Abstract
A case is presented of a male with drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by carbamazepine intake. The patient presented all the elements of DRESS syndrome: Skin reaction, fever, enlargement of the lymph nodes, increased eosinophils and lymphocytes, with associated organ dysfunctions. The patient was admitted with acute laryngeal edema and imminence of respiratory insufficiency. The escalation of symptoms for this syndrome is typical, even after the administering of the the culprit medicine has ceased. However, in this case, the most difficult aspect was the complex treatment scheme prior to admission. All medical compounds involved in the background treatment were substituted with other substances in order to control the immune response. Current knowledge regarding DRESS is reviewed and possible influence of various etiologies over the present case are discussed. Clinicians should be aware of this rare situation with life-threatening potential. We benefited from the advantage of reuniting the knowledge of a complex team of experts from various tertiary emergency units in Romania.
Keywords: DRESS syndrome, eosinophilia, respiratory distress, carbamazepine, addiction
Introduction
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome represents an severe adverse reaction after medication and is defined by the following elements: Skin symptoms, fever, lymph node enlargement, eosinophilia and modified lymphocytes, as well as internal organs dysfunction (1,2). This syndrome appears frequently after anticonvulsant therapy, usually after a period of up to 6 weeks following the beginning of the treatment and the possibility of worsening the symptoms after drug withdrawal (3,4). In addition, there are other drugs responsible for DRESS, such as nonsteroidal anti-inflammatory compounds, antidepressants and some antimicrobial substances (5,6).
The difficulty in diagnosing DRESS resides in the fact that eosinophilia is inconstant and skin and systemic symptoms are variable (7,8). It is a life-threatening condition and it requires early diagnosis with immediate withdrawal of the drug, as well as hospitalization (9,10).
Case report
We encountered a male patient aged 49, with a history of morbid obesity undergoing bariatric surgery and alcohol addiction. Patient informed consent for publication of the data/images associated with the manuscript was obtained. The authors followed the international and national regulations in accordance with the Declaration of Helsinki and all identifying information was removed.
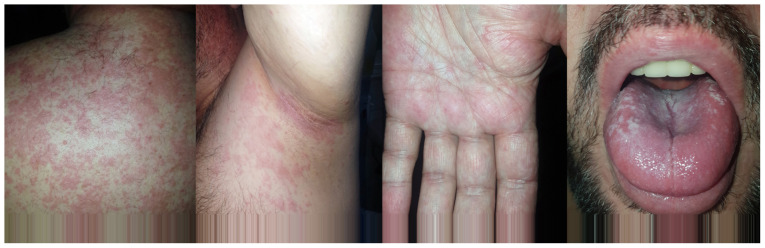
The patient received chronic treatment with carbamazepine, diazepam, zolpidem and cipralex (escitalopram) for 1 month in order to treat his alcohol addiction. Upon admission, the patient presented right ear pain for 7 days, recurrent fever and satellite enlarged lymph nodes on the inferior parotid gland pole. He had self-administered amoxicillin 500 mg every 12 h for 2 days, without previous allergic reactions. Subsequently, he developed a generalized skin reaction with erythema and pruriginous papules. Over the previous 24 h, he had trouble swallowing and cough (Fig. 1).
Figure 1.
Case images on admission: Dorsal, axillar, palmar skin eruption without migration on the body; oral enanthem and edema with impaired breathing.
Blood results revealed marked eosinophilia without leukocytosis: 6,010 leucocytes, 14.7% eosinophils, 125,000/mm3 platelets. In addition, he presented respiratory alkalosis, due to hyperventilation, with pH of 7.434, 32 mmHg pCo2 and 53.9 mmHg pO2. Respiratory distress signaled with 85% O2 saturation in breathing air and 91% with oxygen mask. Liver enzymes ALT=114 U/l, AST=66 U/l and reactive C protein above 30 mg/l; with coagulation status of INR 1.37 and PT 63%.
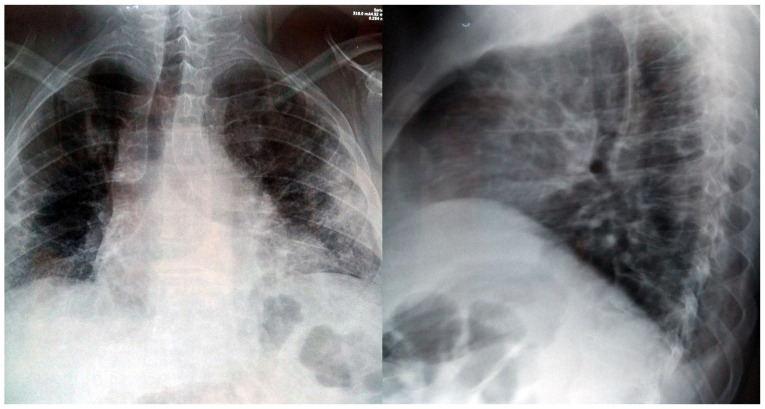
The thoracic X-ray underlined diminished transparency in both lungs, with associated alveolar opacities. In the right side, the authors observed a small transparency in the hilum, indicating a possible old cavern, as well as enlarged cardiac opacity through enlargement of the inferior left arch (Fig. 2).
Figure 2.
Chest X-ray; frontal and lateral view revealing accentuated basal interstitial accumulation of edema.
On admission, the patient presented altered general status with whole body skin reaction and creasing of palms and legs. General data: Body weight 147 kg, height 185 cm and body temperature 36.2 Celsius degrees.
The ENT clinical exam recorded a right parotid lymph node with pain on palpation, an edema of the epiglottis without the possibility of visualizing the vocal cords and inflammation of oral and lingual mucosa.
The patient was admitted to our ENT clinic under the suspicion of acute edema of the epiglottis due to an allergic reaction and generalized skin reaction after medication. Systemic treatment with dexamethasone at 12 h was provided, arnetin (ranitidine) at 12 h, desloratadine at 12 h, clindamycin 300 mg at 8 h, as indicated by the specialist of infectious diseases. The allergy examination recommended stopping the administering of carbamazepine due to a DRESS suspicion. The psychiatry examination diagnosed a background of depression and chronic alcohol consumption and changed the medication scheme to zolpidem once per day, diazepam 10 mg distributed in three fractions per day, Depakine (valproic acid) 150 mg twice per day, as well as stopping carbamazepine. The internal medicine specialist recommended the continuation of antibiotics, plus corticoid and antihistaminic medication. The case had a favorable evolution after the first 24 h, with lowering of the eosinophils and remission of the laryngeal edema.
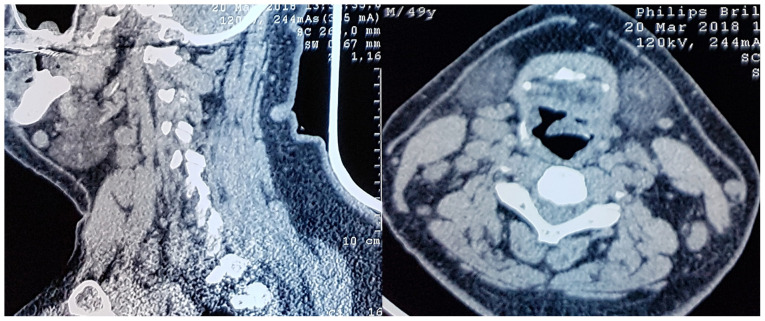
A thorax CT scan was performed without contrast media, which revealed interstitial densities with honeycomb aspect in the periphery without pleural reaction. However, various mediastinum lymph nodes of up to 12 mm in diameter were noted. This raised the suspicion of an infectious process overlying the idiopathic interstitial pneumonia (Fig. 3).
Figure 3.
Neck CT scans in sagittal and axial planes, showing the presence of reactive lymph nodes and the laryngeal edema with imminent respiratory distress.
After 5 days, worsening of the skin condition was observed with a novel increase of eosinophils: up to 20.2% of 8,500 leucocytes/mm3. Antibody levels of EBV, CMV, rubella, toxoplasma, toxocara were in the normal range. The laryngeal edema was in remission. In addition, this episode associated a fever spike of 38.1 degrees Celsius.
Taking into consideration the joint opinion of our allergy, dermatology and infectious diseases specialists, the authors referred the case to a tertiary university dermatology clinic for further treatment.
Discussion
This case reunited all the criteria for carbamazepine-induced DRESS: Acute skin reaction, fever above 38 degrees Celsius, and the presence of enlarged lymph nodes both in the neck and mediastinum, increased liver enzymes and eosinophilia (11). Moreover, there was lung involvement due to eosinophilic interstitial infiltration.
Additional criteria, such as hospital admission and the reaction triggered by medication were also present (12). The patient received carbamazepine for 3 weeks, in addition to the previous antidepressant therapy and the self-administering of penicillin-derived antibiotics, taken 1 week prior to the skin reaction.
Taking into consideration the chest X-ray, the authors also initiated antibiotic treatment with clindamycin, which can be used even in severe cases of penicillin anaphylactic shock (13). The most difficult aspect of this case was the progression towards acute respiratory distress through acute epiglottis edema. This case also presented increased progression of symptoms, despite the suspected drug retrieval and a period of ~72 h of systemic corticoid and antihistaminic therapy. Such late response to common treatment for controlling systemic immune response is considered extremely rare and life-threatening (14).
This case tested negative for tuberculosis, but the authors could not ascertain the presence of HHV type 6, which has been previously associated with drug sensitivity reactions by Pereira de Silva et al (15).
The major difficulty in this case resided in the complex drug scheme self-administered by the patient prior to admission, as well as the necessity to withdraw all of these compounds and to replace them with other drugs while controlling associated pathologies and comorbidities.
In conclusion, the elements defining DRESS and the pathology mechanisms are not fully understood and any new reported case brings new data to this puzzle. The case presented unites the criteria of DRESS after carbamazepine, including additional aspects. Among the specific elements were the pulmonary interstitial reaction and the acute epiglottis edema with the prospect of acute respiratory insufficiency. This is a life-threatening situation with evolution even after the drug withdrawal. The authors consider that hospital admission is mandatory and the systemic treatment with corticoids and antihistamines is broadly approved.
Acknowledgements
Professional editing, linguistic and technical assistance was provided by Irina Radu, Individual Service Provider, certified translator in Medicine and Pharmacy (certificate credentials: Series E no. 0048).
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DV and MD treated the patient, performed literature research and wrote the manuscript. AS treated the patient and was also involved in the conception of the study. AN, GM and EAN offered second opinion during the patient treatment, performed literature research and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study followed the international and national regulations in accordance with the Declaration of Helsinki.
Patients consent for publication
Patient informed consent for publication of the data/images associated with the manuscript was obtained. The authors followed the international and national regulations in accordance with the Declaration of Helsinki and all identifying information was removed.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cho YT, Yang CW, Chu CY. Drug reaction with eosinophilia and systemic symptoms (DRESS): An interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18:1243–1264. doi: 10.3390/ijms18061243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu X, Yang F, Chen S, Xiong H, Zhu Q, Gao X, Xing Q, Luo X. Clinical, viral and genetic characteristics of drug reaction with eosinophilia and systemic symptoms (DRESS) in Shanghai, China. Acta Derm Venereol. 2018;98:401–405. doi: 10.2340/00015555-2867. [DOI] [PubMed] [Google Scholar]
- 3.El Omairi N, Abourazzak S, Chaouki S, Atmani S, Hida M. Drug reaction with eosinophilia and systemic symptom (DRESS) induced by carbamazepine: A case report and literature review. Pan Afr Med J. 2014;18(9) doi: 10.11604/pamj.2014.18.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiransuthikul A, Rattananupong T, Klaewsongkram J, Rerknimitr P, Pongprutthipan M, Ruxrungtham K. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS): 11 years retrospective study in Thailand. Allergol Int. 2016;65:432–438. doi: 10.1016/j.alit.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Musette P, Janela B. New insights into drug reaction with eosinophilia and systemic symptoms pathophysiology. Front Med (Lausanne) 2017;4(179) doi: 10.3389/fmed.2017.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira AM, Carvalho R, Martins A, Reis J. Acute hepatitis in the DRESS syndrome. GE Port J Gastroenterol. 2016;23:304–308. doi: 10.1016/j.jpge.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Li LF. Difficult clinical management of antituberculosis DRESS syndrome complicated by MRSA infection: A case report. Medicine (Baltimore) 2017;96(e6346) doi: 10.1097/MD.0000000000006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munch M, Peuvrel L, Brocard A, Saint Jean M, Khammari A, Dreno B, Quereux G. Early-onset vemurafenib-induced DRESS syndrome. Dermatology. 2016;232:126–128. doi: 10.1159/000439272. [DOI] [PubMed] [Google Scholar]
- 9.Anghel AG, Anghel I, Dumitru M, Soreanu CC. Respiratory and phonatory impairment due to iatrogenic vocal fold paralysis and paresis. A retrospective study of 188 patients. Rev Med Leg. 2012;20:287–290. [Google Scholar]
- 10.Thongsri T, Chularojanamontri L, Pichler WJ. Cardiac involvement in DRESS syndrome. Asian Pac J Allergy Immunol. 2017;35:3–10. doi: 10.12932/AP0847. [DOI] [PubMed] [Google Scholar]
- 11.Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced DRESS syndrome: A systematic review. Mayo Clin Proc. 2016;91:787–801. doi: 10.1016/j.mayocp.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Misthaq AR, Pirasath S, Sugathapala AG. DRESS syndrome associated with sulfasalazine therapy. Jaffna Med J. 2019;31:46–47. [Google Scholar]
- 13.Ozdemir O, Genc G. Drug reaction with eosinophilia and systemic symptoms syndrome associated with ampicillin-sulbactam and clindamyc in: A case report. Istanb Med J. 2019;20:256–260. [Google Scholar]
- 14.Kang SY, Kim J, Ham J, Cho SH, Kang HR, Kim HY. Altered T cell and monocyte subsets in prolonged immune reconstitution inflammatory syndrome related with DRESS (drug reaction with eosinophilia and systemic symptoms) Asia Pac Allergy. 2020;10(e2) doi: 10.5415/apallergy.2020.10.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira de Silva N, Piquioni P, Kochen S, Saidon P. Risk factors associated with DRESS syndrome produced by aromatic and non-aromatic antipiletic drugs. Eur J Clin Pharmacol. 2011;67:463–470. doi: 10.1007/s00228-011-1005-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.