Abstract
Aortic stenosis (AS) management is classically guided by symptoms and valvular metrics. Yet the natural history of AS is dictated by coupling of the left ventricle, aortic valve, and vascular system. We investigated if metrics of ventricular and vascular state add to the appreciation of AS state above valve gradient alone. Seventy patients with severe symptomatic AS were prospectively followed from baseline to 30 days post-transcatheter aortic valve replacement (TAVR). Quality of life (QOL) was assessed by Kansas City Cardiomyopathy Questionnaire (KCCQ). Left ventricular stroke work (SWLV) and vascular impedance spectrums were calculated noninvasively using in-house models based on central blood pressure waveforms, along with hemodynamic parameters from echocardiograms. Patients with higher pre-procedural SWLV and lower vascular impedance were more likely to experience improved QOL after TAVR. Patients fell into two categories – those who did and those who did not exhibit an increase in blood pressure 30-days post-procedure. In patients who developed hypertension (19%), vascular impedance increased and SWLV remained unchanged (impedance at zeroth harmonic - Z0, from 3964.4 to 4851.8 dyne·sec/cm3, P = 0.039; characteristic impedance – Zc, from 376.2 to 603.2 dyne·sec/cm3, P = 0.033). SWLV dropped only in patients who did not develop new hypertension post-TAVR (from 1.58 to 1.26 Joules, P < 0.001). Reduction in valvular pressure gradient after TAVR did not predict change in SWLV (r=0.213, P = 0.129). Reduction of SWLV after TAVR may be an important metric in management of AS, rather than relying solely on the elimination of transvalvular pressure gradients.
Introduction
The symptoms of aortic stenosis (AS) were likely first presented some 350 years ago by the school of Rivierii(1). The dyspnea and heart failure they described remained an essential part of aortic stenosis for years - making its way into the mortality triad that Ross and Braunwald published in 1968(2). Over the last 50 years, the appearance of symptoms has become guide for intervention. The PARTNER 1B trial(3) however taught us that AS is more morbid than appreciated some half-century ago: current AS populations are profoundly different, older, and sicker than the patients described by Ross and Braunwald, and subjective reporting of symptoms could prevent optimal timing for intervention(4).
Quantifiable metrics could replace symptoms, adding precision and more timely triggers of intervention. For years clinicians have sought to leverage the pressure gradient in AS, but the gradient alone cannot be used as determinant of extent of disease or need to intervene, and elimination of gradients does not always assure restoration of health. Aortic valve replacement virtually eliminates the trans-aortic pressure gradients in patients with AS, and yet, quality of life (QOL) does not improve in all patients. Some 35% of patients report no QOL benefit one year after transcatheter aortic valve replacement (TAVR)(5).
Accordingly, the field is searching for additional metrics to follow and use to discriminate subpopulations within the aortic stenosis population. Aortic valve stenosis is a complex, systemic disease that is not solely limited to the aortic valve but is also dependent on the left ventricular (LV) state, vascular load, and ventricular-vascular coupling (6–10). The concept of low gradient AS and the discriminatory potential of dobutamine(11) and nitroprusside(12) have further emphasized the importance of this paradigm in the pathophysiology of AS. Thus, many have called for creation of a quantitative framework that integrates the interactive coupling of the ventricle, valve, and vasculature(6–8) to guide decision-making and treatment optimization.
Here we performed a pilot study to test the hypothesis that interaction between the main components governing systemic perfusion - the LV, aortic valve, and arterial system - can more fully define AS state and the response to TAVR than valve gradient alone. We applied advanced computational models to calculate left ventricular stroke work (SWLV) and vascular impedance in patients before and after TAVR. SWLV represents the work the LV performs by displacing blood against the impedances of the aortic valve and the vascular system(13–15). It is a measure of the energetic state of the LV directly influenced by the valvular and vascular compartments and might represent a comprehensive hemodynamic metric of LV energetic state in patients with AS before and after intervention. Vascular impedance is the load of the proximal aorta and distal arterioles: it adds to SWLV demands both before and after TAVR. We followed changes in SWLV and vascular impedance from baseline to 30 days after TAVR and evaluated the utility of these metrics in predicting improvement in QOL after TAVR. Taken together with valve gradients, SWLV and impedance might refine our understanding of AS.
Results
Baseline characteristics
From April 2016 to April 2017, 70 patients with severe AS who underwent TAVR were prospectively enrolled from two large referral medical centers. The average age was 81 years and 53% were female (Table 1). The majority had symptoms of heart failure at baseline [97% had New York Heart Association (NYHA) functional class ≥ II, and 88% had Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score > 20]. None had clinically significant (≥ moderate to severe) aortic regurgitation (AR), and only eight (11.4%) had left ventricular ejection fraction (LVEF) ≤ 45%. Low gradient severe aortic stenosis (LGAS) (mean gradient < 40 mmHg and aortic valve area < 1 cm2) was found in 26 patients (37%), of which 13 had low flow, defined as stroke volume index (SVi) < 35 ml/m2. Sixty-two (88%) received a balloon-expandable valve and 8 (12%) a self-expandable valve. One patient (1.4%) died within 30 days of procedure from cardiovascular causes. Clinical and echocardiographic data were available in all other patients at the 30-day post-TAVR time point. Noninvasive central blood pressures measurements and related analysis were available in 52 patients (74%).
Table 1. Baseline clinical and echocardiographic characteristics.
Values presented as Mean ± SD or n (%).
| Baseline characteristics | All cohort (N = 70) |
|---|---|
| Age – year | 80.7 ± 9.5 |
| Female Gender | 37 (52.9%) |
| Body mass index, kg/m2 | 28.5 ± 7.7 |
| NYHA class I | 2 (2.9%) |
| class II | 25 (35.7%) |
| class III | 39 (55.7%) |
| class IV | 4 (5.7%) |
| KCCQ - Overall summary score | 48.2 ± 21.1 |
| STS risk score, % | 5.5 ± 2.9 |
| Diabetes | 27 (39.1%) |
| Hypertension | 29 (41.4%) |
| Creatinine >2 gr/dl | 5 (7.1%) |
| Smoker | 13 (18.6%) |
| Previous stroke | 5 (7.1%) |
| Peripheral vascular disease | 26 (37.1%) |
| Previous myocardial infarction | 13 (18.6%) |
| Previous CABG | 18 (25.7%) |
| Previous PCI | 20 (28.6%) |
| Atrial fibrillation/flutter | 24 (34.8%) |
| Echocardiographic finding | |
| Ejection fraction, % | 59.09 ± 11.43 |
| Aortic valve area, cm2 | 0.76 ± 0.22 |
| Mean gradient | 44.1 ± 13.7 |
| < 40 mmHg | 26 (37.1%) |
| > 40 mmHg | 44 (62.9%) |
| Stroke volume index | 41.32 ± 13.46 |
| < 35 ml/m2 | 24 (34.3%) |
| > 35 ml/m2 | 46 (65.7%) |
| Mitral regurgitation ≥ moderate | 18 (25.7%) |
NYHA: New York Heart Association; KCCQ: Kansas City Cardiomyopathy Questionnaire; STS: Society of Thoracic Surgeons; CABG: coronary-artery bypass grafting, PCI: percutaneous coronary intervention.
Changes in hemodynamic metrics from baseline to 30 days after TAVR
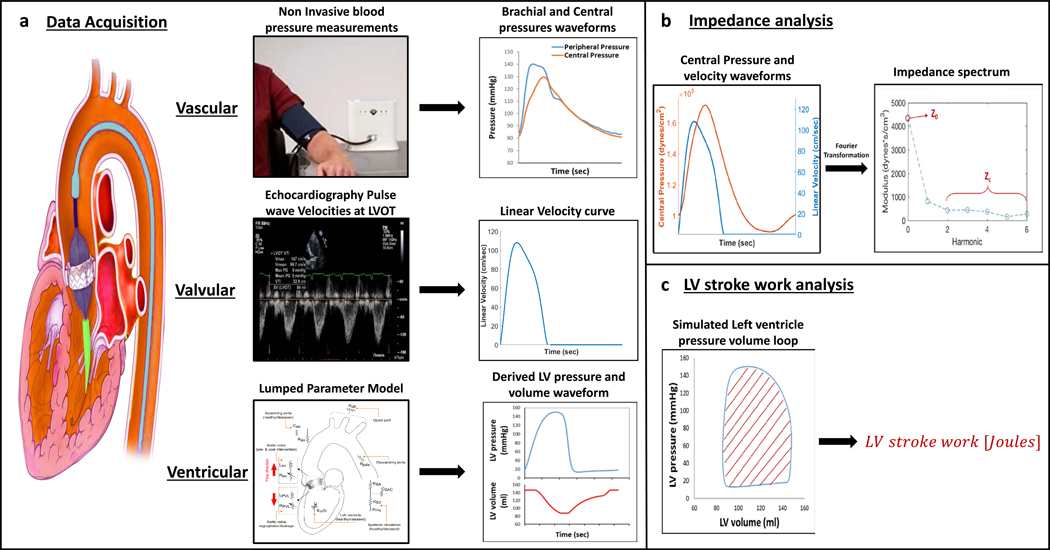
In addition to classic valvular load indices, SWLV and the vascular impedance spectrum were determined before and 30 days after TAVR for each subject using noninvasive models (Fig. 1). Changes of ventricular, valvular, and vascular indices from baseline to 30 days after TAVR are presented in Table 2. As expected, valvular metrics improved significantly after TAVR (P<0.001). There was no change in ejection fraction, SVi, or cardiac index from baseline to 30 days after TAVR. At 30 days post TAVR only 1 patient had paravalvular leak > grade 2 and 2 patients had aortic pressure gradient > 20 mmHg.
Fig. 1. Analysis of ventricular, valvular, and vascular metrics.
(A) Schematic of a heart and great vessels undergoing transcatheter aortic valve replacement and data acquisition workflow. Vascular: Photograph of noninvasive brachial blood pressure measurements captured with the SphygmoCor XCEL device, used to derive peripheral (brachial) and central (aortic) pressure waveforms. Representative waveforms are shown. Valvular: Echocardiographic pulse wave Doppler tracings captured at the left ventricular outflow tract (LVOT), used to derive central velocity waveforms. Representative waveform is shown. Ventricular: Computer-based lumped parameter model used patient-specific echocardiographic data to derive left ventricular (LV) pressure and volume waveforms. Representative waveforms are shown (magnified image of the lumped parameter model can be seen in fig. S2). (B) Vascular impedance analysis. The aortic input impedance spectrum was derived using Fourier decomposition of the noninvasive central pressure and LVOT velocity waveforms. Z0 is the impedance modulus at the zero harmonic. Characteristic impedance (Zc) was calculated as the average of frequencies 2–10 Hz. (C) Left ventricular stroke work analysis. Left ventricle pressure-volume loop was constructed from the computer-based lumped parameter model. LV stroke work (SWLV) was calculated as the area of the simulated pressure-volume loop.
Table 2. Changes in hemodynamic metrics from baseline to 30 days after TAVR.
Values presented as Mean ± SD.
| Before TAVR (n = 52) | 30-days after TAVR (n = 52) | P value | |
|---|---|---|---|
| Ventricular indices | |||
| Ejection fraction, % | 59.4 ± 11.9 | 61.1 ± 11.2 | 0.096 |
| Heart rate, bpm | 68.2 ± 13.3 | 67.7 ± 14.7 | 0.656 |
| Stroke volume Index, ml/m2 | 42.2 ± 14.6 | 40.8 ± 13.6 | 0.521 |
| Cardiac index, L/min/m2 | 2.8 ± 0.9 | 2.7 ± 0.9 | 0.103 |
| Valvular indices | |||
| Aortic valve area, cm2 | 0.8 ± 0.2 | 1.6 ± 0.5 | <0.001 |
| Mean gradient, mmHg | 42.8 ± 14.3 | 11.9 ± 6.5 | <0.001 |
| Peak velocity, cm/sec | 399.5 ± 70.5 | 221.9 ± 56.2 | <0.001 |
| Vascular indices | |||
| Brachial systolic BP, mmHg | 137.6 ± 24.2 | 137.9 ± 20.6 | 0.928 |
| Brachial pulse pressure, mmHg | 70.8 ± 19.5 | 70.4 ± 18.6 | 0.865 |
| Central systolic BP, mmHg | 127.5 ± 21.9 | 123.6 ± 19.9 | 0.228 |
| Central pulse pressure, mmHg | 58.9 ± 16.7 | 54.41 ± 17.3 | 0.048 |
| Combined Metrics | |||
| Vascular impedance (Z0), dyne·sec/cm3 | 4543.6 ± 1795.0 | 4842.7 ± 2350.4 | 0.722 |
| Characteristic impedance (Zc), dyne·sec/cm3 | 494.9 ± 343.3 | 486.0 ± 255.1 | 0.862 |
| LV stroke work, Joule | 1.57 ± 0.51 | 1.29 ± 0.51 | <0.001 |
| Valvulo-arterial impedance (Zva), mmHg·m2/ml | 4.7 ± 1.4 | 4.0 ± 1.2 | 0.001 |
| Systemic arterial compliance, ml/mmHg·m2 | 0.8 ± 0.4 | 0.8 ± 0.3 | 0.425 |
| Total peripheral resistance, dyne·sec/cm5 | 1504.7 ± 514.4 | 1590.0 ± 502.5 | 0.241 |
TAVR: transcatheter aortic valve replacement; BP: blood pressure, Z0: vascular impedance at zeroth harmonic; Zc: characteristic impedance; LV: left ventricle; Zva: valvulo-arterial impedance.
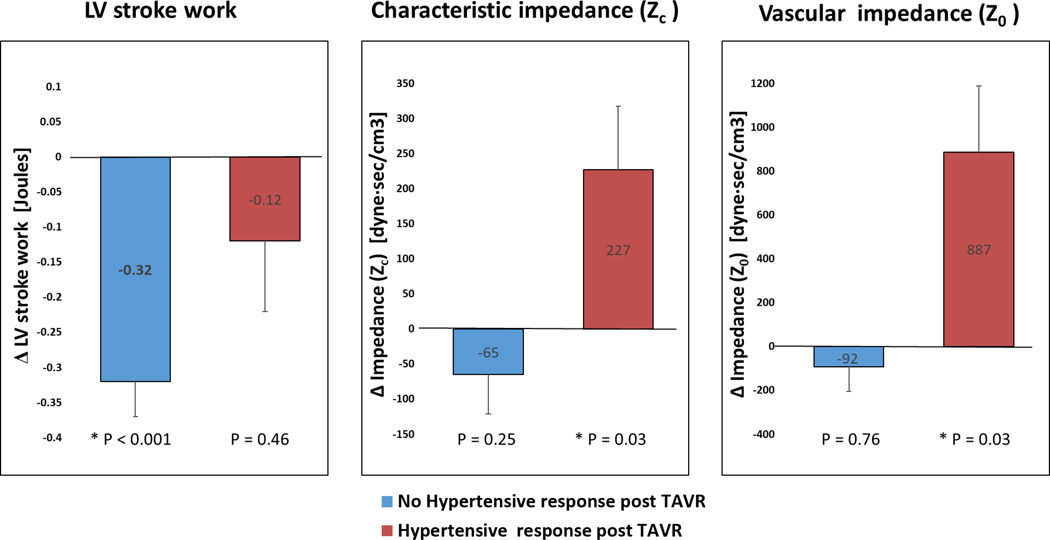
Systolic blood pressure (BP) fell more than 15 mmHg from baseline in 12 patients (23%) and rose above 140 mmHg in 10 patients (19%). In the newly hypertensive patients, vascular impedance metrics, including impedance at zeroth harmonic (Z0), characteristic impedance (Zc), as well as total peripheral resistance (TPR), significantly rose (P= 0.033, 0.039, 0.036 respectively), but they did not change in the rest of the cohort (Fig. 2, Table 3).
Fig. 2. Left ventricle stroke work and vascular impedance metrics in patients developing hypertension after TAVR.
Bar charts comparing (A) LV stroke work (SWLV), (B) characteristic impedance (Zc), and (C) vascular impedance (Z0) in patients who developed new hypertension after TAVR (n = 10; 19%, red) versus the rest of the cohort (n = 42; 81%, blue). Data are presented as the mean changes (Δ) from baseline to 30 days post-TAVR. Error bars represent standard deviation. P values represent the statistical significance of change from baseline to 30 days post-TAVR and was tested with paired samples t-test).
Table 3. Changes in left ventricular stroke work and vascular impedance metrics in patients developing hypertension after TAVR.
Values presented as Mean ± SD. Hypertensive response post-TAVR was defined as systolic blood pressure (BP) >140mmHg or diastolic BP>90mmHg 30-day after TAVR not present at baseline (28).
| No hypertensive response post-TAVR (n = 42) | Hypertensive response post-TAVR (n = 10) | |||||
|---|---|---|---|---|---|---|
| Baseline | 30 days post TAVR | P value | Baseline | 30 days post-TAVR | P value | |
| LV Stroke work, Joule | 1.58 ± 0.51 | 1.26 ± 0.54 | <0.001 | 1.54 ± 0.51 | 1.42 ± 0.35 | 0.460 |
| Vascular impedance (Z0), dyne·sec/cm3 | 4627.7 ± 1922.1 | 4534.9 ± 1598.6 | 0.253 | 3964.4 ± 916.1 | 4851.8 ± 1007.4 | 0.033 |
| Characteristic impedance (Zc), dyne·sec/cm3 | 523.1 ± 317.3 | 458.1 ± 234.6 | 0.765 | 376.2 ± 142.7 | 603.2 ± 314.9 | 0.039 |
| Total peripheral resistance, dyne·sec/cm5 | 1583.5 ± 527.4 | 1587.4 ± 498.1 | 0.956 | 1173.8 ± 286.3 | 1600.9 ± 548.3 | 0.036 |
TAVR: transcatheter aortic valve replacement; LV: left ventricle; Z0: vascular impedance at zeroth harmonic; Zc: characteristic impedance.
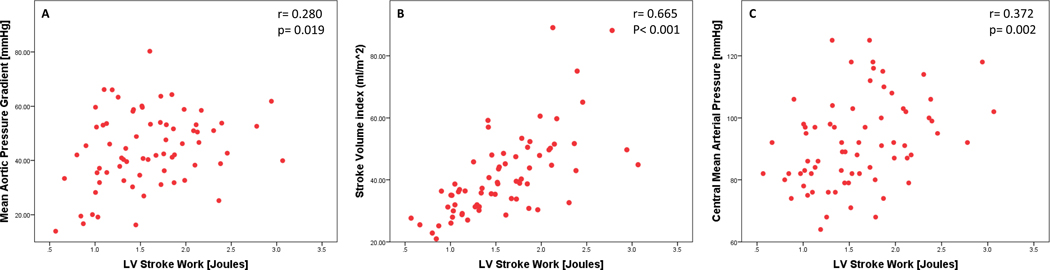
In concert with the pathophysiological definition of SWLV in patients with AS, baseline SWLV correlated with central mean aortic pressure (r=0.280, P=0.019), with SVi (r=0.665, P<0.001), and with mean valvular pressure gradient (r=0.372, P=0.002) (Fig. 3). Change in SWLV from baseline to 30 days after TAVR did not correlate with change in mean valvular pressure gradients (r=0.213, P=0.129) (Fig. 4), whereas it did correlate with change in central mean aortic pressure (r=0.432, P=0.001) and change in SVi (r=0.735, P<0.001) (Fig. S1). All the patients reduced their valve gradient after TAVR. SWLV was reduced in most patients (from 1.6 to 1.3 Joules, P<0.001), but not in the 19% of our cohort who developed new hypertension after TAVR (1.5 to 1.4 Joules, P=0.460) (Fig. 2; Table 3).
Fig. 3. Properties of pre-procedural left ventricular (LV) stroke work.
Correlation of pre-procedural LV stroke work with pre-procedural mean transvalvular pressure gradient (A), stroke work index (B), and central mean aortic pressure (C). R represents the Pearson correlation coefficient; (n = 70).
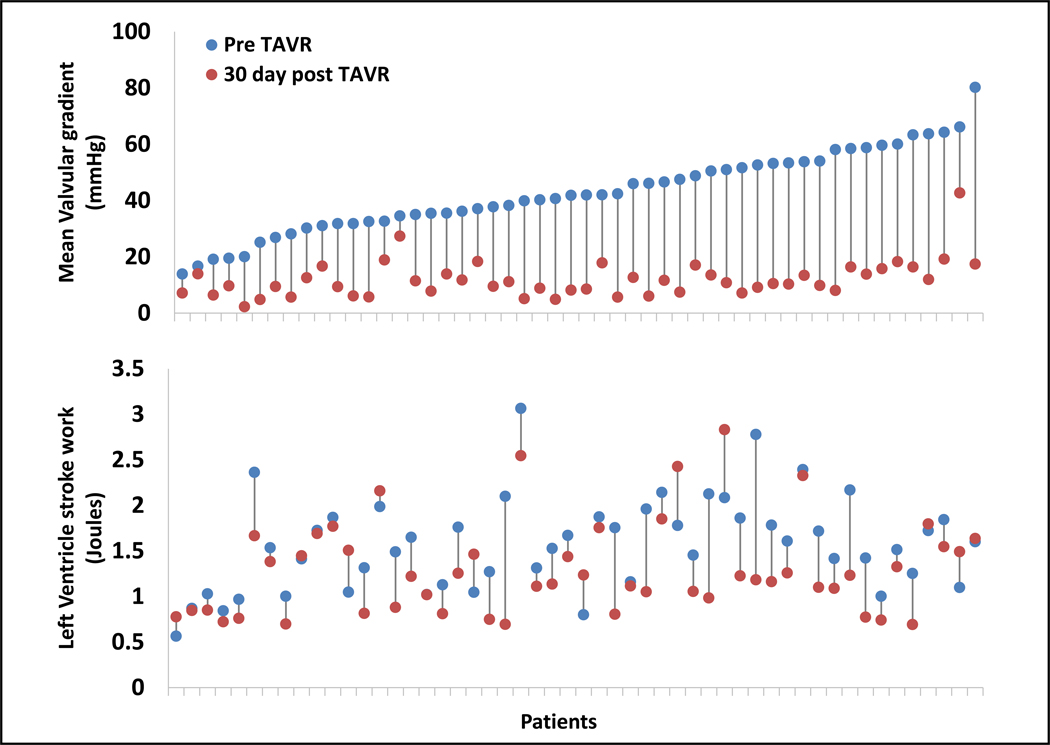
Fig. 4. Changes in left ventricle stroke work and valvular pressure gradients.
Minimum-maximum plots representing patient-specific changes from baseline to 30 days post-TAVR in mean valvular gradient (upper panel) and in left ventricle stroke work (lower panel) (Each data point represent average of two measurements of a single patient). There is no correlation between the decrease in transvalvular pressure gradient after TAVR and the change in left ventricle stroke work (R = 0.213, P = 0.129; n=52; fig S2). R represents the Pearson correlation coefficient
Predictors for QOL improvement 30 days after TAVR
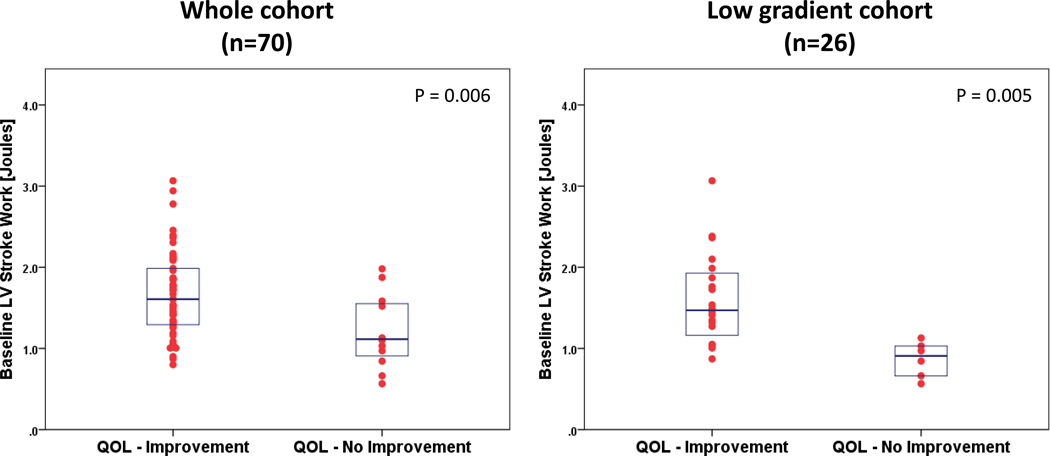
Using a KCCQ-based QOL improvement endpoint, 58 patients (83%) improved and 12 patients (17%) did not improve 30 days post-TAVR (Table 4). Patients whose QOL improved had higher baseline SWLV values (1.6 vs. 1.2 Joules, P=0.006) (Fig. 5). Other baseline metrics associated with QOL improvement were lower vascular impedance at the zeroth harmonic (Z0) (4,368.5 vs. 6,272.8 dyne·sec/cm3, P<0.001), and a higher body mass index (29.3 vs. 24.5 kg/m2, P=0.040). Low baseline Z0 was associated with QOL improvement at 30 days after TAVR whereas baseline BP measurements did not (Table 4). Pre-procedural ejection fraction, mean trans aortic pressure gradient, NYHA functional class, and noninvasive central blood pressure measurements did not differ significantly between the groups distinguished by QOL changes, although those with higher pre-procedural KCCQ were more likely to have QOL improvement at 30-days post-procedure.
Table 4. Baseline differences between quality of life improvement groups.
Values presented as Mean ± SD or n (%). QOL improvement was defined as increase ≥10 points at KCCQ score from baseline to 30-days post procedure.
| No QOL improvement (n = 12) | QOL improvement (n = 58) | P value | |
|---|---|---|---|
| Clinical metrics | |||
| Age, year | 83.8 ± 5.9 | 80.0 ± 9.9 | 0.239 |
| Body mass index, kg/m2 | 24.5 ± 3.9 | 29.3 ± 8.1 | 0.040 |
| STS risk score, % | 6.5 ± 3.6 | 5.3 ± 2.7 | 0.326 |
| Diabetes | 3 (25%) | 25 (43%) | 0.244 |
| Hypertension | 3 (25%) | 26 (45%) | 0.204 |
| Creatinine >2 gr/dl | 1 (8%) | 4 (7%) | 0.860 |
| Ventricular indices | |||
| Ejection fraction, % | 55.0 ± 16.4 | 59.9 ± 10.1 | 0.565 |
| Heart rate, bpm | 65.8 ± 16.9 | 67.7 ± 11.5 | 0.645 |
| Stroke volume Index, ml/m2 | 34.9 ± 10.1 | 42.7 ± 13.7 | 0.043 |
| Cardiac index, L/min/m2 | 2.3 ± 0.8 | 2.8 ± 0.9 | 0.016 |
| Valvular indices | |||
| Aortic valve area, cm2 | 0.66 ± 0.13 | 0.78 ± 0.23 | 0.105 |
| Mean gradient, mmHg | 38.5 ± 18.0 | 45.2 ± 12.6 | 0.126 |
| Peak velocity, cm/sec | 386.2 ± 111.9 | 406.6 ± 63.9 | 0.553 |
| Vascular indices | |||
| Brachial systolic BP, mmHg | 135.9 ± 22.6 | 138.3 ± 24.1 | 0.749 |
| Brachial pulse pressure, mmHg | 67.3 ± 19.7 | 70.2 ± 19.3 | 0.233 |
| Central systolic BP, mmHg | 127.2 ± 19.6 | 128.3 ± 22.3 | 0.869 |
| Central pulse pressure, mmHg | 56.5 ± 15.1 | 58.4 ± 17.3 | 0.725 |
| Pulse wave velocity, m/sec | 8.0 ± 1.9 | 7.7 ± 2.2 | 0.669 |
| Augmentation Index, % | 24.7 ± 12.1 | 19.5 ± 16.4 | 0.304 |
| Combined Metrics | |||
| Vascular impedance (Z0), dyne·sec/cm3 | 6272.8 ± 1841.9 | 4368.5 ± 1514.9 | <0.001 |
| Characteristic impedance (Zc), dyne·sec/cm3 | 530.4 ± 300.1 | 473.4 ± 323.0 | 0.427 |
| LV stroke work, Joule | 1.19 ± 0.45 | 1.66 ± 0.52 | 0.006 |
| Valvulo-arterial impedance (Zva), mmHg·m2/ml | 5.2 ± 1.1 | 4.6 ± 1.3 | 0.147 |
| Systemic arterial compliance, ml /mmHg·m2 | 0.6 ± 0.1 | 0.8 ± 0.5 | 0.073 |
| Total peripheral resistance, dyne·sec/cm5 | 2003.1 ± 613.8 | 1460.9 ± 420.9 | <0.001 |
QOL: Quality Of Life, STS: Society of Thoracic Surgeons; BP: blood pressure; Z0: vascular impedance at zeroth harmonic; Zc: characteristic impedance; LV: left ventricle; Zva: valvulo-arterial impedance.
Fig. 5. LV stroke work in quality of life (QOL) improvement groups.
Individual dot plot and overlaid box plot of baseline (pre-TAVR) LV stroke work segregated by QOL improvement groups. Box plots portray median values and 25–75 percentiles for the whole cohort (n =70) and for the low gradient aortic stenosis cohort (n =26). (Independent samples t-test was used to compare the means).
Of the 26 patients with low gradient aortic stenosis (LGAS), 20 (77%) improved their QOL and 6 (23%) did not. As in the whole cohort, in this pre-specified LGAS cohort, patients who showed improvements in their QOL had higher baseline SWLV values (1.60 vs. 0.86 Joules, P=0.005) (Fig. 5).
Discussion
Our study was based on the premise that optimal definition of patients with AS cannot rely on the emergence of symptoms or valve gradient alone, but rather should combine hemodynamic metrics of the valve, left ventricle, and arterial subsystems. The increasing appreciation by the field that classifying the contribution of each subsystem to AS helps provide an integrated view of patient state may provide objective data as to when to intervene and how to optimize intervention with adjunctive therapies.
In this study, we used patient-specific data and computational analysis to include metrics of the ventricle proximal and vasculature distal to the stenosed aortic valve. These metrics allowed us to demonstrate that LV decompensation and recovery are not simply driven by valve hemodynamics, but also by the vasculature. There is therefore a subset of patients with new hypertension after TAVR due to increased vascular impedance that have persistently high LV stroke work (SWLV) even after valve replacement. Furthermore, we have demonstrated that these metrics impact QOL.
As a composite metric, SWLV represents the LV energetic state and incorporates ventricular geometry and its mechanical properties, as well as valvular and vascular impedances(13–15). Accordingly, we observed that SWLV prior to TAVR correlated with pre-procedural SVi, mean valvular gradient, and mean arterial pressure. As such, SWLV might represent a hemodynamic metric that can be tracked in patients with aortic stenosis before and after intervention. Indeed, in our study the higher the SWLV at baseline the greater the QOL improvement post TAVR. In other words, patients with ventricles that can function at a higher energetic state before valve replacement might have a higher likelihood of improving their QOL after TAVR. The baseline values, and not the degree of change in SWLV, predicted improvement; changes in SWLV from baseline to 30-days post-TAVR can only be appreciated once vascular impedance is incorporated, as they cannot be explained by reduction in mean valvular pressure gradient alone. In fact, SWLV only fell in the cohort who did not show increased BP or vascular impedance after TAVR. In those patients with new hypertension, Z0 rose, hypertension emerged, and there was no fall in SWLV – the energetic load on the heart remained elevated even after the valve gradient was removed.
Herein lies an important distinction with the work of Perlman et al.(16) and Lindman et al.(17), who both reported on the benefit of hypertension post-TAVR, and of the work of Yotti et al.(18), who demonstrated an increase in vascular load after TAVR associated with limited acute afterload relief. We add the full hemodynamic description, adding the coupling of vascular impedance with SWLV as well as implication on QOL at the 30-day time point, and in contrast to previous studies describing hypertension in general, our focus is on the emergence of hypertension and its associated failure of SWLV restoration.
The metrics of vascular impedance we present here are derived from analysis of the frequency domain of the pressure and flow waveforms rather than the temporal domain alone. The use of impedance extracted from the frequency domain of the signal has the advantage of providing additional information regarding vascular load in the setting of pulsatile physiological flow and not just steady load, thus allowing better characterization of load opposing the LV.
The valvulo-arterial impedance (Zva) developed by the group of Philippe Pibarot (9, 19, 20) is a measure of total afterload, acknowledging the contribution of the vascular state to the physiology of AS. In our study, although Zva changed significantly after TAVR (P=0.001) it did not discriminate between patients who improved their QOL and those who did not (P=0.147). We speculate that the Zva index is very sensitive to aortic valve gradient changes that always reduce after TAVR.
Valvular and vascular impedances contribute to LV afterload in AS(9), and elevations in the latter might challenge SWLV even following successful valve replacement. In some patients, valvular stenosis might even mask the effects of vascular stiffness, which only emerges once flow is restored with removal of valve stenosis. Such thinking potentially explains how vascular impedance rose and SWLV did not fall as expected after TAVR in the cohort who developed hypertension post-procedure.
Patients with low gradient AS (LGAS) pose a diagnostic challenge in our daily practice (12, 21). This group can have depressed or preserved LV function, and low or high transvalvular flows. Some patients are truly limited by the valve whereas other are limited by the ventricular energetic state or by the compliance of the vascular tree. We believe that the integration of metrics that describes the state of the ventricular and vascular systems will improve our ability to manage these patients. Indeed, in our LGAS subpopulation patient who did not improved their QOL after TAVR had significant lower baseline SWLV (P=0.005). We also believe that SWLV could serve as an important metric in evaluating patient with AS and reduced EF. Since our cohort included mainly patients with normal EF, further studies are warranted.
These data support the emerging call to classify AS in more rigorous and precise terms that incorporate the individual contributions of the valve, ventricle, and distal vasculature. Temporal tracking of SWLV and vascular impedance metrics might direct the optimal timing of intervention (surgical AVR or TAVR) and help define the nature of adjunctive medical care thereafter. Moreover, these metrics might also identify patients who may not benefit from valve replacement, as the ventricle may not be likely to recover due to a patient’s increased vascular impedance.
Incorporating these data together allows us to appreciate that there are likely several subpopulations of AS patients. In some the primary pathology is isolated to the valve – as in younger patients with bicuspid valves, where LV outlet flow is so restrictive that functional limitation can be attributed to the valve alone. Similarly, there are those patients for whom pathologies in the valve are linked and dramatically exacerbated by LV failure or by excessive afterload. Accordingly, aortic stenosis involves pathologic contributions from the ventricle and vasculature as well as the valve. Along this paradigm, the pathology aortic stenosis in its most extreme and highly symptomatic form extends to all three elements, while in its earliest phases one can define disease subsets where one aspect dominates. It is conceivable then that progressive validation of quantitative metrics will enable classification of AS with valve, ventricular or vascular dominance well before the three combine to create end-stage disease. This perception might inform us as to what metrics to follow and what adjunctive therapies to apply with valve intervention(9, 18, 22).
There were several limitations to our study that warrant consideration. The relatively small sample size did not allow for investigation of clinical event rates after TAVR or for subgroup analyses. Clinical outcome measurement and improvement post-TAVR are not straightforward to discern. We used QOL assessment that was shown to be an important endpoint in the current TAVR population. We acknowledge that 30-days QOL change might be early to fully appreciate the benefit or futility of valve replacement. However, a recent large study showed that QOL metrics that improved after TAVR at 30 days persisted for at least 1 year(5); in other words, the TAVR related benefit in terms of symptoms relief, functional capacity, and quality of life observed at 12 months was totally attained at 30 days. Nonetheless, further studies with longer follow up are needed. Such future studies might use more refined physiological assessments such as 6-minute walk, exercise stress test, or VO2 max. There are multiple means of measuring vascular stiffness. We sought a non-invasive method that derives central blood pressure from peripheral measurements, using the SphygmoCor XCEL device. Although this device has been used in other AS studies(23), it has not been fully validated in this population (24, 25). We did not have full data regarding out of hospital medication change during the first 30 days post TAVR. Nevertheless, the statistically significant increase in impedance in the newly hypertensive patients held whether patients were taking new BP medication.
In this pilot, hypothesis-generating study, our objective was to explore refined and comprehensive metrics to better characterize different sub populations of patient with aortic valve stenosis. We further aimed to support the field in searching for advanced physiologic metrics that can become a part of the evaluation of patients with aortic stenosis and may help guide clinicians about benefit and timing of intervention. LV stroke work and vascular impedance appear to be important metrics to classify patients with AS, as well as meaningful discriminants of response to TAVR. Reduction of SWLV may be an important target in AS rather than reliance solely on the elimination of transvalvular pressure gradients. As metrics of valve dynamics, ventricular function, and vascular impedance can readily and regularly be calculated, their use may become an essential part of the evaluation of AS patients. Additional, larger-scale studies are warranted to further validate these metrics and implement them in clinical practice.
Materials and Methods
Study design
We designed a study which examined the hypothesis that physiological state of patients with AS can be more precisely defined by analyzing the interaction of the LV, aortic valve, and arterial system with specific metrics of each element. To evaluate this hypothesis, we prospectively enrolled patients with severe AS undergoing TAVR at the Massachusetts General Hospital or Brigham and Women’s Hospital. Patients were evaluated pre-procedure and 30-day post-TAVR. Data collection included Quality of life assessments as well as dedicated echocardiography and noninvasive central blood pressure allowing us to calculate left ventricular stroke work (SWLV) and vascular impedance spectrums using in-house computational models. We investigated how dynamics in physiological metrics correlated with changes in QOL metrics. Informed consent was obtained as approved by institutional review board. Eligibility for TAVR was determined by the local heart team.
Data acquisition
Demographic and procedural data were collected from local TAVR databases and patients’ records. KCCQ was collected at baseline and 30 days after TAVR. QOL improvement was defined as an increase in total KCCQ score of more than 10 points (5). Hypertensive response post-TAVR was defined as systolic blood pressure (BP) > 140 mmHg or diastolic BP > 90 mmHg 30 days after TAVR that was not present at baseline(16).
Echocardiograms were reviewed in blinded fashion by two senior cardiologists. Low gradient severe aortic stenosis was defined as mean transvalvular gradient < 40 mmHg and aortic valve area < 1 cm2. Low flow state was defined as stroke volume index (SVi) < 35 ml/m2. The left ventricular outflow track (LVOT) velocity waveform was extracted from the raw echocardiographic DICOM images by an in-house tool using the MATLAB software package (MathWorks, Inc.). (Fig. 1A).
Noninvasive central pressure was captured with the SphygmoCor XCEL device (AtCor Medical). This device uses a generalized transfer function and standard BP measurements to derive the central (aortic) pressure waveform from the brachial volume displacement waveform. Pulse wave contours were formed by averaging several heartbeats. For those patients with a history of arrhythmias, care was taken during measurement to ensure waveforms that were chosen were best representing of sinus rhythm (Fig. 1A).
Protocol for noninvasive central pressure measurements with SphygmoCor XCEL device
A simple blood pressure (BP) cuff connected to the XCEL device was placed on a subject’s arm and an automated sequence measured pulse wave contours in the following manner. Three consecutive BP measurements were taken, and the average of the final two were used for pulse waveform calibration. The cuff was then re-inflated to a sub-diastolic pressure and the pulse wave contours of several heart beats were recorded and averaged. This averaged waveform was digitized and saved for offline processing and analysis.
Vascular impedance calculation
Aortic input impedance spectrums were calculated in the frequency domain using non-invasive central pressure waveforms recorded from the SphygmoCor and a velocity tracing from pulsed wave Doppler measured in the LVOT. Each waveform was decomposed into its Fourier harmonics. Moduli and phase at each harmonic were used to calculate input impedance via an in-house MATLAB program. Z0 was defined as the impedance moduli at the zeroth harmonic. Characteristic impedance (Zc) was calculated as the average of frequency 2–10 Hz, with frequencies greater than three times the median excluded (Fig. 1B). To allow for comparison of these vascular impedance metrics to other vascular load metrics used in the literature, we used standard equations to calculate total peripheral resistance (TPR), systemic arterial compliance (SAC), and valvulo-arterial impedance (Zva).
Impedance is defined as resistance to pulsatile flow. This is the ratio between the frequency harmonics of the pressure waveform to those of the blood flow waveform(26).
Mathematically this can be defined as:
Where Zn is the Aortic input impedance at the nth harmonic. Pn and Qn, are the pressure and flow harmonics at the nth harmonic.
The phase of the two waves with respect to each other can be calculated as
where Phase(n) is the phase of the flow at the nth harmonics, and similarly for pressure.
Lumped parameter model and left ventricle stroke work calculation
A lumped parameter model of the left heart (Fig. 1A) incorporated the left ventricle, aortic valve, extent of aortic regurgitation, and systemic circulation. Each sub-model was validated with in vivo cardiac catheterization and MRI data(27–29). All input parameters were obtained as patient-specific echocardiographic measurements. Using this lumped parameter model, LV pressures and volumes waveforms were extracted. SWLV was calculated as the area of the simulated pressure-volume loop (Fig. 1C).
HEART-ARTERIAL MODEL
The ventricle was filled by a normalized physiological mitral flow waveform adjusted for the required stroke volume. Coupling between left ventricle pressure and volume was performed through a time varying elastance E(t), a measure of cardiac muscle stiffness,
| (1) |
where PLV(t), V(t), and V0 are left ventricular time-varying pressure, time-varying volume, and unloaded volume, respectively. The amplitude of E(t) can be normalized with respect to maximal elastance, Emax, the slope of the end-systolic pressure-volume relationship, giving EN(tN)=E(t)/Emax. Time then can be normalized with respect to the time to reach peak elastance, TEmax (tN=t/TEmax).
| (2) |
A normalized curve of EN(tN) can be described using Fourier series. Therefore, the relationship between PLV(t) and V(t) can be determined for the left ventricle.
MODELING AORTIC VALVE
Aortic stenosis (AS) was modeled using Equation 3. This formulation expresses the instantaneous net pressure gradient across the stenotic valve (after pressure recovery) as a function of the instantaneous flow rate and the energy loss coefficient and links the LV pressure to the aorta pressure:
| (3) |
and
| (4) |
Where , and Q are the valvular energy loss coefficient, the effective orifice area, ascending aorta cross sectional area, the fluid density, and the transvalvular flow rate, respectively. Variable aortic valve resistance (Rav) and constant aortic valve inductance (Lav) in the lumped parameter model are and respectively.
MODELING AORTIC VALVE REGURGITATION
Aortic regurgitation (AR) was modeled using the same formulation as aortic stenosis. AR pressure gradient is the difference between aortic pressure and LV pressure during diastole.
| (5) |
and
| (6) |
Where ELCo|AR, REOA and ALVOT are regurgitation energy loss coefficient, regurgitant effective orifice area, and LVOT area, respectively. Variable aortic valve regurgitation resistance (Rav) and constant aortic valve regurgitation inductance (L ) in the model are and , respectively.
DETERMINING ARTERIAL COMPLIANCE AND PERIPHERAL RESISTANCE
The total systemic resistance was computed as the quotient of the average brachial pressure and the cardiac output [assuming a negligible peripheral venous pressure (mean ~ 5 mmHg) compared to aortic pressure (mean ~ 100 mmHg)]. This total systemic resistance represents the electrical equivalent resistance for all resistances in the current model. Because what the left ventricle faces is the total systemic resistance and not the individual resistances, for the sake of simplicity we considered the aortic resistance, Rao, and systemic venous resistance, RSV, as constants and adjusted the systemic arterial resistance, RSA, according to the obtained total systemic resistance. For each degree of hypertension, we fit the predicted pulse pressure to the actual pulse pressure (known by arm cuff sphygmomanometer) obtained from clinical study by adjusting compliances [aorta (Cao) and systemic (CSAC)].
COMPUTATIONAL ALGORITHM
A lumped parameter model(27–29) was analyzed numerically by creating and solving a system of ordinary differential equations in Matlab Simscape (MathWorks, Inc.), enhanced by adding additional codes to meet demands of cardiac model in circuit. A Fourier series representation of an experimental normalized elastance curve for human adults was used to generate a signal to be fed into the main program. Simulations start at the onset of isovolumic contraction. Left ventricle volume, V(t), is calculated using left ventricle pressure, PLV, and time varying elastance values (equation 1). Matlab’s ode23t trapezoidal rule variable-step solver was used to solve system of differential equations with initial time step of 0.1 milliseconds. The convergence residual criterion was set to 10−5 and initial voltages and currents of capacitors and inductors set to zero.
Equation for hemodynamics parameters
- Total Peripheral Resistance (TPR) was calculated as:
- Systemic Arterial Compliance (SAC) was calculated as:
- Valvulo-arterial impedance (Zva)(19) was calculated as :
“Stroke work loss index” was not included in the analysis as it underestimates the hemodynamic significance of aortic stenosis in patients with hypertension (30).
Statistical analysis
Continuous variables, presented as means (± standard deviations), were tested with the Student’s t-test or Mann-Whitney rank sum test. Categorical variables are presented as number (percentage) and compared using chi-square or Fisher’s exact test. Normal distribution was assessed with the Shapiro-Wilk test. A bivariate Pearson correlation coefficient was computed to assess the relationship between continues variables. All analyses were considered significant using 2-tailed test with a p-value of < 0.05. The SPSS statistical package 20 was used.
Supplementary Material
Fig. S1. Properties of change in left ventricular stroke work (SWLV) from baseline to 30 days after TAVR
Fig. S2. Schematic diagram of the lumped parameter model
Acknowledgments:
We thank Prof. M. O’Rourke for guidance and insight; C. Ricciardi, C. Steinmetz, T. Urman of the MIT/IMES Clinical Research Center for protocol management and data collection; and D. Furman, S. L. Capano, S. R. Devireddy and B. J. Coronis for protocol management and patient data analysis.
Funding: Supported in part by a research grant from Edwards Lifesciences to E.R.E, and a US National Institutes of Health (R01 49039) grant to E.R.E. Edwards Lifesciences had no influence on the design, data collection or analysis of the study.
Footnotes
Competing interests: F.K. is an employee of Edwards Lifesciences; S.E. receives research funds from Edwards Lifesciences and received consulting fees from Medtronic and AstraZeneca; E.R.E. receives research support from Abiomed, Boston Scientific, Edwards Lifesciences, and Medtronic, and is a founder of BioDevek and Panther Therapeutics. The other authors report no potential conflicts of interest.
Data and materials availability: All data associated with this study are present in the paper or supplementary materials. Code was deposited in Harvard Dataverse, available at: https://doi.org/10.7910/DVN/H9FAKE.
References
- 1.Rivierii L, Opera Medica Universa. Observationum medicarum, centuria IV, observatio XXI, Cordis palpitatio pulsus inaequalitas. Lugduni: Sumptibus Antoni, Cellier, 83 (1663). [Google Scholar]
- 2.Ross J, Braunwald E, Aortic Stenosis, Circulation, V61–67 (1968). [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, PARTNER Trial Investigators, Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery, N. Engl. J. Med. 363, 1597–1607 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM, Thompson A, 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Circulation 135, e1159–e1195 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Arnold SV, Spertus JA, Vemulapalli S, Li Z, Matsouaka RA, Baron SJ, Vora AN, Mack MJ, Reynolds MR, Rumsfeld JS, Cohen DJ, Quality-of-Life Outcomes After Transcatheter Aortic Valve Replacement in an Unselected Population, JAMA Cardiol. 2, 409–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto CM, Valvular Aortic Stenosis. Disease Severity and Timing of Intervention J. Am. Coll. Cardiol. 47, 2141–2151 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Pibarot P, Dumesnil JG, Assessment of aortic stenosis severity: check the valve but don’t forget the arteries!, Heart 93, 780–782 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dweck MR, Boon NA, Newby DE, Calcific aortic stenosis: A disease of the valve and the myocardium, J. Am. Coll. Cardiol. 60, 1854–1863 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Pibarot P, Dumesnil JG, Improving assessment of aortic stenosis, J. Am. Coll. Cardiol. 60, 169–180 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Garcia D, A ventricular-vascular coupling model in presence of aortic stenosis, AJP Hear. Circ. Physiol. 288, H1874–H1884 (2004). [DOI] [PubMed] [Google Scholar]
- 11.deFilippi CR, Willett DL, Brickner ME, Appleton CP, Yancy CW, Eichhorn EJ, Grayburn PA, Usefulness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients., Am. J. Cardiol. 75, 191–4 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Lloyd JW, Nishimura RA, Borlaug BA, Eleid MF, Hemodynamic Response to Nitroprusside in Patients With Low-Gradient Severe Aortic Stenosis and Preserved Ejection Fraction, J. Am. Coll. Cardiol. (2017), doi: 10.1016/j.jacc.2017.07.736. [DOI] [PubMed] [Google Scholar]
- 13.Knaapen P, Germans T, Knuuti J, Paulus WJ, Dijkmans PA, Allaart CP, Lammertsma AA, Visser FC, Myocardial energetics and efficiency: Current status of the noninvasive approach Circulation 115, 918–927 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Walley KR, Left ventricular function: time-varying elastance and left ventricular aortic coupling, Crit. Care 20, 270 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ky B, French B, Khan A. May, Plappert T, Wang A, Chirinos JA, Fang JC, Sweitzer NK, Borlaug BA, Kass DA, St. John Sutton M, Cappola TP, Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure, J. Am. Coll. Cardiol. 62, 1165–1172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlman GY, Loncar S, Pollak A, Gilon D, Alcalai R, Planer D, Lotan C, Danenberg HD, Post-Procedural Hypertension Following Transcatheter Aortic Valve Implantation, JACC Cardiovasc. Interv. 6, 472–478 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Lindman BR, Otto CM, Douglas PS, Hahn RT, Elmariah S, Weissman NJ, Stewart WJ, Ayele GM, Zhang F, Zajarias A, Maniar HS, Jilaihawi H, Blackstone E, Chinnakondepalli KM, Tuzcu EM, Leon MB, Pibarot P, Blood Pressure and Arterial Load After Transcatheter Aortic Valve Replacement for Aortic Stenosis, Circ. Cardiovasc. Imaging 10, e006308 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Yotti R, Bermejo J, Gutiérrez-Ibañes E, del Villar C. Pérez, Mombiela T, Elízaga J, Benito Y, González-Mansilla A, Barrio A, Rodríguez-Pérez D, Martínez-Legazpi P, Fernández-Avilés F, Systemic Vascular Load in Calcific Degenerative Aortic Valve Stenosis, J. Am. Coll. Cardiol. 65, 423–433 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Hachicha Z, Dumesnil JG, Pibarot P, Usefulness of the Valvuloarterial Impedance to Predict Adverse Outcome in Asymptomatic Aortic Stenosis, J. Am. Coll. Cardiol. 54, 1003–1011 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P, Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment, J. Am. Coll. Cardiol. 46, 291–298 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Pibarot P, Dumesnil JG, Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction, J. Am. Coll. Cardiol. 60, 1845–1853 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Inada T, Nagao K, Murakami T, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino-Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Maeda C, Jinnai T, Morikami Y, Saito N, Minatoya K, Kimura T, High- Versus Low-Gradient Severe Aortic Stenosis, Circ. Cardiovasc. Interv. 10, e004796 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Rajani R, Chowienczyk P, Redwood SR, Guilcher A, Chambers JB, The noninvasive estimation of central aortic blood pressure in patients with aortic stenosis., J. Hypertens. 26, 2381–2388 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Shoji T, Nakagomi A, Okada S, Ohno Y, Kobayashi Y, Invasive validation of a novel brachial cuff-based oscillometric device (SphygmoCor XCEL) for measuring central blood pressure., J. Hypertens. 35, 69–75 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Ring M, Eriksson MJ, Zierath JR, Caidahl K, Arterial stiffness estimation in healthy subjects: a validation of oscillometric (Arteriograph) and tonometric (SphygmoCor) techniques, Hypertens. Res. 37, 999–1007 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Nichols W, O’Rourke M, Charalambos V, Eds., McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles (CRC Press, Sixth Edit., 2011). [Google Scholar]
- 27.Keshavarz-Motamed Z, Edelman ER, Motamed PK, Garcia J, Dahdah N, Kadem L, The role of aortic compliance in determination of coarctation severity: Lumped parameter modeling, in vitro study and clinical evaluation, J. Biomech. 48, 4229–4237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keshavarz-Motamed Z, Motamed PK, Maftoon N, Non-invasive determination of transcatheter pressure gradient in stenotic aortic valves: An analytical model, Med. Eng. Phys. 37, 321–327 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Keshavarz-Motamed Z, Nezami F. Rikhtegar, Partida RA, Nakamura K, Staziaki PV, Ben-Assa E, Ghoshhajra B, Bhatt AB, Edelman ER, Elimination of Transcoarctation Pressure Gradients Has No Impact on Left Ventricular Function or Aortic Shear Stress After Intervention in Patients With Mild Coarctation, JACC Cardiovasc. Interv. 9, 1953–1965 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong RC-C, Yeo TC, Stroke-work loss underestimates hemodynamic significance of aortic stenosis in patients with hypertension., Echocardiography 24, 673–6 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Properties of change in left ventricular stroke work (SWLV) from baseline to 30 days after TAVR
Fig. S2. Schematic diagram of the lumped parameter model