackley S, Stewart JM. Incidence of and risk factors for developing idiopathic macular hole among a diverse group of patients throughout the United States. JAMA Ophthalmol. 2017; 135: 299, doi: 10.1001/jamaophthalmol.2016.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. James M, Feman SS. Risk factors for idiopathic macular holes. The Eye Disease Case-Control Study Group. Am J Ophthalmol. 1994; 118: 754–761. http://www.ncbi.nlm.nih.gov/pubmed/7977602.. Accessed July 19, 2019. [PubMed] [Google Scholar]
- 20. James M, Feman SS. Macular holes. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980; 215: 59–63. http://www.ncbi.nlm.nih.gov/pubmed/6906170.. Accessed July 19, 2019. [DOI] [PubMed] [Google Scholar]
- 21. Evans JR, Schwartz SD, McHugh JDA, et al.. Systemic risk factors for idiopathic macular holes: a case-control study. Eye. 1998; 12: 256–259, doi: 10.1038/eye.1998.60. [DOI] [PubMed] [Google Scholar]
- 22. Segal O, Ferencz JR, Mimouni M, Nesher R, Cohen P, Nemet AY. Lamellar macular holes associated with end-stage exudative age-related macular degeneration. Isr Med Assoc J. 2015; 17: 750–754, http://www.ncbi.nlm.nih.gov/pubmed/26897976.. Accessed July 19, 2019. [PubMed] [Google Scholar]
- 23. von Rückmann A, Fitzke FW, Gregor ZJ. Fundus autofluorescence in patients with macular holes imaged with a laser scanning ophthalmoscope. Br J Ophthalmol. 1998; 82: 346–351, doi: 10.1136/bjo.82.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morgan CM, Schatz H. Involutional macular thinning: a pre-macular hole condition. Ophthalmology. 1986; 93: 153–161, doi: 10.1016/S0161-6420(86)33767-9. [DOI] [PubMed] [Google Scholar]
- 25. Takahashi A, Nagaoka T, Yoshida A. Stage 1-A macular hole: a prospective spectral-domain optical coherence tomography study. Retina. 2011; 31: 127–147, doi: 10.1097/IAE.0b013e3181e7997b. [DOI] [PubMed] [Google Scholar]
- 26. Han DP, Croskrey JA, Dubis AM, Schroeder B, Rha J, Carroll J. Adaptive optics and spectral-domain optical coherence tomography of human photoreceptor structure after short-duration pascal macular grid and panretinal laser photocoagulation. Arch Ophthalmol. 2012; 130: 518–521, doi: 10.1001/archophthalmol.2011.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rabiolo A, Sacconi R, Giuffrè C, et al.. Self-inflicted laser handheld laser-induced maculopathy: a novel ocular manifestation of factitious disorder. Retin Cases Brief Rep. 2018; 2(Suppl 1): S46–S50, doi: 10.1097/ICB.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 28. Mrejen S, Sarraf D, Chexal S, Wald K, Freund KB. Choroidal involvement in acute posterior multifocal placoid pigment epitheliopathy. Ophthalmic Surg Lasers Imaging Retin. 2016; 47: 20–27, doi: 10.3928/23258160-20151214-03. [DOI] [PubMed] [Google Scholar]


