Abstract
Facing the ongoing pandemic caused by SARS-CoV-2, there is an urgent need for serological assays identifying individuals with on-going infection as well as past coronavirus infectious disease 2019 (COVID-19). We herein evaluated the analytical performances of the CE IVD-labeled Abbott SARS-CoV-2 IgG assay (Des Plaines, IL, USA) carried out with the automated Abbott Architect™ i2000 platform at Hôpital Européen Georges Pompidou, Paris, France, using serum sample panels obtained from health-workers with COVID-19 history confirmed by positive nucleic acid amplification-based diagnosis and from patients randomly selected for whom serum samples were collected before the COVID-19 epidemic. The Abbott SARS-CoV-2 IgG assay showed sensitivity of 94 % and specificity of 100 %, demonstrating high analytical performances allowing convenient management of suspected on-going and past-infections. In addition, the SARS-CoV-2 IgG positivity rates were compared in COVID-19 positive and COVID-19 free areas from our hospital. Thus, the frequency of SARS-CoV-2-specific IgG was around 10-fold higher in COVID-19 areas than COVID-19 free areas (75 % versus 8%; P < 0.001). Interestingly, several inpatients hospitalized in COVID-19 free areas suffering from a wide range of unexplained clinical features including cardiac, vascular, renal, metabolic and infectious disorders, were unexpectedly found seropositive for SARS-CoV-2 IgG by systematic routine serology, suggesting possible causal involvement of SARS-CoV-2 infection. Taken together, these observations highlight the potential interest of SARS-CoV-2-specific serology in the context of COVID-19 epidemic, especially to assess past SARS-CoV-2 infection as well as possible unexpected COVID-19-associated disorders.
Keywords: SARS-CoV-2, COVID-19, Serology, Diagnosis, Abbott SARS-CoV-2 IgG assay
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 was declared by the World Health Organization (WHO) as global pandemic on March 11, 2020 [[1], [2], [3]].
Controlling the outbreak in the community and in hospitals mainly relied on the availability and the sensitivity and specificity of RT-PCR testing [4,5]. Rapidly, it was demonstrated that serological testing looking for specific SARS-CoV-2 IgG and/or IgM could increase the sensitivity of the diagnosis [[6], [7], [8], [9], [10]]. On March 2, 2020, the WHO recommended serological testing in addition to molecular diagnosis for the diagnosis of strongly suspected patients of SARS-CoV-2 infection with negative RT-PCR [11].
At April, the Abbott SARS-CoV-2 IgG assay (Abbott GmbH, Rungis, France) received CE-IVD label and was installed on our automated i2000 platform (Abbott Architect™ i2000) at Hôpital Européen Georges Pompidou (HEGP). Our hospital, belonging to the Assistance Publique-Hôpitaux de Paris, which represents the largest group of university hospitals in Europe, has been organized since mid-March to attend to patients with COVID-19 related conditions (COVID-positive area) in distinct areas from the patients without COVID-19 related conditions (COVID-free area). To analytically and clinically validate the Abbott SARS-CoV-2 IgG assay, we tested pre-epidemic sera, sera from pauci-symptomatic health-worker with SARS-CoV-2 positive RT-PCR and sera from hospitalized patients from both the COVID-positive area and the COVID-free area. To date, few data are available on serology testing, focusing mainly on the time of seroconversion after the onset of symptoms and the neutralizing capability of the produced antibodies [9,[12], [13], [14]].
We herein report on lessons learned from our analytical and clinical validation of the Abbott SARS-CoV-2 IgG assay, including unexpected diagnosis of COVID-19 associated disorders by SARS-CoV-2-specific serology assay.
2. Material and methods
2.1. SARS-CoV-2 serology
Abbott SARS-CoV-2 IgG assay detecting IgG against SARS-CoV-2 nucleoprotein was used on Architect analyzer (Abbott Architect™ i2000), according to manufacturer's instructions. Index value threshold for positivity was 1.4. Qualitative results as well as index values were used for analysis.
2.2. Serum samples
Left over sera from pre-epidemic period (collected from October 2019 to January 2020), available at the virology laboratory of HEGP, were used for specificity evaluation. Some of these sera came from patients with recent clinical history of viral respiratory infection including common coronaviruses (229E; NL63; OC43) as well as clinical history of malaria.
Sensitivity was assessed using sera from hospital staff who had a history of positive SARS-CoV-2 RT-PCR at least one month before serology testing. Specimens were collected by occupational medicine.
Finally, sera were also obtained from patients attending the hospital during the pandemic period, in April 2020, either for COVID-19 related conditions in COVID-positive area of the hospital or for non-COVID-19 related conditions in COVID-free area, for further SARS-COV-2 IgG serological testing.
2.3. Statistical analysis
The index value results and the time between the onset of symptoms and the serological test were compared between seropositive and seronegative subjects using the Wilcoxon-Mann-Whitney test. The seroprevalence was compared between subjects hospitalized for COVID-19 related and non-COVID-19 related conditions using the Chi-square test.
2.4. Ethical statement
Our non-interventional study was carried out in accordance with the Declaration of Helsinki without extra sample collection compared to usual procedures. In particular, serum sample specimens and clinical data were obtained only for standard diagnostic following medical prescriptions and care. Under these conditions, the study was exempted from informed consent application, according to the French public health code (CSP, article L 1121-1.1; https://www.legifrance.gouv.fr/). Data analyses were carried out using anonymized database.
3. Results
3.1. Abbott SARS-CoV-2 IgG assay specificity
Out of 117 pre-epidemic sera collected from October 2019 to January 2020, none tested positive for SARS-CoV-2 IgG. The specificity of the Abbott SARS-CoV-2 IgG assay was 100 % (95 % CI: 97 %–100 %). Median index value was 0.03 (IQR = 0.04). Among the 117 sera, 6 and 7 patients had a recent history of malaria and common coronavirus upper respiratory tract infection (4 coronavirus NL63, 2 coronavirus 229E and one coronavirus OC43), respectively. Only 4 results showed index value results above 0.7 (0.7, 0.77, 0.77 and 0.78 respectively), 2 of which had a recent history of malaria (0.78 and 0.77), the first one having also a recent history of coronavirus 229E upper respiratory tract infection.
3.2. Abbott SARS-CoV-2 IgG assay sensitivity
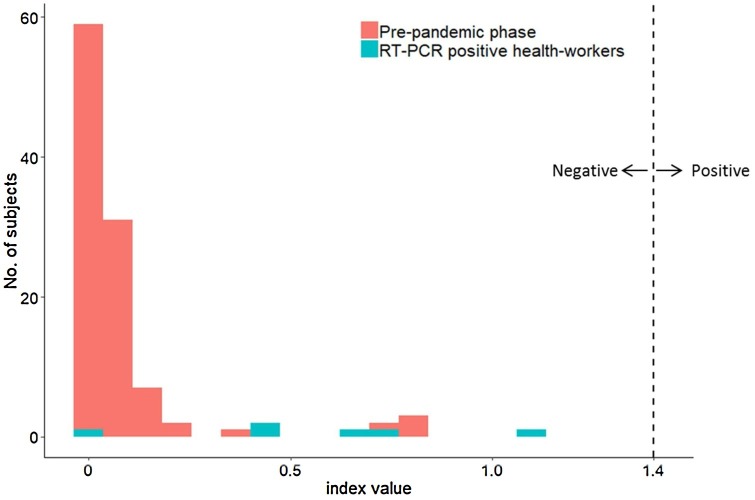
Hundred sera collected from hospital health-workers previously positive for SARS-CoV-2 RT-PCR were tested for SARS-CoV-2 IgG. In this group of health-workers, 69 % were female and the median age was 34 (IQR = 19.5 years) (Table 1 ). Median delay between RT-PCR and serology was 39.5 days (IQR = 9.25 days). Ninety-four serum tested positive for SARS-CoV-2 IgG. The sensitivity of the Abbott SARS-CoV-2 IgG assay was 94 % (95 % IC: 87 %–98 %) (Table 1). Median index value of SARS-CoV-2 IgG positive samples was 5.35 (IQR = 3.51) while the median index value of SARS-CoV-2 IgG negative samples was 0.535 (IQR = 0.31, P = 0.00004). Difference between median index value of pre-epidemic sera and median index value of these negative sera was statistically significant (0.03 versus 0.53; P=0.0069) (Fig. 1 ). All of the negative serum from hospital staff with positive RT-PCR were collected at least 30 days after the initial diagnosis (mean = 44 days; range, 37–52).
Table 1.
Summary of demographic data and SARS-CoV-2 serology results according to patient status.
| COVID + health-workers | Patients from COVID + area | Patients from COVID-free area | Total Hospitalized patients | P | |
|---|---|---|---|---|---|
| Number | 100 | 63 | 96 | 159 | |
| Median age (IQR) | 34 (19.5) | 64 (21.5) | 58 (33.5) | 60 (25) | 2.85 × 10−17a |
| Female / Male % | 69 / 31 | 30 / 70 | 31 / 69 | 31/69 | 1.94 × 10−8a |
| Positive serology n (%) | 94 (94 %) | 47 (75 %) | 8 (8%) | 55 (35 %) | 2.22 × 10−18b |
Total hospitalized patient versus COVID + health-workers.
Patients from COVID + versus patients from COVID-free area.
Fig. 1.
Distribution of Abbott SARS-CoV-2 IgG assay index values from pre-epidemic patients and seronegative health-workers.
3.3. SARS-CoV-2 IgG positivity from hospitalized patients during epidemic period according to COVID-19 hospitalization status
During the epidemic, specific geographical sectors of the hospital were used to attend COVID-19 positive patients while patients without COVID-19 related conditions were hospitalized in other parts of the hospital.
The median age of the hospitalized patients was 60 years (IQR = 25) and 31 % of them were female. The difference between the demographic data (age and sex) within the health workers and the hospitalized patients were statistically significant (P=2.85 × 10−17 and P=1.94 × 10-8, respectively) (Table 1).
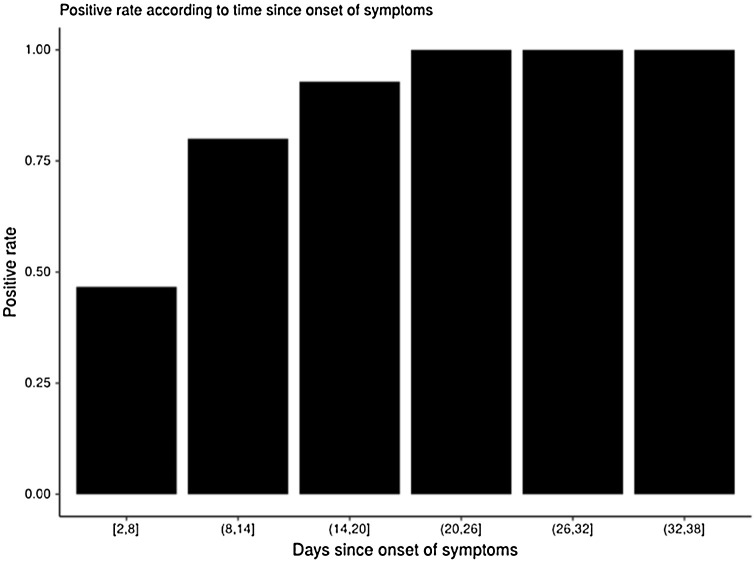
Out of the 63 patients hospitalized for COVID-19 related conditions that were tested for SARS-CoV2 IgG, 47 (74.6 %) tested positive (Table 1). The delay between the onset of symptoms and the time of sampling was statistically different between the serology positive patients and the serology negative patients (median delay = 17.2 days, IQR = 8.5 days, versus median delay = 6.5 days, IQR = 3.8 days, respectively; P = 0.0005). All patients tested at least 20 days after the onset of symptoms were positive for SARS-CoV-2 IgG (Fig. 2 ).
Fig. 2.
SARS-CoV-2 IgG positive rate since onset of symptoms.
Out of the 96 patients hospitalized for non-COVID-19 related conditions that were tested for SARS-COV2 IgG, 8 (8.3 %) tested positive. The difference with COVID-19 positive hospitalized patients was statistically significant (P < 0.0001). Six patients out of 8 were tested for SARS-CoV-2 by RT-PCR, all of them tested negative.
3.4. Unexpected potential COVID-19 related disorders
Out of the 8 patients hospitalized in non-COVID-19 area that showed positive SARS-COV-2 IgG serology, 2 presented renal failure, 2 presented cardiac related disorders, 2 presented vascular related disorders, the last 2 patients were hospitalized for hyperglycemia and pneumonia, respectively (Table 2 ).
Table 2.
Synthesis of patients with unexpected COVID-19 associated disorder.
| COVID-19 associated disorder | Patients ID | Sex | Age | RT-PCR results | SARS-CoV-2 IgG Index value | Reason for admission |
|---|---|---|---|---|---|---|
| Renal | 1 | M | 34 | N | 7.2 | Acute renal failure with nephrotic syndrome |
| 2 | M | 33 | ND | 7.7 | Severe renal insufficiency with nephrotic syndrome | |
| Cardiac | 3 | M | 65 | ND | 7.1 | Cardiac arrest |
| 4 | M | 22 | N | 1.5 | Aortic root replacement (Bentall procedure) | |
| Vascular | 5 | M | 68 | N | 8.9 | Acute limb ischemia |
| 6 | M | 75 | N | 2.6 | Recent claudication of the left calf | |
| Endocrine | 7 | F | 65 | N | 6.2 | Diabetic disorder |
| Infectious | 8 | M | 32 | N | 2.1 | Streptococcus pneumoniae acute pneumonia |
The original clinical presentations of these unexpected COVID-19 associated disorders are described below:
-
✓
Patient #1. A 34-year-old Senegalese male, presented with acute renal failure and nephrotic syndrome. He had a history of stage 4 chronic renal failure (baseline serum creatinine 310 μmol/L) secondary to hypertensive nephrosclerosis with congenital unique kidney. Two weeks before hospital admission, the patient presented flu-like syndrome that was treated with paracetamol. Ten days later he developed edema of the lower limbs and aqueous diarrhea. Laboratory test showed acute renal failure with serum creatinine of 989 μmol/L with nephrotic range proteinuria. Kidney biopsy revealed focal segmental glomerulosclerosis with podocytes hypertrophy and hyperplasia associated with interstitial fibrosis. He partially recovered with a serum creatinine at discharge at 742 μmol/L. SARS-CoC-2 RT-PCR was negative at the time of hospital admission and index value of SARS-CoV-2 IgG was 7.2.
-
✓
Patient #2. A 33-year-old Ivorian male, was referred by his general practitioner for incidental finding of severe renal insufficiency (serum creatinine 2,086 μmol/L) associated with nephrotic syndrome. His past medical history was notable only for tuberculosis. He only complained of abdominal pain. Physical examination was unremarkable except for high blood pressure. Kidney biopsy revealed extensive fibrosis with collapsing focal segmental glomerulosclerosis. The presentation was consistent with end-stage renal disease and the patient required chronic hemodialysis. RT-PCR was not performed at the time of hospital admission and index value of SARS-CoV-2 IgG was 7.7.
Two other patients suffered from cardiac related disorders:
-
✓
Patient #3. A 65-year-old male, was directly admitted to the intensive care unit after cardiopulmonary resuscitation at home for a cardiac arrest. Patient had no past medical history. Medical evaluation found a massive intracranial hemorrhage likely due to the rupture of a left middle cerebral artery aneurysm. The patient died within 48 h after admission. Index value of SARS-CoV-2 IgG was 7.1.
-
✓
Patient #4. A 21-year-old male was admitted for a scheduled aortic root replacement (Bentall procedure). In the course of his mucopolysaccharidosis type II or Hunter syndrome, he developed a symptomatic severe aortic regurgitation with left ventricle dilatation associated with aortic root aneurysm. Pre-operative SARS-CoV-2 RT-PCR was negative and index value of SARS-CoV-2 IgG was 1.5.
Two other patients presented with vascular related disorders:
-
✓
Patient #5. A 68-year-old male was admitted for acute limb ischemia, with no past medical history. At the emergency room, the patient had acute pain in his left foot with paresthesia and palsy 3 h ago. The left popliteal, posterior tibial and pedal pulses were abolished with pallor and coldness of the foot from the toes to the ankle. The electrocardiogram was in sinus rhythm and the angio-computed tomography revealed a thrombus of the infra-renal aorta, with complete occlusion of the left popliteal artery without any atherosclerotic lesions on the arterial tree. Chest scan findings were suggestive of COVID-19. The patient had no symptoms suggestive of COVID-19. Pre-operative and post-operative SARS-CoV-2 RT-PCR (n = 2) were negative. Vascular surgeons performed immediate limb salvage with catheter embolectomy. At the follow-up consultation one month after surgery, the patient was asymptomatic. Index value of SARS-CoV-2 IgG was 8. 9.
-
✓
Patient #6. A 75-year-old male was admitted for recent claudication of the left calf. The patient had already been treated for peripheral arterial disease with a left femoro-popliteal bypass in 2008. The patient had consulted his cardiologist for new onset of his calf claudication one week ago. The duplex ultrasound found an occlusion of the femoro-popliteal bypass. A recanalization of the native left superficial femoral artery was rapidly scheduled. After 48 h, recanalization was still patent and the patient was discharged with a dual antiplatelet therapy. He had no symptom suggestive of COVID-19. Pre-operative and post-operative SARS-CoV-2 RT-PCR (n = 2) were negative. Index value of SARS-CoV-2 IgG was 2.6.
Finally, the last 2 patients showed hyperglycemia and pneumonia, respectively:
-
✓
Patient #7. A 64-year-old female was admitted for hyperglycemia, weakness and diarrhea. Her past medical history included hypertension and type 2 diabetes. Two weeks before hospital admission, she presented with cough and rhinorrhea. She progressively recovered, but complained of moderate abdominal pain, diarrhea without blood nor mucus, nausea and hyperglycemia within the next days. Physical examination showed mild tenderness around the upper abdomen and minor basal crackles in the left lung. Laboratory tests showed moderate acute kidney injury with serum creatinine of 111 μmol/l. Her usual medication was suspended and physiological saline solution was administrated intravenously. The patient’s condition rapidly improved and she was discharged from the hospital 2 days after her admission. Nasopharyngeal SARS-CoV-2 RT-PCR was negative at the time of hospital admission and index value of SARS-CoV-2 IgG was 6.2.
-
✓
Patient #8. A 31 years old man was admitted for fever and shortness of breath. He had been coughing for several months. Previous medical history included dilated and hypertrophic cardiomyopathy, and renal transplant for IgA nephropathy. Physical examination revealed bronchi and crackles in lung basis. SARS-CoV-2 RT-PCR was negative. He was discharged. He came back four weeks later for similar symptoms. Chest CT-scan findings were only suggestive of acute bronchitis. SARS-CoV-2 RT-PCR was negative again, while index value of SARS-CoV-2 IgG was 2.1.
4. Discussion
To our knowledge, this is the second study assessing the analytical performances of the Abbott SARS-CoV-2 IgG assay, the first one with samples collected in Europe. In the first study, both specificity (99.9 %) and sensitivity (100 % after 17 days since the onset of symptoms) were excellent [14]. Our results confirmed the excellent specificity of the assay (100 %) but our sensitivity results were not as good as expected, especially in the health-worker group (sensitivity = 94 %). Our results from the patients hospitalized for COVID-19 were more comparable to the ones observed in the study of Bryan et al. [14]. Indeed, the median delay before the detectability of SARS-CoV-2 IgG since the onset of symptoms was 17 days, as it has previously been described, and every tested patient after 20 days were positive. Various hypotheses could be given to explain this discrepancy between hospitalized patients and health-workers. Firstly, among health workers, only 2 were hospitalized and 98 were not hospitalized and had only little symptoms. Furthermore, the median ages as well as the sex ratio between these 2 groups were statistically different. Whether any of these factors (age, sex and symptoms) could be responsible for the relatively low sensitivity (94 %) in our health workers group would need further investigations. One could also hypothesize that the sensitivity of the test might depend on the SARS-CoV-2 strain and that some specific antibodies might be not detected by the assay. Furthermore, out of the 6 health workers that tested negative with positive RT-PCR, only one had a very low index value at 0.1, all the others had at least an index value of 0.4, which could indicate that some antibodies are produced but are weakly detected. Among these 6 health-workers, one was tested for sero-neutralizing antibodies in a research protocol context. He was positive with a low titer according to the technique (data not shown), which is in agreement with the fact that some antibodies are produced.
In our study, we took advantage of our hospital organization, which was structured in COVID-positive area and COVID-free area to test some patients from the COVID-free area. IgG positivity in this area was 8.3 % which is much higher when compared to the 1.79 % described by Bryan et al. [14]. This could be explained by the fact that our samples were collected by mid-April, at the time of the epidemic peak in France and that a greater percentage of the population had been exposed to the virus at this time. Nevertheless, these results confirmed that the organization in COVID-positive and COVID-free area was efficient as the IgG positivity was highly different between both sectors.
Finally, our work demonstrated that SARS-CoV-2 serology could be a useful tool to retrospectively diagnose COVID-19 infections with 8 cases of unexpected COVID-19 diagnosis in the COVID-19 free area. Clinical features of these 8 patients were in agreement with the wide variety of organs that could be affected by SARS-CoV-2 [[15], [16], [17], [18], [19]]. Indeed, 2 patients presented with nephropathy, 2 patients with cardiac symptoms, 2 patients with vascular symptoms, one patient with hyperglycemia and the last one with bacterial pneumonia. To our knowledge, this is the first study that underlines the retrospective clinical value of SARS-CoV-2 specific serology, especially when recent history of SARS-CoV-2 infection was not obvious. There is no doubt that generalizing the serology diagnosis would reveal unexpected SARS-CoV-2 infections associated with various organ disorders.
Authors contribution
HP, BV, LB, DV designed the research; HP, BV, RV, LB, DV performed statistical analysis; ND, NK, TM, HL, GV, ED, DL provided clinical care and specimens. HP, RV, TM, HL, GV, ED, LB, DV drafted the manuscript. All authors read and approved the final manuscript.
Funding
None.
Declaration of Competing Interest
None.
Acknowledgments
We thank the patients, the nurses and clinical staff who are providing care for the patients. The authors gratefully thank the Clinical Biochemistry department of Hôpital Européen Georges Pompidou, especially the technicians from the serological platform.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J., Liang Z., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. Nat. Res. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 5.Péré H., Podglajen I., Wack M., Flamarion E., Mirault T., Goudot G., Hauw-Berlemont C., Le L., Caudron E., Carrabin S., Rodary J., Ribeyre T., Bélec L., Veyer D. Nasal swab sampling for SARS-CoV-2: a convenient alternative in times of nasopharyngeal swab shortage. J. Clin. Microbiol. NLM (Medline) 2020;58(6):e00720–e00721. doi: 10.1128/JCM.00721-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., Bermudez-Gonzalez M., Kleiner G., Aydillo T., Miorin L., Fierer D.S., Lugo L.A., Kojic E.M., Stoever J., Liu S.T.H., Cunningham-Rundles C., Felgner P.L., Moran T., García-Sastre A., Caplivski D., Cheng A.C., Kedzierska K., Vapalahti O., Hepojoki J.M., Simon V., Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26(7):1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H., Stratton C.W., Tang Y.W. An evolving approach to the laboratory assessment of COVID-19. J. Med. Virol. NLM (Medline) 2020 doi: 10.1002/jmv.25954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petherick A. Developing antibody tests for SARS-CoV-2. Lancet. 2020;395:1101–1102. doi: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., Wang D.Q., Hu Y., Ren J.H., Tang N., Xu Y.Y., Yu LH Mo Z., Gong F., Zhang X.L., Tian W.G., Hu L., Zhang X.L., Xiang J.L., Du HX Liu H.W., Lang C.H., Luo X.H., Wu S.B., Cui X.P., Zhou Z., Zhu M.M., Wang J., Xue C.J., Li X.F., Wang L., Li Z.J., Wang K., Niu C.C., Yang Q.J., Tang X.J., Zhang Y., Liu X.M., Li Z.J., Zhang D.C., Zhang F., Liu P., Yuan J., Li Q., Hu J.L., Chen J., Huang A.L. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 10.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. J. Am. Med. Assoc. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. American Medical Association. [DOI] [PubMed] [Google Scholar]
- 11.Organization W.H . World Health Organization; 2020. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance, 2 March 2020. https://apps.who.int/iris/handle/10665/331329. [Google Scholar]
- 12.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41(5):355–359. doi: 10.1016/j.it.2020.03.007. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fafi-Kremer S., Bruel T., Madec Y., Grant R., Tondeur L., Grzelak L., Staropoli I., Anna F., Souque P., Mutter C., Collongues N., Bolle A., Velay A., Lefebvre N., Mielcarek M., Meyer N., Rey D., Charneau P., Hoen B., De Seze J., Schwartz O., Fontanet A. Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryan A., Pepper G., Wener M.H., Fink S.L., Morishima C., Chaudhary A., Jerome K.R., Mathias P.C., Greninger A.L. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J. Clin. Microbiol. 2020;58(8) doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batlle D., Soler M.J., Sparks M.A., Hiremath S., South A.M., Welling P.A., Swaminathan S. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J. Am. Soc. Nephrol. 2020:1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020:368. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. American Medical Association. [DOI] [PubMed] [Google Scholar]
- 19.Koralnik I.J., Tyler K.L. COVID-19: a global threat to the nervous system. Ann. Neurol. 2020:1–11. doi: 10.1002/ana.25807. ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]