Abstract
The apoplast comprises the intercellular space, the cell walls, and the xylem. Important functions for the plant, such as nutrient and water transport, cellulose synthesis, and the synthesis of molecules involved in plant defense against both biotic and abiotic stresses, take place in it. The most important molecules are ROS, antioxidants, proteins, and hormones. Even though only a small quantity of ROS is localized within the apoplast, apoplastic ROS have an important role in plant development and plant responses to various stress conditions. In the apoplast, like in the intracellular cell compartments, a specific set of antioxidants can be found that can detoxify the different types of ROS produced in it. These scavenging ROS components confer stress tolerance and avoid cellular damage. Moreover, the production and accumulation of proteins and peptides in the apoplast take place in response to various stresses. Hormones are also present in the apoplast where they perform important functions. In addition, the apoplast is also the space where microbe-associated molecular Patterns (MAMPs) are secreted by pathogens. In summary, the diversity of molecules found in the apoplast highlights its importance in the survival of plant cells.
Keywords: apoplast, plant defense, ROS, antioxidants, proteins, peptides, hormones, MAMPs
1. Introduction
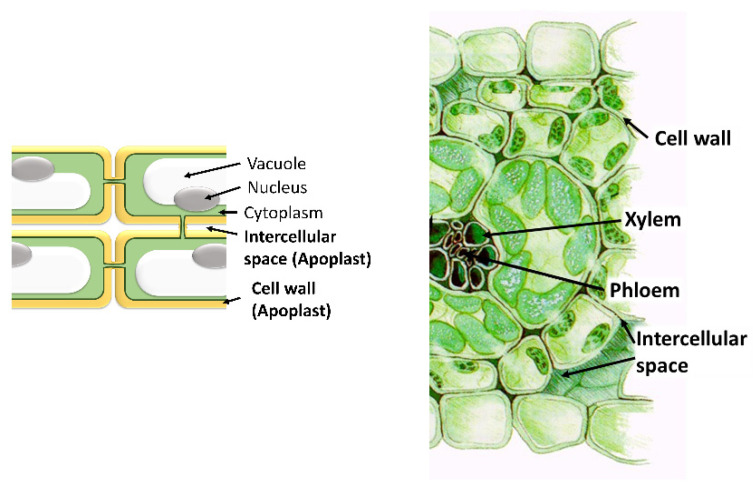
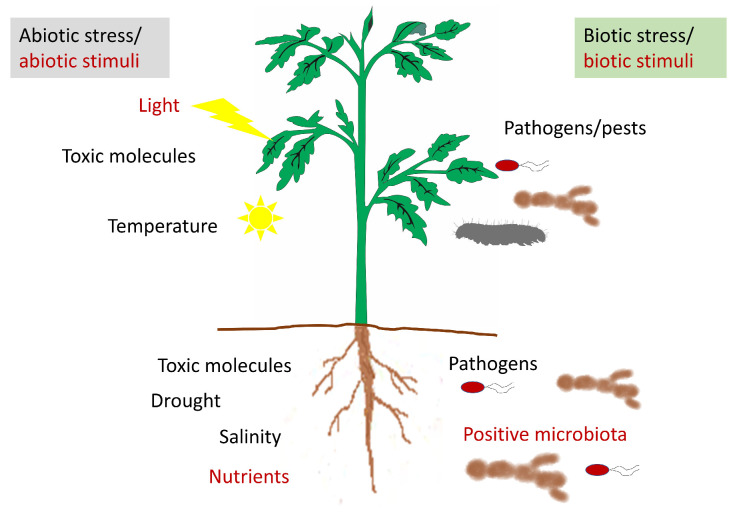
The term apoplast was coined by the German scientist E. Münch in 1930 [1]. He considered the apoplast as the intercellular space involved only in water and nutrient transport and separated the “dead” apoplast from the “living” symplast. Nowadays, the apoplast is defined as the intercellular space filled with gas and water, contained between cell membranes, the interfibrillar and intermicellar space of the cell walls, and the xylem extending to the rhizoplane and cuticle of the outer plant surface (Figure 1). The apoplast is involved in a vast number of functions, since its dynamic nature allows for many reactions of great importance for the plant to take place in it. Among these functions, we can mention nutrient and water transport and the syntheses of components of the cell wall and other molecules. Among them, the synthesis of molecules involved in plant defense against both biotic (phytoalexins, PR, proteins, enzymes) and abiotic stresses are important, since it is in the apoplast where the environmental changes are detected (Figure 2).
Figure 1.
Spaces and structures that form the apoplast in plants. Adapted from: Mluisalozanopulido (CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)).
Figure 2.
Representative diagram of the stresses and stimuli perceived by the plant that could modify the apoplastic content.
The synthesis of the cell wall takes place in the apoplast, after the liberation of the cell wall components through the plasma membrane. This produces the modification, degradation, and reorganization of cell wall polymers, depending on the state of cell development and differentiation. The main components of the cell wall are pectin, cellulose, and hemicellulose, but other components such as xyloglucan or phenolic compounds can be part of it. It is known that some of the steps involved in wall assembly are enzymatic, although there are other steps that seem to simply occur by chemical changes produced in the structure of its components that allow their interaction or deposition [2,3,4]. The content of the cell wall is constantly changing and can be modified according to the stimuli that the plant cell is receiving through the apoplast. That way, both growth activation and the direction of growth or its arrest are regulated by the presence of certain molecules in this intercellular space [5]. In addition to the general constituents of the cell wall, when the plant faces adverse stimuli such as different pathogens, the cell wall can be reinforced with the deposition of callose.
Since the apoplast represents the first barrier between the cell and the surrounding environment, its role in cell nutrition is also of great importance. It has been shown that the apoplast is involved in the transport of glucose produced during photosynthesis and of amino acids to the phloem [6,7]. In the same way, Sattelmacher [8] reviewed the role of the apoplast in plant mineral nutrition, analyzing its role in the acquisition of nutrients in root cells, and important aspects such as Na+ toxicity and Al3+ tolerance. Moreover, he also defined its role in long-distance transport and maintenance of the microbiota through the xylem, and short-distance storage of nutrients in the leaves. The apoplastic content of the leaves is very rich in different molecules and may vary according to the state of the plant, which points to its fundamental role in plant response to external stimuli such as the presence of pathogens [9,10,11].
Hoson [12] attributed an important role to the apoplast in the communication of plants with the external environment, not only at the level of response but also at the level of stimuli perception and their transduction, enabling, that way, plant adaptation to stress. One of the abiotic stresses to which the plant may be subjected is the presence of toxic molecules in the environment. Related to this, it was observed that the apoplastic content plays an important role in overcoming aluminum toxicity, especially through modification of its components avoiding aluminum binding to the wall [13,14]. That way, the changes in the composition prevent the inhibition of root elongation and confer resistance against this stress. It was also shown that other abiotic stresses such as drought or salinity can induce changes in the apoplastic content that could help the plant to overcome them. Moreover, Geilfus [15] suggested that the alkalinization of the pH of the apoplast after exposure to these stresses could play an important role since the transient increase in the pH of the apoplast reduces the stomatal aperture or even may inhibit stomatal opening via effects related to abscisic acid (ABA).
The apoplast also serves as a niche for microbes [7,16]. On the one hand, the endophytic organisms, which tend to be present at low levels, are beneficial for the plant. It has been demonstrated recently by different authors that this symbiosis with endophytic microorganisms play an important role in growth and production, and can help the plant to overcome different stresses [17,18,19].
On the other hand, the content of the apoplastic fluid is rich in nutrients that allow for populations of pathogenic microorganisms to develop in it [9]. However, it has been shown that changes in nutrient composition and metabolism within the apoplast itself may favor pathogen control [11,20]. The apoplastic defense is related to the set of molecules that are either present in the apoplast or secreted in the presence of the pathogen that contributes to plant defense against it [21]. These include reactive oxygen species, toxic compounds, and anti-pathogenic protein molecules such as “pathogenesis related” (PR), other proteins, peptides, and small molecules that are secreted into the apoplastic space. In the plant defense system, through the ETI (effectors triggered immunity) plants are able to recognize the effector molecules secreted by the pathogen through immunoreceptors that are nucleotide-binding leucine-rich repeat proteins (NLR), the synthesis of which leads to a strong apoplastic defense. The interaction between apoplastic defense and control in stomatal closure has been studied, showing that NLR function is cell autonomous in guard cells for stomatal closure defense and non-cell autonomous for apoplastic defense and in the latter case it could affect the defense of the entire plant [22].
This review was written to gather an overview of the recent advances in the field and to gain novel insights into key mechanisms and components, such as ROS, antioxidants, proteins/peptides, and hormones that determine apoplastic immunity and modulate plant–pathogen interactions.
2. Apoplastic ROS Production and Their Role to Overcome Stress
Reactive oxygen species (ROS) are natural products generated in the cells by many metabolic reactions [23]. When first discovered, they were considered deleterious to the cell biomolecules. Nowadays, it is known that ROS play an important role in plant signaling, controlling not only vital processes such as plant growth and development but also plant response to both biotic and abiotic stresses [24,25,26,27].
ROS includes singlet oxygen (1O2), superoxide ion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH), and other organic molecules, such as organic hydroperoxides (ROOH) [28]. However, H2O2 is the most stable molecule and acts as a signal to trigger several cellular responses, such as the modulation of root development or the response against different stresses [29,30].
ROS are reported to be produced in different subcellular compartments, such as the plasma membrane, mitochondria, chloroplasts, and peroxisomes. Different studies also mention the apoplast as a site for ROS generation, although at much lower levels than in the cell [31,32]. The apoplastic ROS (apROS) is produced by several groups of enzymes, such as amine oxidases, lipoxygenases, and oxalate oxidases, but the most recognized enzymes are the cell wall peroxidases and the plasma membrane NADPH oxidases [33].
Apoplastic peroxidases (also known as POXs, Prx, POD, Px or PER) are a class of enzymes that belong to a large class III peroxidases, responsible for the formation and degradation of ROS induced by stress [34]. They are heme oxidoreductases that catalyze O2 conversion to O2•− or H2O2 using apoplastic reductants or •OH production from H2O2 and O2•− [33]. They play an important role in a broad range of developmental and physiological processes, such as cell elongation, auxin metabolism, lignin and suberin formation, cross-linking of cell wall components, and synthesis of phytoalexins; and they participate in ROS and RNS (reactive nitrogen species) metabolism [35]. Moreover, an increase in the activity of class III peroxidases was observed after exposure to various environmental stresses, such as salt, wounding, temperature, phosphate starvation, or potassium deficiency [36,37,38]. For example, Arabidopsis thaliana overexpressing the peroxidase Prx3 showed increased tolerance to salt. In the same way, the expression of CrPrx1 and CrPrx from Catharanthus roseus in Nicotiana tabacum lead to enhanced tolerance to cold stress treatment. In addition, POXs are a well-known class of PR proteins, being induced in host plant tissues by pathogen infection. They belong to the PR-protein 9 subfamily and help to limit the spreading of the infection through the formation of physical barriers or by counterattacking with a large production of ROS. POXs can create physical barriers to restrict pathogen invasion in host tissues by catalyzing the cross-linking of cell wall components which finally leads to cell wall rigidification [39]. This process also occurs in response to wounding or to environmental constraints or simply as a part of the normal cell wall development during growth, differentiation, and senescence [40,41]. Therefore, POX expression results in plant defense either by building up stronger walls or by production of ROS against different stress factors. Related to this, it was observed that knockdown of Prx33 and Prx34 genes in A. thaliana caused increased susceptibility to both fungal and bacterial pathogens, with an impaired oxidative burst, while expression of CaPO2 (CaPrx2) from Capsicum annuum in A. thaliana was found to enhance disease resistance. Furthermore, ROS produced by class III POXs was reported to play an important role in A. thaliana PAMP-triggered immunity (PTI), while the overexpression of these peroxidases provides resistance against Botrytis cinerea infection [37]. In the same way, a positive correlation between peroxidase activity and resistance to Pseudomonas syringae pv. tabaci disease was observed in tobacco plants [38].
Plant NADPH oxidases, named respiratory burst oxidase homologs (RBOHs), are located in the plasma membrane and catalyze the production of apoplastic O2•− by transferring electrons from cytosolic NADPH or NADH to apoplastic O2 [42]. The produced O2•− can further be converted to H2O2 by superoxide dismutase (SOD) [42]. NADPH oxidases have been implicated in abiotic and biotic stress responses, and in the development in different plant species. These enzymes have been studied in detail in Arabidopsis thaliana where ten isoforms have been identified [43]. Among them, RBOHD and RBOHF seem to play crucial roles in the generation of ROS in response to pathogen attack and abiotic stress, while RBOHC, RBOHH and RBOHJ are more related to development. Deficient-mutants in RBOHs such as rbohD and rbohF have been a valuable tool in the study of ROS-abiotic stress interactions [44]. The activation of RBOH enzymes such as RBOHD requires an increase of intracellular calcium, and it can be regulated by ABA, which has been previously described; that regulates ROS production through the RBOH enzymes (RBOHD and RBOHF) [28]. As mentioned above, these enzymes are involved in ROS production in response to biotic and abiotic stresses and they are required for initiation and rapid propagation as systemic signaling between cells, while being dependent on H2O2 accumulation in the apoplast to produce a “ROS wave” [45,46,47,48]. This wave can prime neighboring cells in joint action with other molecules such as hormones, mediating, that way, plant acclimation to abiotic stresses [45]. For example, [49] demonstrated that, in tomato plants, this acclimation-induced cross-tolerance process was related to an increase in H2O2 production dependent on RBOH1 at the apoplast and the subsequent activation of the mitogen-activated protein kinases. That way, NH4+-fed tomato plants displayed basal stomatal closure produced by H2O2 from enhanced CuAO and RBOH1 gene expression, which contributes to protecting the plant against Pseudomonas syringae, reducing disease symptoms and inducing the oxidative burst upon the infection [50]. Moreover, this NH4+-induced oxidative burst seems to be alleviated by the below-mentioned antioxidant enzyme Mn-SOD activity [51].
Oxalate oxidases belong to the germin-like protein family (GLP) and catalyze the oxidation of oxalate to H2O2 and CO2. Plants with higher oxalate oxidase activity were found to be more resistant to plant pathogens, such as the enhanced resistance to Rhizoctonia solani with an overexpressed oxalate oxidase 4 (Osoxo4) gene in rice plants [52,53]. Moreover, oxalate oxidase-mediated H2O2 production in root cells was shown to be important for drought stress acclimation [44].
Apoplastic amine oxidases (AOs) catalyze the oxidative deamination of the essential compounds polyamines (PAs), producing aldehydes, ammonia, and H2O2. They include the copper-containing amine oxidases (CuAOs) and the flavin-containing PA oxidases (PAOs). Both enzymes have been shown to play a key role as a source of H2O2 during plant development and differentiation and in defense mechanisms against pathogens, abiotic stress, and symbiotic interactions [54,55]. The stress signaling can lead to an enhancement in PA levels inside the cell that are released into the apoplast and oxidized producing H2O2. If this H2O2 concentration is high, the programmed cell death (PCD) event occurs, while a slight H2O2 production induces ROS-scavenging enzymes’ action [56]. For example, in chickpeas, the resistance against the fungus Ascochyta rabiei was related to higher expression of CuAO compared with susceptible cultivars [57]. However, both CuAO and PAO could act as PA back-converters in peroxisomes [58].
Other sources of apoplastic O2 are the lipoxygenases (LOX), which are nonheme iron-containing dioxygenases [59]. LOX activity can produce lipid peroxidation, leading to the formation of polyunsaturated fatty acids (PUFA) which can generate ROS [33]. It was observed that LOX activity and lipid peroxidation was increased in maize leaves during low temperatures, suggesting that lipoxygenase-mediated peroxidation of membrane lipids contributes to the oxidative damage occurring in chill-stressed maize leaves [60]. Moreover, apoplastic lipoxygenases were shown to be involved in the oxidative burst together with peroxidases and amine oxidases in Pisum sativum seedlings upon wounding [61]. Besides, an elevated LOX activity was observed during infection of pea roots with the cyst nematode Heterodera goettingiana [62].
Even though apoplastic ROS (apROS) represents only a minor part of the total ROS level of cells, it has great importance since it regulates whole-plant growth by controlling cell division rate and cell elongation [33]. Moreover, an important function of apROS is the regulation of stomatal movement, and cell wall reinforcement by inducing lignin biosynthesis [28,63]. In the same way, apROS are involved in signal transduction from extracellular spaces to the cell interior and may directly eliminate invading pathogens [33]. This is achieved through the activation of immune receptors localized in the plasma membrane such as the receptor-like kinases (RLKs). It was observed that apROS production often occurs following activation of RLK signaling in different cellular processes [64]. Moreover, these ROS can further enter inside the plant cell as H2O2 through membrane aquaporin channels [64]. ApROS were also found to regulate callose deposition at the cell wall, increasing, that way, plant resistance to fungal pathogens. In addition, ROS’ ability to modify the structures of several proteins which are known to play important roles in plant immunity has also been reported [28].
3. Antioxidants in the Apoplast
As mentioned above, ROS can be at the same time beneficial and deleterious for the plant, since they can act as secondary messengers in different physiological processes [65]. However, they can also induce oxidative damage under several environmental stress conditions, such as salinity, drought, cold, heavy metals, and UV irradiation. In the latter case, ROS accumulation may cause many cellular damages that consist of degradation of several biomolecules such as pigments, proteins, lipids, carbohydrates, and DNA, which finally leads to PCD. It was revealed that a high concentration of ROS has a toxic role in plant cells, so the actions of ROS scavengers and antioxidant enzymes are required to avoid its toxicity [65]. The components of the antioxidant machinery can be broadly divided into enzymatic and non-enzymatic. The relevant ROS-scavenging enzymes are superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) (Table 1). Moreover, the non-enzymatic antioxidants such as ascorbate, glutathione, proline, phenolic compounds, and polyamines are also required to avoid cell toxicity [66] (Table 2). Superoxide dismutases (SOD) are enzymes that convert O2•− into H2O2, which is less harmful to the plant. Therefore, they are considered as the first line of detoxification of ROS. SODs are classified according to the metal cofactor used by the enzyme, as manganese (Mn-SOD), iron (Fe-SOD), and copper–zinc (Cu/Zn-SOD) dependent, although Cu/ZnSOD was identified as the most active apoplastic isoform. Furthermore, many studies have reported that these enzymes confer resistance to abiotic stress. These studies highlight the fact that the tolerance level of plants is positively correlated with SOD activity and with the number of SOD isoforms. SOD is up-regulated in many plant species by various types of abiotic stress-inducing factors, such as drought, salt, and heavy metals, in a large number of crops, such as, tomato, wheat, barley, and citrus [67]. Related to this, it was shown that apROS production in wheat root decreased upon a copper treatment, which was correlated with an increase in SOD activity [68]. Moreover, Garcia et al. [69] found a positive correlation between SOD activity and salt-tolerance when studying the response of the apoplastic antioxidant systems in root and leaf tissues from a salt-sensitive and a salt-resistant onion genotype in response to salinity. Furthermore, increased SOD activity was also observed in response to biotic stress. Vanacker et al. [70] showed that the activities of several antioxidant enzymes such as SOD, CAT, APX, DHAR, MDHAR, and GR were induced in the apoplasts of barley and oat leaves 24 h after inoculation with the biotrophic fungal pathogen Blumeria graminis [71]. Furthermore, Trichoderma harzianum inoculated sunflower plants were more resistant to Rhizoctonia solani. This resistance was accompanied by an increase in SOD activity in root apoplast seven days post-inoculation [72].
Table 1.
Schematic overview of the enzymatic antioxidants.
| ENZYMATIC ANTIOXIDANTS | ||||
|---|---|---|---|---|
| Enzyme | Chemical Reaction | Involved in | Cellular Location | Ref. |
| Superoxide Dismutase (SOD) | O2•−+O2•−+2H+→2H2O2+O2 | Regulation of oxidative stress. Stress resistance or tolerance mechanisms |
Apoplast, cytosol, mitochondria, chloroplast, peroxisomes | [23,69] |
| Catalase (CAT) | H2O2→H2O+(1/2) O2 | Regulation of oxidative stress. Stress resistance or tolerance mechanisms. Plant metabolism |
Apoplast, cytosol, chloroplast, mitochondria, peroxisomes | [23,27,73] |
| Ascorbate Peroxidase (APX) | H2O2+Asc→2H2O+DHA | Regulation of oxidative stress. Stress resistance or tolerance mechanisms Plant growth and physiology |
Apoplast, cytosol, mitochondria, peroxisomes, chloroplast | [74,75] |
| Monodehydroascorbate Reductase (MDHAR) | MDHA+NADPH→Asc+NADP+ | Regulation of oxidative stress. Stress resistance or tolerance mechanisms |
Apoplast, cytosol, mitochondria, chloroplast | [23,76,77] |
| Dehydroascorbate Reductase (DHAR) | DHA+2GSH→Asc+GSSG | Regulation of oxidative stress Stress resistance or tolerance mechanisms Plant growth and development |
Apoplast, cytoplasm, mitochondria, chloroplast, peroxisomes | [23,78,79] |
| Glutathione Reductase (GR) | GSSG+NADPH→2GSH+NADP+ | Regulation of oxidative stress. Stress resistance or tolerance mechanisms |
Apoplast, cytoplasm, mitochondria, chloroplast | [80] |
Table 2.
Schematic overview of the non-enzymatic antioxidants.
| NON-ENZYMATIC ANTIOXIDANTS | |||
|---|---|---|---|
| Enzyme | Functions | Location | Ref. |
| Ascorbic Acid (AsA) | -Stress perception -Redox homeostasis -Regulation of oxidative stress -Improvement of plant stress tolerance |
Apoplast, cytosol mitochondria, chloroplast, vacuoles, peroxisomes, nucleus | [23,81] |
| Glutathione (GSH) | -Protect membranes. -Prevent protein oxidative denaturation under stress conditions -Substrate for glutathione peroxidase and gluthatione S-transferase -Metal chelator |
Apoplast, cytosol, chloroplast, mitochondria, vacuole, peroxisome, nucleus | [81] |
| Proline (Pro) | -Osmoprotectant activity -Antioxidant capacity -Metal chelator -Signalling under abiotic and biotic stresses -Plant growth and development |
Apoplast, cytosol, mitochondria, chloroplast | [82,83] |
| Phenolic Compounds | -Antioxidant activity -Metal chelator -Protective and signalling functions against different stresses -Plant growth and development |
Ubiquitous | [84] |
| Polyamines | -Antioxidant capacity -Plant growth and development. -Biotic and abiotic stress responses. -Osmotic adjustment ability |
Ubiquitous | [85,86] |
Catalases (CAT) are the only and the main scavenging enzymes that do not require any reductant for ROS decomposition and directly decompose H2O2 to water and O2 [33]. Moreover, it seems that there is a link between CAT activity and ROS production by RBOH and POXsin the apoplast [87]. In addition, CAT seems to have the highest enzyme activity in this intercellular space, reaching around 0.2% to 2% of the total [70,71,88]. Several studies have shown that an increase of CAT is essential for plant defense against different stresses, since its correct functioning could avoid the damages produced by ROS accumulation. For example, the overexpression of a catalase gene isolated from Cucumis sativum (CsCAT3) in E. coli could increase the tolerance to cold, heat, osmotic, or salinity stresses [89]. Moreover, it was also suggested that SA binds this enzyme, reducing its activity such as it occurs in NO3− treated citrus plants exposed to salinity compared to NH4+ treated plants that showed an increase in CAT activity, allowing plants to tolerate better the salinity-induced oxidative damage [90].
Within the antioxidant system in plants, the ascorbate–glutathione (Asc-GSH) pathway plays a major role in regulating ROS. Four enzymes are engaged in this pathway: ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR), and glutathione reductase (GR). These enzymes catalyze the reduction of H2O2 to water using ascorbate (Asc) and glutathione (GSH) as electron donors. In the Asc-GSH pathway the reduction of H2O2 is ultimately linked to NAD(P)H oxidation. All the enzymes of the Asc-GSH pathway were found in the apoplasts of barley, oat, and pea leaves, but their activity was much lower when compared to their symplastic activity [70,71]. However, in the apoplasts of other plants, not all Asc-GSH pathway enzymes were found to be active; therefore, only certain enzymatic reactions may take place [91].
Ascorbate peroxidase (APX) is a key enzyme of the Asc-GSH pathway [92]. APX catalyzes the reduction of H2O2 to water using Asc as an electron donor. Based on amino acid composition, five isoforms of APX have been identified in plants: cytosolic (cAPX), mitochondrial (mitAPX), chloroplastic (chlAPX: stromal-APX and thylakoidal-APX), and peroxisomal/glyoxysomal (mAPX) [93]. In Arabidopsis only the cytosolic isoform was found to be secreted to the apoplast [94]. It was observed that the expression of APX encoding genes takes place under abiotic stresses (salt, drought, heat, cold, UV radiations, oxidative stress, etc.) and under biotic stresses (pathogen attack, herbivory). The role of APX isoforms concerning different types of abiotic stresses is reviewed in detail by Pandey et al. [93]. An increase in APX in the leaf apoplast of Ctenanthe setosa in response to drought stress was observed [95]. Related to biotic stress, in the Plum pox virus-resistant apricot cultivar Stark Early Orange, a rise in class I APX and strong increases in POX and SOD activities were noticed in the apoplastic compartment [96].
Monodehydroascorbate reductase (MDHAR) is responsible for regenerating Asc from monodehydroascorbate (MDHA) using NADPH as a reducing agent. Therefore, MDHAR is an important enzyme responsible for maintaining the reduced Asc pool, by optimizing the recycling of oxidized Asc and is a key component in the stress tolerance profile of a plant. The activity of MDHAR is induced by various stress conditions [97,98,99]. Although MDHAR activity has been detected in several cell compartments, such as chloroplasts, mitochondria, peroxisomes, and the cytosol, little evidence exists about its presence in the apoplast. Related to this, Bindschedler et al. have shown that one of the six MDHAR isoforms found in Arabidopsis seems to be localized in the apoplastic space [76].
Dehydroascorbate reductase (DHAR) reduces dehydroascorbate (DHA) to Asc using reduced GSH as an electron donor [99]. Therefore, DHAR is involved together with MDHAR in regenerating the Asc pool, maintaining, that way, the Asc levels in both symplast and apoplast. Overexpression of DHAR has highlighted its important roles in ascorbate regeneration and responses to environmental stresses. DHAR functions in plant defense, growth, and development were reviewed in detail by Dingl et al. [78].
Glutathione reductase (GR) is also an important component of the antioxidant machinery. GR catalyzes the reduction of glutathione disulfide (GSSG) to GSH using NADPH as a reductant, which is of great importance for maintaining the cellular redox balance of GSH/GSSG. GR was found to play a positive role in tolerance to abiotic stress [79]. For example, an increase in GR in the leaf apoplast of Ctenanthe setosa in response to drought stress was described [95]. As mentioned above, Vanacker et al. [71] observed an increase in GR in barley and oat apoplast upon Blumeria graminis infection.
Ascorbic acid (AsA) or vitamin C is an antioxidant that plays an important role in plant growth and environmental stress tolerance. The physiologically active form of AsA is termed ascorbate (Asc). The most abundant pool of Asc is found in the cytoplasm, while in the apoplastic space of most plants, the Asc content was estimated to reach up to 10% of the total cellular pool. Despite this, the apoplastic fraction of AsA is considered of great importance for oxidative stress signaling. The antioxidant capacity of the apoplast is generally low and despite the presence of other antioxidants, it is highly dependent on AsA pools [100]. To protect against the deleterious effects of ROS, the AsA pools are required to be maintained in a reduced state. For this purpose, Asc is oxidized to MDHA by the action of APX and then to DHA by non-enzymatic dissociation. The oxidized forms of Asc are converted back to reduced Asc by MDHAR using NADH or NADPH as an electron donor and by DHAR using reduced GSH as an electron donor, respectively. Due to its apoplastic localization, AsA plays an important role in stress perception, redox homeostasis, and regulation of oxidative stress and plant responses under normal or abiotic stress conditions. AsA has been shown to be effective in improving stress tolerance in plants. Several examples can be found in Akram et al. [100].
Apart from the AsA, there are many non-enzymatic compounds that intercept and complete free radical reactions and possess the capacity to decrease substrates for antioxidant enzymes, reducing the deleterious effects of ROS in plants. Several examples of these antioxidants are glutathione, proline, phenolic compounds, and polyamines, among others. GSH is known as a powerful scavenger that has diverse functions, such as protecting cell membranes, preventing oxidative denaturation of proteins, and being a substrate for glutathione peroxidase and S-transferase [101]. Thus, GSH is also involved in stress responses, as was shown in Arabidopsis thaliana plants, where an increase of GSH was related to drought and salt tolerance [102]. Moreover, a relation of GSH with heat tolerance has been suggested, since an accumulation of GSH levels was also observed in wheat plants under heat stress [103], and a high GSH was observed in heat-tolerant wheat genotypes [104,105]. In addition, proline is a proteinogenic amino acid that possesses osmoprotective activity, and an antioxidant, a metal chelator, and a signaling molecule during stresses [106]. When this amino acid was exogenously supplied, a reduction of ROS was observed in the roots of Arabidopsis thaliana [107]. Thereby, proline application alleviates salinity stress in Vicia faba plants [108]. Moreover, an accumulation of proline was observed in drought-tolerant and salinity-tolerant chickpea and rice plants, respectively [109,110]. Finally, increased concentrations of proline in response to drought stress were detected in both the apoplastic and symplastic compartments of leaves from drought-tolerant and drought-sensitive cultivars of the common bean [82]. In the same way, an increase in the apoplastic proline content was observed by Saglam et al. [111] in Ctenanthe setosa plants submitted to drought stress. Other non-enzymatic compounds are phenolic compounds, secondary metabolites that donate electrons or atoms to ROS, and inhibiting oxidizing enzyme activities [112,113]. The accumulation of these compounds in different crops growing under heavy metals, salt, or drought stresses has been widely studied [114]. Finally, the role of polyamines should be pointed out, since they are small aliphatic amines involved in the antioxidant response [56], but as mentioned above, their catabolism also produces H2O2 by AOs action in the apoplast [58].
To sum up, it is important to mention that there is a relation between the different antioxidants mentioned, and the combined action of the whole set of antioxidant defense compounds produces an enhancement of the response to cope with environmental stresses.
4. Apoplastic Proteins and Peptides in Modulating Plant–Pathogen Interactions. Microbe-Associated Molecular Patterns (MAMPs) of Proteic Nature
The most abundant proteins related to defense are the pathogen-related proteins (PR-proteins) that represent 23–33% of the total apoplastic fluid proteins (AFPs) [115]. The production and accumulation of PR-proteins are produced by various stresses, including biotic/biological stresses and abiotic/non-biological stresses [116]. The plant PR-proteins are divided into 17 groups (Table 3). Moreover, based on the amino acid sequence and the biochemical activity identified during the last decade, they also include novel peptide families. The PR peptides include proteinase inhibitors (PR-6 family), plant defensins (PR-12 family), thionins (PR-13 family), and lipid transfer proteins (PR-14 family) [117,118].
Table 3.
Classification of pathogenesis-related proteins and peptides.
| Families | Properties | References |
|---|---|---|
| PR-1 | Antifungal | [119] |
| PR-2 | β-1,3-glucanase | [120] |
| PR-3 | Chitinase type I, II, IV, V, VI, VII | [121] |
| PR-4 | Chitinase type I, II | [122] |
| PR-5 | Thaumatin- like | [39] |
| PR-6 | Proteinase- inhibitor | [123] |
| PR-7 | Endoproteinase | [124] |
| PR-8 | Chitinase type III | [125] |
| PR-9 | Peroxidase | [126] |
| PR-10 | Ribonuclease like | [127,128] |
| PR-11 | Chitinase, type I | [129] |
| PR-12 | Defensin | [130] |
| PR-13 | Thionin | [131] |
| PR-14 | Lipid- transfer protein | [132] |
| PR-15 | Oxalate oxidase | [133] |
| PR-16 | Oxalate oxidase-like | [123] |
| PR-17 | PRp27 Unknown | [134] |
Besides the plant PR-peptides mentioned above, the regulation of signaling pathways by small peptides is a central theme in molecular biology. The apoplast is also the space where the microbe-associated molecular patterns (MAMPs), by plant cell surface-localized pattern recognition receptors (PRRS), are initially determined [135].
4.1. Apoplastic Proteins Related to Plant Defense
Nowadays, PR-proteins, based on their protein sequences similarities and other biological features, show diverse functions, such as β-1,3-glucanase (PR-2), chitinases (PR-3), thaumatin-like (PR-5), and peroxidases (PR-9) [118,136].
Despite their differential fungicidal activity, PR-1 proteins are often used as markers for salicylic acid (SA)-mediated disease resistance [115,119]. PR-1 proteins have been identified in Oryza sativa, Nicotiana tabacum, and Arabidopsis, and often have a molecular weight of 14 to 17 kDa [137]. Although PR-1 proteins can have different properties and differ substantially in biological activity, all of them have a similar structure and are classified in the same family based on sequence homology [39].
Plant β-1,3-glucanases are grouped in the PR-2 family of PR proteins and play an imperative role in plant defense, and the usual processes of plant growth. These proteins have a size from 33 to 44 kDa, with both acidic and basic isoforms [120]. Based on the amino acid sequencing, structural features, and cellular localizations, the glucanases have been classified into three major classes and two minor classes [138,139,140]. Most of classes II, III, and IV of β-1,3-glucanases acidic proteins were found and secreted into the extracellular spaces. Moreover, several studies have shown that the synthesis of β-1,3-glucanases is stimulated by pathogen infections and it makes the plant resistant to fungal pathogens either alone or in association with other proteins, including chitinases, peroxidases, and thaumatin-like proteins [141,142,143]. Furthermore, it has been suggested that β-1,3-glucanases act as the key enzymes in the lysis of phytopathogenic fungal cell walls during the antagonistic action by hydrolyzing the O-glycosidic linkages of β-glucan chains [144,145]. Besides, the results shown by Floerl et al. [10] demonstrated PR-2 apoplastic activity in susceptible primary leaves of Arabidopsis thaliana 25 days after inoculation with Verticillum longisporum. Additionally, in the related article they reported that β-1, 3-glucanases are increased in wheat plants (Triticum aestivum) in response to Zymoseptoria tritici infection [122].
Chitinases are a huge and diverse group of enzymes that have been classified into two main categories—endochitinases and exochitinases, and based on their primary structures, plant chitinases have been divided into seven classes, class I through VII [146,147]. Plant chitinases which represent a major group of PR-proteins are expressed in response to environmental stresses and the attack of phytopathogens and secreted in extracellular space [148,149,150,151,152]. Moreover, several studies have shown that extracellular chitinases induce chitinase biosynthesis by detecting fungal elicitors and block the spreading hyphae invading intercellular spaces as well. For example, Petriccione, et al. [153] reported that extracellular chitinase was induced in infected Actinidia deliciosa leaves with Pseudomonas syringae. Furthermore, Pechanova et al. [154] showed that class IV chitinase and acidic class III chitinase were accumulated in leaves of poplar upon water stress. They demonstrated that three different type III chitinases were detected in leaf apoplasts, while the poplar stem apoplast contains only one single type III chitinase. In addition, apoplastic chitinases are involved in the destruction of the bacterial cell wall, and in the production of ROS by the production of chitin oligomers [155]. Chitinase induction has been reported against bacterial (mainly Pseudomonas) and fungal pathogens in Actinidia deliciosa and Brassica napus subsp. Napus, respectively [153,156]. Additionally, leaf apoplastic chitinases and 1, 3-β-glucanases increased in response to Cladosporium fulvum and Septoria tritici diseases [157,158]. Moreover, Han et al. [159] described the apoplastic Cys-rich repeat protein 1 (CRR1), a protector of chitinase, helping the plant resistance to the pathogen releasing VdSSEP1 protease.
On the other hand, thaumatin-like proteins (TLPs) are classified as the PR-5 protein family [39]. Several studies have shown that plant TLPs are secreted in the apoplast and are involved in plant protection against biotic and abiotic stresses [160,161,162,163]. Furthermore, various studies have demonstrated that TLPs exhibit antifungal activity. For example, Shatters Jr. et al. [164] revealed that PR-5 proteins can affect fungi, either by disrupting its membranes or by hydrolyzing β-1,3-glucans of the cell walls. Additionally, Wang et al. [163] have shown that the expression of TLP genes in plants infected by fungi and bacteria is significantly increased.
Germin–like proteins (GLPs) are a large gene family that belongs to the cupin superfamily [165]. GLPs are involved not only in plant development but also in plant defense against biotic and abiotic stresses, including bacteria [166], fungi [167,168], salt pressures [169], and drought stresses [170]. Moreover, ADP–glucose pyrophosphatase or phosphodiesterase (AGPPas), oxalate oxidase (OxO), and superoxide dismutase (SOD) are three enzymes which are associated with GLPs [171]. In general, the primary reactions detectable in plant–pathogen recognition are the opening of specific ion channels and the formation of reactive oxygen intermediates [172]. It seems that SODs in association with GLPs protect the plant from the effect of oxidative stress by quickly converting O2− and H2O into H2O2 and O2 [173,174]. Furthermore, GLPs also seem to be related to phytohormones, since the transcript levels of some GLPs are enhanced after application of phytohormones, including SA and ABA [175]. Additionally, a GLP with serine protease inhibitory activity (GLP-serine protease inhibitor) has been detected in the wheat apoplast and suggested to be part of a defense system against insect and bacterial proteases [157]. Moreover, GLPs are also expressed in abiotic stress. For example, expression of GLPs in tomato and Nicotiana tabacum plants in response to salt stress has been reported by Amini et al. [176], and Dani et al. [169], respectively.
The regulation of secreted proteases and protease inhibitors (PIs) plays an important role in apoplastic immunity [177]. It seems that proteases increase after pathogen infection systemically and locally [178]. Specifically, subtilisin-like serine proteases or subtilases play an essential role in responses to environmental stress [179]. For example, the apoplastic PR protease (P69B) is required for the induction of tomato immunity against the pathogen Phytophthora infestans [180]. Moreover, it has been also described that the Arabidopsis SBT3.3 gene, encoding an extracellular subtilase homologous to the tomato P69C, also plays a role in immune priming by induction SA-responsive gene [181].
4.2. Apoplastic Peptides Related to Plant Defense
PR gene families PR-6, PR- 12, PR-13, and PR-14, which contain protease inhibitors, defensins, thionins, and lipid transfer proteins respectively, are the so-called antimicrobial peptides (AMPs). These AMPs are usually cysteine-rich molecules that possess potential and a broad range of antimicrobial activities [118,132]. Specifically, the peptides belonging to the PR-6 family have shown effective antimicrobial activity against fungal pathogens [182]. Apoplastic papain-like cysteine proteases (PLCP) play an important role in apoplastic immunity by releasing small peptides that serve as damage-associated molecular patterns (DAMPs) [183]. In addition, it is worth noting that PLCPs and serine hydrolases (SHs) protease families are up-regulated upon pathogen infection or targeted by pathogen-derived inhibitors [184]. For example, Ziemann et al. [185] pointed out that Zip1 peptide, which is released in the presence of PLCP, leads to SA accumulation in maize leaves. Moreover, Schulze Hüynck et al. [186] identified a specific PLCP named CP1C in maize root. Surprisingly, CP1C which shares sequence homology to the Arabidopsis RD21 subfamily did not show higher activity after SA treatment of maize roots. Moreover, RCR3 is an apoplastic PLCP that acts as PR protein against fungal pathogens and nematodes [187,188].
Plant defensins and thionins are small, cysteine-rich peptides (around 5 kDa) which are grouped into plant-specific antimicrobial peptides [189,190]. There is strong evidence that some defensins are also induced upon pathogen attack, environmental stimuli, and jasmonate treatment [130,191]. For example, the defensin gene MtDef4.2 of Medicago truncatula plants provides strong resistance to the fungus Puccinia triticina in transgenic wheat [192]. Additionally, the Arabidopsis thaliana apoplastic defensin type 1.1 (AtPdF1.1) acts to mediate defense against Pectobacterium carotovorum subsp. Carotovorum by activation of ethylene pathway in result of iron-deficiency [193]. Moreover, an increasing level of Tad1 which is a plant defensin-like has been detected in wheat in response to pathogen attack and cold temperatures. Interestingly, the Tad1 expression is an independent defense signal and it is not regulated by the methyl jasmonate acid pathway [194].
Lipid transfer proteins (LTPs) are small, cationic, cysteine-rich peptides, which are recognized based on their role and ability to transport lipids between cell membranes. They usually have below 10 kDa molecular weights [195,196]. Extracellular LTPs have been found in various plant species, such as tobacco, cowpea (Vigna unguiculata), Medicago, and sunflower [197,198,199]. Researchers have been interested in LTPs for three main reasons: for their ability of transferring and binding to lipids, because they are one of the components of plant innate immunity, and for their clinical features [200]. Apoplastic LTPs were shown to be involved as major players in cutin formation [201]. However, much evidence showed that LTPs play a key role in plant defense mechanisms [202,203]. For example, Dani et al. [169] detected two LTPs in the leaf apoplasts of tobacco in response to salt stress. It seems that the induction of LTPs during this, stress causes deposition of cuticular material decreasing, and that way, leaf transpiration. Moreover, defective-mutants in the induced resistance gene DIR1, which encodes apoplastic LTPs, can still promote the SA-mediated pathway, but they have disabled the induction of systemic acquired resistance (SAR), which is one of the resistance mechanisms in plants [203,204].
4.3. Microbe-Associated Molecular Patterns of Proteic Nature
The recognition of microbe-associated molecular patterns (MAMPs) triggers several cellular response processes, such as alteration of ionic current between cell membranes which alkalizes intercellular space, increasing the cytoplasmic calcium ion concentration [184], and the biosynthesis of ethylene stress hormone which is activated by 1-aminocyclopropane-1-carboxylic acid-synthase (ACC-synthase). Other primary responses include activation of mitogen-activated protein kinases (MAPKs), which in turn activate transcription factors [205]. This is followed by transcription of protein-coding genes such as defensins and other antimicrobial metabolites [206].
Demonstrating the action of bacterial flagellin (a type of protein subunit of the bacterial flagellum) in the plant was the turning point in understanding innate immunity [207]. Previous studies have shown that flagellin, or more precisely flg22, inhibits the growth of Arabidopsis and leads to callose deposition in leaf tissue [208]. Flg22 led to alkalization of the tobacco cell culture suspension, and stimulated the production of ROS in the tobacco leaves. The receptors responsible for flagellin perception by Arabidopsis are the leucine-rich repeat (LRR) transmembrane receptor kinase flagellin sensitive 2 (FLS2) and the co-receptor brassinosteroid insensitive 1-associated kinase 1 (BAK1) [209,210]. It was determined that the message sent by FLS2 was through a cascade of MAPK, including AtMPK3 and AtMPK6, and led to an induction of the expression of WRKY22 and WRKY29 genes [205]. Moreover, when plants were treated with flg22 it protected them against a subsequent pathogen challenge, providing direct evidence that it drives an effective immune response in the plant. In addition, it seems that the flagellin perception on leaf surface may be important in stimulating the active defense response because the Arabidopsis fls2 mutants, which had lost the flagellin receptor, were more susceptible to Pseudomonas syringae pv DC3000 infection [211].
The elongation factor thermal unstable, abbreviated as Ef-Tu, is another widely described MAMP protein which plays an unavoidable role in protein biosynthesis, so its presence is inevitable for bacteria. The protein was isolated and identified from E. coli, which had been shown to have severe MAMP activity even by disrupting its flagellin gene [212]. The receptor of this protein, called elongation factor receptor (EFR), like FLS2 also belongs to the LRR family [213].
Moreover, many plant species of the Solanaceae family detect the highly conserved nucleic acid binding motif RNP-1 of bacterial cold-shock proteins (CSPs), represented by the peptide csp22, as a MAMP. The recipient of this MAMP, which is a kind of plant receptor-like kinase (RLK), was identified in tomato and named CORE. Transfer and expression of cold shock protein receptor to Arabidopsis thaliana can increase plant resistance to P. syringae pv. tomato [214].
Unlike flagellin, EF-Tu, and CSP, the fungal protein ethylene-inducing xylanase (EIX) activity cannot be attributed to a peptide. Fungal xylanase—by releasing cell wall fragment, and therefore, the production of DAMPs—can activate various defense responses such as alkalization of the extracellular space, ethylene biosynthesis, and the production of ROS in tomato and tobacco [215,216,217]. EIX is recognized by the tomato leucine-rich repeat receptor-like proteins with a signal for receptor-mediated endocytosis, LeEIX1, and LeEIX2, of which only the latter mediates a hypersensitive response [218].
Besides proteic MAMPs, structural components of bacterial cell wall such as lipopolysaccharides (LPS) and peptidoglycan (PGN) can also act as MAMPs in the plant [219,220,221].
5. Hormones Found in the Apoplast and Their Functions
As mentioned above in the introduction, the apoplast consists of the intercellular space, the cell walls, and xylem. However, this space is not homogeneous throughout the plant, since a clear difference in its composition can be observed depending on the organ where it is localized and within each organ this composition varies according to the characteristics of the tissue and its function in the plant.
The hormonal levels in the apoplast are significantly lower than inside the cell and depend on the presence of transmembrane efflux carriers [222]. The main question is whether these molecules perform important functions in the apoplast. Already in 1998, Sakurai [201] mentioned the presence of auxins and cytokines in the apoplast that seemed to be involved in the transport pathway. Moreover, the concentration of these hormones was found to vary according to the different parts of the plant where the apoplast was localized and also according to the different stages of development of the plant that helped to postulate their role as growth regulators. Kramer [222] who analyzed the movement of auxins, ABA, and gibberellins in the apoplast, highlights the fact that these hormones are usually removed from the apoplast and concentrated in the cytoplasm of plant cells, playing, that way, an important role in the intracellular communication. He also showed that when reentering the extracellular, space these hormones can travel only a limited distance before reentering a cell. Although according to this author, the presence of these hormones in the apoplast does not seem to have a specific function, the auxin role in controlling shoot growth through apoplastic pH change has long been demonstrated [223]. Recently Barbez et al. [224] have demonstrated the auxin role in root growth, since endogenous auxin levels could trigger apoplastic acidification and cell elongation in A. thaliana. In contrast, when increasing the levels of endogenous or exogenous auxin, they observed an inhibitory effect on root growth due to the induction of a transient alkalinization of the apoplast and the subsequent reduction of cellular elongation.
On the other hand, Hartung et al. [225] showed how ABA, which is considered one of the main hormones involved in plant response to stress, moves through the xylem from the root to the different parts of the shoot where it regulates water loss and leaf growth. The ABA present in the apoplast can come from two endogenous sources, on one hand from the synthesis in the root cells and on the other hand from the synthesis in the mesophyll cells. The ABA synthesized in the mesophyll cells can be loaded to the phloem, from where it will be transported to other parts of the plant. Moreover, it is known that abiotic stresses like salinity or drought can produce an increase in the levels of ABA in the apoplast and that after acting on stomatal closure, ABA is rapidly degraded. Therefore, it seems that free ABA does not persist for a long time in the apoplast. Instead, it can be found in conjugated form as ABA-glucose (ABA-GE). Related to this, Sauter et al. [226] showed that ABA-GE levels in the leaf apoplast increase 7 times under stress conditions. One of the situations in which the role of ABA in the apoplast has been studied in detail is the activation of the synthesis of hydrogen peroxide in the apoplast, demonstrating its regulatory role concerning other hormones such as salicylic acid, jasmonic acid, and ethylene [33].
The role played by the hormones SA, JA/ET, and ABA in plant defense against biotic stresses is well known. The presence of these hormones in the apoplast is of great importance for pathogen control since many of them develop the first stages of infection in the apoplast. Scalschi et al. [11] observed that, although the content of the hormones JA, SA, OPDA, and ABA was much lower in the apoplast when compared to the content observed in leaves, it increased significantly in the presence of the pathogen. Moreover, considering that SA can act directly on the bacteria and that there are bacteria such as P. syringae, or R. solanacearum that live in the apoplast, it could well be that SA acts directly on them at this level.
One of the most studied pathosystems at the apoplast level is the Arabidopsis-Pseudomonas syringae pv tomato. It is well known that the earliest events of P. syringae infection occur in the apoplast. The interaction between auxins and cytokinins in this pathosystem has been studied [227]. Both hormones can be present in the apoplast and they usually interact antagonistically at many levels. Cytokinin can activate SA pathway in the presence of the pathogen by binding to the AHK proteins (Arabidopsis histidine kinase) present in the apoplast. This induces the phosphorylation of AHP proteins (Arabidopsis histidine phosphotransfer protein), and together with it the activation of SA signaling cascade [227] which helps the plant to defend itself against the pathogen. On the other hand, it has been shown that Pseudomonas is able to induce auxin synthesis through small molecules called effectors that the bacterium injects into the plant cell through the type III secretion system. Besides, the bacterium can induce the transport of free auxins in the apoplast through the AUX1 system (auxin input transporter). The presence of auxins in the cytoplasm can induce the JA/ET pathway that acts as an antagonist of SA pathway, therefore plant response against the bacteria will no longer be effective [228].
Finally, the redox status of the apoplast has been shown to be fundamental for the control of hormonal responses and the signaling cascades of the different defense pathways [229]. In general, it seems that the oxidation of the apoplast deactivates the hormones present in it. SA was shown to induce ROS accumulation during pathogen responses [230]. In addition, hormones belonging to the indole family, such as auxins, can act as antioxidants [231] since their effect is greater under reduced conditions, being able to promote the generation of hydroxyl radicals (•OH) in the apoplast and contribute to plant growth [232].
6. Concluding Remarks
In this review we have collected the current knowledge about the apoplastic response against plant stresses, including the hormones, ROS production, antioxidants, and defensive proteins and peptides. Nevertheless, in order to fully clarify the role of the apoplast in the response to plant stress, it is necessary to continue the research in extracellular compartment responses to understand the perception and transduction of the signals and the modification of metabolites. In their natural environment, plants are exposed to different abiotic and biotic stresses, and have developed an array of adaptive mechanisms, which are based on a combination of signaling pathways that allow them to react to adverse conditions and survive pathogen attacks. Due to its extracellular nature, the apoplast is involved not only in the response but also in the perception and transduction of environmental signals. This space is also considered as the first layer of stress recognition and the first battlefield against pathogens. However, the information about the presence and modulation of antioxidants, hormones, and other metabolites related to plant defense is still limited, since most of the research is focused on the response of the whole tissue and does not single out the apoplastic response.
Funding
This work was supported by Plan de Promoción de la Investigació de la Universitat Jaume I (UJI-B2017-30 and UJI-A2019-19) and by the Spanish Ministry of Science and Innovation (AGL2017-85987-C3-1-R) E.L. is the holder of a “Juan de la Cierva-Incorporación” fellowship from MINECO.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Münch E. Die Stoffbewegungen in der Pflanze. G. Fischer; Schaffhausen, Switzerland: 1930. [Google Scholar]
- 2.Boyer J.S. Cell wall biosynthesis and the molecular mechanism of plant enlargement. Funct. Plant Biol. 2009;36:383. doi: 10.1071/FP09048. [DOI] [PubMed] [Google Scholar]
- 3.Pauly M., Keegstra K. Biosynthesis of the Plant Cell Wall Matrix Polysaccharide Xyloglucan. Annu. Rev. Plant Biol. 2016;67:235–259. doi: 10.1146/annurev-arplant-043015-112222. [DOI] [PubMed] [Google Scholar]
- 4.Voiniciuc C., Pauly M., Usadel B. Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol. 2018;176:2590–2600. doi: 10.1104/pp.17.01776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q., Luo L., Zheng L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018;19:335. doi: 10.3390/ijms19020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch W., Kwart M., Laubner M., Heineke D., Stransky H., Frommer W.B., Tegeder M. Reduced amino acid content in transgenic potato tubers due to antisense inhibition of the leaf H+/amino acid symporter StAAP1. Plant J. 2003;33:211–220. doi: 10.1046/j.1365-313X.2003.01618.x. [DOI] [PubMed] [Google Scholar]
- 7.McCully M.E. Niches for bacterial endophytes in crop plants: A plant biologist’s view. Aust. J. Plant Physiol. 2001;28:983–990. doi: 10.1071/PP01101. [DOI] [Google Scholar]
- 8.Sattelmacher B. The apoplast and its significance for plant mineral nutrition. New Phytol. 2001;149:167–192. doi: 10.1046/j.1469-8137.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 9.Rico A., Preston G.M. Pseudomonas syringae pv. tomato DC3000 Uses Constitutive and Apoplast-Induced Nutrient Assimilation Pathways to Catabolize Nutrients That Are Abundant in the Tomato Apoplast. Mol. Plant-Microbe Interact. 2008;21:269–282. doi: 10.1094/MPMI-21-2-0269. [DOI] [PubMed] [Google Scholar]
- 10.Floerl S., Majcherczyk A., Possienke M., Feussner K., Tappe H., Gatz C., Feussner I., Kües U., Polle A. Verticillium longisporum Infection Affects the Leaf Apoplastic Proteome, Metabolome, and Cell Wall Properties in Arabidopsis thaliana. PLoS ONE. 2012;7:e31435. doi: 10.1371/journal.pone.0031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scalschi L., Llorens E., González-Hernández A.I., Valcárcel M., Gamir J., García-Agustín P., Vicedo B., Camañes G. 1-Methyltryptophan Modifies Apoplast Content in Tomato Plants Improving Resistance Against Pseudomonas syringae. Front. Microbiol. 2018;9:2056. doi: 10.3389/fmicb.2018.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoson T. Apoplast as the site of response to environmental signals. J. Plant Res. 1998;111:167–177. doi: 10.1007/BF02507163. [DOI] [PubMed] [Google Scholar]
- 13.Horst W.J., Wang Y., Eticha D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann. Bot. 2010;106:185–197. doi: 10.1093/aob/mcq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horst W.J. The role of the apoplast in aluminium toxicity and resistance of higher plants: A review. Zeitschrift für Pflanzenernährung und Bodenkd. 1995;158:419–428. doi: 10.1002/jpln.19951580503. [DOI] [Google Scholar]
- 15.Geilfus C.M. The pH of the Apoplast: Dynamic Factor with Functional Impact Under Stress. Mol. Plant. 2017;10:1371–1386. doi: 10.1016/j.molp.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblueth M., Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Mol. Plant-Microbe Interact. 2006;19:827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- 17.Bulgarelli D., Rott M., Schlaeppi K., Ver Loren van Themaat E., Ahmadinejad N., Assenza F., Rauf P., Huettel B., Reinhardt R., Schmelzer E., et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 2012;488:91–95. doi: 10.1038/nature11336. [DOI] [PubMed] [Google Scholar]
- 18.Romero F.M., Rossi F.R., Gárriz A., Carrasco P., Ruíz O.A. A Bacterial Endophyte from Apoplast Fluids Protects Canola Plants from Different Phytopathogens via Antibiosis and Induction of Host Resistance. Phytopathology. 2019;109:375–383. doi: 10.1094/PHYTO-07-18-0262-R. [DOI] [PubMed] [Google Scholar]
- 19.Turner T.R., James E.K., Poole P.S. The plant microbiome. Genome Biol. 2013;14:1–10. doi: 10.1186/gb-2013-14-6-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Leary B.M., Neale H.C., Geilfus C.M., Jackson R.W., Arnold D.L., Preston G.M. Early changes in apoplast composition associated with defence and disease in interactions between Phaseolus vulgaris and the halo blight pathogen Pseudomonas syringae Pv. phaseolicola. Plant Cell Environ. 2016;39 doi: 10.1111/pce.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doehlemann G., Hemetsberger C. Apoplastic immunity and its suppression by filamentous plant pathogens. New Phytol. 2013;198:1001–1016. doi: 10.1111/nph.12277. [DOI] [PubMed] [Google Scholar]
- 22.Yan J., Yu H., Li B., Fan A., Melkonian J., Wang X., Zhou T., Hua J. Cell autonomous and non-autonomous functions of plant intracellular immune receptors in stomatal defense and apoplastic defense. PLoS Pathog. 2019;15:e1008094. doi: 10.1371/journal.ppat.1008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das K., Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014;2:53. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- 24.Pogány M., Harrach B.D., Hafez Y.M., Barna B., Király Z., Páldi E. Role of reactive oxygen species in abiotic and biotic stresses in plants. Acta Phytopathol. Entomol. Hungarica. 2006;41:23–35. doi: 10.1556/APhyt.41.2006.1-2.3. [DOI] [Google Scholar]
- 25.Foyer C.H., Noctor G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxidants Redox Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- 26.Mittler R. ROS Are Good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Noctor G., Reichheld J.P., Foyer C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018;80:3–12. doi: 10.1016/j.semcdb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Qi J., Wang J., Gong Z., Zhou J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017;38:92–100. doi: 10.1016/j.pbi.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Huang H., Ullah F., Zhou D.X., Yi M., Zhao Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhary A., Kumar A., Kaur N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020;42:33–43. doi: 10.1016/j.pld.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jubany-Marí T., Munné-Bosch S., López-Carbonell M., Alegre L. Hydrogen peroxide is involved in the acclimation of the Mediterranean shrub, Cistus albidus L., to summer drought. J. Exp. Bot. 2009;60:107–120. doi: 10.1093/jxb/ern274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roychoudhury A., Basu S. Oxidative Stress Plants Causes, Consequences Toler. I.K. International Publishing House Pvt. Ltd.; New Delhi, India: 2012. Ascorbate-glutathione and plant tolerance to various abiotic stresses; pp. 177–258. [Google Scholar]
- 33.Podgórska A., Burian M., Szal B. Extra-cellular but extra-ordinarily important for cells: Apoplastic reactive oxygen species metabolism. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minibayeva F., Kolesnikov O., Chasov A., Beckett R.P., LÜthje S., Vylegzhanina N., Buck F., BÖttger M. Wound-induced apoplastic peroxidase activities: Their roles in the production and detoxification of reactive oxygen species. Plant. Cell Environ. 2009;32:497–508. doi: 10.1111/j.1365-3040.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- 35.Almagro L., Gómez Ros L.V., Belchi-Navarro S., Bru R., Ros Barceló A., Pedreño M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009;60:377–390. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- 36.Shigeto J., Tsutsumi Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016;49:1395–1402. doi: 10.1111/nph.13738. [DOI] [PubMed] [Google Scholar]
- 37.Survila M., Davidsson P.R., Pennanen V., Kariola T., Broberg M., Sipari N., Heino P., Palva E.T. Peroxidase-generated apoplastic ROS impair cuticle integrity and contribute to DAMP-elicited defenses. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandey V.P., Awasthi M., Singh S., Tiwari S., Dwivedi U.N. A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem. Anal. Biochem. 2017;6:308. doi: 10.4172/2161-1009.1000308. [DOI] [Google Scholar]
- 39.Van Loon L.C., Rep M., Pieterse C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 40.Passardi F., Cosio C., Penel C., Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- 41.Passardi F., Penel C., Dunand C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 2004;9:534–540. doi: 10.1016/j.tplants.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Kadota Y., Shirasu K., Zipfel C. Regulation of the NADPH Oxidase RBOHD During Plant Immunity. Plant Cell Physiol. 2015;56 doi: 10.1093/pcp/pcv063. [DOI] [PubMed] [Google Scholar]
- 43.Torres M.A., Jones J.D.G., Dangl J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choudhury F.K., Rivero R.M., Blumwald E., Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–867. doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- 45.Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. ROS signaling: The new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Sagi M., Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–340. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marino D., Dunand C., Puppo A., Pauly N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012;17:9–15. doi: 10.1016/j.tplants.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Hu C.-H., Wang P.-Q., Zhang P.-P., Nie X.-M., Li B.-B., Tai L., Liu W.-T., Li W.-Q., Chen K.-M. NADPH Oxidases: The Vital Performers and Center Hubs during Plant Growth and Signaling. Cells. 2020;9:437. doi: 10.3390/cells9020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J., Xia X.-J., Zhou Y.-H., Shi K., Chen Z., Yu J.-Q. RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J. Exp. Bot. 2013;65:595–607. doi: 10.1093/jxb/ert404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernández-Crespo E., Scalschi L., Llorens E., García-Agustín P., Camañes G. NH4+ protects tomato plants against Pseudomonas syringae by activation of systemic acquired acclimation. J. Exp. Bot. 2015;66:6777–6790. doi: 10.1093/jxb/erv382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.González-Hernández A.I., Fernández-Crespo E., Scalschi L., Hajirezaei M.-R., von Wirén N., García-Agustín P., Camañes G. Ammonium mediated changes in carbon and nitrogen metabolisms induce resistance against Pseudomonas syringae in tomato plants. J. Plant Physiol. 2019;239:28–37. doi: 10.1016/j.jplph.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X.Y., Nie Z.H., Wang W.J., Leung D.W.M., Xu D.G., Chen B.L., Chen Z., Zeng L.X., Liu E.E. Relationship between Disease Resistance and Rice Oxalate Oxidases in Transgenic Rice. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0078348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang B., Oakes A.D., Newhouse A.E., Baier K.M., Maynard C.A., Powell W.A. A threshold level of oxalate oxidase transgene expression reduces Cryphonectria parasitica-induced necrosis in a transgenic American chestnut (Castanea dentata) leaf bioassay. Transgenic Res. 2013;22:973–982. doi: 10.1007/s11248-013-9708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angelini R., Cona A., Federico R., Fincato P., Tavladoraki P., Tisi A. Plant amine oxidases “on the move”: An update. Plant Physiol. Biochem. 2010;48:560–564. doi: 10.1016/j.plaphy.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Tavladoraki P., Cona A., Angelini R. Copper-containing amine oxidases and FAD-dependent polyamine oxidases are key players in plant tissue differentiation and organ development. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saha J., Brauer E.K., Sengupta A., Popescu S.C., Gupta K., Gupta B. Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 2015;3:21. doi: 10.3389/fenvs.2015.00021. [DOI] [Google Scholar]
- 57.Angelini R., Bragaloni M., Federico R., Infantino A., Porta-Pugua A. Involvement of Polyamines, Diamine Oxidase and Peroxidase in Resistance of Chickpea to Ascochyta rabiei. J. Plant Physiol. 1993;142:704–709. doi: 10.1016/S0176-1617(11)80906-5. [DOI] [Google Scholar]
- 58.Wang W., Paschalidis K., Feng J.C., Song J., Liu J.H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porta H., Rocha-Sosa M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002;130:15–21. doi: 10.1104/pp.010787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma P., Jha A.B., Dubey R.S., Pessarakli M., Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012;2012:1–26. doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- 61.Roach T., Colville L., Beckett R.P., Minibayeva F.V., Havaux M., Kranner I. A proposed interplay between peroxidase, amine oxidase and lipoxygenase in the wounding-induced oxidative burst in Pisum sativum seedlings. Phytochemistry. 2015;112:130–138. doi: 10.1016/j.phytochem.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Leone A., Melillo M.T., Bleve-Zacheo T. Lipoxygenase in pea roots subjected to biotic stress. Plant Sci. 2001;161:703–717. doi: 10.1016/S0168-9452(01)00458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denness L., McKenna J.F., Segonzac C., Wormit A., Madhou P., Bennett M., Mansfield J., Zipfel C., Hamann T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in arabidopsis. Plant Physiol. 2011;156:1364–1374. doi: 10.1104/pp.111.175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janku M., Luhová L., Petrivalský M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants. 2019;8:105. doi: 10.3390/antiox8040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caverzan A., Casassola A., Patussi Brammer S. Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives. InTech; Nappanee, IN, USA: 2016. Reactive Oxygen Species and Antioxidant Enzymes Involved in Plant Tolerance to Stress. [Google Scholar]
- 66.Kangasjärvi S., Kangasjärvi J. Towards Understanding Extracellular ROS Sensory and Signaling Systems in Plants. Adv. Bot. 2014;2014:538946. doi: 10.1155/2014/538946. [DOI] [Google Scholar]
- 67.Berwal M.K., Ram C. Abiotic and Biotic Stress in Plants. IntechOpen; London, UK: 2019. Superoxide Dismutase: A Stable Biochemical Marker for Abiotic Stress Tolerance in Higher Plants. [Google Scholar]
- 68.Sgherri C., Quartacci M.F., Navari-Izzo F. Early production of activated oxygen species in root apoplast of wheat following copper excess. J. Plant Physiol. 2007;164:1152–1160. doi: 10.1016/j.jplph.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 69.García G., Clemente-Moreno M.J., Díaz-Vivancos P., García M., Hernández J.A. The apoplastic and symplastic antioxidant system in onion: Response to long-term salt stress. Antioxidants. 2020;9:67. doi: 10.3390/antiox9010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanacker H., Harbinson J., Ruisch J., Carver T.L.W., Foyer C.H. Antioxidant defences of the apoplast. Protoplasma. 1998;205:129–140. doi: 10.1007/BF01279303. [DOI] [Google Scholar]
- 71.Vanacker H., Carver T.L.W., Foyer C.H. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998;117:1103–1114. doi: 10.1104/pp.117.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh B.N., Singh A., Singh S.P., Singh H.B. Trichoderma harzianum- mediated reprogramming of oxidative stress response in root apoplast of sunflower enhances defence against Rhizoctonia solani. Eur. J. Plant Pathol. 2011;131:121–134. doi: 10.1007/s10658-011-9792-4. [DOI] [Google Scholar]
- 73.Sharma I., Ahmad P. Oxidative Damage to Plants: Antioxidant Networks and Signaling. Elsevier Inc.; Amsterdam, The Netherlands: 2014. Catalase: A Versatile Antioxidant in Plants; pp. 131–148. [Google Scholar]
- 74.Caverzan A., Passaia G., Rosa S.B., Ribeiro C.W., Lazzarotto F., Margis-Pinheiro M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012;35:1011–1019. doi: 10.1590/S1415-47572012000600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharova E.I., Medvedev S.S., Demidchik V.V. Ascorbate in the Apoplast: Metabolism and Functions. Russ. J. Plant Physiol. 2020;67:207–220. doi: 10.1134/S1021443720020156. [DOI] [Google Scholar]
- 76.Bindschedler L.V., Palmblad M., Cramer R. Hydroponic isotope labelling of entire plants (HILEP) for quantitative plant proteomics; an oxidative stress case study. Phytochemistry. 2008;69:1962–1972. doi: 10.1016/j.phytochem.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Chen Z., Gallie D.R. Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol. 2006;142:775–787. doi: 10.1104/pp.106.085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding H., Wang B., Han Y., Li S. The pivotal function of dehydroascorbate reductase in glutathione homeostasis in plants. J. Exp. Bot. 2020 doi: 10.1093/jxb/eraa107. [DOI] [PubMed] [Google Scholar]
- 79.Yousuf P.Y., Hakeem K.U.R., Chandna R., Ahmad P. Abiotic Stress Responses in Plants. Springer; New York, NY, USA: 2012. Role of Glutathione Reductase in Plant Abiotic Stress; pp. 149–158. [Google Scholar]
- 80.Zechmann B. Subcellular distribution of ascorbate in plants. Plant Signal. Behav. 2011;6:360–363. doi: 10.4161/psb.6.3.14342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hasanuzzaman M., Borhannuddin Bhuyan M.H.M., Anee T.I., Parvin K., Nahar K., Al Mahmud J., Fujita M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8:384. doi: 10.3390/antiox8090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saruhan Güler N., Sağlam A., Demiralay M., Kadioğlu A., Saruhan Güler N., Sağlam A., Demiralay M., Kadioğlu A. Apoplastic and symplastic solute concentrations contribute to osmotic adjustment in bean genotypes during drought stress. Turk J Biol. 2012;36:151–160. [Google Scholar]
- 83.Dar M.I., Naikoo M.I., Rehman F., Naushin F., Khan F.A. Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies. Springer; Delhi, India: 2015. Proline accumulation in plants: Roles in stress tolerance and plant development; pp. 155–166. [Google Scholar]
- 84.Babenko L.M., Smirnov O.E., Romanenko K.O., Trunova O.K., Kosakivska I.V. Phenolic compounds in plants: Biogenesis and functions. Ukr. Biochem. J. 2019;91:5–18. doi: 10.15407/ubj91.03.005. [DOI] [Google Scholar]
- 85.Chen D., Shao Q., Yin L., Younis A., Zheng B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019;9:1945. doi: 10.3389/fpls.2018.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takahashi T., Kakehi J.-I. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2009;105:1–6. doi: 10.1093/aob/mcp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salguero J., Böttger M. Secreted catalase activity from roots of developing maize (Zea mays L.) seedlings. Protoplasma. 1995;184:72–78. doi: 10.1007/BF01276903. [DOI] [Google Scholar]
- 88.Parra-Lobato M.C., Fernandez-Garcia N., Olmos E., Alvarez-Tinaut M.C., Gómez-Jiménez M.C. Methyl jasmonate-induced antioxidant defence in root apoplast from sunflower seedlings. Environ. Exp. Bot. 2009;66:9–17. doi: 10.1016/j.envexpbot.2009.01.002. [DOI] [Google Scholar]
- 89.Zhou Y., Liu S., Yang Z., Yang Y., Jiang L., Hu L. CsCAT3, a catalase gene from Cucumis sativus, confers resistance to a variety of stresses to Escherichia coli. Biotechnol. Biotechnol. Equip. 2017;31:886–896. doi: 10.1080/13102818.2017.1360797. [DOI] [Google Scholar]
- 90.Fernández-Crespo E., Gómez-Pastor R., Scalschi L., Llorens E., Camañes G., García-Agustín P. NH4 + induces antioxidant cellular machinery and provides resistance to salt stress in citrus plants. Trees. 2014;28:1693–1704. doi: 10.1007/s00468-014-1078-y. [DOI] [Google Scholar]
- 91.De Pinto M.C., De Gara L. Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J. Exp. Bot. 2004;55:2559–2569. doi: 10.1093/jxb/erh253. [DOI] [PubMed] [Google Scholar]
- 92.Asada K. Ascorbate peroxidase—A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992;85:235–241. doi: 10.1111/j.1399-3054.1992.tb04728.x. [DOI] [Google Scholar]
- 93.Pandey S., Fartyal D., Agarwal A., Shukla T., James D., Kaul T., Negi Y.K., Arora S., Reddy M.K. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Brien J.A., Daudi A., Finch P., Butt V.S., Whitelegge J.P., Souda P., Ausubel F.M., Bolwell G.P. A Peroxidase-Dependent Apoplastic Oxidative Burst in Cultured Arabidopsis Cells Functions in MAMP-Elicited Defense. Plant Physiol. 2012;158:2013–2027. doi: 10.1104/pp.111.190140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saruhan N., Terzi R., Saǧlam A., Kadioǧlu A. Scavenging of reactive oxygen species in apoplastic and symplastic areas of rolled leaves in Ctenanthe setosa under drought stress. Acta Biol. Hung. 2010;61:282–298. doi: 10.1556/ABiol.61.2010.3.5. [DOI] [PubMed] [Google Scholar]
- 96.Diaz-Vivancos P., Rubio M., Mesonero V., Periago P.M., Barceló A.R., Martínez-Gómez P., Hernández J.A. The apoplastic antioxidant system in Prunus: Response to long-term plum pox virus infection. J. Exp. Bot. 2006;57:3813–3824. doi: 10.1093/jxb/erl138. [DOI] [PubMed] [Google Scholar]
- 97.Pandey P., Singh J., Achary V.M.M., Mallireddy Reddy K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015;3:25. doi: 10.3389/fenvs.2015.00025. [DOI] [Google Scholar]
- 98.Negi B., Salvi P., Bhatt D., Majee M., Arora S. Molecular cloning, in-silico characterization and functional validation of monodehydroascorbate reductase gene in Eleusine coracana. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0187793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eltayeb A.E., Kawano N., Badawi G.H., Kaminaka H., Sanekata T., Shibahara T., Inanaga S., Tanaka K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta. 2007;225:1255–1264. doi: 10.1007/s00425-006-0417-7. [DOI] [PubMed] [Google Scholar]
- 100.Akram N.A., Shafiq F., Ashraf M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017;8:613. doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hasanuzzaman M., Nahar K., Anee T.I., Fujita M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants. 2017;23:249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng M.C., Ko K., Chang W.L., Kuo W.C., Chen G.H., Lin T.P. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015;83:926–939. doi: 10.1111/tpj.12940. [DOI] [PubMed] [Google Scholar]
- 103.Hasanuzzaman M., Nahar K., Alam M.M., Roychowdhury R., Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013;14:9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sairam R.K., Srivastava G.C., Saxena D.C. Increased antioxidant activity under elevated temperatures: A mechanism of heat stress tolerance in wheat genotypes. Biol. Plant. 2000;43:245–251. doi: 10.1023/A:1002756311146. [DOI] [Google Scholar]
- 105.Dash S., Mohanty N. Response of seedlings to heat-stress in cultivars of wheat: Growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity. J. Plant Physiol. 2002;159:49–59. doi: 10.1078/0176-1617-00594. [DOI] [Google Scholar]
- 106.Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012:7. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cuin T.A., Shabala S. Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant. Cell Environ. 2007;30:875–885. doi: 10.1111/j.1365-3040.2007.01674.x. [DOI] [PubMed] [Google Scholar]
- 108.Gadallah M.A.A. Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999;42:249–257. doi: 10.1023/A:1002164719609. [DOI] [Google Scholar]
- 109.Mafakheri A., Siosemardeh A., Bahramnejad B., Struik P.C., Sohrabi Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010;4 [Google Scholar]
- 110.Roychoudhury A., Basu S., Sarkar S.N., Sengupta D.N. Comparative physiological and molecular responses of a common aromatic indica rice cultivar to high salinity with non-aromatic indica rice cultivars. Plant Cell Rep. 2008;27:1395–1410. doi: 10.1007/s00299-008-0556-3. [DOI] [PubMed] [Google Scholar]
- 111.Saglam A., Terzi R., Nar H., Saruhan N., Faik A.A., Kadioglu A. Inorganic and organic solutes in apoplastic and symplastic spaces contribute to osmotic adjustment during leaf rolling in Ctenanthe setosa. Acta Biol. Cracoviensia Ser. Bot. 2010;52:37–44. doi: 10.2478/v10182-010-0005-9. [DOI] [Google Scholar]
- 112.Amarowicz R., Weidner S. Grapevine Molecular Physiology and Biotechnology. 2nd ed. Springer; Dordrecht, The Netherlands: 2009. Biological activity of grapevine phenolic compounds; pp. 389–405. [Google Scholar]
- 113.Caverzan A., Piasecki C., Chavarria G., Stewart C.N., Vargas L. Defenses against ROS in crops and weeds: The effects of interference and herbicides. Int. J. Mol. Sci. 2019;20:86. doi: 10.3390/ijms20051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sharma A., Shahzad B., Rehman A., Bhardwaj R., Landi M., Zheng B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules. 2019;24:2452. doi: 10.3390/molecules24132452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guerra-Guimarães L., Tenente R., Pinheiro C., Chaves I., do Céu Silva M., Cardoso F.M.H., Planchon S., Barros D.R., Renaut J., Ricardo C.P. Proteomic analysis of apoplastic fluid of Coffea arabica leaves highlights novel biomarkers for resistance against Hemileia vastatrix. Front. Plant Sci. 2015;6:478. doi: 10.3389/fpls.2015.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tuzun S., Somanchi A., der Hoorn R. Multigenic and Induced Systemic Resistance in Plants. Volume 59 SRC- Springer; Berlin, Germany: 2008. The Possible Role of PR proteins in Multigenic and Induced Systemic Resistance; pp. 112–142. [Google Scholar]
- 117.Dempsey D.A., Silva H., Klessig D.F. Engineering disease and pest resistance in plants. Trends Microbiol. 1998;6:54–61. doi: 10.1016/S0966-842X(97)01186-4. [DOI] [PubMed] [Google Scholar]
- 118.Sels J., Mathys J., De Coninck B.M.A., Cammue B.P.A., De Bolle M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008;46:941–950. doi: 10.1016/j.plaphy.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 119.Breen S., Williams S.J., Outram M., Kobe B., Solomon P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017;22:871–879. doi: 10.1016/j.tplants.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 120.Hong T., Meng M. Biochemical characterization and antifungal activity of an endo-3-beta-glucanase of Paenibacillus sp. Isol. from Gard. soil. Appl. Microbiol. Biotechnol. 2003;61:472–478. doi: 10.1007/s00253-003-1249-z. [DOI] [PubMed] [Google Scholar]
- 121.Yang Q., Gong Z. Purification and characterization of an ethylene-induced antifungal protein from leaves of guilder rose (Hydrangea macrophylla) Protein Expr. Purif. 2002;24:76–82. doi: 10.1006/prep.2001.1551. [DOI] [PubMed] [Google Scholar]
- 122.Yang Y.-X., Ahammed G., Wu C., Fan S., Zhou Y.-H. Crosstalk among Jasmonate, Salicylate and Ethylene Signaling Pathways in Plant Disease and Immune Responses. Curr. Protein Pept. Sci. 2015;16:450–461. doi: 10.2174/1389203716666150330141638. [DOI] [PubMed] [Google Scholar]
- 123.Naz R., Bano A., Wilson N.L., Guest D., Roberts T.H. Pathogenesis-related protein expression in the apoplast of wheat leaves protected against leaf rust following application of plant extracts. Phytopathology. 2014;104:933–944. doi: 10.1094/PHYTO-11-13-0317-R. [DOI] [PubMed] [Google Scholar]
- 124.Tornero P., Conejero V., Vera P. Identification of a new pathogen-induced member of the subtilisin-like processing protease family from plants. J. Biol. Chem. 1997;272:14412–14419. doi: 10.1074/jbc.272.22.14412. [DOI] [PubMed] [Google Scholar]
- 125.Simmons C. The Physiology and Molecular Biology of Plant 1,3-β-D-Glucanases and 1,3;1,4-β-D-Glucanases. Crit. Rev. Plant Sci. 1994;13:325–387. [Google Scholar]
- 126.Van Loon L.C. Induced resistance in plants and the role of pathogenesis-related proteins. Eur. J. Plant Pathol. 1997;103:753–765. doi: 10.1023/A:1008638109140. [DOI] [Google Scholar]
- 127.Sugawara T., Trifonova E.A., Kochetov A.V., Kanayama Y. Expression of an extracellular ribonuclease gene increases resistance to Cucumber mosaic virus in tobacco. BMC Plant Biol. 2016;16 doi: 10.1186/s12870-016-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Filipenko E., Kochetov A., Kanayama Y., Malinovsky V., Shumny V. PR-proteins with ribonuclease activity and plant resistance against pathogenic fungi. Russ. J. Genet. Appl. Res. 2013;10 SRC-:474–480. doi: 10.1134/S2079059713060026. [DOI] [Google Scholar]
- 129.Bravo J.M., Campo S., Murillo I., Coca M., San Segundo B. Fungus- and wound-induced accumulation of mRNA containing a class II chitinase of the pathogenesis-related protein 4 (PR-4) family of maize. Plant Mol. Biol. 2003;52:745–759. doi: 10.1023/A:1025016416951. [DOI] [PubMed] [Google Scholar]
- 130.Penninckx I.A., Eggermont K., Terras F.R., Thomma B.P., De Samblanx G.W., Buchala A., Métraux J.P., Manners J.M., Broekaert W.F. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stec B. Plant thionins--the structural perspective. Cell. Mol. Life Sci. 2006;63:1370–1385. doi: 10.1007/s00018-005-5574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kido E.A., Pandolfi V., Houllou-Kido L.M., Andrade P.P., Marcelino F.C., Nepomuceno A.L., Abdelnoor R.V., Burnquist W.L., Benko-Iseppon A.M. Plant Antimicrobial Peptides: An Overview of SuperSAGE Transcriptional Profile and a Functional Review. Curr. Protein Pept. Sci. 2010;11:220–230. doi: 10.2174/138920310791112110. [DOI] [PubMed] [Google Scholar]
- 133.Hu X., Bidney D.L., Yalpani N., Duvick J.P., Crasta O., Folkerts O., Lu G. Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol. 2003;133:170–181. doi: 10.1104/pp.103.024026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Christensen A.B., Cho B.H.O., Næsby M., Gregersen P.L., Brandt J., Madriz-Ordeñana K., Collinge D.B., Thordal-Christensen H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant Pathol. 2002;3:135–144. doi: 10.1046/j.1364-3703.2002.00105.x. [DOI] [PubMed] [Google Scholar]
- 135.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 136.Van Loon L.C., Van Strien E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999;55:85–97. doi: 10.1006/pmpp.1999.0213. [DOI] [Google Scholar]
- 137.Liu Q., Xue Q. Computational identification of novel PR-1-type genes in Oryza sativa. J. Genet. 2006;85:193–198. doi: 10.1007/BF02935330. [DOI] [PubMed] [Google Scholar]
- 138.Payne G., Ward E., Gaffney T., Goy P.A., Moyer M., Harper A., Meins F., Ryals J. Evidence for a third structural class of β-1,3-glucanase in tobacco. Plant Mol. Biol. 1990;15:797–808. doi: 10.1007/BF00039420. [DOI] [PubMed] [Google Scholar]
- 139.Leubner-Metzger G., Meins F. 3. Functions and Regulation of Plant ß-1,3-glucanases (PR-2) CRC Press LLC; Boca Raton, FL, USA: 1999. [Google Scholar]
- 140.Dyakov Y.T., Dzhavakhiya V.G., Korpela T. Comprehensive and Molecular Phytopathology. Elsevier; Amsterdam, The Netherlands: 2007. [Google Scholar]
- 141.Castresana C., de Carvalho F., Gheysen G., Habets M., Inzé D., Van Montagu M. Tissue-specific and pathogen-induced regulation of a Nicotiana plumbaginifolia beta-1,3-glucanase gene. Plant Cell. 1990;2:1131–1143. doi: 10.1105/tpc.2.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alonso E., de Carvalho Niebel F., Obregon P., Gheysen G., Inze D., Van Montagu M., Castresana C. Differential in vitro DNA binding activity to a promoter element of the gn1 beta-1,3-glucanase gene in hypersensitively reacting tobacco plants. Plant J. 1995;7:309–320. doi: 10.1046/j.1365-313X.1995.7020309.x. [DOI] [PubMed] [Google Scholar]
- 143.Balasubramanian V., Vashisht D., Cletus J., Sakthivel N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012;34:1983–1990. doi: 10.1007/s10529-012-1012-6. [DOI] [PubMed] [Google Scholar]
- 144.Vázquez-Garcidueñas S., Leal-Morales C.A., Herrera-Estrella A. Analysis of the beta-1,3-Glucanolytic System of the Biocontrol Agent Trichoderma harzianum. Appl. Environ. Microbiol. 1998;64:1442–1446. doi: 10.1128/AEM.64.4.1442-1446.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ait-Lahsen H., Soler A., Rey M., De La Cruz J., Monte E., Llobell A. An Antifungal Exo-α-1,3-Glucanase (AGN13.1) from the Biocontrol Fungus Trichoderma harzianum. Appl. Environ. Microbiol. 2001;67:5833–5839. doi: 10.1128/AEM.67.12.5833-5839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kasprzewska A. Plant chitinases--regulation and function. Cell. Mol. Biol. Lett. 2003;8:809–824. [PubMed] [Google Scholar]
- 147.Saikia R., Singh B.P., Kumar R., Arora D.K. Detection of pathogenesis-related proteins-chitinase and β-1,3-glucanase in induced chickpea. Curr. Sci. 2005;84:659–663. [Google Scholar]
- 148.Garg N., Gupta H. Isolation and purification of fungal pathogen (Macrophomina phaseolina) induced chitinase from moth beans (Phaseolus aconitifolius) J. Pharm. Bio Allied Sci. 2010;2:38. doi: 10.4103/0975-7406.62708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fukamizo T., Chie S., Masahiro T. Plant chitinases: Structure-function relationships and their physiology. Foods Food Ingredients J. 2003;208:631–632. [Google Scholar]
- 150.Gooday G.W. Aggressive and defensive roles for chitinases. EXS. 1999;87:157–169. doi: 10.1007/978-3-0348-8757-1_11. [DOI] [PubMed] [Google Scholar]
- 151.Afroz A., Ali G.M., Mir A., Komatsu S. Application of proteomics to investigate stress-induced proteins for improvement in crop protection. Plant Cell Rep. 2011;30:745–763. doi: 10.1007/s00299-010-0982-x. [DOI] [PubMed] [Google Scholar]
- 152.Cantu D., Vicente A.R., Labavitch J.M., Bennett A.B., Powell A.L.T. Strangers in the matrix: Plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008;13:610–617. doi: 10.1016/j.tplants.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 153.Petriccione M., Salzano A.M., Di Cecco I., Scaloni A., Scortichini M. Proteomic analysis of the Actinidia deliciosa leaf apoplast during biotrophic colonization by Pseudomonas syringae pv. actinidiae. J. Proteom. 2014;101:43–62. doi: 10.1016/j.jprot.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 154.Pechanova O., Hsu C.-Y., Adams J.P., Pechan T., Vandervelde L., Drnevich J., Jawdy S., Adeli A., Suttle J.C., Lawrence A.M., et al. Apoplast proteome reveals that extracellular matrix contributes to multistress response in poplar. BMC Genom. 2010;11:674. doi: 10.1186/1471-2164-11-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Delaunois B., Colby T., Belloy N., Conreux A., Harzen A., Baillieul F., Clément C., Schmidt J., Jeandet P., Cordelier S. Large-scale proteomic analysis of the grapevine leaf apoplastic fluid reveals mainly stress-related proteins and cell wall modifying enzymes. BMC Plant Biol. 2013;13:24. doi: 10.1186/1471-2229-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Floerl S., Druebert C., Majcherczyk A., Karlovsky P., Kües U., Polle A. Defence reactions in the apoplastic proteome of oilseed rape (Brassica napus var. napus) attenuate Verticillium longisporum growth but not disease symptoms. BMC Plant Biol. 2008;8:129. doi: 10.1186/1471-2229-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Segarra C.I., Casalongué C.A., Pinedo M.L., Ronchi V.P., Conde R.D. A germin-like protein of wheat leaf apoplast inhibits serine proteases. J. Exp. Bot. 2003;54:1335–1341. doi: 10.1093/jxb/erg139. [DOI] [PubMed] [Google Scholar]
- 158.Joosten M.H.A.J., De Wit P.J.G.M. Identification of Several Pathogenesis-Related Proteins in Tomato Leaves Inoculated with Cladosporium fulvum (syn. Fulvia fulva ) as 1,3-β-Glucanases and Chitinases. Plant Physiol. 1989;89:945–951. doi: 10.1104/pp.89.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Han L.B., Li Y.B., Wang F.X., Wang W.Y., Liu J., Wu J.H., Zhong N.Q., Wu S.J., Jiao G.L., Wang H.Y., et al. The Cotton Apoplastic Protein CRR1 stabilizes chitinase 28 to facilitate defense against the fungal pathogen verticillium dahliae. Plant Cell. 2019;31:520–536. doi: 10.1105/tpc.18.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hon W.-C., Griffith M., Mlynarz A., Kwok Y.C., Yang D.S. Antifreeze proteins in winter rye are similar to pathogenesis-related proteins. Plant Physiol. 1995;109:879–889. doi: 10.1104/pp.109.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rajam M.V., Chandola N., Goud P.S., Singh D., Kashyap V., Choudhary M.L., Sihachakr D. Thaumatin gene confers resistance to fungal pathogens as well as tolerance to abiotic stresses in transgenic tobacco plants. Biol. Plant. 2007;51:135–141. doi: 10.1007/s10535-007-0026-8. [DOI] [Google Scholar]
- 162.Islam M.A., Sturrock R.N., Holmes T.A., Ekramoddoullah A.K.M. Ultrastructural studies of Phellinus sulphurascens infection of Douglas-fir roots and immunolocalization of host pathogenesis-related proteins. Mycol. Res. 2009;113:700–712. doi: 10.1016/j.mycres.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 163.Wang X., Tang C., Deng L., Cai G., Liu X., Liu B., Han Q., Buchenauer H., Wei G., Han D., et al. Characterization of a pathogenesis-related thaumatin-like protein gene TaPR5 from wheat induced by stripe rust fungus. Physiol. Plant. 2010;139:27–38. doi: 10.1111/j.1399-3054.2009.01338.x. [DOI] [PubMed] [Google Scholar]
- 164.Shatters R.G., Boykin L.M., Lapointe S.L., Hunter W.B., Weathersbee A.A. Phylogenetic and structural relationships of the PR5 gene family reveal an ancient multigene family conserved in plants and select animal taxa. J. Mol. Evol. 2006;63:12–29. doi: 10.1007/s00239-005-0053-z. [DOI] [PubMed] [Google Scholar]
- 165.Dunwell J.M., Khuri S., Gane P.J. Microbial Relatives of the Seed Storage Proteins of Higher Plants: Conservation of Structure and Diversification of Function during Evolution of the Cupin Superfamily. Microbiol. Mol. Biol. Rev. 2000;64:153–179. doi: 10.1128/MMBR.64.1.153-179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Godfrey D., Able A.J., Dry I.B. Induction of a grapevine germin-like protein (VvGLP3) gene is closely linked to the site of Erysiphe necator infection: A possible role in defense? Mol. Plant. Microbe. Interact. 2007;20:1112–1125. doi: 10.1094/MPMI-20-9-1112. [DOI] [PubMed] [Google Scholar]
- 167.Regente M., Pinedo M., San Clemente H., Balliau T., Jamet E., de la Canal L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017;68:5485–5495. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- 168.Wojtaszek P., Stobiecki M., Bolwell G.P. Changes in the composition of exocellular proteins of suspension-cultured Lupinus albus cells in response to fungal elicitors or CuCl2. J. Exp. Bot. 1997;48:2015–2021. doi: 10.1093/jxb/48.12.2015. [DOI] [Google Scholar]
- 169.Dani V., Simon W.J., Duranti M., Croy R.R.D.R.D. Changes in the tobacco leaf apoplast proteome in response to salt stress. Proteomics. 2005;5:737–745. doi: 10.1002/pmic.200401119. [DOI] [PubMed] [Google Scholar]
- 170.Jaswanthi N., Krishna M.S.R., Sahitya U.L., Suneetha P. Apoplast proteomic analysis reveals drought stress-responsive protein datasets in chilli (Capsicum annuum L.) Data Br. 2019;25:104041. doi: 10.1016/j.dib.2019.104041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dunwell J.M., Gibbings J.G., Mahmood T., Saqlan Naqvi S.M. Germin and germin-like proteins: Evolution, structure, and function. CRC. Crit. Rev. Plant Sci. 2008;27:342–375. doi: 10.1080/07352680802333938. [DOI] [Google Scholar]
- 172.Somssich I., Hahlbrock K. Pathogen defence in plants — a paradigm of biological complexity. Trends Plant Sci. 1998;3:86–90. doi: 10.1016/S1360-1385(98)01199-6. [DOI] [Google Scholar]
- 173.Bowler C., Montagu M.V., Inze D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992;43:83–116. doi: 10.1146/annurev.pp.43.060192.000503. [DOI] [Google Scholar]
- 174.Khuri S., Bakker F.T., Dunwell J.M. Phylogeny, function, and evolution of the cupins, a structurally conserved, functionally diverse superfamily of proteins. Mol. Biol. Evol. 2001;18:593–605. doi: 10.1093/oxfordjournals.molbev.a003840. [DOI] [PubMed] [Google Scholar]
- 175.Wang T., Chen X., Zhu F., Li H., Li L., Yang Q., Chi X., Yu S., Liang X. Characterization of Peanut Germin-Like Proteins, AhGLPs in Plant Development and Defense. PLoS ONE. 2013;8:e61722. doi: 10.1371/journal.pone.0061722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Amini F., Ehsanpour A.A., Hoang Q.T., Shin J.S. Protein pattern changes in tomato under in vitro salt stress. Russ. J. Plant Physiol. 2007;54:464–471. doi: 10.1134/S102144370704005X. [DOI] [Google Scholar]
- 177.Van der Hoorn R.A.L. Plant Proteases: From Phenotypes to Molecular Mechanisms. Annu. Rev. Plant Biol. 2008;59:191–223. doi: 10.1146/annurev.arplant.59.032607.092835. [DOI] [PubMed] [Google Scholar]
- 178.Tian M., Win J., Song J., Van Der Hoorn R., van der Knaap E., Kamoun S. A Phytophthora infestans Cystatin-Like Protein Targets a Novel Tomato Papain-Like Apoplastic Protease. Plant Physiol. 2006;143:364–377. doi: 10.1104/pp.106.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Figueiredo A., Monteiro F., Sebastiana M. Subtilisin-like proteases in plant–pathogen recognition and immune priming: A perspective. Front. Plant Sci. 2014;5:739. doi: 10.3389/fpls.2014.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tian M., Benedetti B., Kamoun S. A second Kazal-like protease inhibitor from Phytophthora infestans inhibits and interacts with the apoplastic pathogenesis-related protease P69B of tomato. Plant Physiol. 2005;138:1785–1793. doi: 10.1104/pp.105.061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ramírez V., López A., Mauch-Mani B., Gil M.J., Vera P. An Extracellular Subtilase Switch for Immune Priming in Arabidopsis. PLoS Pathog. 2013;9:e1003445. doi: 10.1371/journal.ppat.1003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Terras F.R.G., Schoofs H.M.E., Thevissen K., Osborn R.W., Vanderleyden J., Cammue B.P.A., Broekaert W.F. Synergistic enhancement of the antifungal activity of wheat and barley thionins by radish and oilseed rape 2S albumins and by barley trypsin inhibitors. Plant Physiol. 1993;103:1311–1319. doi: 10.1104/pp.103.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Boller T., Felix G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 184.Planas-Marquès M., Bernardo-Faura M., Paulus J., Kaschani F., Kaiser M., Valls M., Van Der Hoorn R.A.L., Coll N.S. Protease Activities Triggered by Ralstonia solanacearum Infection in Susceptible and Tolerant Tomato Lines. Mol. Cell. Proteom. 2018;17:1112–1125. doi: 10.1074/mcp.RA117.000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ziemann S., van der Linde K., Lahrmann U., Acar B., Kaschani F., Colby T., Kaiser M., Ding Y., Schmelz E., Huffaker A., et al. An apoplastic peptide activates salicylic acid signalling in maize. Nat. Plants. 2018;4:172–180. doi: 10.1038/s41477-018-0116-y. [DOI] [PubMed] [Google Scholar]
- 186.Schulze Hüynck J., Kaschani F., van der Linde K., Ziemann S., Müller A.N., Colby T., Kaiser M., Misas Villamil J.C., Doehlemann G. Proteases underground: Analysis of the maize root apoplast identifies organ specific papain-like cysteine protease activity. Front. Plant Sci. 2019;10:473. doi: 10.3389/fpls.2019.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Krüger J., Thomas C.M., Golstein C., Dixon M.S., Smoker M., Tang S., Mulder L., Jones J.D.G. A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science. 2002;296:744–747. doi: 10.1126/science.1069288. [DOI] [PubMed] [Google Scholar]
- 188.Lozano-Torres J.L., Wilbers R.H.P., Gawronski P., Boshoven J.C., Finkers-Tomczak A., Cordewener J.H.G., America A.H.P., Overmars H.A., Van ’t Klooster J.W., Baranowski L., et al. Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode. Proc. Natl. Acad. Sci. USA. 2012;109:10119–10124. doi: 10.1073/pnas.1202867109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lay F., Anderson M. Defensins - Components of the Innate Immune System in Plants. Curr. Protein Pept. Sci. 2005;6:85–101. doi: 10.2174/1389203053027575. [DOI] [PubMed] [Google Scholar]
- 190.Bohlmann H. The role of thionins in plant protection. CRC. Crit. Rev. Plant Sci. 1994;13:1–16. doi: 10.1080/07352689409701905. [DOI] [Google Scholar]
- 191.Broekaert W.F., Terras F.R.G., Cammue B.P.A., Osborn R.W. Plant defensins: Novel antimicrobial peptides as components of the host defense system. Plant Physiol. 1995;108:1353–1358. doi: 10.1104/pp.108.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kaur J., Fellers J., Adholeya A., Velivelli S.L.S., El-Mounadi K., Nersesian N., Clemente T., Shah D. Expression of apoplast-targeted plant defensin MtDef4.2 confers resistance to leaf rust pathogen Puccinia triticina but does not affect mycorrhizal symbiosis in transgenic wheat. Transgenic Res. 2017;26:37–49. doi: 10.1007/s11248-016-9978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hsiao P.Y., Cheng C.P., Koh K.W., Chan M.T. The Arabidopsis defensin gene, AtPDF1.1, mediates defence against Pectobacterium carotovorum subsp. carotovorum via an iron-withholding defence system. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-08497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Koike M., Okamoto T., Tsuda S., Imai R. A novel plant defensin-like gene of winter wheat is specifically induced during cold acclimation. Biochem. Biophys. Res. Commun. 2002;298:46–53. doi: 10.1016/S0006-291X(02)02391-4. [DOI] [PubMed] [Google Scholar]
- 195.Boutrot F., Chantret N., Gautier M.F. Genome-wide analysis of the rice and arabidopsis non-specific lipid transfer protein (nsLtp) gene families and identification of wheat nsLtp genes by EST data mining. BMC Genom. 2008;9 doi: 10.1186/1471-2164-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kader J.-C. LIPID-TRANSFER PROTEINS IN PLANTS. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:627–654. doi: 10.1146/annurev.arplant.47.1.627. [DOI] [PubMed] [Google Scholar]
- 197.De O. Carvalho A., de S. Teodoro C.E., Da Cunha M., Okorokova-Facanha A.L., Okorokov L.A., Fernandes K.V.S., Gomes V.M. Intracellular localization of a lipid transfer protein in Vigna unguiculata seeds. Physiol. Plant. 2004;122:328–336. doi: 10.1111/j.1399-3054.2004.00413.x. [DOI] [Google Scholar]
- 198.Kusumawati L., Imin N., Djordjevic M.A. Characterization of the secretome of suspension cultures of medicago species reveals proteins important for defense and development. J. Proteome Res. 2008;7:4508–4520. doi: 10.1021/pr800291z. [DOI] [PubMed] [Google Scholar]
- 199.Pagnussat L., Burbach C., Baluska F., de la Canal L. An extracellular lipid transfer protein is relocalized intracellularly during seed germination. J. Exp. Bot. 2012;63:6555–6563. doi: 10.1093/jxb/ers311. [DOI] [PubMed] [Google Scholar]
- 200.Finkina E.I., Melnikova D.N., Bogdanov I.V., Ovchinnikova T.V. Lipid Transfer Proteins As Components of the Plant Innate Immune System: Structure, Functions, and Applications. Acta Naturae. 2016;8:47–61. doi: 10.32607/20758251-2016-8-2-47-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sakurai N. Dynamic function and regulation of apoplast in the plant body. J. Plant Res. 1998;111:133–148. doi: 10.1007/BF02507160. [DOI] [Google Scholar]
- 202.Lee S.B., Go Y.S., Bae H.J., Park J.H., Cho S.H., Cho H.J., Lee D.S., Park O.K., Hwang I., Suh M.C. Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein gene altered cuticular lipid composition, increased plastoglobules, and enhanced susceptibility to infection by the fungal pathogen alternaria brassicicola. Plant Physiol. 2009;150:42–54. doi: 10.1104/pp.109.137745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sarowar S., Kim Y.J., Kim K.D., Hwang B.K., Ok S.H., Shin J.S. Overexpression of lipid transfer protein (LTP) genes enhances resistance to plant pathogens and LTP functions in long-distance systemic signaling in tobacco. Plant Cell Rep. 2009;28:419–427. doi: 10.1007/s00299-008-0653-3. [DOI] [PubMed] [Google Scholar]
- 204.Maldonado A.M., Doerner P., Dixonk R.A., Lamb C.J., Cameron R.K. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature. 2002;419:399–403. doi: 10.1038/nature00962. [DOI] [PubMed] [Google Scholar]
- 205.Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.-L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 206.Nürnberger T., Brunner F., Kemmerling B., Piater L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol. Rev. 2004;198 doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- 207.Felix G., Duran J.D., Volko S., Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313X.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 208.Gómez-Gómez L., Felix G., Boller T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 1999;18:277–284. doi: 10.1046/j.1365-313X.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- 209.Gómez-Gómez L., Boller T. FLS2: An LRR Receptor–like Kinase Involved in the Perception of the Bacterial Elicitor Flagellin in Arabidopsis. Mol. Cell. 2000;2:1003–1011. doi: 10.1016/S1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 210.Sun Y., Li L., Macho A.P., Han Z., Hu Z., Zipfel C., Zhou J.M., Chai J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science. 2013;342:624–628. doi: 10.1126/science.1243825. [DOI] [PubMed] [Google Scholar]
- 211.Zipfel C., Robatzek S., Navarro L., Oakeley E.J., Jones J.D.G., Felix G., Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 212.Kunze G., Zipfel C., Robatzek S., Niehaus K., Boller T., Felix G. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell. 2004;16:3496–3507. doi: 10.1105/tpc.104.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mott G.A., Middleton M.A., Desveaux D., Guttman D.S. Peptides and small molecules of the plant-pathogen apoplastic arena. Front. Plant Sci. 2014;5:677. doi: 10.3389/fpls.2014.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang L., Albert M., Einig E., Fürst U., Krust D., Felix G. The pattern-recognition receptor CORE of Solanaceae detects bacterial cold-shock protein. Nat. Plants. 2016;2 doi: 10.1038/nplants.2016.185. [DOI] [PubMed] [Google Scholar]
- 215.Felix G., Grosskopf D.G., Regenass M., Basse C.W., Boller T. Elicitor-induced ethylene biosynthesis in tomato cells: Characterization and use as a bioassay for elicitor action. Plant Physiol. 1991;97:19–25. doi: 10.1104/pp.97.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yano A., Suzuki K., Uchimiya H., Shinshi H. Induction of hypersensitive cell death by a fungal protein in cultures of tobacco cells. Mol. Plant-Microbe Interact. 1998;11:115–123. doi: 10.1094/MPMI.1998.11.2.115. [DOI] [Google Scholar]
- 217.Enkerli J., Felix G., Boller T. The enzymatic activity of fungal xylanase is not necessary for its elicitor activity. Plant Physiol. 1999;121:391–397. doi: 10.1104/pp.121.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ron M., Avni A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell. 2004;16:1604–1615. doi: 10.1105/tpc.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ranf S., Gisch N., Schäffer M., Illig T., Westphal L., Knirel Y.A., Sánchez-Carballo P.M., Zähringer U., Hückelhoven R., Lee J., et al. A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 2015;16:426–433. doi: 10.1038/ni.3124. [DOI] [PubMed] [Google Scholar]
- 220.Willmann R., Lajunen H.M., Erbs G., Newman M.-A., Kolb D., Tsuda K., Katagiri F., Fliegmann J., Bono J.-J., Cullimore J.V., et al. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc. Natl. Acad. Sci. USA. 2011;108:19824–19829. doi: 10.1073/pnas.1112862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gust A.A., Willmann R., Desaki Y., Grabherr H.M., Nürnberger T. Plant LysM proteins: Modules mediating symbiosis and immunity. Trends Plant Sci. 2012;17:495–502. doi: 10.1016/j.tplants.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 222.Kramer E.M. How far can a molecule of weak acid travel in the apoplast or xylem? Plant Physiol. 2006;141:1233–1236. doi: 10.1104/pp.106.083790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hager A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003;116:483–505. doi: 10.1007/s10265-003-0110-x. [DOI] [PubMed] [Google Scholar]
- 224.Barbez E., Dünser K., Gaidora A., Lendl T., Busch W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2017;114:E4884–E4893. doi: 10.1073/pnas.1613499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hartung W., Sauter A., Hose E. Abscisic acid in the xylem: Where does it come from, where does it go to? J. Exp. Bot. 2002;53:27–32. doi: 10.1093/jexbot/53.366.27. [DOI] [PubMed] [Google Scholar]
- 226.Sauter A., Hartung W. Radial transport of abscisic acid conjugates in maize roots: Its implication for long distance stress signals. J. Exp. Bot. 2000;51:929–935. doi: 10.1093/jexbot/51.346.929. [DOI] [PubMed] [Google Scholar]
- 227.Moubayidin L., Di Mambro R., Sabatini S. Cytokinin-auxin crosstalk. Trends Plant Sci. 2009;14:557–562. doi: 10.1016/j.tplants.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 228.Naseem M., Dandekar T. The Role of Auxin-Cytokinin Antagonism in Plant-Pathogen Interactions. PLoS Pathog. 2012;8:e1003026. doi: 10.1371/journal.ppat.1003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pignocchi C., Kiddle G., Hernández I., Foster S.J., Asensi A., Taybi T., Barnes J., Foyer C.H. Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signaling and orchestration of defense processes in tobacco. Plant Physiol. 2006;141:423–435. doi: 10.1104/pp.106.078469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen Z., Silva H., Klessig D.F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- 231.Cano A., Alcaraz O., Arnao M.B. Free radical-scavenging activity of indolic compounds in aqueous and ethanolic media. Anal. Bioanal. Chem. 2003;376:33–37. doi: 10.1007/s00216-003-1848-7. [DOI] [PubMed] [Google Scholar]
- 232.Schopfer P., Plachy C., Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001;125:1591–1602. doi: 10.1104/pp.125.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]