Abstract
OBJECTIVE
To review available options of assessing murine bladder function and to evaluate a non-invasive technique suitable for long-term recording.
METHODS
We reviewed previously described methods to record rodent bladder function. We used modified metabolic cages to capture novel recording tracings of mouse micturition. We evaluated our method in a pilot study with female mice undergoing partial bladder outlet obstruction or sham operation, respectively; half of the partial obstruction and sham group received treatment with an S6K-inhibitor, targeting the mTOR pathway, which is known to be implicated in bladder response to obstruction.
RESULTS
Our non-invasive method using continuous urine weight recording reliably detected changes in murine bladder function resulting from partial bladder outlet obstruction or treatment with S6K-inhibitor. We found obstruction as well as treatment with S6K-inhibitor to correlate with a hyperactive voiding pattern.
CONCLUSIONS
While invasive methods to assess murine bladder function largely disturb bladder histology and intrinsically render post-cystometry gene expression analysis of questionable value, continuous urine weight recording is a reliable, inexpensive, and critically non-invasive method to assess murine bladder function, suitable for a long-term application.
Keywords: animal model, bladder physiology, murine micturition assessment, cystometry, VSOP, S6K, bladder hyperactivity, partial bladder outlet obstruction
INTRODUCTION
Animal models are an essential part of research in bladder physiology and pathophysiology. Drug-effects, structural and functional changes resulting from experimental procedures, or phenotypic changes based on genetic manipulations can be assessed with regards to bladder physiology in rodents. However, the physiologic parameters measured in rodent bladders have only limited translational value to human bladder pathology. To avoid confusion, the terms used to describe animal bladder physiology should be clearly defined in experimental studies and terms describing human bladder physiology should be avoided [1]. Despite these translational limitations, rodents have been used extensively in research of LUTS [2,3]. A research project’s hypothesis, methods involved, and the planned endpoints determine the choice of species to be used in a study. Using mice as experimental animals has the powerful advantage of the full availability of transgenic manipulation and generally lower cost than larger animals. While histologic workup and molecular analysis of bladder tissue are routinely done in all species used, there are three main techniques within a spectrum of options to assess murine bladder function.
Continuous urine weight recording
Continuous urine weight recording (CUWR) as assessment of bladder physiology has been used in different studies with some variation in the technical set-ups and was first published in 2001 [4,5]. However, the basic principle of collecting and weighing urine directly under a mouse cage remains. Also, measurement of post-void residual urine (PVR) at the time of bladder harvest is commonly done in studies using CUWR. Although the method is simple and requires little specialized equipment, it was not widely applied in the past. CUWR is a common method to record micturition pattern in rats, whereby urine is funneled in a measuring cup while solid material such as feces or chow dropped by the study animal gets collected separately. However, the same equipment is unsuitable for quantification of micturitions in smaller rodents such as mice, as their much smaller voided volume would adhere to the funnel and thus not reach the measuring cup [6,7].
Voided stain on paper
Voided stain on paper (VSOP), alternatively named “voided stain assay” (VSA) was first described using automated paper propulsion, which allowed for recordings of up to 24 h duration [8]. The more recent publications used VSOP either with static paper underneath a mouse cage or continuously transported paper with the addition of automated scanning and quantification of urine spots (aVSOP) [9].
Cystometrogram
Invasive cystometry or the cystometrogram (CMG) probably yield the broadest spectrum of functional parameters, and it was first described in the early 1970s [10], while the addition of concomitant bladder ultrasound was only recently suggested [11]. However, CMG jeopardized bladder histology and RNA expression patterns [1,12]. Also, cystometric studies in mice are much more challenging to obtain than in larger animals. As an extension of cystometry, video-urodynamics have recently been described as well, combining CMG with bladder ultrasonograpy [11]; image-derived measurement of bladder volume seems to be the added advantage of that technique.
Among these 3 principal approaches to assess murine bladder function, mouse cystometry is the most commonly applied method with 78 PubMed-indexed studies between 2007 and 2016 whereas 25 studies utilized VSOP or VSA to assess murine bladder function. aVSOP was applied in 5 studies. Only 3 studies used the weight-based micturition recording during the same 10-year period. Here, we present our experience with novel tracing analyses derived by CUWR to determine bladder physiologic parameters in mice. We also tested our set-up of CUWR and applied it in a pilot study, evaluating the functional effect of treatment with an mTOR pathway inhibitor (S6K-inhibitor; PF-4708671) [13,14] in female mice with partial bladder outlet obstruction (pBOO). We also provide a review of other described options to assess murine bladder physiology and compare it to CUWR, a method that is non-invasive efficient, low cost, not labor intensive, and that can be done over extended periods of time.
MATERIAL AND METHODS
Cages and recording equipment
We adapted metabolic cages available in our animal facility that were designed for rats (Fig. 1); in the original configuration, voided urine was funneled into a measuring cup, while feces were collected in a separate container. Despite hydrophobic coating of the funnel, some drops of urine from each void would stick to the funnel, making this method unsuitable for micturition recording in mice. To circumvent this problem, we removed the funnel and let all excrements drop on a paper-covered plate residing on a weight scale underneath the cage. We evaluated polytetrafluoroethylene (PTFE) mesh as well as different sizes of stainless steel mesh treated with hydrophobic spray to keep feces from dropping onto the scale while letting urine pass. We directly compared recordings with and without a mesh above the scale and consequently removed the mesh and adopted a new approach. We developed a novel tracing analysis algorithm further described below. The algorithm can discriminate whether a weight-increase was caused by solid or fluid material based on the acute peak of the former and evaporation of the latter (Fig. 2A and 2B). Essentially, analyses included tracing shape, frequency per unit time, and the amount of urine weight gain for each event as detected by the scale.
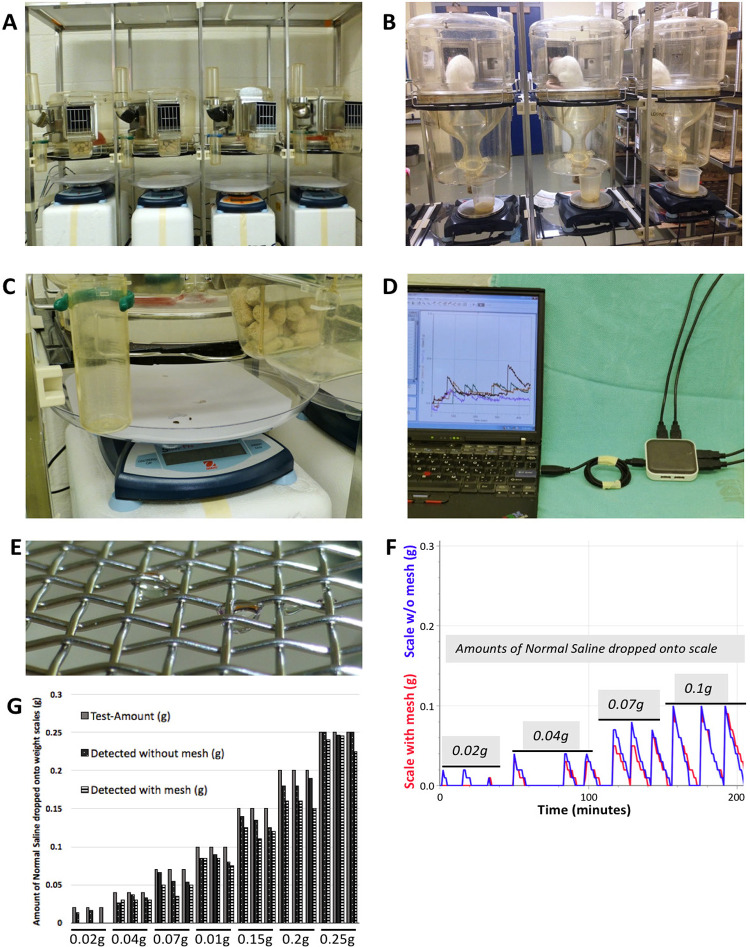
Figure 1.
Equipment for weight-based micturition assessment in mice. A. Metabolic cages initially designed for rats were adjusted so that all excrements could be collected and weighed directly without loss of urine sticking to a screen interposed between the animal and the scale. B. Original set-up of metabolic cages to assess micturition parameters in rats with urine funnelled into a cup residing on a scale. C. Urine and faecal pellets were allowed to drop directly onto the paper-lined plate on the weight scale, where fluid components evaporate. D. Several channels were recorded simultaneously connecting the scales to a laptop running LoggerPro® software via a USB hub. E. Example of urine drop with droplets remaining on mesh. F. Traces of weight-recording from one scale with and another scale without mesh above, with identical amounts of normal saline (NS) pipetted on them; volumes were repeated 3 times. G. Detected amounts of normal saline dropped on a scale with mesh were consistently lower than amounts detected by a scale without mesh above.
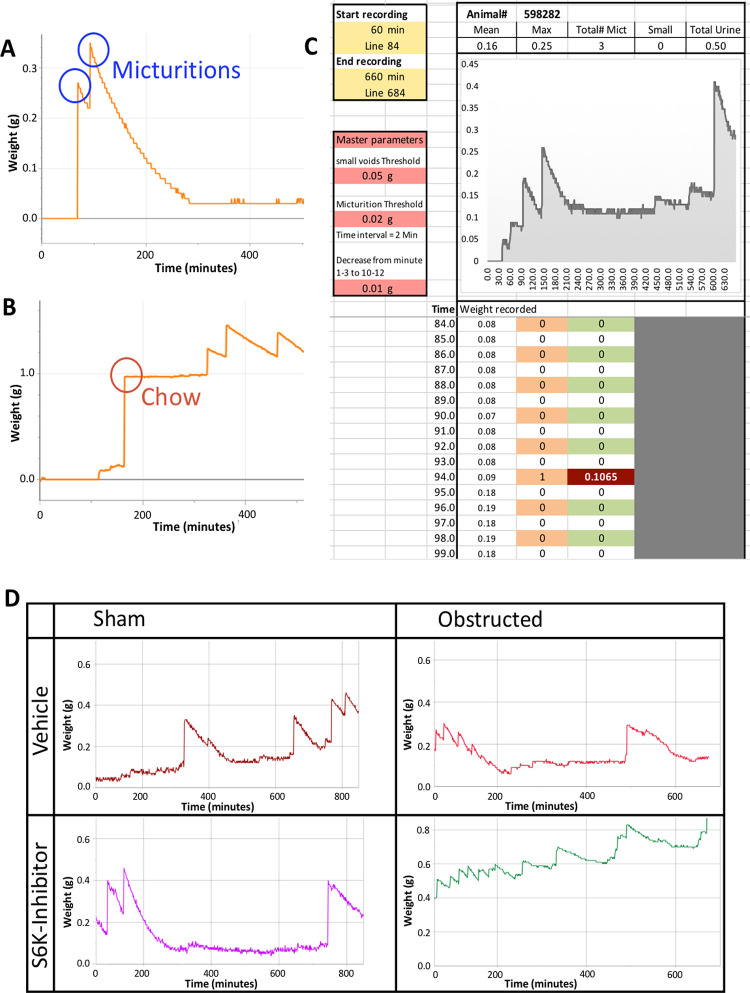
Figure 2.
Data recording and discrimination of urine-induced increase in recorded weight. A. Micturitions are recorded as steep increase, followed by a slightly curved decrease caused by evaporation of urine. B. If a solid particle such as a chow pellet happens to drop on the scale, the weight increase is sustainable. C. Example of recorded trace with analysis results. D. Sample traces from all 4 groups of animals; sham operated animals showed very few small voids, whereas, obstructed animals—drug treated or vehicle—demonstrated an increase in small voids, indicative of hyper-activity, similar to over-activity in obstructed patients.
The weight scales (Scout®Pro, Ohaus® Corporation, Parsippany, NJ, USA) had a resolution of 10 mg and were connected via USB hub to a lap top computer running a recording software (Logger Pro®, Vernier Software &Technology®, Beaverton, OR, USA). The scales were placed 15 cm underneath the cages so that mice’s tail would not touch the scale and thus lead to artifacts. To describe the effect of a mesh placed underneath a metabolic cage, normal saline (NS) ranging from 20 μl to 250 μl was pipetted onto 2 paper-covered weight scales, with one of them having a wire mesh 5 cm above it and weight was recorded once per minute; weight increases recorded by the scale with mesh were for the most part lower than the increase recorded by the scale without mesh (Fig. 1F); the weights as detected by the analysis algorithm were slightly lower than the actual amounts dropped onto the scales, which was a result of averaging recordings immediately before and after a weight increase (Fig. 1G).
Based on our observation of frequent voiding after an initial period of about 45 to 60 min from the mouse being placed in the cage, we started recording after allowing for one hour of adaptation. Since we aimed to capture the phase where bladder storage capacity is highest [15,16], we recorded micturitions for 10 h during the animals’ sleep cycle. The duration of measurement was limited by the duration approved by our institution’s animal care committee.
Data analysis algorithm
The recorded data was transferred to a spread sheet (Excel®, Microsoft corporation, USA) and we devised a simple macro that would detect a weight increase, under the condition that it was followed by a decrease (also validated on MatLab®; Fig. 2C and Fig. S1). This prerequisite would allow distinction between fluid/evaporative and solid material dropped onto the scale (Fig. 2A and 2B). The calculation also averaged cell values to account for minor fluctuations in recordings caused by airflow. Additionally, we programmed a software macro to avoid double counting of a single micturition; based on the column featuring the raw weight recordings, the macro detected micturitions in a first step, while it would edit the actual micturition-induced weight increase in a second step. Parameters setting the various thresholds referenced by the macro are listed as “Master parameters” and would be identical throughout the project. Resulting variables were mean micturition volume, maximum micturition volume, total number of voids, number of small voids (i.e., less than 50 μl), and total urine volume over 10 h of recording; the ratio of small voids was calculated as the number of small voids divided by total number of voids and served as a surrogate for hyperactive bladder.
Surgical procedure and drug treatment
We evaluated our method of assessing murine bladder physiology by continuous urine weight recording in a pilot study, examining the effect of S6K-inhibition on mice with pBOO. To this end, 28 female C57/Bl6 mice weighing 17–20 g were randomly divided into two groups, and 16 underwent pBOO as previously described [17], while 12 mice underwent the respective sham procedure. Two mice of the first group were inadvertently completely obstructed and consequently had to be euthanized. Half of each group received daily intraperitoneal injections with the S6K-Inhibitor PF-4708671 at 75 mg/kg body mass, while the other half received vehicle [18]. Bladders were harvested after 2 weeks, and bladder mass was recorded. Mice were housed in groups under standard conditions and had free access to food and water. All experiments with animals undergoing micturition assessment were approved by the institutions animal care committee.
Literature search
We also screened the recent literature describing methods to assess murine bladder physiology; for the three main options, namely CUWR, VSOP or aVSOP as a variation, and cystometry. We determined numbers of publications over a 10-year period from 2007 to 2016 and for their respective first description including the articles citing the respective method.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 24 (SPSS Inc., Chicago, IL). Direct comparison between 2 groups was done using two-tailed t-test, or Welch t-test where equality of variances was not given, respectively. P values < 0.001 were considered highly significant. Numerical results were summarized graphically using boxplots, the box encompassing the interquartile range (IQR) from the first quartile (Q1) to the third quartile (Q3), and the bold transverse bar representing the median. The whiskers mark maximum and minimum values. Outliers represented by a circle-symbol (o) are more than a 1.5-fold IQR away from Q1 or Q3, respectively.
RESULTS
Micturition recording and analysis method
We found a wire mesh with 11 × 11 by square inch grid (opening size of 2 mm; wire diameter 0.45 mm) to reliably retain mouse feces, larger mesh size would allow feces to pass through while smaller sizes would increase the amount of urine sticking to the mesh despite treatment with hydrophobic coating such as silicone spray (Fig. 1E). In a direct comparison of identical amounts of NS pipetted onto a scale without and on another scale with wire mesh above, the scale with the mesh failed to pick up small voids of 20 μl and almost consistently led to lower amounts of recorded weight-increase (Fig. 1F and 1G).
Also, when recording for several hours, contamination of the mesh by dried urine as well as the increasing amount of feces on the mesh would potentially cause progressively more urine to be absorbed over time. We also evaluated a PTFE mesh with a similar size as the wire mesh; however, due to its highly hydrophobic capacity, the urine drops would mostly not even pass the mesh but stay on top of it. We, therefore, did not use PTFE mesh in further trials. Since any mesh seemed to reduce the amount of urine detected, we decided to leave the mesh aside and recorded all material falling underneath the cage, i.e. urine, feces, occasional pellets of chow, when a mouse could move one out of the food tray (Fig. 2A and 2B). We also weighed feces pellets which were around 20 to 30 mg. Since they had only a slow evaporation-related mass decrease, feces led to changes in recording that were not followed by the same decrease in recorded weight as voided urine. We also noted that airflow reduction in the measurement room visibly reduced the extent of random fluctuations in recorded weight. The iterative changes to a simple macro allowed the recorded weight-traces to be analyzed by an optimized algorithm, which reliably detected micturitions while weight increases caused by solid material dropped onto the scale were not classified as micturition. The smallest voided volume we were able to detect weighed 20–30 mg, but airflow-induced fluctuations in recorded weight as well as maximum resolution of the scales used did not allow us to detect smaller voids.
Functional changes associated with pBOO as well as treatment with S6K-inhibitor are detected using novel analysis algorithm
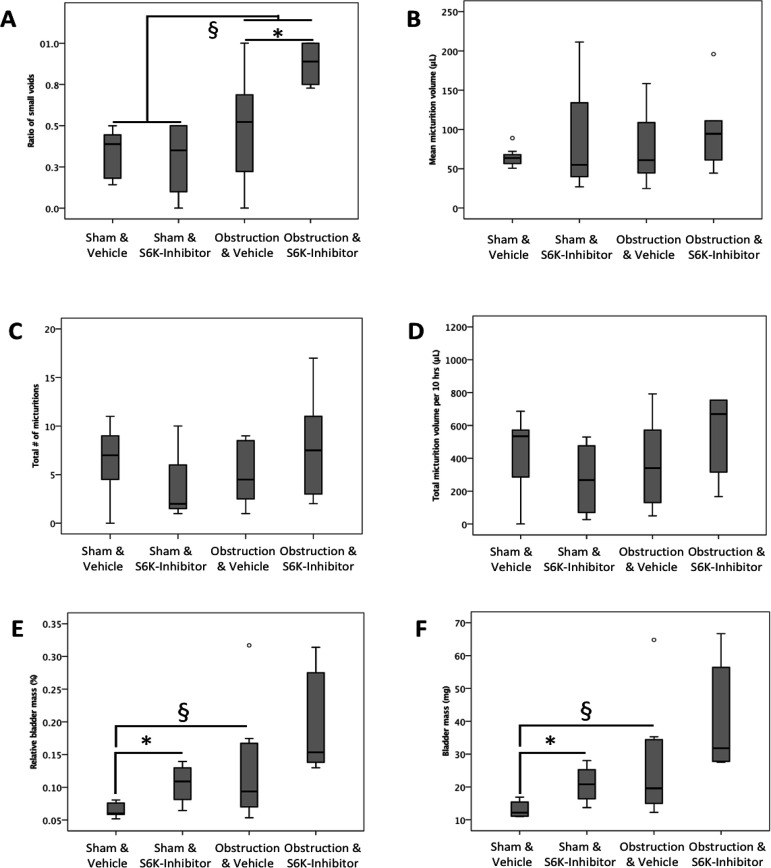
The ratio of small was almost double in obstructed and drug treated mice compared to obstructed and vehicle treated mice (P = 0.023); similarly, ratio of small voids was increased by 2-fold in all obstructed mice compared to all sham operated mice (P = 0.011; Fig. 3A). Two weeks after pBOO or sham procedure, respectively, we found that obstruction tended to lead to a 2-fold increase in relative bladder mass (P = 0.082; Fig. 3E) and absolute bladder mass (P = 0.066; Fig. 3F). In sham animals, treatment with S6K-inhibitor induced an increase in relative bladder mass by almost 60% (P = 0.014; Fig. 3E) as well as an increase in absolute bladder mass of over 50% compared to sham operated and vehicle treated animals (P = 0.016; Fig. 3F). The changes in bladder mass among the obstructed groups were not statistically significant. The total number of micturitions, total voided volume, and mean micturition volume over 10 h of recording were similar among all four groups (for details see Fig. 3C and 3D).
Figure 3.
Partial bladder outlet obstruction and treatment with S6K-inhibitor affect bladder mass and lead to a hyperactive voiding pattern. A. Ratio of small voids as a surrogate of bladder hyperactivity was almost double in obstructed and drug treated mice compared to obstructed and vehicle treated mice (*P = 0.023); this ratio was also increased by 2-fold in all obstructed animals pooled compared to all sham operated animals (§P = 0.011). B-D. Mean micturition volume, total number of micturitions, and total voided volume over 10 h of recording were similar among groups (o indicates outliers). E. Relative bladder mass in sham operated mice was increased by treatment with S6K-inhibitor by almost 60% (*P = 0.014) compared to sham operated and vehicle treated animals, while the almost 2-fold increase in relative bladder mass induced by obstruction was not statistically significant (§P = 0.082). F. Obstruction tended to lead to a 2-fold increase in absolute bladder mass (§P = 0.066) in vehicle treated mice, while treatment with S6K-Inhibitor resulted in an over 50% increase in bladder mass (P = 0.016).
DISCUSSION
Animal models—and rodent models in particular—are an essential part in studying bladder physiology and pathophysiology [3]. While models for benign bladder diseases that required a surgical intervention such as pBOO were traditionally for the most part done in rats, there seems to be recent shift towards the use of mice also in this field of research. The full availability of genetic modifications in mice arguably being the leading reason for this trend also highlight the need for accurate functional methods that are non-invasive so as not to disturb underlying the underlying genomic and epigenomic responses while maintaining reliable phenobiology of this field. Morphologically, the mouse bladder also may be more like human bladder since both contain intramural ganglia, as opposed to the rat bladder, which is typically ganglia-free [19]. However, assessment of murine bladder function is technically far more challenging given the small bladder size, delicate tissue architecture, and low micturition volumes. Together, this rationale argues for new non-invasive methodologies to assess murine bladder function.
Equipment and data analysis for CUWR
Our lab previously established a novel, reliable pBOO-model in female mice with a much-reduced signal-to-noise ratio in sham operated animals with very low animal morbidity and mortality [17]. Subsequently, we had to determine a method to assess murine bladder function. The three principle options were cystometry, aVSOP, and CUWR. We considered the latter to be the least labor intensive, the least expensive, and obviously also a non-invasive method. Additionally, CUWR lends itself for micturition assessment over extended periods of time with minimal additional effort and expenses. Based on equipment originally designed to record rat micturition behavior, we modified the method to render it suitable for mice. The challenges in refining CUWR in mice included how to either prevent solid waste elimination from confounding the recording strategy while maximizing the capture of liquid (urine) elimination patterns.
Our CUWR method with the corresponding analysis allowed capture and detection of mouse micturitions weighing as little as 20–30 μg, which is a significantly higher capture resolution than reported in other studies using CUWR, where voided volumes did not seem to be much less than 50 μg [4,5,9]. In one of the studies, this difference might be due to the wire mesh placed underneath the cage to retain feces, but which also retarded capture of some of the voided urine thus causing small voids to remain undetected despite the use of very precise weight scales [4]. In another study employing CUWR, detected micturitions also were greater than 100 μg for the most part [5]; it is unclear whether this finding could be related to the strain of mice used (not indicated), which notoriously affects functional bladder parameters [20]. Indeed, it is important to note that micturitions in mice of both genders and at various animal ages are often less than 50 μl [21]. In addition, our method of CUWR to assess murine bladder function allows for precise detection of an array of parameters including maximum voided volume, micturition frequency, the absolute number of small voids as well as the ratio of small voids, which can serve as a surrogate of hyperactive voiding behavior. CUWR is not labor intensive, allows for parallel recording of multiple animals, and can be done repetitively and over extended periods of time.
We chose to evaluate our CUWR method in a pilot study applying a recently established pBOO model in female mice [17]; additionally, we sought to determine the functional effect of mTOR inhibition during pBOO in mice using an inhibitor of S6K, a downstream signaling partner of the mTOR pathway. In previous studies, our lab showed that S6K-activity was increased in the partially obstructed bladder, which was associated with a loss of smooth muscle cell differentiation [13]. While mechanistic evaluation of the involved pathway itself is beyond the scope of this report, our functional results showed the anticipated increase in bladder mass secondary to obstruction. Also, we found obstruction leading to an increased ratio of small voids, which serves as a surrogate for hyperactive voiding pattern; in this regard, the pBOO model used leads to functional changes similar to pBOO in men with BPH [22]. Interestingly, our CUWR strategy was successful in detecting a further increase in bladder hyperactivity after treatment with S6K-inhibitor, based on algorithm-based analysis of tracing patterns. Our results also show that pBOO as well as S6K-inhibition affect bladder mass and lead to an altered voiding patterns, detected in both cases vs. control animals using our CUWR method. While repeated measurements of the same animal within a short period of time yielded similar results, especially the introduction of pBOO is known to increase variability of bladder functional parameters as well as bladder mass within the same treatment group [23]. Intra-group variability and effect size of treatment or experimental procedure, respectively, determine the number of animals needed per group to allow statistically significant detection of functional differences among groups.
In studies involving bladder physiology, post-void residual urine (PVR) can be assessed using ultrasound [11]; however, it is more commonly determined via direct volume measurement at the time of bladder harvest. While in rats, this parameter is can be determined via needle-aspiration of urine, the friable and much smaller mouse bladder does not allow for the same technique to be used reliably. The most accurate measurement of murine residual urine during bladder harvest can be done by temporarily clamping the bladder at its neck, weighing the urine-containing bladder on a scale, so that the empty bladder mass can then be subtracted to derive the weight of residual urine. While female rats virtually all void during induction of anesthesia, this behavior is much less consistent in mice. Mouse handling around induction of anesthesia clearly affects whether the animals empty their bladder at that time or not [11]; we therefore also recommend that micturition during induction should be observed and recorded.
Overview of options to assess murine bladder function
In general, CUWR yields directly measured parameters such as maximum micturition volume, mean micturition volume, micturition interval/micturition frequency, the number of voids, and the number of small voids (Table 1). Additionally, provided the animals void during induction of anesthesia, PVR can be determined as well. Furthermore, PVR plus maximum voided volume may be considered as maximum bladder capacity. As a surrogate of voiding efficiency, mean micturition fraction is the ratio of average voided volume divided by maximum bladder capacity. The ratio of small to total number of voids, by definition more frequent than normal voiding, is a marker of a hyperactive voiding pattern [23]. The ease of recording and data analysis makes CUWR a non-invasive, efficient and little labor intensive method. Also, besides modified cages, only weight scales and data recording software is necessary to apply CUWR, which makes this method arguably the most affordable of all options discussed here. On the other hand, a limitation of CUWR is obviously that no intravesical pressure data can be recorded and non-voiding contractions go unnoticed.
Table 1.
Overview of methods assessing mouse bladder physiology.
| Method | Parameters measured | Equipment necessary | Cost | Invasive | Reported duration | Ref. |
|---|---|---|---|---|---|---|
| Cystometry | Basal pressure, maximum pressure, threshold pressure, intermicturition pressure, spontaneous activity, non-voiding contractions, calculated bladder capacity, micturition interval (= 1/micturition frequency), micturition volume, calculated PVR (bladder capacity—micturition volume), bladder compliance, leak point pressure, intercontraction interval | Bladder catheter, 3-way valve, pressure transducer, infusion pump, transducer amplifier, data acquisition board | $$$ | Yes | 1–2 h | [1,6,7,25,33,39,40] |
| Video urodynamics | As cystometry Additionally, ultrasonographic measurement of bladder capacity and PVR | As cystometry Additionally, high resolution ultrasound probe | $$$$ | Yes | 1–2 h | [11,41,42] |
| VSOP | Micturition volume, amount of urine per hour, micturition frequency, PVR at time of harvest | Filter paper, image scanning and analysis equipment/software | $ | No | 2–4 h | [24,25,40] |
| aVSOP | As VSOP, enhanced time-resolution | As VSOP Special metabolic cage with automated transport of filter paper and automated image-scanning | $$ | No | Up to 8 d | [9,21,41] |
| CUWR | Micturition volume, ratio of small voids, micturition frequency PVR at time of harvest Calculated bladder capacity and micturition fraction | Modified animal cages Digital weight scales with USB-connection Data recording software | $ | No | Up to 7 d | [4,5] |
VSOP is another non-invasive method to assess murine bladder function (Table 1). Parameters determined by VSOP are similar to CUWR. However, micturition frequency may be less accurate, given the limited duration of measurement of 2–4 h [24,25]. Since stained paper needs to be recovered and scanned with resulting images analyzed to extract micturition data, VSOP is likely more labor intensive than CUWR and only little specialized equipment is necessary. Limitations are the restricted duration of measurement, and the possible overlap of stains from repeated micturitions in the same area, which becomes more likely as the duration of measurement increases. In an attempt to reduce artifacts, some researchers also did not provide water to mice, which further limited the possible duration of VSOP-measurement [25], as well as may affect bladder function both from under filling and increased urine concentration. As a variant of VSOP, a VSOP-method with automated paper propulsion was described in 1978 [8]. A further refined method with automated image processing was described in 2012 and named aVSOP [9]. Parameters determined by aVSOP are again similar to CUWR and virtually the same as in VSOP; however, measurement can be done over extended periods of time and the resolution regarding the time of micturition-event is improved compared to VSOP. With the automated scanning and image processing, aVSOP might be less labor intensive than VSOP, while the equipment necessary as well as quality and quantity of laminated filter paper used almost certainly increase the cost for aVSOP, especially if a protocol requires long-term or repeated measurements. Although accuracy in recording the time of a single micturition is improved in aVSOP, an overlap of urine stains is still of concern and especially small voids might go unnoticed when urine drips on paper in an area of a previous larger void, which limits the accuracy of this method.
Cystometry theoretically offers the broadest spectrum of physiologic parameters of all assessment options (Table 1) [1], whereby direct measurement of MV still requires urine collection in a cup or on filter paper [1,6]. Other authors also used manual collection of urine from bottom of the cage [26]. While the criteria described for CMG in animals appear similar to those registered in human urodynamic studies, terminology should reflect the distinct differences; detrusor over-activity, for example, is defined by ICS as “involuntary detrusor contractions during the filling phase which can be spontaneous or provoked”, or cystometric bladder capacity is defined by ICS as “the bladder volume at the end of the filling cystometrogram, when ‘permission to void’ is usually given” [27]. Both criteria involve active cooperation of the subject studied, which is obviously not possible in animals. Instead of detrusor over-activity, the term bladder hyperactivity should be used when non-voiding contractions are recorded or when increased micturition frequency is observed [1]. Additionally, urodynamic testing is defined as “an interactive diagnostic study of the lower urinary tract composed of a number of tests that can be used to obtain functional information about bladder filling, urine storage and emptying” [28]; considering this definition, the term urodynamic testing may also deserve to be limited to studies in humans. Rats are probably the most widely used species for cystometry, but it is also applicable in rabbits or guinea pigs [29,30]. However, the latter is obviously more expensive to purchase and to hold and anesthesia is more challenging than rats or mice. Additionally, they require more of potentially expensive study drugs based on their greater body mass. Cystometry in mice, on the other hand, is technically more difficult given the small size of their bladder [31,32]. Most commonly, suprapubic catheter insertion is performed for mouse cystometry, and the rarely used transurethral technique in anesthetized mice only yields a limited choice of functional parameters [33]. A recent description of how the entry point of transvesically inserted catheters affects cystometric results also deserves consideration when planning an experiment or comparing results [11]. The small catheters used for mouse cystometry are prone to twisting and occlusion, and they can also lead to irritation of the small bladder which obviously translates into artifactually induced bladder activity [1]. Restraining mice for cystometry reduces the risk of mechanical dysfunction of the catheter; however, the technique increases sympathetic activity and thus increases bladder capacity, again introducing unpredictable and spurious functional measurements [12]. Cystometry can also be done in anesthetized animals, but results have to be interpreted with caution, as virtually any anesthetic confounds bladder functional responses [34]. Catheter implantation and execution of the measurement protocol makes rodent cystometry extremely labor intensive and leads to loss of animals. Considering the equipment necessary, cystometry is also the most expensive method to assess murine bladder physiology.
Despite its popularity, cystometry in rodents also has significant limitations. Histologic work-up of rat bladders 2 d after catheter implantation showed granulation, severe edema, and hemorrhage, while 7 d after the procedure, these changes were still in a repairing stage [12]. Because of these changes, histologic analysis of bladders after CMG is of limited value. Despite the fact that rodent bladders are still in a repair stage one week after catheter implantation, most cystometries are still done within the first 3 d thereafter [1,6]. An indwelling catheter not only affects bladder histology and function [11], it almost certainly also affects gene expression patterns in the bladder [35], especially over the first days after catheter implantation—the active wound healing process after laparotomy and catheter insertion makes results from gene expression analysis difficult if not impossible to interpret. Furthermore, time of recording also seems to be critical in cystometry. During the first days after catheter implantation, bladder activity and pressures seem to be increased along with low micturition volume; these parameters stabilize by day 6 after catheter implantation [34]. The same study also found that after one week, 15% of catheters lost function for reasons such as occlusion dislodgement, biting, stone formation, or bleeding; mean post implantation survival was only 22 d; therefore, CMG with implanted catheters does not seem to be a suitable method for longitudinal observations with repeated measurements. It was also noticed that CMG-results are less consistent across laboratories than non-invasive methods such as VSOP [25]. Besides the variability resulting from slightly different application of a certain measurement technique, mouse strain, sex, age, environmental factors and husbandry practices as well as time point in the animals’ circadian rhythm clearly affect physiologic results as well, which mandates that this kind of information is provided in experimental reports [20,36-38].
In conclusion, among the methods to assess murine bladder function, continuous urine weight recording is critically non-invasive and seems to be the most accurate, efficient, arguably the least expensive, and most suitable option for long-term recording. It can be applied with little specialized equipment, sometimes even with just minor modifications of animal cages available in most animal facilities. With our novel approach, using a software algorithm to discern solid versus liquid material dropping on the weight scale, no urine gets caught in the mesh, allowing for small micturitions to be recorded reliably. Our analysis of tracing patterns discerned changes in bladder function induced by pBOO or pharmacological manipulation. Successful detection of small voided volumes, in fact, is critical in discovering hyperactive voiding patterns such as induced by pBOO. VSOP, on the other hand, is usually limited to short-term measurements and has limited precision in time-resolution of events; aVSOP mitigates these shortcomings, while more specialized and expensive equipment becomes necessary. Invasive measurement methods such as cystometry or video-urodynamic studies offer the advantage of yielding a broad range of parameters, including intravesical pressure data; these methods are obviously a lot costlier and labor intensive than non-invasive methods, and the yielded results are also less consistent between laboratories than non-invasive measurements and thus more challenging to compare. Disturbed histology and limited value of gene expression analysis of bladder tissue after cystometry are significant drawbacks of this method. Choice of the assessment method for murine bladder function is influenced by the anticipated effect of an experimental intervention and the consequential endpoints a study aims to monitor. Since animal strain, gender, age, and husbandry practices as well as measurement technique affect bladder function and measured physiologic parameters, these aspects should be precisely defined in scientific reports to facilitate interpretation of results.
Supplementary information
Macro code for visual basic editor in Excel and code for MatLab®.
Supplementary information of this article can be found online at http://www.bladderj.org/bladder/rt/suppFiles/582.
Acknowledgments
The work was funded by a CHIR operating grant (DJB).
References
- 1.Franken J, Gevaert T, Uvin P, Wauterickx K, Boeve AC, et al. (2015) Urodynamic changes in mice with experimental autoimmune encephalomyelitis correlate with neurological impairment. Neurourol Urodyn 35: 450-456. doi: 10.1002/nau.22742. PMID: [DOI] [PubMed] [Google Scholar]
- 2.Bjorling DE, Wang ZY, Bushman W. (2011) Models of inflammation of the lower urinary tract. Neurourol Urodyn 30: 673-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fry CH, Daneshgari F, Thor K, Drake M, Eccles R, et al. (2010) Animal models and their use in understanding lower urinary tract dysfunction. Neurourol Urodyn 29: 603-608. doi: 10.1002/nau.20903. PMID: [DOI] [PubMed] [Google Scholar]
- 4.Wood R, Eichel L, Messing EM, Schwarz E. (2001) Automated noninvasive measurement of cyclophosphamide-induced changes in murine voiding frequency and volume. J Urol 165: 653-659. doi: 10.1097/00005392-200102000-00089. PMID: [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Takahashi S, Zhong S, Hosoda C, Zheng H, et al. (2005) Function of the lower urinary tract in mice lacking alpha1d-adrenoceptor. J Urol 174: 370-374. doi: 10.1097/01.ju.0000161210.17365.cc. PMID: [DOI] [PubMed] [Google Scholar]
- 6.Hicks AN, Campeau L, Burmeister D, Bishop CE, Andersson K. (2013) Lack of nicotinamide mononucleotide adenylyltransferase 2 (Nmnat2): consequences for mouse bladder development and function. Neurourol Urodyn 32: 1130-1136. doi: 10.1002/nau.22372. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campeau L, Füllhase C, Sawada N, Gratzke C, Hedlund P, et al. (2013) Characterization of bladder function in a cannabinoid receptor type 2 knockout mouse in vivo and in vitro. Neurourol Urodyn 33: 566-570. doi: 10.1002/nau.22454. PMID: [DOI] [PubMed] [Google Scholar]
- 8.Stewart FA, Michael BD, Denekamp J. (1978) Late radiation damage in the mouse bladder as measured by increased urination frequency. Radiat Res 75: 649-659. doi: 10.2307/3574851. PMID: [DOI] [PubMed] [Google Scholar]
- 9.Negoro H, Kanematsu A, Doi M, Suadicani SO, Matsuo M, et al. (2012) Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat Commmun 3: 809. doi: M. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins C, Maruniak JA, Bronson FH. (1973) Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939-941. doi: 10.1126/science.182.4115.939. PMID: [DOI] [PubMed] [Google Scholar]
- 11.Takezawa K, Kondo M, Kiuchi H, Soda T, Takao T, et al. (2014) Combination of bladder ultrasonography and novel cystometry method in mice reveals rapid decrease in bladder capacity and compliance in LPS-induced cystitis. Am J Physiol Renal Physiol 307: F234-F241. doi: 10.1152/ajprenal.00043.2014. PMID: [DOI] [PubMed] [Google Scholar]
- 12.Morikawa K, Kakiuchi M, Fukuoka M, Kato H, Ito Y, et al. (1990) Effects of various drugs on bladder function in conscious restrained-denervated rats placed in a restraining cage and produced by transection of the hypogastric nerve. Jpn J Pharmacol 52: 405-411. doi: 10.1254/jjp.52.405. PMID: [DOI] [PubMed] [Google Scholar]
- 13.Aitken KJ, Tolg C, Panchal T, Leslie B, Yu J, et al. (2009) Mammalian target of rapamycin (mTOR) induces proliferation and de-differentiation responses to three coordinate pathophysiologic stimuli (mechanical strain, hypoxia, and extracellular matrix remodeling) in rat bladder smooth muscle. Am J Pathol 176: 304-319. doi: 10.2353/ajpath.2010.080834. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schröder A, Kirwan TP, Jiang J, Aitken KJ, Bägli DJ. (2013) Rapamycin attenuates bladder hypertrophy during long-term outlet obstruction in vivo: tissue, matrix and mechanistic insights. J Urol 189: 2377-2384. doi: 10.1016/j.juro.2012.12.110. PMID: [DOI] [PubMed] [Google Scholar]
- 15.Dörr W. (1992) Cystometry in mice--influence of bladder filling rate and circadian variations in bladder compliance. J Urol 148: 183-187. doi: 10.1016/S0022-5347(17)36549-7. PMID: [DOI] [PubMed] [Google Scholar]
- 16.Herrera GM, Meredith AL. (2010) Diurnal variation in urodynamics of rat. PLoS One 5 e12298. doi: 10.1371/journal.pone.0012298. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidler M, Aitken KJ, Jiang JX, Bägli DJ. (2017) Nerve-sparing Mid-urethral Obstruction (NeMO) in Female Small Rodents. J Vis Exp : doi: 10.3791/55288. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di R, Wu X, Chang Z, Zhao X, Feng Q, et al. (2012) S6K inhibition renders cardiac protection against myocardial infarction through PDK1 phosphorylation of Akt. Biochem J 441: 199-207. doi: 10.1042/BJ20110033. PMID: [DOI] [PubMed] [Google Scholar]
- 19.Uvelius B, Gabella G. (1995) Intramural neurones appear in the urinary bladder wall following excision of the pelvic ganglion in the rat. Neuroreport 6: 2213-2216. doi: 10.1097/00001756-199511000-00027. PMID: [DOI] [PubMed] [Google Scholar]
- 20.Cornelissen LL, Misajet B, Brooks DP, Hicks A. (2008) Influence of genetic background and gender on bladder function in the mouse. Auton Neurosci 140: 53-58. doi: 10.1016/j.autneu.2008.04.001. PMID: [DOI] [PubMed] [Google Scholar]
- 21.Negoro H, Kanematsu A, Matsuo M, Okamura H, Tabata Y, et al. (2012) Development of diurnal micturition pattern in mice after weaning. J Urol 189: 740-746. doi: 10.1016/j.juro.2012.07.140. PMID: [DOI] [PubMed] [Google Scholar]
- 22.Moss MC, Rezan T, Karaman UR, Gomelsky A. (2017) Treatment of Concomitant OAB and BPH. Curr Urol Rep 18: 1. doi: 10.1007/s11934-017-0649-z. PMID: [DOI] [PubMed] [Google Scholar]
- 23.Sidler M, Aitken K, Jiang J, Bijos D, Belik J, et al. (2017) Finding NeMO-nerve-sparing mid-urethral obstruction: A pathophysiologically accurate model of rodent partial bladder outlet obstruction. Urology 105: e1-e208.e9. doi: 10.1016/j.urology.2017.03.032. PMID: [DOI] [PubMed] [Google Scholar]
- 24.Sugino Y, Kanematsu A, Hayashi Y, Haga H, Yoshimura N, et al. (2008) Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn 27: 548-552. doi: 10.1002/nau.20552. PMID: [DOI] [PubMed] [Google Scholar]
- 25.Bjorling DE, Wang Z, Vezina CM, Ricke WA, Keil KP, et al. (2015) Evaluation of voiding assays in mice: impact of genetic strains and sex. Am J Physiol Renal Physiol 308: doi: 10.1152/ajprenal.00072.2015. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mucignat-Caretta C, Bondì M, Caretta A. (2004) Endocrine status affects bladder size and postvoid residual urinary volume in mice. Horm Behav 46: 11-18. doi: 10.1016/j.yhbeh.2004.02.004. PMID: [DOI] [PubMed] [Google Scholar]
- 27.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, et al. (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61: 37-49. doi: 10.1016/S0090-4295(02)02243-4. PMID: [DOI] [PubMed] [Google Scholar]
- 28.Winters JC, Dmochowski RR, Goldman HB, Herndon CDA, Kobashi KC, et al. (2012) Urodynamic studies in adults: AUA/SUFU guideline. J Urol 188: 2464-2472. doi: 10.1016/j.juro.2012.09.081. PMID: [DOI] [PubMed] [Google Scholar]
- 29.Guven A, Kalorin C, Onal B, Whitbeck C, Chichester P, et al. (2007) Novel biomarkers of bladder decompensation after partial bladder obstruction. Neurourol Urodyn 26: 1036-1042. doi: 10.1002/nau.20433. PMID: [DOI] [PubMed] [Google Scholar]
- 30.Biers SM, Reynard JM, Doore T, Brading AF. (2006) The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int 97: 612-616. doi: 10.1111/j.1464-410X.2005.05988.x. PMID: [DOI] [PubMed] [Google Scholar]
- 31.Pandita RK, Fujiwara M, Alm P, Andersson KE. (2000) Cystometric evaluation of bladder function in non-anesthetized mice with and without bladder outlet obstruction. J Urol 164: 1385-1389. PMID: [PubMed] [Google Scholar]
- 32.Smith PP, Kuchel GA. (2010) Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn 29: 1344-1349. doi: 10.1002/nau.20850. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundbeck F, Djurhuus JC, Vaeth M. (1989) Bladder filling in mice: an experimental in vivo model to evaluate the reservoir function of the urinary bladder in a long term study. J Urol 141: 1245-1249. doi: 10.1016/S0022-5347(17)41231-6. PMID: [DOI] [PubMed] [Google Scholar]
- 34.Yaksh TL, Durant PA, Brent CR. (1986) Micturition in rats: a chronic model for study of bladder function and effect of anesthetics. Am J Physiol 251: doi: 10.1152/ajpregu.1986.251.6.R1177. PMID: [DOI] [PubMed] [Google Scholar]
- 35.Baskin LS, Sutherland RS, Thomson AA, Nguyen HT, Morgan DM, et al. (1997) Growth factors in bladder wound healing. J Urol 157: 2388-2395. PMID: [PubMed] [Google Scholar]
- 36.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, et al. (2014) Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol 306: F1296-F1307. doi: 10.1152/ajprenal.00074.2014. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keil KP, Abler LL, Altmann HM, Bushman W, Marker PC, et al. (2014) Influence of animal husbandry practices on void spot assay outcomes in C57BL/6J male mice. Neurourol Urodyn 35: 192-198. doi: 10.1002/nau.22692. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Zhang L, Hill WG, Yu W. (2017) Evaluating the voiding spot assay in mice: a simple method with complex environmental interactions. Am J Physiol Renal Physiol 313: doi: 10.1152/ajprenal.00318.2017. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wada N, Shimizu T, Takai S, Shimizu N, Kanai AJ, et al. (2016) Post-injury bladder management strategy influences lower urinary tract dysfunction in the mouse model of spinal cord injury. Neurourol Urodyn 36: 1301-1305. doi: 10.1002/nau.23120. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda Y, Zabbarova I, Schaefer CM, Bushnell D, De Groat WC, et al. (2017) Fgfr2 is integral for bladder mesenchyme patterning and function. Am J Physiol Renal Physiol 312: doi: 10.1152/ajprenal.00463.2016. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takezawa K, Kondo M, Kiuchi H, Ueda N, Soda T, et al. (2016) Authentic role of ATP signaling in micturition reflex. Sci Rep 6: 19585. doi: 10.1038/srep19585. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sano T, Kobayashi T, Negoro H, Sengiku A, Hiratsuka T, et al. (2016) Intravital imaging of mouse urothelium reveals activation of extracellular signal-regulated kinase by stretch-induced intravesical release of ATP. Physiol Rep 4: e13033. doi: 10.14814/phy2.13033. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Macro code for visual basic editor in Excel and code for MatLab®.