Abstract
Background
Anesthesia providers are at risk for contracting COVID-19 due to close patient contact, especially during shortages of personal protective equipment. We present an easy to follow and detailed protocol for producing 3D printed face shields and an effective decontamination protocol, allowing their reuse.
Methods
The University of Nebraska Medical Center (UNMC) produced face shields using a combination of 3D printing and assembly with commonly available products, and produced a simple decontamination protocol to allow their reuse. To evaluate the effectiveness of the decontamination protocol, we inoculated bacterial suspensions of E. coli and S. aureus on to the face shield components, performed the decontamination procedure, and finally swabbed and enumerated organisms onto plates that were incubated for 12-24 hours. Decontamination effectiveness was evaluated using the average log10 reduction in colony counts.
Results
Approximately 112 face shields were constructed and made available for use in 72 hours. These methods were successfully implemented for in-house production at UNMC and at Tripler Army Medical Center (Honolulu, Hawaii). Overall, the decontamination protocol was highly effective against both E. coli and S. aureus, achieving a ≥4 log10 (99.99%) reduction in colony counts for every replicate from each component of the face shield unit.
Discussion
Face shields not only act as a barrier against the soiling of N95 face masks, they also serve as more effective eye protection from respiratory droplets over standard eye shields. Implementation of decontamination protocols successfully allowed face shield and N95 mask reuse, offering a higher level of protection for anesthesiology providers at the onset of the COVID-19 pandemic.
Conclusions
In a time of urgent need, our protocol enabled the rapid production of face shields by individuals with little to no 3D printing experience, and provided a simple and effective decontamination protocol allowing reuse of the face shields.
Key Words: COVID-19, Personal protective equipment, Safety
Background
COVID-19 Pandemic
According to the Center for Disease Control and Prevention (CDC), “A pandemic is a global outbreak of disease. Pandemics happen when a new virus emerges to infect people and can spread between people sustainably.”1 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first isolated in human airway epithelial cells from a cluster of patients with pneumonia of unknown etiology in December 2019 from Wuhan, China.2 The novel virus has since spread to every continent, except for Antarctica, infecting greater than 1.5 million people and causing greater than 170,000 deaths as of today. It is estimated that millions of Americans will get infected by the SARS-CoV-2 virus that causes Coronavirus disease 2019 (COVID-19) and that 40% of the healthcare workforce will be infected and removed from the workforce due to exposure to the virus primarily through respiratory droplets emitted by patients.3 , 4
Safety Concerns for the Anesthesia Provider
Anesthesia providers are at increased risk for exposure because of their primary role in airway instrumentation for symptomatic and asymptotic COVID-19 patients during diagnostic, therapeutic, and surgical procedures.5 Surgical masks, the standard personal protective equipment (PPE) before the COVID-19 outbreak for anesthesia providers, do not offer satisfactory protection from COVID-19 during close patient interaction, partly due to the risk for aerosol generation at the time of intubation. Current literature indicate that surgical masks provide insufficient protection against inhalation of viral particles that exist in both respiratory droplets and aerosolized submicron particles generated by infected patients.6 To overcome this challenge, stringent policies and appropriate use of PPE, such as face shields, safety glasses, and N95 masks, are indicated for providers performing aerosol-generating procedures.7 N95 Filtering Face Piece Respirators (FFR) and powered air-purifying respirators (PAPR) are a more sophisticated PPE that provides full face and body coverage, respectively, enhancing the level of protection against aerosolized particles. However, there are several challenges associated with the continuous use of PPE, especially for PAPR and FFR, including the limited supply chain due to the high demand, communication barriers between provider and patient, and discomfort after long-hours of wear.8, 9, 10 Therefore, extending the use of N95 masks, as recommended by the CDC, is an appropriate and suitable alternative in a resource-constrained environment, in which the use of PAPR and FFR is not practical.11
Preservation of PPE
With the fast development of the COVID-19 pandemic and the incredibly high transmission rates of SARS-Cov-2, shortage of PPE has become one of the greatest and most concerning challenges among healthcare professionals. High cost, limited availability, low storage stocks to meet surge capacity, and limited capabilities for reuse of PPE all contribute to unavailability. Additionally, changing recommendations of appropriate PPE use rapidly evolved in response to the pandemic, producing previously unused supply chain requests.5 , 8, 9, 10
The CDC recommends the implementation of procedures that extend the use of N95 masks11 to combat the shortage of PPE. This sentiment is echoed by A Joint Position Statement supported by The American Society of Anesthesiologists, Anesthesia Patient Safety Foundation, American Academy of Anesthesiologist Assistants, and American Association of Nurse Anesthetists.5 One strategy to mitigate the soiling of N95 masks and extend their use is the addition of a face shield that is capable of withstanding decontamination. The physical barrier provided by the face shield provides an added layer of protection of the N95 mask and the face of the provider from respiratory droplets. It prevents the N95 from becoming soiled, allowing for prolonged use.
The challenge of acquiring face shields during COVID-19 pandemic
In response to the COVID-19 Pandemic, The University of Nebraska Medical Center (UNMC), Department of Anesthesiology, mandated that anesthesia providers use face shields during patient care to extend the life of N95 masks and adequately protect providers from infection with SARS-CoV-2. This mandate required the immediate procurement of 112 face shields for approximately 60 clinical providers working at any 1 time. Our goal was to meet the immediate demand for an increased level of provider protection by providing face shields to reduce viral transmission to the provider. The face shields also prevent the soiling of N95 masks, allowing for reuse with a previously developed ultra-violet radiation sterilization protocol recently approved by CDC/NIOSH.12
Producing 3D printed face shields in-house to meet local demands
Due to the high demand and low supply of commercially produced face shields, UNMC turned to in-house 3D printed face shields using publicly available resources. Using this strategy, UNMC was able to quickly and efficiently produce 112 face shields in approximately 72 hours using four relatively inexpensive and readily available 3D printers. The face shields were deployed for use by our clinical anesthesia providers the very next day, along with a sterilization protocol that allowed for the reuse of the face shields.
The methods developed by UNMC as described in this paper was also successfully replicated by the 15th Maintenance Squadron (15th Wing Airmen Joint Base Pearl Harbor-Hickam) and the Combat Logistics Battalion 3 Marines (Marine Corps Base Hawaii) to rapidly produce and supply face shields to healthcare providers (nurses, medics, physicians, intensivists) at Tripler Army Medical Center (Tripler AMC, HI). They were able to produce approximately 100 face shields in 72 hours with follow-up plans to equip greater than 1000 military and military associated healthcare providers and first responders on the island of Oahu.
Development of 3D printing and decontamination protocols
All the information we used to make the face shields was readily available from various sources in the public domain. However, we had to overcome several significant, time-wasting challenges to produce a final, working product. First, we were limited in materials acquisition, to only using locally available materials and previously acquired equipment. Second, we needed to understand and produce a product that had the appropriate dimensions not to impede our providers in clinical care. Finally, we had a significant learning curve to overcome a significant learning curve to produce face shields without prior 3D printing experience. Consequentially, we are providing a complete step-by-step instructional guide to producing 3D printed face shields rapidly. Our protocol is specific to producing a face shield that is sized appropriately to not interfere with commonly performed procedures, such as endotracheal intubation, and are reusable after decontamination. However, the methods we provide can be used to produce and decontaminate face shields for general use at all treatment facilities.
Brief introduction to 3D printing
Additive manufacturing, or what is commonly referred to as 3D printing, is a fabrication process in which layers of material are added successively to form the desired object. Methods of additive manufacturing fall under several different categories, such as filament deposition manufacturing (FDM), also known as fused filament fabrication, stereolithography, digital light processing (DLP), selective laser sintering, or multijet fusion. The key similarity among the methods is the process by which layer-by-layer an object is built through the addition of material. While the same object may be created using any of the methods mentioned above, each has its strengths and limitations.13
For all 3D printing platforms, the electronic file of the object to be printed (commonly a stereolithography or .stl file) can either be created by the user or downloaded from the shared sources on the internet. In order to print an object, the electronic file must first be loaded into the printer software, and potentially altered to be compatible with the specifications of the printer.
Printing platform
A major consideration is the printing platform to use. Printers used for FDM and DLP are commonly used desktop-sized printers. Of these, FDM is the most frequently used 3D printing platform for nonindustrial applications because it is relatively affordable in both the printer and the required thermoplastic filament. FDM printing platforms offer both low-cost filament use and often a more substantial build plate compared to a DLP platform, enabling FDM printers to produce larger or more objects in 1 run. Thus, this was our platform of choice.
A significant disadvantage of FDM is that it results in individual layers of a thermoplastic material that, while fused, may not be air- or watertight. DLP printing utilizes a digital projector screen to flash images of the object on to photosensitive resin in order to cure the resin layer by layer. This process results in objects with the potential for higher-layer resolution and are solid pieces that are air and watertight. The authors recognize that all FDM prints generated for the headband of the face shield are not watertight, allowing water and air to pass through them to some degree. This would make UV sterilization or a simple wipe down with anti-bacterial/viral wipes inappropriate and ineffective. Given this information, we developed a decontamination protocol that utilized a dilute bleach solution that would allow penetration into any of the pores that are generated in the 3D printing process and permit the reuse of the face shields.
The solid headband and chin piece of the face shield we created were 3D printed via FMD, while all other materials, including the transparent face guard, were purchased commercially, and then used to construct the face shield (Table 1 ). The FDM fabrication process, even with a clear PET filament, does not allow for the type of uniformity and consistency that would permit the creation of a truly clear shield that allows good visual acuity for the wearer.
Table 1.
Comprehensive list of supplies used for the face shield
| Item | Equipment needed to acquire | Approximate cost | Sources | |
|---|---|---|---|---|
| 1 | 3D printer capable of using PLA filament, sufficient printing surface area and uses .stl files | Prusa i3 MK3S | $750 | www.Prusa3D.com |
| 2 | Filament for 3D printing of the head band | PLA filament | $20/1 kg spool. This produces approximately 30 headbands. | www.Prusa3D.com, amazon.com, and many other sources |
| 3 | Clear face protector portion of shield | 10 mL thick PVC plastic binding covers, standard 8 ½ x 11 in size, clear | $17/100 sheets | office supply stores, www.amazon.com |
| 4 | Tourniquet for the head strap | Standard type used for venipuncture | $15/100 tourniquets | medical supply sources, www.amazon.com |
| 5 | Paper hole punch | Standard single paper hole punch | $5 | office supply stores, www.amazon.com |
| 6 | Slicer software | Needed to transfer .stl files to 3D printer, or make any modifications | Free. Available in public domain. | www.Prusa3D.com |
| 7 | Face guard template | Used to mark the location of the holes needed in the face guard | Free. Available in public domain. | www.Prusa3D.com |
| Components to 3D Print | ||||
|---|---|---|---|---|
| 8 | Head band and chin pieces for the face shield | .stl file for printing the headband portion of the face shield. | Free. Available in public domain. | www.Prusa3D.com |
Filament choice
FDM filament types include polylactic acid (PLA), acrylonitrile butadiene styrene (ABS), polyethylene terephthalate (PET). PLA requires lower printing temperatures (200°C-210°C) and has less warping than ABS or PET but is more brittle. ABS has higher printing temperatures (230°C-255°C) and more durability, but is more prone to warping and can generate toxic gas fumes during printing. PET has a moderate printing temperature (220°C-235°C) with durability similar to ABS with the ease of use similar to PLA. However, it absorbs water and requires additional care when storing the filament. Nylon is very durable and is a high-temperature (240°C-260°C) filament.
While other thermoplastic filaments are available, the need for high-speed and low-cost prompted the use of PLA in the current protocol. We chose PLA as our printing material due to the fact that the material was readily available, we were very familiar with its use, and the material was low-cost. Moreover, PLA has excellent printing properties, allowing a FDM printer to print at very high speeds for the make and model (150 mm/s on a Prusa MK3 and/or MK3S printer).
Printer selection
After careful consideration, we chose to use a Prusa i3 MK3S model printer for our 3D face shield printing needs. This model of printer is relatively low-cost (approximately $600), handles PLA filament well, and has sufficient printing surface area. Additionally, many files are available in the public domain that are designed for use with this printer, thus lowering the barrier for production for individuals who may have little or no 3D printing experience.
Computer and software selection
The specifications for the computer used for designing, modeling, processing, and printing of the .stl file depends on the 3D printing software used. For the face shields printed at UNMC, we used a standard Dell desktop (XPS) computer running Slic3R software (Table 1). The processed .stl file was then saved to an SD card, and the SD card inserted into the Prusa 3D printer for printing. A wide array of software options is available, ranging from relatively simple such as Google SketchUP (free software) to highly complex such as Autodesk AutoCAD. Mid-range software includes Blender, Autodesk Maya, and Solidworks. As complexity increases, the computer requirements also increase. For example, Google SketchUP requires a 2.1+GHz CPU and 4Gb RAM, while Autodesk AutoCAD requires, at minimum, an Intel Pentium 4 processor with 8Gb RAM.
Special considerations for printing at locations with high-security restrictions
Due to the need to transfer files from computer to 3D printer, downloading and installation of software, downloading of .stl files, there may be limitations/challenges to producing face shields at government and military installations, treatment facilities, or hospitals with secure networks that do not allow for easy transfer of files from computer to printer by an external drive. Additionally, at these locations, there may be restrictions on acquiring or installing 3D printing software onto network computers.
MATERIALS AND METHODS
The successful production of a 3D printed face shield will require the following steps: (1) creating or obtaining the electronic file for the 3D printed parts; (2) printing the face shield parts (Fig 1 ); and (3) assembling the face shields with the additional required supplies.
Fig 1.
The terminology of the face shield components. Items referenced in the assembly direction refer to the items in Table 1.
Step 1: Creating the Electronic File for Printing
First, one must either make or find the file of the idealized model of the desired object. The .slt files used in this paper are located on the Prusa Face Shield website (https://www.prusaprinters.org/prints/25857-prusa-face-shield), under files. This file is optimized for use in the Prusa printer and thus would require minimal, if any, modifications by the user to print a quality product.
Once the .stl file was obtained, it was loaded onto a computer capable of running the slicer software. The slicer software takes the 3D virtual model and determines the process required for the printer to produce the object layer by layer. The slicer printer software can assist in setting the appropriate speed, layer height, and generation of tool paths (the path that the printer extruder follows while printing) for the 3D printer being used. Unlike traditional ink printers, where most settings are universal and ink pages would be printed the same regardless of the printer used, 3D printers will require unique modifications based on the model and brand of printer used.
Step 2: 3D Printing of the Head Band and Chin Piece
The headband print file was adjusted using slicer software to control speed, layer height, support material and the use of other supporting materials. Using the Prusa RC3 Quattro file, a .gcode file was produced that would result in an optimized print speed. Using and 0.2 mm layer height, 2 outer layers and 3 layers for top and bottom layers along with 0% infill, a stack of 4 head and lower pieces could be printed in 7.5 hours. This print file also utilized a raft to provide optimum adhesion to the build plate give the small amount of contact area this design has with the build plate. No support material is used as the chamfered undersides of the headband allow the printer to successively print layers vertically without issue.
Step 3: Assembly of the Face Shield
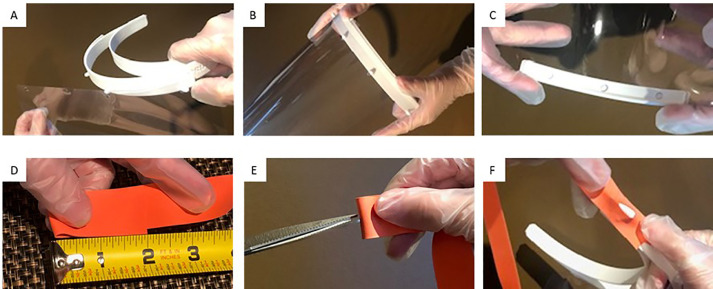
Prepare all components of the face shield for assembly. Figure 1 contains the terminology of the face shield components, whereas Figure 2 depicts a pictorial description for the initial face shield assembly.
-
1
Remove the printed headbands and chins (item 7) from the printing deck of the 3D printer. Separate into individual components.
-
2
Using the face guard template from the Prusa website, item 7 (www.Prusa3D.com), mark the location of the needed holes in the clear PVC binding cover (item 3) that will be used for the face guard, using a permanent marker.
-
3
Using a standard hole punch (item 5), punch the holes marked in the face guard.
-
4
Fold the ends of the IV tourniquet (item 4) over ∼2 inches and cut a ∼0.5 cm slit in the band.
-
5
Attach the clear face guard (item 3) to the headband (item 8) by aligning the punched holes in the clear PVC binding sheet with the pegs on the top of the headband (item 8), Figure 2, panel A and B. Check to see that the “Prusa” logo on the side of the headband is oriented correctly (facing up), to assist with proper orientation.
-
6
Slide chin piece (part of item 8) onto the bottom of the face guard by sliding it onto the bottom of the clear PVC sheet (item 3). It is not necessary (and preferred) to not punch any holes in the PVC sheet to attach this piece. Figure 2, panel C.
-
7
Attach the head strap (IV tourniquet, item 4), to the slides of the headband (item 8) by placing the side attachment bars of the headband through the slit in the tourniquet. Figure 2 Panel D, E, F.
Fig 2.
Pictorial description of how to assemble the face shield for the first time. (A) attaching the face guard to the headband; (B) view after face guard attachment; (C) attaching the chin piece; (D) measuring the head strap; (E) cutting a slit into the head strap; and (F) attaching the head strap to the headband.
Step 4: Sterilization Protocol
Our choice to utilize a high speed and low cost FDM printer with PLA, had a known result of leaving small pores present in the final 3D printed product, making the use of UV light sterilization not possible. To circumvent this challenge, we created a liquid sterilization protocol that would allow reuse of our face shields. In brief, the face shields were dissembled, placed briefly in a dilute bleach solution, and allowed to be air dry. When dry, the clear face guard was wiped to remove any spots, and the face shield reassembled for use. Our detailed decontamination protocol is included in Appendix A.
Experiment to assess effectiveness of the decontamination protocol
Bacteria
Escherichia coli ATCC 8937 and Staphylococcus aureus ATCC 25923 from the American Type Culture Collection (ATCC, Manassas, VA) were selected as model Gram-negative and Gram-positive organisms. Overnight growth of bacteria incubated at 37°C on tryptic soy agar supplemented with 5% sheep's blood agar (BA; Remel, Lenexa, KS) was used to prepare a 0.5 McFarland (1 × 108 CFU/mL) in phosphate buffered solution (PBS).
Inoculation of Face Shields
The surface of each face shield part was cleaned by wiping surfaces with CaviWipes (Metrex, Orange, CA) followed by 70% Ethanol prior to spiking. Following drying, spots to be inoculated were marked with permanent marker. Subsequently, 10 µL of bacterial concentration was applied to each marked spot. As a positive control, organism suspensions were inoculated to each face shield part, allowed to dry, and swabbed without decontamination. PBS was inoculated to each part as a negative control. The droplets were left to air dry for 1 hour.
Assessment of Decontamination
Each face shield part was disinfected according to Appendix A. After adequate drying, a cotton-tipped swab (Puritan Medical Products Company, Guilford, ME) was used to sample each marked spot. Swabs were moistened in sterile PBS and the area was swabbed using a firm sweeping and rotating motion. Organisms were enumerated using the spread-plate technique on BA plates. The plates were incubated at 37°C for 20-24 hours. Experiments were repeated five times per face shield part (head band, head piece, face shield) and organism (E.coli, S. aureus) for a total of 30 experiments not including positive and negative controls. Decontamination effectiveness was evaluated using the average log10 reduction in colony counts.
Assessing the effectiveness of the Appendix A decontamination protocol
To assess the effectiveness of the Appendix A decontamination protocol, we inoculated bacterial suspensions of E. coli ATCC 8937 and S. aureus ATCC 25923 directly onto each part of the face shield unit. All positive organism and PBS controls were as expected. The decontamination protocol effectiveness against E. coli was greater than S. aureus. Two-spiked E. coli spots exhibited growth, 1 colony each, whereas 5-spiked S. aureus spots had characteristic growth. E. coli was observed on the face guard piece, S. aureus was detected from the chin piece and face guard. No organisms were recovered from the head bands. Overall, the decontamination protocol was highly effective against both E. coli and S. aureus, achieving a ≥4 log10 (99.99%) reduction in colony counts for every replicate.
DISCUSSION
Limitations to Consider
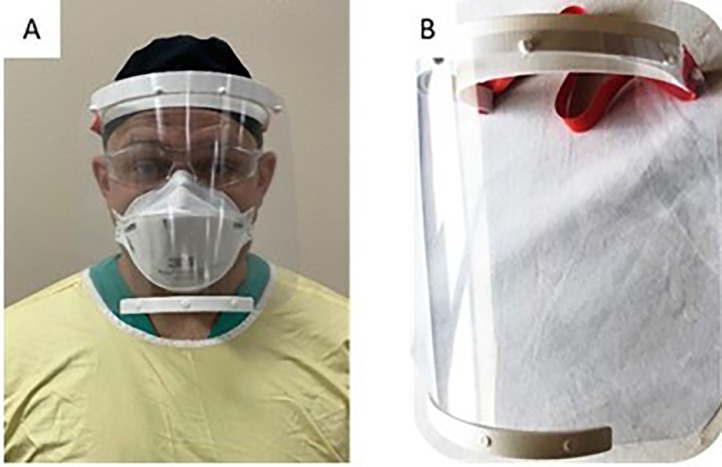
The masks we created are comparable and sometimes superior, to standard commercially available face shields, in terms of the protection area and coverage (Fig 3 ). A known limitation of our face shield design is the gap between the clear shield and the forehead of the wearer. This space is usually occupied by a foam barrier present in several commercially available face shield models. While this foam provides comfort, it limits the ability to extend the use of the product. In order to reduce the possibility of provider contamination from droplets entering this top opening, a bouffant surgical cap can be pulled forward and attached to the 4 pins of the headband holding the face guard in place. Another option to mitigate this concern is to print a cover piece for the headband, which is currently under development.14 However, for our purposes of rapidly producing enhanced PPE in the form of face shields, this design met the needs of our Department. Given the emergent circumstances and perceived time constraints, institutional infection control was notified, provided input, and was responsible for determining the sites for donning, doffing, and disinfection protocol.
Fig 3.
Depiction of the 3D printed face shield. (A) coverage and design, including use of goggles and a standard N95 mask underneath. Note the coverage that extends below the chin; and (B) completed face shield.
Optional Supplies
While the goal of the authors is to provide a detailed protocol and methodology that met the urgent needs of the UNMC Department of Anesthesiology, modifications may be desired, or even necessary, depending on the availability of resources. To that end, we have included popular modifications and additional product resources.
3D Printer
If a Prusa brand printer is unavailable, or undesirable for other reasons, printer options with similar functionality are available. We recommend the Creality CR-10S Pro V2 (https://www.creality3dofficial.com/) or the Creality Ender 5 Series that was used to produce face shields in-house for TAMC personnel.
Printing filament
For this protocol, we recommend using PLA due to its characteristics and compatibility with this project. Other types of filaments could be used; however, they would require significant modifications of this protocol and additional steps.
Foam for the headband
A 10” piece of foam is optional and can be attached to the headband. For the purchase, foam Window Seal can be found at any departmental store. Make sure you have a 1/2 wide by 1/4 thick. To ensure that the foam holds in place, glue the foam into the headband. However, we do not recommend its use since it cannot be sterilized, nor detached from the headband. Hence, if using the foam, the face shield will have to be disposed after a single use, and we therefore elected to not utilize a layer of foam inside the headband.
Head strap
There are several materials that can be used for the head strap. We tried 3 materials: rubber bands, elastic strips with buttonholes, and tourniquets. Rubber bands have the advantage of being readily available, low-cost, ability to be sterilized in liquid and disposable. However, they were very difficult to adjust and tended to slip, making the security of the face shield a concern. However, they are easy to acquire and could be used if the urgency of the situation merited it. The elastic strip with buttonholes was also low-cost and somewhat easy to acquire, requiring a trip to a fabric or craft store. The buttonholes made adjustment and security of the face shield sufficient. However, the fabric-type and porosity of the material would not allow for reliable sterilization and reuse of the face shields. The material of choice for our design was a tourniquet used clinically for the placement of IVs or phlebotomy. This material was readily available, low-cost, able to be sterilized and did not get stuck in hair as easily as rubber bands
Clear face guard
We settled on an 8.5 × 11” clear 10mil polyvinyl chloride binding cover as it was readily available and only created a small amount of distortion to the wearer's vision. The width permitted the holes to be punched to fit the RC3 headband. The edges were trimmed to prevent the lower corners from contacting the wearer's chest if they flexed their neck. The binding covers are offered in fixed width, to be used with standard-sized paper. Nonetheless, if there is a need for adjusting the dimensions and size of the clear shield, one can replace the binding cover with laminating foil or Plexiglas. However, there are limitations to using such materials, such as the need for additional equipment, such as a die cutter for the Plexiglas or a laminating machine for the laminating foil. However, polyvinyl chloride poses no other benefits over these materials if this equipment and expertise in use are already available. Due to the cleaning solution, the clear face guard became slightly blurred with time. Additional clear face guards were available for providers who wanted to replace it. However, the number of times needed for the clear face guard to lose its transparency varied.
Decontamination Protocol Effectiveness
We performed the swab method to recover organisms from the face shield surfaces, this method is commonly used in transfer studies,15 , 16 although other methods exist such as direct elution which may be more efficient in organism recovery from porous surfaces.17 , 18 The swab method was selected because it is simple and less labor-intensive than the direct elution method. It is acknowledged that swabs may retain some portion of organisms during plating.
Conclusion
The rapid manufacturing capacity of commercially available desktop FDM printers paired with open source designed and readily available materials allowed for the creation of sufficient face shields to provide protection until other, more durable shields could be procured. At the time of this writing, these face shields have been in use in the UNMC Anesthesiology Department for 97 days, and at TAMC for 93 days. UNMC is currently awaiting a locally-sourced injection molding type face shield that has a cover over the opening in the top. 3D printing can allow for not only rapid prototyping and iterative changes but can allow the user to manufacturer and augment key components of PPE when providers and first responders are faced with supply shortages.
Acknowledgments
We thank Walker Thomas for assistance in photography of the face shield.
Footnotes
Conflicts of Interests: Support for this project and manuscript was provided solely from institutional and/or departmental sources. The authors declare no competing interests. The views expressed in this abstract are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2020.07.037.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.CDC. Center for disease control and prevention. Coronavirus disease 2019 (COVID-19). Available at:https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/summary.html. Accessed April 8, 2020.
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in china, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams JG, Walls RM. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020;323:1439–1440. doi: 10.1001/jama.2020.3972. [DOI] [PubMed] [Google Scholar]
- 4.The L. COVID-19: protecting healthcare workers. Lancet. 2020;395:922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covid-19 FAQ's. Available at: https://www.asahq.org/about-asa/governance-and-committees/asa-committees/committee-on-occupational-health/coronavirus/clinical-faqs. Accessed August 20, 2020.
- 6.Brosseau L, Sietsema M. 2020. Commentary: Masks-for-all for COVID-19 not based on sound data.https://www.cidrap.umn.edu/news-perspective/2020/04/commentary-masks-all-covid-19-not-based-sound-data Available at: [Google Scholar]
- 7.Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. Available at:https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html. Accessed August 20, 2020.
- 8.Kantor J. Behavioral considerations and impact on personal protective equipment (PPE) use: early lessons from the coronavirus (COVID-19) outbreak. J Am Acad Dermatol. 2020;82:1087–1088. doi: 10.1016/j.jaad.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iacobucci G. Covid-19: doctors still at “considerable risk” from lack of PPE, BMA warns. BMJ. 2020;368:m1316. doi: 10.1136/bmj.m1316. [DOI] [PubMed] [Google Scholar]
- 10.Ong JJY, Bharatendu C, Goh Y, et al. Headaches associated with personal protective equipment - a cross-sectional study among frontline healthcare workers during COVID-19. Headache: The Journal of Head and Face Pain. 2020;60:864–877. doi: 10.1111/head.13811. [DOI] [PubMed] [Google Scholar]
- 11.Recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings. Available at:https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html. Accessed August 20, 2020.
- 12.MacIntyre CR, Chughtai AA, Rahman B, et al. The efficacy of medical masks and respirators against respiratory infection in healthcare workers. Influenza Other Respir Viruses. 2017;11:511–517. doi: 10.1111/irv.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngo TD, Kashani A, Imbalzano G, Nguyen KT, Hui D. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Composites Part B. 2018;143:172–196. [Google Scholar]
- 14.Prusa face shield cover printable components. Available at:https://www.prusaprinters.org/prints/27318-prusa-protective-face-shield-cover-rc1-wip. Accessed April 17th, 2020.
- 15.Rusin P, Maxwell S, Gerba C. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J Appl Microbiol. 2002;93:585–592. doi: 10.1046/j.1365-2672.2002.01734.x. [DOI] [PubMed] [Google Scholar]
- 16.Lopez GU, Gerba CP, Tamimi AH, Kitajima M, Maxwell SL, Rose JB. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl Environ Microbiol. 2013;79:5728–5734. doi: 10.1128/AEM.01030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattar SA, Springthorpe S, Mani S, et al. Transfer of bacteria from fabrics to hands and other fabrics: development and application of a quantitative method using Staphylococcus aureus as a model. J Appl Microbiol. 2001;90:962–970. doi: 10.1046/j.1365-2672.2001.01347.x. [DOI] [PubMed] [Google Scholar]
- 18.Resendiz M, Horseman TS, Hover AJ, Bradley DF, Lustik MB, West GF. Assessment of surgical instrument bioburden after steam sterilization: a pilot study. Am J Infect Control. 2020;48:219–221. doi: 10.1016/j.ajic.2019.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.