Abstract
Neural circuitry can be investigated and manipulated using a variety of techniques, including electrical and optical recording and stimulation. At present, most neural interfaces are designed to accommodate a single mode of neural recording and/or manipulation, which limits the amount of data that can be extracted from a single population of neurons. To overcome these technical limitations, we developed a chronic, multi-scale, multi-modal chamber-based neural implant for use in non-human primates that accommodates electrophysiological recording and stimulation, optical manipulation, and wide-field imaging. We present key design features of the system and mechanical validation. We also present sample data from two non-human primate subjects to validate the efficacy of the design in vivo.
I. Introduction
Recent technological advances enable us to monitor and manipulate neural circuits using an increasingly diverse range of physical modalities: electrical recording and stimulation, wide-field and multiphoton optical imaging and stimulation, magnetic resonance imaging, diffusion-weighted imaging, acoustic imaging and stimulation, and their combinations. Each technique offers complementary advantages for interrogating neural circuits at the micro-scale, large-scale, and brain-scale with different spatial and temporal resolutions. Since no single technique offers access across these scales, probing the same population of neurons using different techniques is necessary in order to understand the integrated function of brain networks.
Traditional non-human primate (NHP) neural implants, however, are designed to use a single experimental modality and therefore a single spatial scale. Systems designed for electrophysiology recordings with penetrating microelectrodes do not permit imaging neural activity optically [1]. Similarly, systems designed for optical imaging do not permit large-scale electrophysiological recording and stimulation [2]. In each case, gaining access to the same population of neurons with another modality requires changing the implanted neural interface due to restrictive implant geometry.
Here, we present a novel modular chronic neural interface for monitoring and manipulating neural activity using both electrical and optical modalities in the same volume of cortical tissue. We design a low-profile chronic chamber implant onto which hardware can be flexibly mounted and removed depending on experimental needs. We describe the experimental hardware designed for use with the chronic implant, explain validation methods used during the design process, and present preliminary in vivo recordings from two non-human primates.
II. System Design
A. Overview and System Development
The driving concept behind the system is modularity. The chronically-implanted portion of the chamber system, the base, is designed to be low-profile, allowing flexibility in experiment-specific hardware. The height of the base is 3 mm, and the inner diameter is 25 mm (Fig. 1a). The base is custom-shaped to match the curvature of an individual animal skull from a three-dimensional reconstruction of the skull from magnetic resonance imaging (MRI) data using BrainSight software (Rogue Research). The base is then surgically implanted onto the surface of the skull (Fig. 1b). The chamber base is manufactured out of biocompatible glass-filled polyetherimide (PEI) plastic (ProtoLabs), which is MRI-compatible. Glass-filled PEI has a much higher yield tensile strength (169 MPa) and flexural strength (228 MPa) than unfilled PEI (110 MPa and 165 MPa, respectively), making glass-filled PEI a better candidate for chronic implantation material [3,4].
Figure 1.
Example chamber system configuration. (A) Schematic of maximum brain access afforded by a single chamber with inner diameter of 25mm. Visible sulci are labeled: PCS-Paracingulate; CS-Central, IPS-Intraparietal; Arc-Arcuate. (B) Bilateral chamber implant configuration targeting primary motor cortex in a non-human primate using BrainSight targeting software (Rogue Research). Closed chamber built up with experimental hardware on the animal’s right, chronically-implanted chamber base on the animal’s left.
After the base is implanted, an experiment-specific assembly can be mounted onto the base either acutely or semi-chronically. Each component of the assembly can be mounted when the animal is awake and head-fixed without the need for another surgical procedure (Fig. 1b). A watertight seal is achieved at the interface between each assembly component using a 0.5 mm silicone rubber gasket (polysiloxane). The gasket thickness and durometer was tested to confirm watertight seals (see Methods IIIa). Gaskets range from 0.2A to 0.4A in durometer. Each assembly features a custom part, the extender, which adapts the base from an animal-specific curved surface to a flat surface. Different experiment modalities are supported by “inserts” that sit on top of the extender or by various “adapters” that can attach to the chamber stack at predefined locations.
To permit sub-dural electrical and optical access, the assembly is compatible for use with an “artificial dura” (AD). ADs have been used in NHP implants to provide optical [5] and electrical [6] access to the pial surface. We molded silicone (Momentive RTV615) using custom-shaped aluminum mold pieces and cured at 65°C.
This chamber design can, in principle, support a wide variety of techniques for measuring and manipulating neural activity. Below, we demonstrate this flexibility with three example configurations and the capabilities they enable.
B. Viral Injections and Optogenetic Stimulation
To optically manipulate cortical brain regions, we designed an interface between our implant system and a Flex MT system (Alpha Omega) that allows us to perform both viral injections and optical stimulation through the chamber (Fig. 2). The Flex MT sits on an adapter made of 3D-printed polylactic acid (PLA), which attaches to the chamber at the extender. Our adapter allows the Flex MT to be positioned in precise locations within the chamber while maintaining stability of the apparatus loaded into the device (i.e. injection hardware, fibers, electrodes, etc).
Figure 2.
Flex MT mount chamber configuration. (A) Schematic of Flex MT mount hardware. (B) Photograph of Flex MT loaded with fiber transmitting blue laser light.
C. Wide-field Imaging
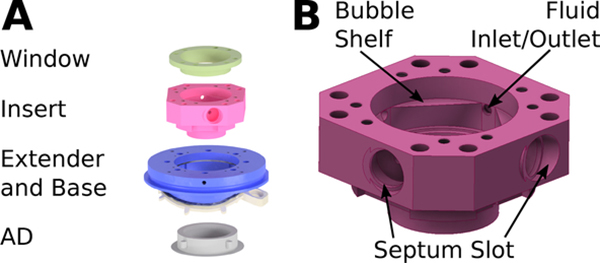
Cortical imaging can be a powerful tool for circuit mapping, physiological feature identification, and visual confirmation of viral expression. To image the cortex, an insert specifically designed for wide-field imaging is placed on top of the extender (Fig. 3a). The insert consists of a body (“imaging insert”) and a window cap (“imaging window”). The window consists of a ring upon which an 18 mm coverslip is bonded using silicone. A gasket seals the cap to the body, which in turn is sealed to the extender. The imaging insert is 3D printed to allow for specific feature geometry that is not manufacturable using standard machining methods. The insert is made of printed titanium (Ti-6A1–4V, ProtoLabs) because of its biocompatibility and very low fluorescence compared to plastics.
Figure 3.
Imaging chamber configuration. (A) Schematic of tall imaging hardware. (B) CAD model highlighting key design features of the insert.
To minimize artifacts due to cardiac and respiratory pulsations during wide-field imaging, the chamber is filled with incompressible fluid. Artificial cerebrospinal fluid (aCSF) is injected and air is removed from the chamber using two to four rubber septa (Resolution Systems) press-fit on the side of chamber insert (Fig. 3b). These allow the insertion and removal of hypodermic needles in the internal space of the chamber without breaking the watertight seal. When filling the chamber, two needles are used: one needle to inject aCSF with a syringe, and another needle to remove air.
The slots into which the septa are press-fit are a key design feature of the insert. The septa are 6 mm in diameter and 3 mm in width when uncompressed, and all four septa need to fit tightly enough in the slots to ensure that the insert seals the interior of the chamber for filling with liquid at nominal pressures. The maximum combined pressure of the brain and the liquid filling the chamber volume was specified to never exceed 1.764 kPa, twice the intracranial pressure of a macaque monkey [7]. The press-fit slot has a 5.5 mm diameter inlet that opens up to a cylindrical pocket (6.5 mm diameter, 2.9 mm height). The slot geometry allows four septa to compress sufficiently for water-tight pressurization of the chamber when closed with the imaging window and necessitates 3D printing of the part. The seal was confirmed by attaching the imaging insert over a flat test piece, closing the insert with a window, and deliberately over-pressurizing the system by filling the closed volume with water. The seal at the flat test piece failed before the seal at the glass coverslip or the septa failed.
When filling the chamber with fluid, air bubbles tend to gravitate toward the highest point in the chamber interior due to buoyant forces. To guide bubbles to a septum port, a square-shaped shelf in the chamber guides bubbles toward one of four possible septum ports (Fig. 3b). This works for any orientation of the chamber that is not vertical with respect to gravity.
D. Wireless Electrophysiology
Once physiological features of exposed cortex are identified, the underlying circuit architecture can be mapped using a variety of electrophysiology methods. The chamber system is capable of housing both a custom-designed subdural micro-electrocorticography (μECoG) array and a commercially-available 32-channel microdrive (Gray Matter Research) simultaneously (Fig. 4a) [6]. The μECoG array is molded into an AD [6] and placed on the pial surface. The array then connects to circuit boards mounted on the extender for data acquisition. The microdrive sits in the electrophysiology insert, which is mounted to the extender (Fig. 4b).
Figure 4.
Wireless electrophysiology chamber configuration. (A) Schematic of wireless electrophysiology hardware. (B) Electrophysiology insert with key features highlighted. (C) Antenna orientation planning in BrainSight targeting software. Head-fixation hardware is shown in green.
The electrophysiology insert contains ports that surround the microdrive seat and permit fluid delivery and evacuation for chronic system maintenance (Fig. 4b). The ports are closed with self-sealing screws (Zago) to maintain fluid-tight seals. Additionally, the electrophysiology insert is designed to allow the microdrive to be placed at a variable height. Spacers between the microdrive and the insert provide up to 5 mm of height adjustment.
To allow for untethered wireless recording of neural data, the protective cap of the chamber system accommodates an interface between the recording devices and a 96-channel wireless recording system (Blackrock Microsystems) (Fig. 4a). The transmitter of the wireless system attaches to the protective cap via an adapter that connects to a cylindrical protrusion that is custom-oriented on the chamber. Custom orientation assures compatibility with other cranial instrumentation (Fig. 4c). The adapter between the cap and the antenna provides a watertight seal on the cap side via a rubber o-ring to protect the cables connecting the recording devices with the antenna. The adapter is held in place with four set screws attached to the cap and four set screws attached to the antenna.
III. Validation Methods
A. Pre-Implantation Bench Tests
Before surgical implantation of the chamber base, bench tests were performed on all chamber hardware to confirm sealing capability. The chamber configuration was fully assembled, closed with an appropriate seal-providing cap, and filled with a measured amount of colored liquid. Depending on the configuration of the chamber, the volume of liquid that can be filled inside the chamber ranges from approximately 3.7 mL to 7.6 mL. Suction was then applied around the outside of the chamber at all gasket joints. The chamber was then left to sit for at least 5 minutes before the liquid was drawn up into a syringe and measured. For a chamber system to pass a seal test, no liquid can escape from any gasket joints when suction is applied, and all liquid placed inside the chamber must be redrawn within a 0.1 mL margin of error.
B. Surgical Implantation
The low-profile chamber base was implanted and used for data collection in two non-human primates (Monkey J and Monkey G, macaca mulatta). The subjects were anesthetized during surgical implantation. The base was fixed to the skull with dental cement (MetaBond, Parknell Inc. and Simplex P, Stryker) and ceramic bone screws (Rogue Research). After the chamber base was affixed to the skull, chamber hardware was stacked on top of the base to a height that was tall enough to allow for seal testing of the chamber in vivo. The chamber was filled with a measured amount of aCSF before suction was applied around the outside of the chamber at the level of every gasket joint to confirm gasket seals. Additional suction was applied at the level of the skull-chamber base interface to confirm the seal provided by the dental cement. After suction was applied, the chamber was left to sit for approximately 15 minutes before measuring the remaining fluid to confirm no leaks were present. Passing seal test qualifications were the same as pre-implantation metrics.
After confirmation of chamber seals, a craniotomy and durotomy were performed to provide brain access, and an AD was implanted within the durotomy. All data collection occurred while the subjects were awake. All animal procedures were approved by New York University’s University Animal Welfare Committee (UAWC).
C. Viral Injection and Optical Stimulation Methods
Monkey J was injected with GFP-tagged channelrhodopsin (AAV5-CaMKII-ChR2-H134R) in motor and premotor cortical areas after chamber implantation. The injections were performed using the Flex MT-compatible chamber configuration while Monkey J was awake and head-fixed in a restraint chair. The Flex MT system then allowed us to confirm viral expression by recording optically-driven neural responses with an acute penetrating electrode.
D. Cortical Image Methods
To verify that the brain could be safely pressure-controlled to eliminate motion artifacts due to physiological brain pulsations, wide-field images were acquired through the imaging-specific chamber configuration of both Monkey J and Monkey G’s implants while the subjects were awake and head-fixed. Acquisition of fluorescent images allows for visual confirmation of ChR2 expression after injections are performed, while acquisition of vascular images of the cortical surface allows for construction of a high-resolution vascular map to serve as a reference for other measurements such as microelectrode insertion.
E. Wireless Recording Methods
Monkey J was implanted with a semi-chronic μECoG array and a semi-chronic Gray Matter Research microdrive after chamber implantation. The two recording devices were then connected to the 96-channel wireless transmitter and the broadband neural signals sampled at 30 kHz were continuously acquired in-cage from the untethered system (Cereplex Direct, Blackrock Microsystems).
IV. Results
A. Optical Stimulation Results
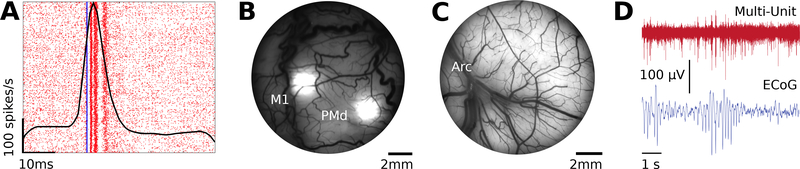
Neural data from Monkey J were successfully collected during acute optogenetic stimulation at ChR2 injection sites (Fig. 5a). Local driven multi-unit responses were observed in the expected cortical areas.
Figure 5.
Sample data collected through each chamber configuration. (A) Spike raster plot with overlaid peristimulus time histogram showing driven multi-unit activity in response to optical stimulation in the post-central gyrus. Blue line indicates time of stimulation. (B) Fluorescent image of viral expression in motor and premotor cortical regions acquired from Monkey J with imaging insert in place. (C) Vascular image of motor cortices rostral to the arcuate cortex acquired from Monkey G with imaging insert in place. (D) Neural traces collected from the wireless electrophysiology configuration.
B. Imaging Results
Fluorescent images from Monkey J were successfully acquired several weeks after ChR2 injections (Fig. 5b). The images provide visual confirmation of viral expression at cortical injection sites. Vascular images from Monkey G were also successfully acquired (Fig. 5c).
C. Wireless Electrophysiology Results
Neural data from Monkey J were successfully collected while the implant was untethered and the animal was unrestrained (Fig. 5d). Here, we provide sample neural traces of simultaneously recorded multi-unit activity recorded with penetrating electrodes and μECoG data.
V. Discussion & Conclusion
We present the design and validation of a chronic modular neural implant system compatible with wireless electrophysiological recording, optical stimulation, and wide-field imaging in non-human primates. We describe how the system was developed and validated, present key design features of each chamber configuration, and provide sample data to demonstrate system viability.
This work builds upon recent work [6] and enables imaging and optical manipulation of the same population of neurons. The design allows changes in chamber hardware needed for different experiments without surgical intervention. At this time, Monkey J has been implanted for 18 months, and Monkey G has been implanted for 4 months. The implants have facilitated optical and electrical measurements and manipulations in both animals.
The chamber system is a step towards enabling large-scale neural recording and manipulation. As depicted in Fig. 1b, two chamber systems fit comfortably on the NHP skull. This allows for simultaneous access to two different cortical regions. Future extensions include two-photon imaging compatibility and large-scale chambers. These will enable large-scale multimodal interrogation of neural circuitry to resolve the mechanisms by which brain networks support complex flexible behaviors.
Acknowledgments
Research supported by the National Institute of Health (U01-NS099967, U01-NS103518) and by the Defense Advanced Research Project Agency contracts W911NF-14-2-0043, N66001-17-C-4002, HR0011-14-C-0102.
Contributor Information
Jessica E. Kleinbart, Center for Neural Science, New York University, New York, NY, 10003, USA..
Amy L. Orsborn, Center for Neural Science, New York University, New York, NY, 10003, USA.
John S. Choi, Center for Neural Science, New York University, New York, NY, 10003, USA.
Charles Wang, Department of Biomedical Engineering, Duke University, Durham, NC 27708, USA..
Shaoyu Qiao, Center for Neural Science, New York University, New York, NY, 10003, USA..
Jonathan Viventi, Department of Biomedical Engineering, Duke University, Durham, NC 27708, USA..
Bijan Pesaran, Center for Neural Science, New York University, New York, NY, 10003, USA..
VI. References
- [1].Dotson NM, Hoffman SJ, Goodell B, Gray CM. A Large-Scale Semi-Chronic Microdrive Recording System for Non-Human Primates. Neuron. 2017. November 15;96(4):769–782.e2. [DOI] [PubMed] [Google Scholar]
- [2].Seidemann E, Chen Y, Bai Y, Chen SC, Mehta P, Kajs BL, Geisler WS, Zemelman BV. Calcium imaging with genetically encoded indicators in behaving primates. Elife. 2016. July 21;5. pii: e16178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].SABIC Innovative Plastics, “ULTEM 2300 Resin,” TDS-4199 datasheet, November 1995. [Revised May 2013]. [Google Scholar]
- [4].SABIC Innovative Plastics, “ULTEM 1000 Resin,” TDS-4195 datasheet, November 1995. [Revised May 2013]. [Google Scholar]
- [5].Arieli A, Grinvald A, and Slovin H, “Dural stubstitute for long-term imaging of cortical activity in behaving monkeys and its clinical applications,” J Neurosci Methods vol. 114(2), pp. 119–133, 2002. [DOI] [PubMed] [Google Scholar]
- [6].Orsborn AL, Wang C, Chiang K, Maharbiz MM, Viventi J, and Pesaran B, “Semi-chronic chamber system for simultaneous subdural electrocorticography, local field potentials, and spike recordings,” 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), 2015, pp. 398–401. [Google Scholar]
- [7].Gücer G and Viernstein LJ, “Intracranial pressure in the normal monkey while awake and asleep,” J Neurosurgery, vol. 51, no. 2, pp. 206–210, 1979. [DOI] [PubMed] [Google Scholar]