Abstract
Purpose
The purpose of this study was to characterize foveal pit morphology in an African (Ghanaian) population, to compare it to that of a Caucasian group and to determine if it varied with age in the two populations.
Methods
The depth, diameter, slope, and volume of the foveal pit were interpolated from optical coherence tomography volume scans recorded in 84 Ghanaian and 37 Caucasian individuals. Their association with age, sex, and ethnicity was investigated using multilevel regression models.
Results
The foveal pit differed significantly in width, slope, and volume between Ghanaian men and women (P < 0.001), but only in width and volume between Caucasian men and women (P < 0.01). In Ghanaians, age was associated with a narrowing of the foveal depression and a reduction of its volume. Overall, these changes were more pronounced in women as compared to men and were largely absent from the Caucasian group. When controlled for age, the foveal pit of Ghanaians was significantly wider and larger in volume as compared to the Caucasian group (P < 0.001).
Conclusions
The morphology of the foveal pit differs between African and Caucasian individuals. These anatomic differences should be considered when examining differences in prevalence and clinical features of vitreoretinal disorders involving the fovea between the two populations.
Translational Relevance
Differences in retinal anatomy may partly explain variations in the prevalence and clinical features of retinal diseases between Africans and Caucasians. Such differences should be adequately considered in diagnoses and monitoring of ocular diseases in patients with African ancestry.
Keywords: retina, fovea, macula, optical coherence tomography, anatomy
Introduction
The prevalence of many ocular disorders, including age-related macular degeneration (AMD),1–3 glaucoma,3,4 retinopathy of prematurity,5 vitreomacular adhesion,6 and hypertensive retinopathy,7 varies dramatically between populations with Caucasian and African ancestries. Clinical features associated with some of these diseases also tend to differ between these two populations. In AMD for instance, drusen are generally distributed pericentrally to the macula in African Americans, whereas they tend to develop within the central 1500 µm macular zone in Caucasian Americans.2,8 Progressions from medium to large-sized drusen1,2,8 and to late-stage disease2,9 are also more frequent in Caucasians. In diabetes, it was reported that individuals with African-Caribbean ancestry had wider retinal arterioles as compared to Caucasians, which might explain the comparatively more severe microvascular damages observed in this population.10 Evidence suggests that optic disc structures, such as disc area, vary between African Americans and Caucasians, which may affect the diagnosing and monitoring of glaucoma in individuals with African ancestry.11 The origin of these differences is unknown, in part because of the lack of understanding of the variations in ocular anatomy between Africans and Caucasians. In addition, these disparities indicate that ethnic specificities should be considered to adequately diagnose and monitor ocular diseases in populations with African ancestry. They are, however, largely overlooked, and the use of normative databases that do not control for ethnicity is widespread, including in optical coherence tomography (OCT) software and devices.12,13 Understanding baseline differences in ocular structures between Africans and Caucasians may also identify predictive markers of disease initiation and progression in the populations where these diseases have a higher prevalence.
Few investigations have sought to characterize specific aspects of retinal anatomy in populations with African ancestry. Some have shown that the macular retina was thinner in African Americans as compared to Caucasian Americans.14,15 This was associated with differences in the morphology of the foveal pit, which was found to be wider15,16 and deeper16 in African Americans. Differences in subfield macular thickness between African American men and women have also been reported16; although the study was limited to young individuals (mean age 25.6 ± 9.9 years old). The main limitation of these studies is that they did not control for age, and that they may not be generalizable to Africans because of differences in risk factors with African Americans.17 The association between age and foveal pit morphology has previously been explored in predominantly Caucasian groups typically consisting of <60 individuals.18,19 Previous reports suggested that foveal pit morphology was independent of sex; however, statistical analyses were carried out without controlling for age.16,19
The fovea is a site of pathology in many vitreoretinal disorders that include AMD, diabetic macular edema, and macular hole; however, differences in its structure between Africans and Caucasians remain largely unexplored. In this paper, we sought to characterize the previously unreported morphology of the foveal pit in an African population from Ghana and to determine if it differed from that of Caucasians. We also aimed to ascertain if the morphology of the foveal pit changed with age in Africans and Caucasians. The morphology of the foveal depression was previously investigated by extracting foveal pit depth,15,18,20 diameter,15,18 volume,19,20 and maximal slope19 from OCT images using automated or semi-automated segmentations15,18,20 or mathematical regression.19 In this study, we used a published and validated method16,21–23 to generate estimates of the foveal pit depth, diameter, maximal slope, and volume in 84 Ghanaians and 37 Caucasians. Associations among foveal pit parameters and age, sex, and ethnicity were then examined using multilevel linear regression models.
Methods
Subjects
Ghanaian subjects were recruited as part of a prospective study carried out at two teaching hospitals in Ghana, the Korle-Bu Hospital (KBTH) in Accra and the Komfo Anokye Teaching Hospital (KATH) in Kumasi. The main aim of the study was to investigate the phenotypic features and genetic etiology of AMD in the previously unreported Ghanaian population. A small subset of participants (260 individuals) underwent an OCT scan in both eyes. Caucasian individuals were recruited from local communities surrounding the Medical College of Wisconsin (Milwaukee, WI). The research adhered to the Tenets of the Declaration of Helsinki and the protocol was approved by the Ethics Review Boards of KBTH (University of Ghana Medical School Protocol ID No: MS-Et/M.4-P./2008/2009) and KATH (KNUST Reference: CHRPE/32/10) for Ghanaian participants and by the Institutional Review Board at the Medical College of Wisconsin for Caucasian individuals.
OCT Imaging and Grading
The same imaging protocol was applied to Ghanaian and Caucasian participants. All patients’ eyes were dilated prior to imaging using tropicamide 1% and phenylephrine 2.5%. For each patient, a volumetric scan of the retina was obtained in at least one eye using a Zeiss Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA). Volumes acquired in all participants were nominally 6 × 6 mm and consisted of at least 128 B-scans (512 A-scans/B-scans). The quality of all scans was seven or higher. Images were graded for any vitreoretinal disorders. Eyes with a history of vision-limiting ocular diseases or presenting any ocular pathology or clinical marker of any vitreoretinal disorder, including AMD, were rejected. In addition, eyes with myopia of more than -4.00D were excluded from the analysis.
Image Segmentation
OCT scans were manually inspected to ensure that the inner limiting membrane and retinal pigment epithelium layers were accurately segmented. No scans were rejected, and parameters describing the morphology of the foveal depression were extracted using a published and validated method.16,21 Briefly, foveal profiles extracted from OCT volumes were fitted to a difference of Gaussians and the depth, diameter, slope, and volume of the foveal pit were then estimated from the interpolated function. A typical B-scan centered on the foveal depression is shown in Figure 1. The center of the foveal pit was identified as the central point with a slope equal to zero. Points A and C correspond to the rims of the foveal pit, where the slope of the pit is also zero. In three-dimensions, the points A and C are connected through a line. Point B corresponds to the point where the slope of the foveal contour is maximal. The diameter of the foveal pit was defined as the distance from rim to rim (distance AC). The slope of the foveal pit corresponded to the maximum slope between the foveal center and the rim of the foveal depression (point B). The depth of the foveal pit consisted of the difference between the average retinal thickness at the rim of the foveal depression and the thickness at the center of the pit. The volume of the foveal pit was defined as the space between the internal limiting membrane and the top of the foveal pit.
Figure 1.
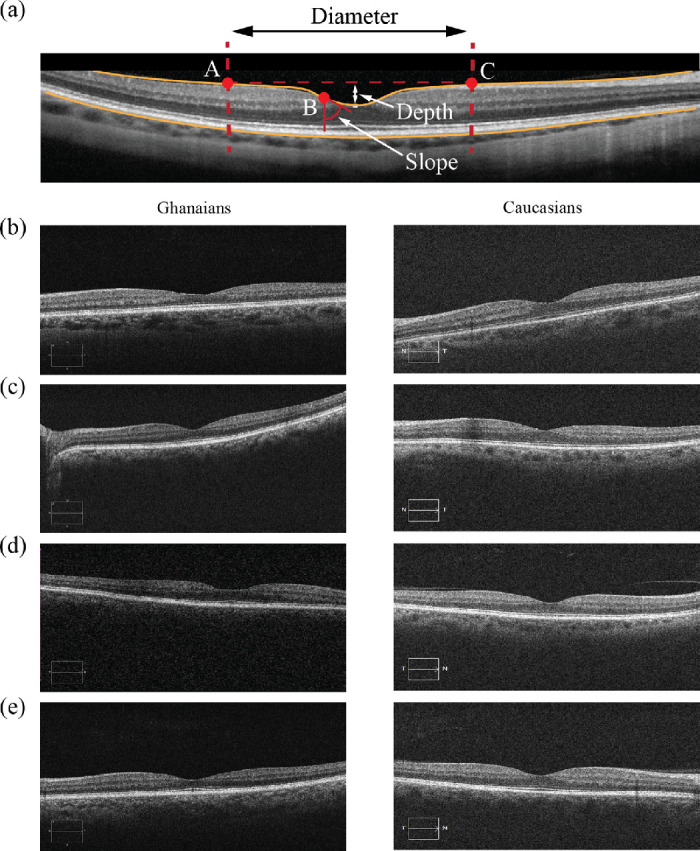
(a) Schematic of the foveal pit diameter, depth and slope interpolated from an SD-OCT B-scan centered on the fovea recorded in a Caucasian individual. Points A and C represent the rim of the foveal pit. Point B corresponds to the point where the slope of the pit is maximal. (b) B-scans taken from Ghanaian (left column) and Caucasian (right column) individuals with foveal pit (b) depth, (c) diameter, (d) maximal slope, and (e) volume that were closest to the population means.
Statistical Analysis
The statistical analysis was carried out using R.24 Bland-Altman plots and Pearson's correlation coefficient (here denoted r) and its confidence interval (CI) were used to identify associations between foveal pit parameters measured in the left and right eyes. Observed dependencies were accounted for by excluding from the analysis one randomly selected eye in patients with measurements in both eyes. Pearson's correlation coefficient was also used to examine associations between foveal pit parameters. Linear regression models were used to assess the association among foveal pit parameters, age, sex, and ethnicity. When predictors had large effects their interactions with other predictors were included in the models. Post hoc power analyses were performed for each predictor in each model using simulations. The simulations included the number of Ghanaian and Caucasian men and women present in the study as inputs. A minimum power of 80% was required for statistically significant associations. The relative importance of age, sex, and ethnicity were assessed using analysis of variance (ANOVA) after confirming normality and homoscedasticity. We assessed the effect of the lack of correction for axial length on transverse measurements and their associations with age, sex, and ethnicity by performing Monte-Carlo simulations. Briefly, a random error term was added to measurements for a large number of simulations, and for each of them a P value for associations with age, sex, or ethnicity (here denoted Psim) was computed. The association was then considered to be robust if the proportion of significant Psim was larger than 99%. Convergence studies were carried out to determine the adequate number of Monte-Carlo simulations to run. The level of significance for all tests was set at α = 0.05.
Results
Subject Demographics
A total of 124 eyes of 84 Ghanaians and 74 eyes of 37 Caucasians met the inclusion criteria. Foveal pit parameters were extracted in both OS and OD for 77 (37%) of Ghanaians and Caucasians. The age of participants ranged from 41 to 85 years old (mean 64.1 ± 10.2 years old). There were no significant differences between the mean age (P = 0.15; t = 1.47) or the proportion of men and women (P = 0.3; χ2 = 1.05) between the Caucasian and Ghanaian groups (see Table 1). Among all participants, measurements taken from the left and right eye were dependent and correlated strongly (depth: r = 0.94; CI = 0.90–0.96; diameter: r = 0.93; CI = 0.90–0.96; slope: r = 0.90; CI = 0.85–0.94; volume: r = 0.95; CI = 0.93–0.97; P < 0.001 for all coefficients).
Table 1.
Baseline Demographics of Caucasians and Ghanaians Included in this Study
| Demographic | Caucasians (n = 37) | Ghanaians (n = 84) | P Value for Differences |
|---|---|---|---|
| Age | |||
| Mean (SD) | 61.9 (11.5) | 65.1 (9.4) | 0.151a |
| Range | 41-85 | 45-82 | |
| Sex | |||
| Females | 28 | 54 | 0.3b |
| Males | 9 | 30 | |
| Eyes (included in analysis) | |||
| OD | 37 (19) | 63 (41) | - |
| OS | 37 (18) | 61 (43) |
Welsh's t-test, t = 1.47, CI = -1.14 to 7.47.
X2 = 1.05.
Morphology of the Foveal Pit in Ghanaians
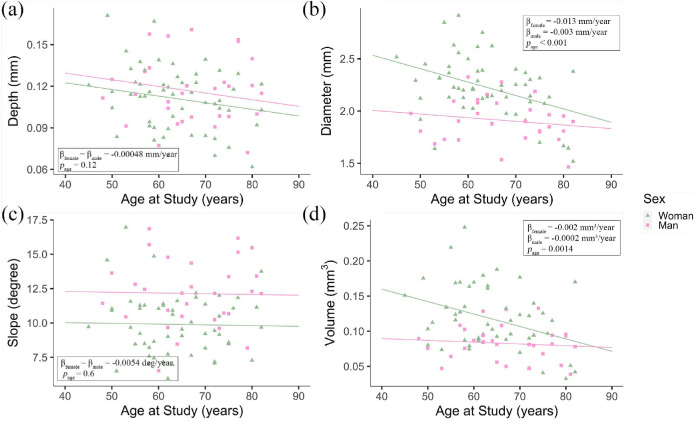
Measurements made in the left and right eye of the same Ghanaian subject were dependent and correlated strongly (0.92 ≤ r ≤ 0.95; CI = 0.85–0.97; P < 0.001). In Ghanaians, foveal pit depth and volume correlated strongly with slope (r = 0.78; CI = 0.68–0.85; P < 0.001) and diameter (r = 0.88; CI = 0.82–0.92; P < 0.001), respectively. Age was significantly associated with foveal pit diameter (P = 1.1 × 10−4) and volume (P = 1.4 × 10−3) but it was not associated with either depth (P = 0.12) or slope (P = 0.6); see Table 2. We sought to further examine the association between pit diameter and volume and age by generating a model including interactions between age and sex. We found that pit diameter decreased with age in both men and women; although this decrease was qualitatively more pronounced in women (β = −0.013 mm/year) as compared to men (β = −0.003 mm/year); see Figure 2b. Pit volume also decreased faster with age in women (β = −0.002 mm3/year) as compared to men (β = −0.002 mm3/year); see Figure 2d. These differences were, however, not statistically different (P > 0.1 for both diameter and volume).
Table 2.
Coefficients of the Multilevel Linear Regressions and Results of the ANOVAs Assessing the Association Among Foveal Pit Depth, Diameter, Slope, Volume, Age, and Sex Within and Between Ethnic Groups
| Ghanaians | Caucasians | Ghanaians vs. Caucasians | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Sex | Sex | Ethnicity | |||||||
| Intercept | (Male) | Age | Intercept | (Male) | Age | Intercept | (Male) | Age | (Ghanaian) | |
| Depth | ||||||||||
| Coefficient | 0.14 | 7.0 × 10−3 | −4.8 × 10−4 | 0.12 | −0.01 | −1.5 × 10−4 | 0.13 | 0.002 | −3.7 × 10−4 | 5.9 × 10−3 |
| Standard error | 1.8 × 10−2 | 5.3 × 10−3 | 2.7 × 10−4 | 0.02 | 0.01 | 4 × 10−4 | 0.014 | 4.7 × 10−3 | 2.2 × 10−4 | 4.7 × 10−3 |
| Regression P value | <0.001 | 0.19 | 0.08 | <0.001 | 0.26 | 0.69 | <0.001 | 0.6 | 0.09 | 0.21 |
| R2 (adjusted) | 0.03 | −0.005 | 0.009 | |||||||
| ANOVA P value | - | 0.19 | 0.12 | - | 0.26 | 0.5 | - | 0.62 | 0.15 | 0.19 |
| Diameter | ||||||||||
| Coefficient | 2.81 | −0.29 | −9.2 × 10−3 | 1.75 | −0.36 | 0.003 | 2.2 | −0.31 | −4.4 × 10−3 | 0.29 |
| Standard error | 0.19 | 0.057 | 0.0029 | 0.18 | 0.08 | 0.003 | 0.14 | 0.048 | 2.2 × 10−3 | 0.05 |
| P value | <0.001 | 3.78 × 10−6 | 0.002 | <0.001 | 2.89 × 10−5 | 0.25 | <0.001 | 3.28 × 10−9 | 0.049 | 2.1 × 10−8 |
| R2 (adjusted) | 0.32 | 0.37 | 0.38 | |||||||
| ANOVA P value | - | 3.78 × 10−6 | 1.1 × 10−4 | - | 2.89 × 10−5 | 0.9 | - | 3.3 × 10−9 | 0.015 | 2.42 × 10−7 |
| Slope | ||||||||||
| Coefficient | 10.24 | 2.26 | −0.0054 | 13.37 | 1.05 | −0.03 | 12.2 | 1.95 | −0.007 | −1.05 |
| Standard error | 1.80 | 0.54 | 0.028 | 2.49 | 1.06 | 0.04 | 1.44 | 0.49 | 0.023 | 0.49 |
| P value | <0.001 | 7.4 × 10−5 | 0.85 | <0.001 | 0.33 | 0.42 | <0.001 | 0.036 | 0.44 | 0.036 |
| R2 (adjusted) | 0.16 | −0.019 | 0.12 | |||||||
| ANOVA P value | - | 7.4 × 10−5 | 0.6 | - | 0.33 | 0.55 | - | 1.16 × 10−4 | 0.77 | 0.076 |
| Volume | ||||||||||
| Coefficient | 0.19 | −0.036 | −0.0012 | 0.08 | −0.04 | 1.4 × 10−4 | 0.13 | −0.033 | −6.8 × 10−4 | 0.03 |
| Standard error | 0.03 | 0.009 | 4.5 × 10−4 | 0.025 | 0.01 | 1.4 × 10−4 | 0.02 | 0.007 | 3.3 × 10−4 | 0.007 |
| P value | <0.001 | 7.0 × 10−4 | 0.0098 | 0.003 | 0.001 | 0.73 | <0.001 | 1.03 × 10−5 | 0.0412 | 2.23 × 10−5 |
| R2 (adjusted) | 0.20 | 0.22 | 0.25 | |||||||
| ANOVA P value | - | 7.0 × 10−4 | 1.4 × 10−3 | - | 0.001 | 0.65 | - | 1.03 × 10−5 | 0.017 | 9.7 × 10−5 |
Figure 2.
Scatter plots showing the variation of (a) foveal pit depth, (b) foveal pit diameter, (c) foveal pit slope, and (d) foveal pit volume with age and the P value for their association with age in 30 Ghanaian men (■) and 54 Ghanaian women (▲). The plain lines correspond to regression lines for Ghanaian men and women, which were calculated separately for men and women in (b) and (d).
When controlling for age, the diameter (P = 3.78 × 10−6), slope (P = 7.4 × 10−5) and volume (P = 7.0 × 10−4) of the foveal pit varied significantly between men and women; however, pit depth did not (P = 0.19). The diameter of the foveal pit was, on average, 0.29 mm smaller in men as compared to women. The slope of the pit was 2.26 degrees smaller in Ghanaian females, on average, and its volume was, on average, 0.036 mm3 smaller in men as compared to women.
Morphology of the Foveal Pit in Caucasians
As in Ghanaians, measurements made in the left and right eye of the same Caucasian subject were dependent and correlated strongly (0.87 ≤ r ≤ 0.95; CI = 0.77–0.98; P < 0.001). Foveal pit depth and volume correlated strongly with slope (r = 0.89; CI = 0.78–0.94) and diameter (r = 0.81; CI = 0.64–0.9), respectively. Age did not associate significantly with either foveal pit depth (P = 0.49), diameter (P = 0.9), slope (P = 0.55), or volume (P = 0.65). Foveal pit diameter and volume were, on average, 0.36 mm (P = 2.89 × 10−5) and 0.04 mm3 (P = 0.001) smaller in men as compared to women, respectively. No significant associations were found between sex and pit depth (P = 0.26) or slope (P = 0.33) when controlling for age.
Differences Between Ghanaians and Caucasians
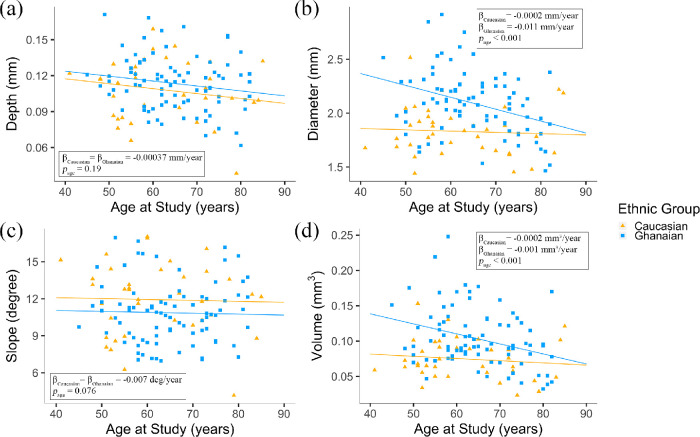
Differences in foveal pit morphology between Ghanaians and Caucasians were investigated using linear regression models that included age, sex, and ethnicity as predictors. Age was significantly associated with foveal pit diameter (P = 0.017) and volume (P = 0.017), but not with depth (P = 0.015) or slope (P = 0.077). Based on the analyses carried out separately for Ghanaians and Caucasians, the association between age and pit diameter or volume were largely driven by the Ghanaian group. Linear regression models, including age, ethnicity, and their interactions as predictors, revealed that the diameter of the foveal pit decreased with age in Ghanaians but not in Caucasians (β = –0.011 mm/year vs. β = –0.002 mm/year); see Figure 3b. We found this difference to be statistically significant (P = 0.02). The volume of the foveal pit decreased with age in both Ghanaians and Caucasians. This change was qualitatively more prominent in Ghanaians as compared to Caucasians (β = –0.001 mm3/year vs. β = –0.0002 mm3/year, see Figure 3d); however, this difference did not reach statistical significance (P = 0.052).
Figure 3.
Scatter plots showing the variation of (a) foveal pit depth, (b) foveal pit diameter, (c) foveal pit slope, and (d) foveal pit volume with age and the P value for their association with age in 37 Caucasians (▲) and 84 Ghanaians (■). The plain lines correspond to the regression lines for Caucasians and Ghanaians, which were generated by combining men and women in each ethnic group. They were calculated separately for Caucasians and Ghanaians in (b) and (d).
When controlling for age, sex was significantly associated with pit diameter, slope, and volume. The diameter and volume of the foveal pit were, on average, smaller in men as compared to women, whereas the slope of the foveal pit was, on average, smaller in women (P < 0.0001 for all three parameters). Because we found no association between sex and foveal pit slope in Caucasians, this difference was largely driven by Ghanaians. Foveal pit diameter and volume were, on average, 0.29 mm (P = 2.42 × 10−7) and 0.03 mm3 (P = 9.7 × 10−5) larger in Ghanaians, respectively. The slope of the pit was, on average, 1.05 degrees smaller in Ghanaians; however, this difference did not reach statistical significance (P = 0.076).
Discussion
As previously reported in Caucasians and African Americans,16,18,21 we found that the structure of the foveal pit is highly symmetrical between eyes of the same individual in Ghanaians. When controlling for age, the foveal pit is wider in Ghanaians as compared to Caucasians; this difference was also noted between Caucasian and black Americans.15,16 We found no significant differences in foveal pit depth between Caucasians and Ghanaians. In fact, age, sex, and ethnicity explained very little of the variability in pit depth among either Ghanaians or Caucasians. In comparison, the foveal pit was reported to be deeper in African Americans as compared to Caucasian Americans.16 We found that the slope of the foveal pit was less steep in the Ghanaian group as compared to the Caucasian group, which indicates that Ghanaians have generally flatter foveal depressions. However, in line with previous reports,16 this difference was not statistically significant. Finally, we found that the volume of the foveal pit is larger in Ghanaians as compared to Caucasians. To the best of our knowledge, no other study has considered variations in foveal pit volume between populations with Caucasian and African ancestry. Discrepancies between our results and investigations comparing Caucasians and blacks in the United States may be due to the small number of African Americans that these studies generally included (<30 individuals)15,16 or the lack of control for age. They could also be the result of significant mixing between populations with diverse ancestries in North America over the past 500 years.25
The post hoc power analyses that we performed allowed us to confirm that the associations that we report were not caused by the unequal number of Ghanaian and Caucasian men and women included in our study. When controlling for age, we found the foveal depression to be significantly narrower in men as compared to women in both Ghanaians and Caucasians. The foveal pit is significantly flatter (i.e. less steep) in Ghanaian women as compared to Ghanaian men; in comparison, pit slope did not differ significantly between Caucasian men and women. A previous study suggested that the characteristics of the foveal pit morphology were independent of sex16; however, statistical analyses were carried out without controlling for age.
Few studies have investigated age-related changes in the structure of the foveal pit. A study carried out on a mixed population composed of 57 individuals found that age did not correlate with foveal shape or structure.18 Previous work using a predominantly Caucasian group of individuals and mathematical models showed that the fovea was less symmetric and had steeper slopes in older subjects.19 We found that age was significantly associated with foveal morphology in Ghanaians, but not in Caucasians. In Ghanaians, increasing age was associated with a narrowing of the foveal depression, a steepening of its slope and a reduction of its volume. We found that pit depth was largely independent of age. The decrease in the volume of the foveal depression with age is likely driven by the narrowing of the pit, as suggested by the strong correlation between these two parameters. In our cohort, these age-related changes are more prominent in Ghanaian women as compared to men, which challenges previous reports that the characteristics of the foveal pit morphology are independent of sex.16,19 We found that age-related changes in foveal pit morphology were less pronounced in Caucasians as compared to Ghanaians, which has not been reported before. Because we did not find any significant interaction between age and sex in Ghanaians, our data indicate that the differences in the age effect that we report between Ghanaians and Caucasians are unlikely to be contributed solely by women of the Ghanaian group. A larger sample size would be necessary to further investigate interactions between age and sex among Ghanaians and Caucasians.
The fovea is a region of the retina specialized for diurnal high acuity vision. It has been postulated that one function of the foveal depression was to modulate the refractivity index of the retina at the fovea and that the foveal pit allowed for a tighter packing of cones.26 Under these assumptions, it seems plausible that in Ghanaians the shape of the foveal pit should be adjusted to increased melanin levels, which may attenuate the light signal.27 This is further supported by the observation that wider foveae, which we found to be characteristic of Ghanaians as compared to Caucasians, are associated with a secondary peak of the macular pigment spatial profile.28 The mechanisms involved in the changes in foveal pit morphology with age are unknown, largely because very few studies have investigated them. The formation of the foveal pit is driven by biomechanical processes29 and by cellular events30 that include an increase in the packing density of foveal cones.31 Decreases in vitreoretinal adhesion32 and foveal cone density33 with age might, therefore, drive the reduction in foveal pit volume that we report in Ghanaians. Under this assumption, our study would suggest that the pattern of change in pit morphology associated with cone loss and decrease in vitreoretinal adhesion differs between African and Caucasian populations. A previous study carried out in a dominantly Caucasian population found that pit volume did not correlate with peak cone density23; however, the study did not control for age. More work is needed to elucidate the functional significance of the volume of the foveal pit and the shape of the foveal depression as a whole, and our study is an important step in that direction.
In line with previous studies,15,18–20 transverse measurements were not corrected for axial length. Axial length was shown to minimally affect foveal pit depth and slope measurements; however, it may introduce an error in the diameter of the foveal pit that can reach 12% to 13%.18 We sought to assess whether this lack of correction affected the significance of the associations that we report. To do so, we performed Monte-Carlo simulations that introduced a random variation of the foveal pit diameter in Ghanaians and Caucasians by up to 13%. We found that the associations between foveal pit diameter and sex in Ghanaians and Caucasians and between pit diameter and ethnicity remained significant for more than 99.5% of the simulations (see Table 3). Possible reductions in axial length with age34 may introduce an additional error that was not accounted for in our models. However, a previous cross-sectional study showed that axial length only decreased by 2% on average between the ages of 40 and 80,34 which indicates that the effect of its variation on our measurements was marginal.
Table 3.
Results of the Monte-Carlo Simulations Carried Out to Assess the Effect of the Lack of Correction for Axial Length on Associations Between Foveal Pit Diameter and Age, Sex, or Ethnicity
| Ghanaians | Caucasians | Ghanaians vs. Caucasians | |||||
|---|---|---|---|---|---|---|---|
| Sex | Sex | Sex | Ethnicity | ||||
| (Male) | Age | (Male) | Age | (Male) | Age | (Ghanaian) | |
| P value (no error term) | 3.78 × 10−6 | 1.1 × 10−4 | 2.89 × 10−5 | 0.9 | 3.3 × 10−9 | 0.015 | 2.42 × 10−7 |
| Min psim | 8.2 × 10−9 | 3.06 × 10−7 | 4.1 × 10−8 | 0.02 | 2.0 × 10−11 | 1.79 × 10−5 | 8.9 × 10−10 |
| Max psim | 0.03 | 0.17 | 0.08 | 0.99 | 0.002 | 0.89 | 0.01 |
| Mean psim | 3.2 × 10−4 | 0.003 | 0.002 | 0.65 | 4.2 × 10−6 | 0.07 | 6.7 × 10−5 |
| %psim < 0.05 | 100% | 99.6% | >99.9% | 0.1% | 100% | 58% | 100% |
A simulated P value (denoted psim) was produced for each of the 10,000 simulations. The table indicates the proportion of simulations for which the associations remained significant.
Although our calculations showed that the lack of correction for axial length was unlikely to affect the significance of the associations that we report, it limits our ability to compare our measurements with other studies. A consensus on ways to correct for axial length should ideally be reached to ensure that foveal pit parameters can be robustly compared between studies and populations in health and disease. The small number of Caucasian subjects that our study included limited our ability to look at interactions between predictors within this group. Larger sample sizes would allow us to consider more predictors and their interactions and ultimately to identify both strong and weak determinants of foveal pit morphology. An important limitation of our study is that it did not include any predictor that reflected differences in environmental factors between the Ghanaian and Caucasian groups. Environmental factors are likely to introduce population-specific variabilities that are generally difficult to account for,1 and that will be considered in future investigations. Finally, the measures that we considered to describe the structure of the foveal depression—pit depth, diameter, maximal slope, and volume—may not fully account for local variations in pit morphology. For instance, our methodology did not allow us to explore local variations in the slope and depth of the foveal pit that may be associated with asymmetries previously reported in normal aged foveae.19
Future work will focus on assessing how differences in foveal pit morphology relate to vitreoretinal disease prevalence and clinical features in African and Caucasian populations. Foveal hypoplasia has a broad spectrum of clinical phenotypes and is not always associated with impaired vision.35,36 Wider and shallower foveal pits were described in Caucasian individuals with mild retinopathy of prematurity20; although the functional consequences are unclear. The changes in foveal pit morphology that we observed with age in Ghanaians but not in Caucasians might be protective or adaptive against vitreomacular adhesion and vitreomacular traction. This might explain the comparatively lower incidence of these conditions among African Americans.6 If, as some suggested, the specialization of the retina at the fovea confers an increased vulnerability to age-related macular degeneration,37 differences in the structure of the foveal pit between Caucasians and Africans may explain part of the variability in susceptibility and clinical features for this disease between the two populations.
Although genetic variants associated with retinal thickness in healthy eyes were recently reported,38 variants associated with the formation of the foveal pit are yet to be identified. Inherited disorders that are associated with variations in foveal pit morphology, such as albinism,39 may help identify some of these variants. Future studies will investigate associations between foveal pit morphology and genetic risk for AMD. Our aim will be to determine whether genetic variants associated with a reduced risk for AMD in Africans are also associated with differences in foveal pit morphology. An important limitation is that the genetic etiology of AMD was discovered and described in case-control populations of largely European descent, and evidence suggest that it may not be generalizable to African populations.40 More work is, therefore, needed to understand this disease and other retinal disorders in populations with African ancestry. In addition, we hope to further characterize how modifiable risk factors, including smoking and hypertension,41 alter the natural course of AMD in Caucasian and African populations and how they relate to changes in retinal morphology.
Acknowledgments
The authors thank the following for help and support: Paul Bernstein, MD, PhD, Albert T. Vitale, MD, Christopher Ricks, MD, and Susie Choi, PhD, from the John A. Moran Eye Center, Department of Ophthalmology & Visual Sciences, University of Utah, Salt Lake City; Mike Feilmeier, MD, and Jessica Feilmeier, MD, from the University of Nebraska Medical Center, Omaha; Lisa Nichols, Norma Miller, BS, and Stacie Matthews from the Sharon Eccles Steele Center for Translational Medicine, John A. Moran Eye Center, Department of Ophthalmology & Visual Sciences, University of Utah, Salt Lake City; Lisa S. Hancox, BS, from the Department of Microbiology and Immunology, University of Iowa, Iowa City; Benjamin Abaidoo, MD, from the University of Ghana Medical School, Accra; Sheri McCormick, Laura Cushley, Coilin Ferrin, Courtney Hageman, Albert Asiedu, Ezekiel Brikrong, Paul Lartey, Karima Zouache, PhD, and Joyce Bamford-Addo (Former Speaker of the Parliament of Ghana from 2009 to 2013).
This work was supported in part by the National Eye Institute of the National Institutes of Health under award numbers R01EY024969 (J.C.) and R24EY017404 (G.S.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support from an Unrestricted Grant from Research to Prevent Blindness, New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah.
Disclosure: M.A. Zouache, None; G. Silvestri, None; W.M. Amoaku, None; V. Silvestri, None; W.C. Hubbard, None; C. Pappas, None; S. Akafo, None; S. Lartey, None; R.R. Mastey, None; J. Carroll, None; G.S. Hageman, Voyant Biotherapeutics, LLC (C, I)
Amended May 26, 2020: Textual changes that the authors had requested prior to the original publication have now been made to the published article.
References
- 1. Chang MA, Bressler SB, Munoz B, West SK. Racial differences and other risk factors for incidence and progression of age-related macular degeneration: Salisbury Eye Evaluation (SEE) Project. Invest Ophthalmol Vis Sci. 2008; 49: 2395–2402. [DOI] [PubMed] [Google Scholar]
- 2. Bressler SB, Muñoz B, Solomon SD, West SK, Salisbury Eye Evaluation (SEE) Study Team. Racial differences in the prevalence of age-related macular degeneration: the Salisbury Eye Evaluation (SEE) Project. Arch Ophthalmol. 2008; 126: 241–245. [DOI] [PubMed] [Google Scholar]
- 3. Congdon N, O'Colmain B, Klaver CCW, et al.. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004; 122: 477–485. [DOI] [PubMed] [Google Scholar]
- 4. Friedman DS, Wolfs RCW, O'Colmain BJ, et al.. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004; 122: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saunders RA. Racial variation in retinopathy of prematurity. Arch Ophthalmol. 1997; 115: 604. [DOI] [PubMed] [Google Scholar]
- 6. Rodman JA, Shechtman D, Sutton BM, Pizzimenti JJ, Bittner AK, VAST Study Group. Prevalence of vitreomacular adhesion in patients without maculopathy older than 40 years. Retina (Philadelphia, Pa.). 2018; 38: 2056–2063. [DOI] [PubMed] [Google Scholar]
- 7. Wong TY, Klein R, Duncan BB, et al.. Racial differences in the prevalence of hypertensive retinopathy. Hypertension. 2003; 41: 1086–1091. [DOI] [PubMed] [Google Scholar]
- 8. Sadigh S, Luo X, Cideciyan AV, et al.. Drusen and photoreceptor abnormalities in African-Americans with intermediate non-neovascular age-related macular degeneration. Curr Eye Res. 2015; 40: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schachat AP. Features of age-related macular degeneration in a black population. Arch Ophthalmol. 1995; 113: 728. [DOI] [PubMed] [Google Scholar]
- 10. Mahal S, Strain WD, Martinez-Perez ME, Thom SAM, Chaturvedi N, Hughes AD. Comparison of the retinal microvasculature in European and African-Caribbean people with diabetes. Clin Sci. 2009; 117: 229–236. [DOI] [PubMed] [Google Scholar]
- 11. Girkin CA. Differences in optic nerve structure between individuals of predominantly African and European ancestry: implications for disease detection and pathogenesis. Clin Ophthalmol. 2008; 2: 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan A, Duker JS, Ko TH, Fujimoto JG, Schuman JS. Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol. 2006; 124: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grover S, Murthy RK, Brar VS, Chalam KV. Normative data for macular thickness by high-definition spectral-domain optical coherence tomography (spectralis). Am J Ophthalmol. 2009; 148: 266–271. [DOI] [PubMed] [Google Scholar]
- 14. Kashani AH, Zimmer-Galler IE, Shah SM, et al.. Retinal thickness analysis by race, gender, and age using Stratus OCT. Am J Ophthalmol. 2010; 149: 496–502.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bafiq R, Mathew R, Pearce E, et al.. Age, sex, and ethnic variations in inner and outer retinal and choroidal thickness on spectral-domain optical coherence tomography. Am J Ophthalmol. 2015; 160: 1034–1043.e1. [DOI] [PubMed] [Google Scholar]
- 16. Wagner-Schuman M, Dubis AM, Nordgren RN, et al.. Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011; 52: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewallen S, Courtright P.. Blindness in Africa: present situation and future needs. Br J Ophthalmol. 2001; 85: 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tick S, Rossant F, Ghorbel I, et al.. Foveal shape and structure in a normal population. Invest Ophthalmol Vis Sci. 2011; 52: 5105–5110. [DOI] [PubMed] [Google Scholar]
- 19. Nesmith B, Gupta A, Strange T, Schaal Y, Schaal S. Mathematical analysis of the normal anatomy of the aging fovea. Invest Ophthalmol Vis Sci. 2014; 55: 5962–5966. [DOI] [PubMed] [Google Scholar]
- 20. Hammer DX, Iftimia NV, Ferguson RD, et al.. Foveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2008; 49: 2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubis AM, McAllister JT, Carroll J. Reconstructing foveal pit morphology from optical coherence tomography imaging. Br J Ophthalmol. 2009; 93: 1223–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubis AM, Hansen BR, Cooper RF, Beringer J, Dubra A, Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012; 53: 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilk MA, Dubis AM, Cooper RF, Summerfelt P, Dubra A, Carroll J. Assessing the spatial relationship between fixation and foveal specializations. Vision Res. 2017; 132: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: Available at: https://www.r-project.org/about.html. [Google Scholar]
- 25. Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015; 96: 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walls GL. Significance of the foveal depression. Arch Ophthalmol. 1937; 18: 912–919. [Google Scholar]
- 27. Chauhan DS, Marshall J.. The interpretation of optical coherence tomography images of the retina. Invest Ophthalmol Vis Sci. 1999; 40: 2332–2342. [PubMed] [Google Scholar]
- 28. Kirby ML, Galea M, Loane E, Stack J, Beatty S, Nolan JM. Foveal anatomic associations with the secondary peak and the slope of the macular pigment spatial profile. Invest Ophthalmol Vis Sci. 2009; 50: 1383–1391. [DOI] [PubMed] [Google Scholar]
- 29. Springer AD, Hendrickson AE.. Development of the primate area of high acuity, 3: temporal relationships between pit formation, retinal elongation and cone packing. Vis Neurosci. 2005; 22: 171–185. [DOI] [PubMed] [Google Scholar]
- 30. Provis JM, Diaz CM, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol. 1998; 54: 549–580. [DOI] [PubMed] [Google Scholar]
- 31. Yuodelis C, Hendrickson A.. A qualitative and quantitative analysis of the human fovea during development. Vision Res. 1986; 26: 847–855. [DOI] [PubMed] [Google Scholar]
- 32. Creveling CJ, Colter J, Coats B. Changes in vitreoretinal adhesion with age and region in human and sheep eyes. Front Bioeng Biotechnol. 2018; 6: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Retinal photoreceptor density decreases with age. Ophthalmology. 1995; 102: 1853–1859. [DOI] [PubMed] [Google Scholar]
- 34. Leighton DA, Tomlinson A.. Changes in axial length and other dimensions of the eyeball with increasing age. Acta Ophthalmol. (Copenh.) 1972; 50: 815–826. [DOI] [PubMed] [Google Scholar]
- 35. Marmor MF, Choi SS, Zawadzki RJ, Werner JS. Visual insignificance of the foveal pit: reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol. 2008; 126: 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCafferty BK, Wilk MA, McAllister JT, et al.. Clinical insights into foveal morphology in albinism. J Pediatr Ophthalmol Strabismus. 2015; 52: 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Provis JM, Penfold PL, Cornish EE, Sandercoe TM, Madigan MC. Anatomy and development of the macula: specialisation and the vulnerability to macular degeneration. Clin Exp Optom. 2005; 88: 269–281. [DOI] [PubMed] [Google Scholar]
- 38. Gao XR, Huang H, Kim H. Genome-wide association analyses identify 139 loci associated with macular thickness in the UK Biobank cohort. Hum Mol Genet. 2019; 28: 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilk MA, McAllister JT, Cooper RF, et al.. Relationship between foveal cone specialization and pit morphology in albinism. Invest Ophthalmol Vis Sci. 2014; 55: 4186–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Restrepo NA, Spencer KL, Goodloe R, et al.. Genetic determinants of age-related macular degeneration in diverse populations from the PAGE study. Invest Ophthalmol Vis Sci. 2014; 55: 6839–6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000; 107: 2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]