Abstract
The pandemic situation with the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from China has endangered human lives. Coronavirus disease 2019 (COVID-19) is presented with asymptomatic, mild, or severe pneumonia-like symptoms. COVID-19 patients with diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular diseases (CVD), hypertension, malignancies, HIV, and other comorbidities could develop a life-threatening situation. SARS-CoV-2 utilizes ACE-2 receptors found at the surface of the host cells to get inside the cell. Certain comorbidities are associated with a strong ACE-2 receptor expression and higher release of proprotein convertase that enhances the viral entry into the host cells. The comorbidities lead to the COVID-19 patient into a vicious infectious circle of life and are substantially associated with significant morbidity and mortality. The comorbid individuals must adopt the vigilant preventive measure and require scrupulous management. In this review, we rigorously focused on the impact of common morbidities in COVID-19 patients and recapitulated the management strategies with recent directions. We found limited resources describing the association of comorbidities in COVID-19; however, our review delineates the broader spectrum of comorbidities with COVID-19 patients.
Keywords: SARS-CoV-2, Comorbidities, COVID-19, Diabetes, Chronic obstructive pulmonary disease, Cardiovascular diseases
Introduction
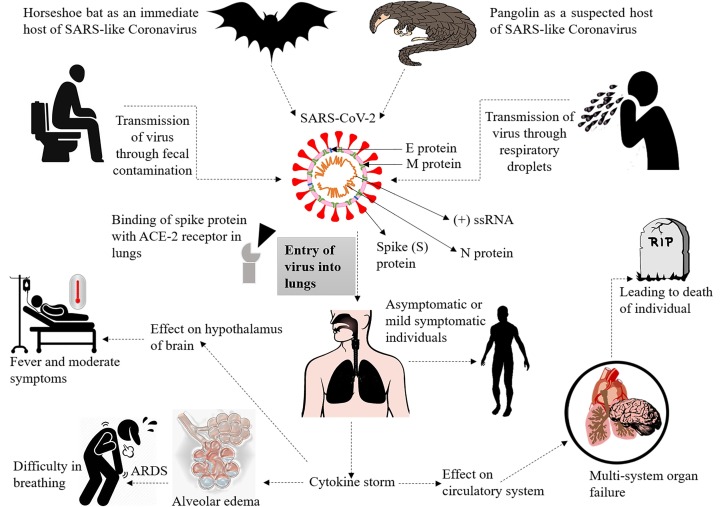
The Wuhan City of China evidenced unknown etiology pneumonia cases at the end of December 2019. On 7 January 2020, the causative agent was identified as a novel coronavirus (2019-nCoV), currently referred to as SARS-CoV-2, and coronavirus disease as COVID-19 [1]. The disease overrun entire China and surpassed international borders in no time, extending the world tally to >7 million confirmed cases and >0.4 million deaths [2]. Coronaviruses (CoVs) classified into four genera: α-CoV, β-CoV, γ-CoV, and δ-CoV. Among these genera, only α-CoV and β-CoV are known to cause diseases in mammals. β-CoVs were known to cause terrible life-threatening respiratory disorders such as severe acute respiratory syndrome (SARS) in 2003 and the Middle East respiratory syndrome (MERS) in 2012 [3]. SARS-CoV-2 also belongs to β-CoV, an enveloped virus with a positive sense-RNA genome is culpable for COVID-19 [4]. Genomic analysis of SARS-CoV-2 confirmed 96% of its genome similarity with bat CoV RaTG13. Early reports on phylogenetic analysis of SARS-CoV also indicate its similarity with Rhinolophus affinis, Bat-SL-CoVZC21, and then Bat-SL-CoVZC45, confirming its origin from Chinese chrysanthemum bat. The genomic sequence and evolutionary of analysis SARS-CoV-2 presented 79.5% genome resembled with SARS-CoV, and the bats have suggested as potential reservoirs that transferred the virus to humans via an unidentified intermediate host. Lately, pangolin found to share 99% genome similarity with SARS-CoV-2 and suggesting to play an essential role in viral transmission and infection [5]. SARS-CoV-2 is transmitted through zoonotic animals or human interaction through respiratory droplets (Fig. 1 ).
Fig. 1.
This figure depicts the pathogenesis of SARS-CoV-2 as it is transmitted from bat through pangolin as an intermediate host and transferred from human to human. The virus utilizes the ACE-2 receptor present in alveolar cells in the lungs, hepatocytes, and kidneys, and affects the host's biochemistry by entering cells. SARS-CoV-2 causes acute respiratory distress syndrome (ARDS) by entering into the lungs and generating cytokine storms, which can affect the circulatory system that leads to morbidity and mortality.
Analyzing the clinical and epidemiological data of COVID-19 from the last few weeks suggest that specific comorbidities increase the risk of infection with worse lung injury and death. The most common comorbidities reported up till now are hypertension, cardiovascular diseases, and diabetes [6]. Also, a high proportion of COVID-19 patients and other conditions in admitted ICU cases suggest comorbidities as a potential risk factor for COVID-19 patients [7]. In this review, we have highlighted the association of COVID-19 with some comorbidities, including hypertension, diabetes, obesity, chronic obstructive pulmonary disease (COPD), asthma, cardiovascular diseases (CVD), liver diseases, malignancy, human immunodeficiency viruses (HIV) and renal diseases. We have also briefly observed the morbidity, mortality, and management of COVID-19 comorbid patients.
Risks of and pathogenesis of COVID-19
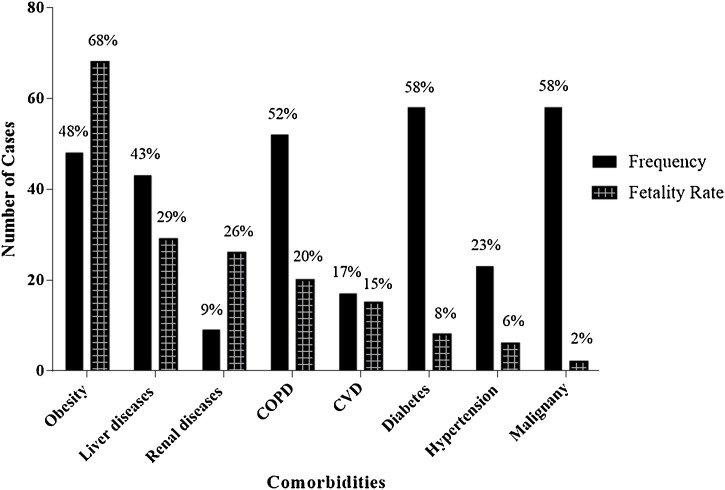
SARS-CoV-2 infects people of all age groups, but individuals aged above 60 years, along with comorbidities such as diabetes, chronic respiratory disease, and cardiovascular diseases, are at a higher risk of developing infection [2]. The underlying mechanism of SARS-CoV-2 remains elusive; however, it is established that the virus utilizes ACE-2 receptors, which are found on the surface of the host cells to get inside the cell [5]. High-plasma pro-inflammatory cytokines, lymphopenia, and atypical respiratory manifestations are the attributes of COVID-19 patients with high-grade fever and breathing problems. Several metabolic and infectious diseases impact the severity of COVID-19 and play a pivotal role in establishing complex symptoms. A summary of pathogenesis is depicted in Fig. 1 while the comorbidities and their fatalities are shown in Fig. 2 . The mortalities, symptoms, and target sites in various morbidities in COVID-19 are mentioned in Tables 1 and 2 .
Fig. 2.
The frequency of comorbidity and its fatality in COVID-19 infections [13,29,32,34,37,41,51,52].
Table 1.
Mortality rate of COVID-19 patients with comorbidities.
| S. No. | Disease | Country with mortality % |
References | ||
|---|---|---|---|---|---|
| China | Italy | USA | |||
| 1 | Hypertension | 9.5 | 73.8 | Not reported | [13,41,53] |
| 2 | Diabetes | 7.4 | 35.5 | 58 | [13,52,53] |
| 3 | COPD | 7 | 13.7 | 4 | [13,32,53] |
| 4 | CVD | 7.3 | 42.5 | 9 | [41,53,54] |
| 5 | Liver diseases | 2.4 | 3.7 | 0.6 | [[53], [54], [55]] |
| 6 | Obesity | 13 | 8.5 | 55 | [15,41,51] |
| 7 | Renal diseases | 0.7 | 20.2 | 21 | [13,52,53] |
| 8 | Malignancy | 2 | 5 | 9.5 | [15,46,49] |
Table 2.
Comorbidities, symptoms, and targets concerning SARS-CoV-2.
| S. No. | Disease | SARS-CoV-2 targets | Symptoms | References |
|---|---|---|---|---|
| 1 | Hypertension | Upregulate ACE-2 expression | Increased blood pressure with pneumonia | [20] |
| 2 | COPD | Upregulate ACE-2 expression | Severe hypoxemia | [19] |
| 3 | CVD | Impaired immune system | Myocardial injury, heart attack | [12,29] |
| 4 | Liver diseases | ACE-2 expression in liver cells, i.e., cholangiocytes, endothelial cells hepatocytes, and Kupffer cells | Elevated serum aminotransferases | [31,32] |
| 5 | Malignancy | Impaired immune system | Adult respiratory distress syndrome | [32,49] |
| 6 | Asthma | Delayed innate antiviral immune response and delayed secretion of IFN-λ | Chronic respiratory diseases along with pneumonia-like symptoms | [22,56] |
| 7 | Renal diseases | Increase secretion of enzymes, dipeptidyl peptidase-4 and angiotensin-converting enzyme (ACE-2) | Acute kidney injury (AKI) | [40,52] |
| 8 | HIV | Antiretroviral therapy (ART) with the impaired immune system and ACE-2 receptor in the lungs | Pneumonia like symptoms with jaundice | [57] |
| 9 | Obesity | The abnormal secretions of cytokines, adipokines, and interferons | Chronic low-grade inflammation of abdominal obesity with effect on bronchi and lung parenchyma | [17,51] |
| 10 | Diabetes | ACE-2 expression, impaired T-cell function and increased interleukin-6 (IL-6) | Pneumonia like symptoms | [11,58] |
Diabetes and COVID-19
People with diabetes are inclined to get infections due to impaired phagocytic cell capabilities. Further, several other factors increase the risk of COVID-19 in diabetic patients. An elevated level of ACE-2 receptors found to be causally related to diabetes by Mendelian randomization analysis; this might prejudice people with diabetes to SARS-CoV-2 infection [8]. Furin is a type 1 membrane-bound protease expressed in high levels in diabetic patients [9]. This proprotein convertase involved in the entry of the virus inside the host cell by decreasing the SARS-CoV-2 dependency on human proteases. The SARS-CoV-2 spike (S) protein attach to the ACE-2 receptors is activated by the enormous furin levels. This pre-activation of S protein allows the viral entry into the cell and escapes from the human immune system [10]. Hence, a dysregulated immune response with increased ACE-2 receptors and furin expression may lead to a higher lung inflammation rate and lower insulin levels. The convenient entry of virus leads to a life-threatening situation for diabetic patients [8,9]. Moreover, the impaired function of T-cell and elevated levels of interleukin-6 (IL-6) also plays a decisive role in developing COVID-19 disease in diabetics [11]. Emerging data about COVID-19 suggests that 11–58% of all COVID-19 patients have diabetes, and an 8% COVID-19 fatality rate has been reported in diabetic patients [12,13]. The risk for ICU admissions in COVID-19 individuals with diabetic comorbidity is 14.2% higher than individuals without diabetes [7].
Obesity and COVID-19
Obesity (BMI ≥ 30 kg/m2) is linked with reduced oxygen saturation of blood by compromised ventilation at the base of the lungs. Additionally, some other characteristic features of low-grade inflammation due to obesity may occur, such as the abnormal secretions of cytokines, adipokines, and interferon consequences in compromised immune response [14]. Surprisingly, obesity was not a risk factor for COVID-19 in the early reports from China, Italy, and the United States [13,15,16]. Nevertheless, the high number of COVID-19 cases observed in the regions with more obese people from Europe and North America [17]. Thus, it is needed to explore the relationship of obesity with the frequency of COVID-19. Obesity is one of the less highlighted comorbidities in COVID-19 infections. Though, 47.6% of obese people get infected with COVID-19 and out of these patients, 68.6% receive ventilation in a critical situation [18]. Hence, a high body mass index (BMI) is a risk factor in COVID-19 severity, and obese peoples should take extra care to prevent themselves in this current pandemic.
COPD and COVID-19
COVID-19 illness can lead to the development of hypoxemia in 15–20% of the patients, which require ventilator support in adverse conditions [19]. The transition in the inflammatory response, microbiome imbalance, weak immunity, continual mucus production, use of respiratory corticosteroids, and structural damages are involved in establishing COPD. COPD and other chronic disorders were also associated with SARS (1.4%) and MERS (13%) infections [3]. Although earlier studies did not report a high number of COVID-19 cases with COPD, the expression of ACE-2 receptors is increased in this disease, contributing to the establishment of severe symptoms among COVID-19 individuals, including structural damage to lungs, weak immunity and hyper mucous production [20]. COPD observed in 50–52.3% of the total ICU admitted COVID-19 cases, lead to high mortality among these patients with increased mucous production and blockage of air passages [21].
Asthma and COVID-19
It is known for almost 18 years that asthmatic people are more prone to develop viral infections. If left uncontrolled, these viral infections can develop severe symptoms. People with asthma have a delayed innate antiviral immune response and impaired secretion of IFN-λ, which makes people more susceptible to develop severe complications [22]. Asthma, along with other pulmonary chronic diseases, were associated with SARS (1.4%) and MERS (13%), which induced severe symptoms [3]. Based on history, it is assumed that asthma could be among a potent risk factor of COVID-19; however, we did not find any specific evidence of SARS-CoV-2 in asthmatic patients. A comparative analysis of critical and non-critical COVID-19 patients in Wuhan revealed no significant association of SARS-CoV-2 with asthma and other self-reported allergies, such as food allergy, atopic dermatitis, and allergic rhinitis. However, the risk of developing severe disease in COVID-19 patients is associated with asthmatic smokers, particularly geriatric individuals [23]. Though asthma is not directly associated with COVID-19 infections, people with other complications and respiratory diseases are more likely to become entangled during asthma.
Hypertension and COVID-19
Uncontrolled blood pressure is associated with COVID-19 infection and also with a high case fatality rate (CFR). In China, 23% of hypertensive COVID-19 cases were reported with 6% CFR, and the number continuously inclined due to pandemic anxiety [24]. In patients suffering from hypertension, ACE-2 inhibitors, and angiotensin receptor blockers (ARBs) are frequently used for the treatment purpose. These inhibitors, when used in a high amount, upregulate expression of the ACE-2 receptor, thereby leading to increased susceptibility to SARS-CoV-2 infection [25]. Higher expression of receptor cells on the lungs makes the infection more vulnerable, and chances of severe lung injury and increased chances of respiratory failure.
On the other hand, experimental studies suggest ACE-2 is a potent anti-inflammatory agent and protects against lung injury, kidney injury, and respiratory distress syndrome, which are the common severe complications in COVID-19. The use of ACE inhibitors and ARBs enhance ACE 2, which reduces the inflammatory action of angiotensin II [26]. It is not clear either the use of ACE inhibitors or ARB is harmful or beneficial, but it is recommended to use these molecules to maintain the normal blood pressure. The steps in controlling blood pressure should remain an essential consideration in COVID-19 patients to reduce disease burden.
CVD and COVID-19
CVD had a strong relationship with SARS (8%) and MERS (30%) [27,28]. Similarly, the increased prevalence of CVD observed in COVID-19 patients, most notably among those with severe signs and symptoms. A study in Wuhan noted 6.8% CVD non-survivors from 191 COVID-19 patients, while another research observed that 17% of the COVID-19 non-survivors had CVD [6,29]. Although the mechanism behind the association between CVD and COVID-19 is not precise, whether it is a direct or indirect relationship, most of these COVID-19 patients reported with the compromised immune system that is common in patients with CVDs [12]. High risk of COVID-19 in pre-existing CVD patients might be due to ACE-2 receptors' presence on cardiac muscle cells, suggesting the potential involvement of the cardiovascular system in SARS-CoV-2 infection. Patients with CVD have a higher risk of developing acute coronary syndrome in acute infections. This syndrome escalates the myocardial demand, which eventually led to myocardial injury or infarction. Moreover, an increased rate of inflammatory cytokines in COVID-19 cases mediate atherosclerosis, procoagulant activation, and hemodynamic instability leading to ischemia and thrombosis [30]. Cardiovascular comorbidities are common among COVID-19 patients who need immediate care to reduce morbidity and mortality.
Liver diseases and COVID-19
Liver injuries and abnormal liver biochemistry were reported in SARS, MERS, and now in COVID-19 infections. It implies that there is a relationship between abnormal liver enzyme secretion and coronavirus infection. ACE-2 receptors present on liver cells mediate the entry of SARS-CoV-2 inside the liver cells [31]. Among the COVID-19 cases, 43.4% found with the abnormal secretion of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactic dehydrogenase (LDH) [32]. However, no patient observed with the characteristic intrahepatic cholestasis or hepatic failure. Another study reported that 39.1% of COVID-19 patients exhibit elevated ALT and AST levels, and 6% have increased bilirubin levels [33]. Around 29% of the COVID-19 patients demonstrate liver injury and develop severe complications during later stages of infections [34]. Besides abnormal liver function tests in COVID-19, elevated enzymes may also be released from cardiac and body muscles. The changes in blood chemistry usually return to normal without significant hepatic morbidity. The liver damage is presented as a temporarily raised level of ALT and AST without hepatic failure in most of the patients; however, this could be detrimental in severe cases of COVID-19. Psychological stress, systemic inflammatory response, drug toxicity, and preceding hepatic diseases could be the underlying mechanisms of liver damage in SARS-CoV-2 infection [35]. Currently, it is not evident that the SARS-CoV-2 is associated with hepatocellular damage or intrahepatic cholestasis pathophysiology.
Malignancy and COVID-19
Patients suffering from any malignancy are at a higher risk of developing COVID-19 infection due to the weak immune response. SARS-CoV-2 gets an efficient replication environment in these individuals' to initiate infection. It has been found that 58.3% of the COVID-19 patients in a study had lung carcinoma, and 41.7% of them were taking immunotherapy, chemotherapy, or radiotherapy. However, none of these patients required ICU care during the hospital stay [32]. A total of 2% fatality rate observed among the COVID-19 cases who already had malignancies [7].
HIV and COVID-19
A strain of CoV OC43 was isolated from HIV positive patients in 2003, and COVs have a firm history in HIV patients [36]. People suffering from HIV infection have a high risk of developing COVID-19 disease because of the compromised immune system. After the first report of HIV affected patient positive for SARS-CoV-2, it was presumed that HIV infection is vulnerable comorbidity with COVID-19 infection [4]. However, no significant correlation observed between HIV positive individuals having COVID-19 infections. As the outbreak expands, few more cases of COVID-19 were reported in HIV patients; nevertheless, all patients had mild disease without ICU admissions. There is no correlation between HIV and COVID-19 was observed in Thailand, which is one of the most HIV affected areas [4]. Formerly, it was also speculated that antiretroviral drugs have potent activity against SARS-CoV-2, which could be a reason behind fewer cases of SARS-CoV-2 in HIV patients [37].
Renal diseases and COVID-19
SARS-CoV-2 affects the kidneys by direct cellular injury or sepsis, leading to a cytokine storm. Recently, in Guangzhou, China scientists successfully isolated SARS-CoV-2 from the urine sample of an infected patient, which suggests the kidneys are also a potential target for SARS-CoV-2 [38]. Acute kidney injury (AKI) observed in 3–9% of the COVID-19 cases while it was reported in SARS (5%) and MERS (15%) patients with a 60%–90% mortality rate [38,39]. There are chances of mortalities in addition to the risk of AKI in COVID-19. Besides, the raised levels of blood urea nitrogen, studies suggest that 26.7% of patients develop hematuria, 34% albuminuria, 63% proteinuria [40,41]. Patients with renal diseases are more likely to suffer from COVID-19 infection due to an increase in ACE-2 expression.
Patient management and challenges
Knowing that effective antiviral medications and SARS-CoV-2 vaccines are not yet available, treating a COVID-19 patient is a major challenge for health care staff. COVID-19 with comorbidities leads to a vicious circle, enormous morbidity, and higher mortality in affected patients. The exposure to SARS-CoV-2 in comorbid individuals such as suffering from diabetes (lung inflammation and higher ACE-2 expression), CVDs (impaired heart and immune functions), and COPD (mucous production and inflammatory response) is detrimental to lungs, heart, kidneys, and liver. The complications end up with a deleterious effect on the patient due to multiple organ failure, shock, acute respiratory distress syndrome, heart failure, arrhythmias, renal failure, and, eventually, mortality [42,43]. World Health Organization (WHO) and the National Institute of Health (NIH) have issued recommendations based on clinical evidence and expert guidance for optimal care of COVID-19 patients [44]. Management strategies vary according to the signs and symptoms of COVID-19 patients, e.g., those with no visible symptoms but positive for COVID-19 should be isolated at home. Patients with mild symptoms (absence of pneumonia and hypoxia) should start intervention, and the decision of inpatient and outpatient settings vary on case to case. While patients with severe symptoms of COVID-19 (respiratory distress) require intensive care using a ventilator and other supportive management. Therefore, efficient management can be accomplished by following these guidelines to manage further transmission and reduced mortality.
Although most COVID-19 patients develop mild disease, about 20% of patients need hospitalization, and 5–8% develop severe symptoms and need intensive care and ICU admission [45]. Differences in ICU admission rates in different countries are based on clinical practice and ICU admission requirements in that area; moreover, predisposing factors such as age and comorbidity often influence the ICU administration rates. Different countries have a variable proportion of patients admitted to ICU as China reported 7–26%, 5–12% in Italy, and the highest rate recorded in the United States, 81% [46,47,48]. Accurate data related to the duration of ventilation is limited, but sustained mechanical ventilation for two weeks or more is required to make patients breathe normally.
Underlying diseases, such as hypertension, CVD, diabetes, malignancy, COPD, and asthma, have been reported as risk factors for severe disease and also increased the mortality rate, therefore better management with special consideration must be given to these patients. Most of the COVID-19 patients die due to pre-existing comorbidity; therefore, accurate evaluation is required at the time of hospital admission. Patients with and without comorbidity must be separated into two groups, and different guidelines should be designed for these patients. The treatment for underlying diseases while treating COVID-19 must continue without any interruption [49].
A vigorous handwashing, social distancing, and personal hygiene ameliorate the prevention from the COVID-19. The individuals with comorbidities should conscientiously apply personal protective strategies. The use of the influenza vacci+ne reduces 43%–55% risk of pneumonia in diabetic patients and could be used to differentiate influenza and COVID-19 symptoms [50]. Due to the compromised immunity in comorbid patients, it is advised as long as the patient is suffering from mild or moderate symptoms that can be controlled at home, these patients should stay in home isolation. In addition to isolation and social distancing, the communication between patient and physician should be optimized for the better management of COVID-19 comorbid patients. This study's strength is our more comprehensive focus on the clinically significant commodities common in our society with an association with COVID-19, which have not probably described in a single study. It would be instructive for the health professionals and the community regarding the precautionary measures, comprehend the risk of comorbidities in COVID-19 and establishing management strategies to combat the pandemic situation. However, we found limited data from several counties on mortalities associated with comorbid COVID-19 patients. The mechanisms and pathophysiology of some comorbidities in COVID-19 patients yet need further understanding.
Conclusion
SARS-CoV-2 affected globally a large population with pneumonia-like symptoms, and the patients with other comorbidities are utmost at the risk of infection. Critical situations develop in individuals with hypertension, diabetes, COPD, heart diseases, malignancies, and HIV. COPD patients develop substantially severe symptoms and comparatively higher mortality rates. The meticulous management of COVID-19 patients with comorbidities in contrast to without comorbidities is emphasized to control the jeopardy of life. The comorbid individuals must undertake vigilant preventive measures to protect themselves during the pandemic. The SARS-CoV-2 infection becomes detrimental when it confronts a person with comorbidity, and the management of these patients with appropriate medical care is an imperative step towards their survival. The use of the influenza vaccine would help to differentiate the influenza-like symptoms in COVID-19 and protects comorbid patients from influenza. The individuals with the comorbidities should be vaccinated on a first priority subject to the availability of SARS-CoV-2 vaccine.
Funding
No funding sources.
Competing interest
None declared.
Ethical approval
Not required.
References
- 1.WHO . 2020. Novel coronavirus (2019-nCoV) situation report — 1. Geneva, Switzerland: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4. [Accessed 21 January 2020] [Google Scholar]
- 2.WHO . 2020. Coronavirus disease 2019 (COVID-19) Situation report — 141. Geneva, Switzerland: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200609-covid-19-sitrep-141.pdf?sfvrsn=72fa1b16_2. [Accessed 9 June 2020] [Google Scholar]
- 3.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu F., Cao Y., Xu S., Zhou M. Reply to comments on’ Co-infection of SARS – CoV-2 and HIV in a patient in Wuhan city, China’. J Med Virol. 2020;25838:1–4. doi: 10.1002/jmv.25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao S., Lau A., So H.C. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a Mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care. 2020;2020 doi: 10.2337/dc20-0643. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez C., Rysa J., Almgren P., Nilsson J., Engstrom G., Orho-Melander M., et al. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med. 2018;284(4):377–387. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulcsar K.A., Coleman C.M., Beck S.E., Frieman M.B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20) doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region-case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Zheng J., Zhang L., Liu Y., Chen G.P., Wang L., et al. Systemic inflammation mediates the detrimental effects of obesity on asthma control. Allergy Asthma Proc. 2018;39(1):43–50. doi: 10.2500/aap.2018.39.4096. [DOI] [PubMed] [Google Scholar]
- 15.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/nejmoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan Dh, Ravussin E., Heymsfield S. COVID 19 and the patient with obesity—the editors speak out. Obesity. 2020;28(5):847. doi: 10.1002/oby.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . 2020. Clinical management of severe acute respiratory infection when COVID-19 is suspected. Geneva, Switzerland: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. [Accessed 13 March 2020] [Google Scholar]
- 19.Qiu H., Tong Z., Ma P., Hu M., Peng Z., Wu W., et al. Intensive care during the coronavirus epidemic. Intensive Care Med. 2020;46:576–578. doi: 10.1007/s00134-020-05966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7) doi: 10.1128/jvi.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W., Tao Z.W., Wang L., Yuan M.L., Liu K., Zhou L., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contoli M., Message S.D., Laza S.V., Edwards M.R., Wark P.A., Bartlett N.W., et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N., et al. The impact of COPD and smoking history on the severity of Covid-19: a systemic review and meta-analysis. J Med Virol. 2020;2020:25889. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W.W., Gao R.L., Liu L.S., Zhu M.L., Wang W., Wang Y.J., et al. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol. 2020;17(1):1–8. doi: 10.11909/j.issn.1671-5411.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/s2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiffrin E.L., Flack J.M., Ito S., Muntner P., Webb R.C. Hypertension and COVID-19. Am J Hypertens. 2020;33(5):373–374. doi: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan J., Ng C., Chan Y., Mok T., Lee S., Chu S., et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58(8):686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 31.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., et al. Tissue-based map of the human proteome. Science. 2015;347(6220) doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 32.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Q., Huang D., Ou P., Yu H., Zhu Z., Xia Z., et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;14309:1–11. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y., Sun W., Li J., Chen L., Wang Y., Zhang L., et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. MedRxiv. 2020:1–28. doi: 10.1101/2020.02.17.20024166. [DOI] [Google Scholar]
- 36.Pene F., Merlat A., Vabret A., Rozenberg F., Buzyn A., Dreyfus F., et al. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis. 2003;37(7):929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64(5) doi: 10.1128/aac.00399-20. e00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes Infect. 2020;9(1):991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y.T., Shao S.C., Lai E.C., Hung M.J., Chen Y.C. Mortality rate of acute kidney injury in SARS, MERS, and COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):439. doi: 10.1186/s13054-020-03134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z., Wu M., Guo J., Yao J., Liao X., Song S., et al. Caution on kidney dysfunctions of 2019-nCoV patients. MedRxiv. 2020:1–11. doi: 10.1101/2020.02.08.20021212. [DOI] [Google Scholar]
- 42.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epidemiology working group for NCIP epidemic response, Chinese center for disease control and prevention. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 44.WHO . 2020. Clinical management of COVID-19. Geneva, Switzerland: https://www.who.int/publications-detail/clinical-management-of-covid-19. [Accessed 27 May 2020] [Google Scholar]
- 45.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 46.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 48.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang T., Du Z., Zhu F., Cao Z., An Y., Gao Y., et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228):e52. doi: 10.1016/s0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel A., Jernigan D.B. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak-United States, December 31, 2019–February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(5):140. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;2020:22831. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 54.Chow N., Fleming D.K., Gierke R., Hall A., Hughes M., Pilishvili T., et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019–United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong X., Cao Y.Y., Lu X.X., Zhang J.J., Du H., Yan Y.Q., et al. Eleven faces of coronavirus disease 2019. Allergy. 2020;14289:1–11. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yasri S., Wiwanitkit V. Dose prediction of lopinavir/ritonavir for 2019-novel coronavirus (2019-nCoV) infection based on mathematic modeling. Asian Pac J Trop Med. 2020;13(3):137–138. doi: 10.4103/1995-7645.277815. [DOI] [Google Scholar]
- 58.Maddaloni E., Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;31 doi: 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]