Abstract
Sunlight contains a significant amount of ultraviolet (UV) ray, which leads to various effects on homeostasis in the body. Defense strategies to protect from UV rays have been extensively studied, as sunburn, photoaging, and photocarcinogenesis are caused by excessive UV exposure. The primary lines of defense against UV damage are melanin and trans-urocanic acid, which are distributed in the stratum corneum. UV rays that pass beyond these lines of defense can lead to oxidative damage. However, cells detect changes due to UV rays as early as possible and initiate cell signaling processes to prevent the occurrence of damage and repair the already occurred damage. Cosmetic and dermatology experts recommend using a sunscreen product to prevent UV-induced damage. A variety of strategies using antioxidants and anti-inflammatory agents have also been developed to complement the skin’s defenses against UV rays. Researchers have examined the use of plant-derived materials to alleviate the occurrence of skin aging, diseases, and cancer caused by UV rays. Furthermore, studies are also underway to determine how to promote melanin production to protect from UV-induced skin damage. This review provides discussion of the damage that occurs in the skin due to UV light and describes potential defense strategies using plant-derived materials. This review aims to assist researchers in understanding the current research in this area and to potentially plan future studies.
Keywords: sunlight, ultraviolet, oxidative damage, antioxidant, inflammation, melanin, photoprotection, photoaging, photocarcinogenesis, plant extract, cosmetics
1. Introduction
Solar energy is a major factor in the environment that interacts with life and has a positive or negative effect on the birth, growth, aging, and death of organisms [1]. Sunlight that passes through the atmosphere and reaches the earth’s surface mainly comprises visible light and some ultraviolet (UV) and infrared rays [2,3]. Of these, visible light and infrared rays are relatively safe for life and only have a harmful effect under special conditions, such as the presence of photosensitizers. However, high-levels of UV rays can lead to direct damage to living organisms.
In humans, melanin and trans-urocanic acid perform the primary defense functions in the skin by absorbing UV rays [4,5]. The body is equipped with various enzymatic and non-enzymatic measures to protect from the UV rays that pass through the primary lines of defense [6,7]. Damage is inevitable if UV rays are too intense or the defenses in the skin are not sufficient, leading to negative consequences, such as oxidative damage and disease. Therefore, enhancing the external defenses against UV rays, rather than relying only on the skin’s own defense capabilities, is essential to prevent UV-associated damage.
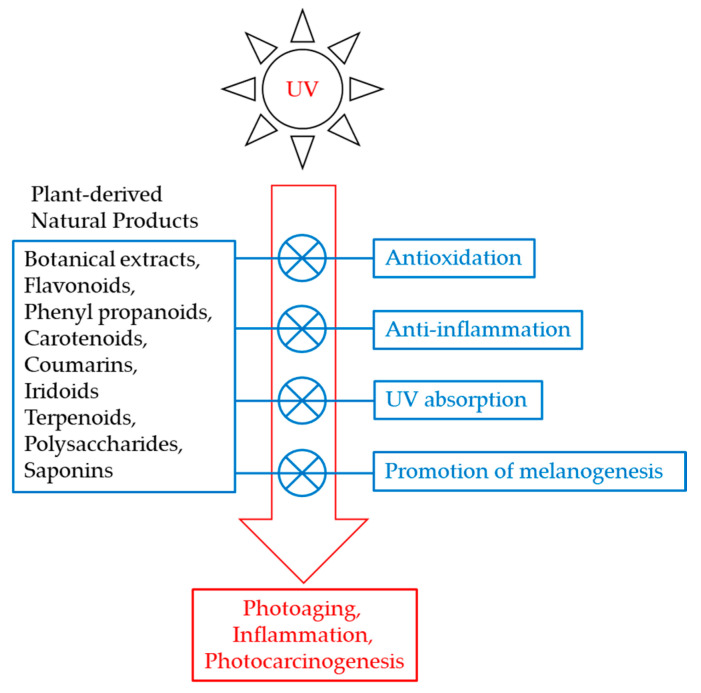
Defense strategies to protect the skin against the harmful effects of UV rays have been widely studied [8]. In this review, we will introduce these studies, with a focus on protection against UV-induced toxicity using plant-derived natural products. These products are divided into several categories, including UV absorbers, antioxidants, anti-inflammatory agents, and promoters of melanin synthesis. Figure 1 shows the scope of this review. The aim of this review is to assist the development of novel research plans and industrial application strategies to reduce skin damage caused by UV rays.
Figure 1.
Scope of this review article. This review will cover emerging strategies to protect the skin from UV-induced toxic effects using plant-derived natural products which can act as UV absorbers, antioxidants, anti-inflammatory agents, and promoters of melanin synthesis.
2. UV-Induced Toxicity in the Skin
UV rays are categorized into UVA (315–400 nm), UVB (280–315 nm), and UVC (200–280 nm) rays, depending on the wavelength range (ISO 21348 Definitions of Solar Irradiance Spectral Categories). UV rays are a major cause of skin photoaging and photocarcinogenesis [9,10]. Overexposure to UV radiation, particularly the UVB component, causes erythema, edema, hyperplasia, hyperpigmentation, photoaging, immunosuppression, and skin cancer [11,12]. Overexposure of the skin to UV rays stimulates the production of reactive oxygen species (ROS); increases oxidative damage of biomolecules, such as lipids, nucleic acids, and proteins; and decreases endogenous antioxidants in the cutaneous tissues [13,14,15]. Approximately 9–14% of solar UVB rays reach the dermis in the skin and can induce inflammatory responses, such as erythema and edema [12]. Sunburn reactions are mediated by the tumor suppressor p53 [16,17]. The p53 arrests cell cycle, allowing cells to properly repair the damaged DNA or to remove the damaged cells, thereby reducing the risk of cancer development. Previous studies have demonstrated that exogenous antioxidants can prevent photocarcinogenesis [18].
Intrinsic aging of the human skin, also called natural or chronological aging, is dependent on time and genetics, whereas extrinsic skin aging is affected by environmental factors, such as solar radiation [19]. UV radiation is a major cause of skin photoaging, which is characterized by wrinkles, laxity, blister formation, roughness, and loss of skin tone [12,20,21]. Apart from intrinsic aging, which is currently inevitable, photoaging can be reduced by minimizing UV exposure and maintaining proper skin care [20]. The main issues that are targeted in the cosmetics field include wrinkles and unwanted pigmentation, which are associated with inflammation or oxidative stress due to overexposure to UV rays [12,20].
Skin photoaging involves alterations in the extracellular matrix composition of the dermis. UV rays induce and activate matrix metalloproteinases (MMPs), a group of zinc endopeptidases that degrade extracellular matrix macromolecules, including type I collagen [22,23]. MMPs secreted from both the dermal fibroblasts and epidermal keratinocytes participate in collagen metabolism in the skin [24,25]. MMPs play key roles in connective tissue remodeling in UV-exposed skin and cause wrinkles and other phenotypes in photo-aged skin [26,27].
Gene expression of MMPs, such as MMP-1, -2, -3, and -9, is upregulated in UV-exposed human dermal fibroblasts [26,27]. The cell signaling pathways involve UV-induced activation of cytokine receptors and the subsequent activation of mitogen-activated protein kinases (MAPK), such as extracellular signal-regulated kinase (ERK), c-Jun-N-terminal kinase (JNK), and p38 kinase [28,29,30]. The promoters of the MMP-1 and MMP-3 genes can be transactivated by activator protein-1 (AP-1) complexes [29,31]. Although the initial events of this signal cascade are not fully understood, evidence suggests that UV-damaged DNA acts as a trigger that initiates this process [32].
Keratinocytes account for 95% of the mass of cells in the human epidermis and play an important role in maintaining skin homeostasis, through both their autocrine and paracrine effects [33]. Under normal conditions, the constitutive production of cytokines and other soluble factors in human keratinocytes is low; however, various stimuli, such as UV rays and endotoxins, can trigger the expression of pro-inflammatory cytokines [34]. Certain cytokines or cell components that are secreted from UVB-irradiated epidermal keratinocytes can regulate gene expression of dermal fibroblasts through paracrine effects. For example, interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and stratifin are released from UV-exposed keratinocytes and stimulate MMP-1 expression in fibroblasts [35,36,37].
Apoptotic cell death is involved in skin photoaging [38], and typically involves changes in the expression of the pro-apoptotic (Bax, Bak, and Bid) and anti-apoptotic (Bcl-2 and Bcl-x) members of the Bcl-2 protein family [39]. UV-induced apoptosis is mediated by caspases in keratinocytes [40]. UV rays induce apoptosis of keratinocytes via intrinsic pathways, involving direct DNA damage; extrinsic pathways, involving activated cell membrane death receptors; and other ROS-mediated pathways [38,40]. Apoptosis can be detected using various markers, including DNA laddering, changes in the expression of pro-apoptotic (Bax, Bak, and Bid) and anti-apoptotic (Bcl-2 and Bcl-x) members of the Bcl-2 protein family, and activation of caspases [41,42,43].
3. Melanin as an Endogenous UV Filter
Melanin is a polymeric dark pigment produced by melanocytes [44]. Pheomelanin and eumelanin are the major forms of melanin that are found in the skin, hair, iris of eyes, and the stria vascularis of the inner ear, whereas neuromelanin is found in the brain. In human skin, epidermal melanocytes are present at the junction of the dermis and epidermis [44]. The number of melanocytes per unit area of skin does not vary greatly among individuals; however, melanocytes from individuals with different skin colors have different activities that lead to more or less production of pheomelanin or eumelanin [12,45].
There is a close relationship between the melanogenic activity and human skin color [46,47]. The vertical and horizontal distribution of melanin in the skin can also change the appearance of the skin color [48]. Skin color also appears to be associated with genetic background, e.g., mutations in the SLC24A5 and SLC45A2, which encode solute carrier proteins [49,50]. Single nucleotide polymorphisms in these genes alter the activity of the potassium-dependent sodium–calcium exchanger and the biogenesis of melanosomes [51,52]. Other intrinsic and extrinsic factors contribute to skin color by regulating the expression of melanin-related genes [53].
Abnormal melanin metabolism can lead to skin pigment disorders, which are categorized into either hyperpigmentation or hypopigmentation [53,54]. Hyperpigmentation occurs when melanin excessively accumulates owing to various internal and external stimulatory factors [55,56]. Meanwhile, hypopigmentation occurs when melanin production is reduced by genetic or epigenetic factors, as observed in albinism or vitiligo [57,58].
Melanin plays an important role in the regulation of epidermal homeostasis which is associated with the behavior of melanocytes [45,59,60]. Melanin absorbs UV radiation and dissipates energy in the form of heat, providing protection against UV radiation in the skin [10]. In our in vitro study, small interfering RNA (siRNA)-mediated knockdown of tyrosinase (TYR) resulted in decreased melanin content and viability of melanocytes exposed to UV rays [61]. The incidence of malignant melanoma is known to be significantly lower in dark-skinned people than in fair-skinned people [62].
4. Trans-Urocanic Acid and Sunscreen Products
trans-Urocanic acid is a major acid-soluble UV-absorbing compound in the stratum corneum [63]. The photon energy absorbed by trans-urocanic acid is dissipated in the form of heat in a reversible isomerization reaction to its cis isomer [64,65,66]. Histidase-deficient mice that cannot produce urocanic acid are more prone to UV-induced DNA damage and apoptotic cell death than the wild type littermates, supporting an essential role of urocanic acid in UV protection [67]. However, there is a controversy surrounding whether topically applied urocanic acid has a beneficial or detrimental effect when the skin is exposed to UV radiation [68]. Urocanic acid has been shown to mediate an immunosuppressive effect against UV rays [69] and increase photo-carcinogenic risk in hairless mice [70].
Sunscreen products are widely used for the maintenance of skin health and beauty [71]. Cosmetic and dermatology experts recommend using a sunscreen product to assist the skin’s own defense against UV rays. Although current evidence suggests that both inorganic and organic agents in sunscreen products are safe enough for daily use on the skin, there is an increasing concern regarding the penetration of sunscreen agents into the skin and the potential harmful side effects of these products [72,73]. Therefore, there is a need for the development of safer and more effective strategies for UV protection.
5. UV Protection by Botanical Extracts
Numerous plant extracts or constituents have previously been demonstrated to attenuate inflammatory responses due to UV exposure in cells, animals, and humans [74,75]. Selected studies have investigated plant extracts and the key findings of these studies are listed in Table 1.
Table 1.
Protective effects of plant-derived extracts against ultraviolet (UV) radiation-induced toxicity.
| Models | Materials | Key Findings | Literature |
|---|---|---|---|
| C57BL/6 mice, SKH-1 hairless mice | Sasa quelpaertensis | Topically applied plant extracts reduced edema and erythema in mice exposed to UV light. | [76] |
| SKH:hr-1 hairless albino mice | Propolis | The extract reduced cutaneous inflammation, immunosuppression, and lipid peroxidation induced by UV exposure. | [77] |
| SKH-1 hairless mice | Broccoli sprout | Dietary glucoraphanin-rich broccoli sprout extracts protected against UV-induced skin carcinogenesis. | [78] |
| Primary keratinocytes | Blackberry | Anthocyanin-rich fractions of blackberry extracts reduced UV-induced free radicals and oxidative damage in cells. | [79] |
| HaCaT human keratinocytes | Gardenia jasminoides | The extract displayed antioxidant, anti-inflammatory, and anti-apoptotic effects. | [41] |
| Human epidermal keratinocytes, Human dermal fibroblasts | Portulaca oleracea | The extracts protected human keratinocytes and fibroblasts from UV-induced apoptosis. | [80] |
| HaCaT human keratinocytes, Human volunteers | Citrus and Rosemary | The extracts protected UV-induced damage in a skin cell model and in human volunteers. | [81] |
| HaCaT human keratinocytes | Bambusae caulis in Taeniam | The extract enhanced the viabilities of UVB-exposed cells and reduced the number of apoptotic events. | [42] |
| HaCaT human keratinocytes, Humans volunteers | Scutellaria radix | The extract enhanced the sun protection factor (SPF) of a sunscreen product, as determined in human subjects. | [82] |
| HaCaT human keratinocytes, Reconstituted human skin tissue | Propolis | The extract inhibited UV-induced photodamage. | [83] |
In our study, botanical extracts derived from Sasa quelpaertensis, Althaea rosea, and Dryopteris crassi rhizoma attenuated cytotoxicity and melanin synthesis in cultured human epidermal melanocytes exposed to UVB rays [84]. When these plant extracts were topically applied to the ears of C57BL/6 mice or the dorsal skin of the SKH-1 hairless mouse before and after exposure to UVB rays, they prevented an increase in ear thickness or dorsal skin redness, suggesting that they possess anti-inflammatory activity that reduces edema and erythema [84]. Sasa quelpaertensis extract, which contains p-coumaric acid as one of its main constituents, had the most potent activity [76,84].
Bambusae caulis in Taeniam has been used as a health food additive and a traditional medicine for the treatment of atherosclerosis, hyperlipidemia, hypertension, and fatigue, among other conditions [85,86,87]. Its bioactivity is believed to be at least partly related to potent antioxidant properties [24,88]. Bambusae caulis in Taeniam extract, which also contains p-coumaric acid, enhanced the viability of UVB-exposed HaCaT human keratinocytes and attenuated apoptotic events, including the cleavage of procaspase 3 to its active form and an increase in the Bax to Bcl-2 ratio [42]. It also exhibited antioxidant activity by decreasing the generation of ROS and reducing lipid peroxidation in cells exposed to UVB [42]. Additionally, it reduced the expression of MMP1 and phosphorylation of JNK after stimulation with UVB [42].
We have also compared the protective effects of a number of yellow plant extracts, such as Gardenia jasminoides, Phellodendron amurense, and Rheum rhabarbarum, in HaCaT keratinocytes exposed to UVB rays [41]. Of the plant extracts tested, Gardenia jasminoides extract had the lowest cytotoxicity and enhanced the viability of UVB-exposed cells in a dose-dependent manner. The extract also attenuated lipid peroxidation, the gene expression of IL-1β, TNF-α and MMP1, and UVB-induced apoptosis, supporting its antioxidative, anti-inflammatory, and anti-apoptotic effects. Many of these properties that Gardenia jasminoides extract exhibits against UV treatment are attributed to crocin, a water-soluble carotenoid derivative [89]. The pharmacological effects of crocetin and crocin have been widely investigated [90,91].
Scutellaria radix, which is the root of Scutellaria baicalensis Georgi, has been used in traditional medicine in Asia to treat inflammatory and allergic diseases because it contains various flavonoids, such as bailcalein and baicalin (baicalein-7-O-glucuronide) [92]. Scutellaria radix extract and its constituents have been shown to exhibit antioxidant and anti-inflammatory effects in various experimental models [93,94,95]. Scutellaria radix extract also showed UV-protective effects [96,97,98]. In our study, the extract of Scutellaria radix showed high UV absorptivity and free radical scavenging activity, and attenuated UV-induced cell death of HaCaT keratinocytes [82]. The inclusion of the Scutellaria radix extract in sunscreen cream significantly enhanced the sun protection factor (SPF), as determined in human subjects [82].
Propolis is a mixture of pollen, resin, bee wax, and salivary gland secretions produced by honeybees and contains various phenolic compounds [99]. Previous studies have shown that propolis and its constituents exhibited antioxidant effects via mitigating oxidative modifications of biomolecules [77,100]. Propolis extract has also been shown to have immunomodulatory and anti-inflammatory effects under various pathological conditions [77,101,102]. In a recent study, propolis extract significantly lowered the total protein carbonyl content, a marker of protein oxidation, in HaCaT cells exposed to UVB rays [83]. It also attenuated oxidative photodamage due to UVB exposure in a model of reconstituted skin tissue [83].
An extract of Portulaca oleracea, commonly called Purslane [103], has been shown to reduce apoptotic cell death of human fibroblasts and keratinocytes after UVB irradiation, as mitochondrial membrane depolarization was detected by JC-1 staining, phosphatidylserine exposure was detected by annexin V-fluorescein isothiocyanate (FITC) staining, and apoptotic DNA fragmentation was detected using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and electrophoretic DNA ladder assay [80]. Other extracts derived from broccoli sprouts, blackberries, citrus, and rosemary have also been shown to have UV-protective effects in keratinocytes, mice, and humans [78,79,81].
6. Plant-Derived Antioxidants That Protect Melanocytes
Melanocytes localized in the stratum basale of the epidermis can also be exposed to UV rays that can increase ROS generation in the cell and deplete the endogenous pool of antioxidants [104]. UV-induced oxidative stress of melanocytes is related to the occurrence of vitiligo and melanoma [105,106]. Mimicking these conditions by exposing melanocytes to hydrogen peroxide and using various defense strategies with antioxidants have been important research topics in the field of dermatological sciences.
As listed in Table 2, numerous studies have reported that a variety of plant-derived compounds, including flavonoids, such as quercetin, apigenin, (−)-epigallocatechin-3-gallate, hyperoside, afzelin, and baicalein; iridoids, such as geniposide; and terpenoids, such as bilobalide, alleviated oxidative stress and reduced apoptosis in melanocytes exposed to hydrogen peroxide. This experimental evidence supports the working hypothesis that certain plant-derived antioxidants may alleviate the oxidative stress of melanocytes in human skin exposed to UV rays.
Table 2.
Cytoprotective effects of plant-derived antioxidants in melanocytes.
| Materials | Models | Key Findings | Literature |
|---|---|---|---|
Quercetin
|
Mel-Ab melanocytes | Quercetin reduced H2O2-induced cell death. | [107] |
| Normal human epidermal melanocytes | Quercetin attenuated ER dilation and H2O2-induced apoptosis. | [108] | |
Apigenin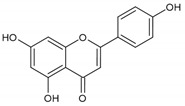
|
Normal human epidermal melanocytes | Apigenin attenuated dopamine-induced apoptosis. | [109] |
Hyperoside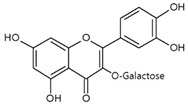
|
Normal human epidermal melanocytes | Hyperoside (quercetin-3-O-galactoside) decreased apoptosis of H2O2-injured melanocytes. | [110] |
(−)-Epigallocatechin-3-gallate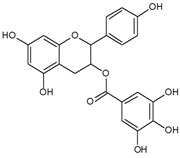
|
Normal human epidermal melanocytes | (−)-Epigallocatechin-3-gallate decreased apoptosis in H2O2-injured melanocytes. | [111] |
Afzelin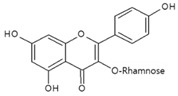
|
Normal human epidermal melanocytes | Afzelin (kaempferol-3-O-rhamnoside) inhibited H2O2-mediated cell death. | [112] |
Baicalein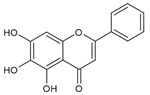
|
Human vitiligo melanocytes | Baicalein inhibited H2O2-induced cytotoxicity and apoptosis. | [113] |
Geniposide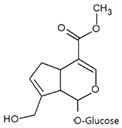
|
Normal human epidermal melanocytes | Geniposide (genipin-1-O-glucoside) decreased the apoptosis rate of H2O2-treated cells. | [114] |
Bilobalide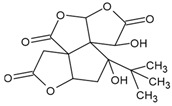
|
Normal human epidermal melanocytes | Bilobalide attenuated H2O2-induced apoptosis and ER stress. | [115] |
7. UV Absorption by Phenyl Propanoids
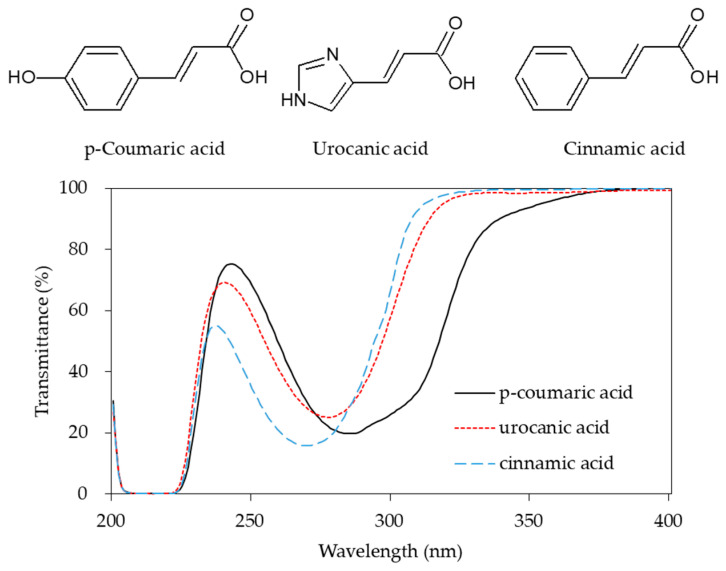
Urocanic acid is synthesized by L-histidine ammonia lyase (also called histidase) from histidine [116]. Botanical compounds, p-coumaric acid, and cinnamic acid are synthesized by L-tyrosine ammonia lyase from L-tyrosine, and by L-phenylalanine ammonia lyase from L-phenylalanine, respectively [117,118]. The chemical structures and UV transmittance spectra of p-coumaric acid, urocanic acid, and cinnamic acid are presented in Figure 2. These compounds can act as effective UV filters, with p-coumaric acid having the broadest spectrum.
Figure 2.
Chemical structures and ultraviolet (UV) transmittance spectra of p-coumaric acid, urocanic acid, and cinnamic acid. The compounds were dissolved in phosphate buffered saline at 30 μM.
p-Coumaric acid is a common secondary metabolite in plants that has been shown to have antioxidant activity in a variety of oxidative stress models, including cell [119] and animal [120,121] models. p-Coumaric acid attenuated UVB toxicity in human epidermal melanocytes [122] and HaCaT human keratinocytes [123]. p-Coumaric acid also reduced erythema induction in hairless mice and human skin exposed to UV [123,124,125]. Certain protein factors released from UV-irradiated keratinocytes induced MMP-1 expression in dermal fibroblasts via paracrine effects [36,126]. Keratinocyte-releasable stratifin was shown to induce MMP-1 expression in target fibroblasts [127,128]. In our study, p-coumaric acid reduced the expression and secretion of stratifin and indirectly attenuated MMP-1 expression in fibroblasts in medium transfer experiments [43].
Cinnamic acid attenuated UVA-induced expression of MMP-1 and -3 and the degradation of type I procollagen through inhibition of AP-1 and induction of nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant gene expression in human dermal fibroblasts [129]. Caffeic acid and ferulic acid showed anti-inflammatory and anticarcinogenic effects in mice exposed to UV [130,131,132,133,134]. The incorporation of ferulic acid in a sunscreen product as an anti-inflammatory additive increased SPF and UVA-protection factor (UVA-PF), as demonstrated in human skin [135]. Selected studies on the protective effects of cinnamic acid, p-coumaric acid, caffeic acid, and ferulic acid against UV rays are listed in Table 3.
Table 3.
Protective effects of phenyl propanoids against UV-induced toxicity.
| Compounds | Models | Key Findings | Literature |
|---|---|---|---|
Cinnamic acid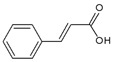 |
Human dermal fibroblasts | Cinnamic acid attenuated UVA-induced metalloproteinase expression through inhibition of AP-1 and activation of Nrf2. | [129] |
p-Coumaric acid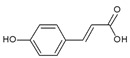
|
Human epidermal melanocytes | p-Coumaric acid inhibited melanin synthesis and attenuated UVB toxicity in melanocytes. | [122] |
| Human epidermal melanocytes, Mice | p-Coumaric acid reduced erythema and pigmentation in the skin of mice exposed to UV rays. | [124] | |
| Humans | p-Coumaric acid reduced erythema and pigmentation in human skin exposed to UV rays. | [125] | |
| HaCaT human keratinocytes, Mice | p-Coumaric acid attenuated UVB toxicity in keratinocytes and reduced erythema and edema in mice skin exposed to UV rays. | [123] | |
Caffeic acid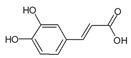
|
Mice | Caffeic acid suppressed UVB radiation-induced expression of interleukin-10 and activation of MAPKs involved in contact hypersensitivity. | [130] |
| Mice | Caffeic acid targeted ERK1/2 to attenuate solar UV-induced skin carcinogenesis. | [131] | |
| Mice | Caffeic acid prevented UVB-induced photocarcinogenesis through regulation of PTEN signaling. | [132] | |
Ferulic acid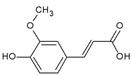 |
Mice | Ferulic acid suppressed UVB-induced MMP-2 and -9 expression in mouse skin. | [133] |
| Mice | Intraperitoneal and topical administration of ferulic acid reduced the incidence of UVB-induced tumors. | [134] | |
| Humans | Ferulic acid incorporated in a sunscreen product increased SPF and UVA-PF in human skin. | [135] |
8. Anti-inflammatory and Anticarcinogenic Effects of Quercetin
Flavonoids are a group of phenolic compounds derived from plants [136]. Various flavonoids have diverse bioactivities depending on their chemical structure, and the uptake of certain flavonoids is believed to have health benefits [137,138]. In this section, studies on the anti-inflammatory and anticarcinogenic effects of quercetin, a representative flavonoid, will be discussed.
In 1997, Steerenberg et al. reported that the oral administration of quercetin had no effect on the onset or growth of non-melanoma skin tumors in SKH hairless mice exposed to sub-erythemal doses of UVB for 17 weeks, although quercetin treatment restored the skin-associated contact hypersensitivity to picryl chloride [139,140]. Subsequent studies by Erden Inal et al. showed that intraperitoneal administration of quercetin reduced oxidative stress in Sprague–Dawley rats exposed to UVA for 9 days [141]. Casagrande et al. reported that the topical application of quercetin, formulated in emulsions, attenuated UVB-induced skin damage in hairless mice (HRS/J) [142].
Quercetin has also been shown to inhibit UV-induced lipid peroxidation in liposomes in vitro, primarily by scavenging UV-generated radical species, although it can also absorb UV radiation [143]. Quercetin decreased UV-induced nuclear factor (NF)-κB activation in HaCaT keratinocytes, thereby suppressing gene expression of inflammatory cytokines, such IL-1β, IL-6, IL-8, and TNF-α [144]. Quercetin has also been shown to lower the levels of ROS generation in HaCaT keratinocytes exposed to UVB and prevent the loss of cell membrane fluidity, mitochondrial membrane depolarization, outflow of cytochrome C, and apoptosis [145].
Quercetin-loaded nanoparticles prepared using poly(D,L-lactide-co-glycolide) (PLGA) and tocopheryl polyethylene glycol 1000 succinate (TPGS), suppressed UVB-induced NF-kB activation and cyclooxygenase (COX) 2 expression in HaCaT keratinocytes [146]. The quercetin-loaded PLGA-TPGS nanoparticles exhibited enhanced skin permeation and protective effects against UVB-induced damage in the skin of mice [146]. Quercetin-loaded chitosan was shown to permeate through the cell membrane and to inhibit the NF-kB/COX-2 signaling pathway in HaCaT keratinocytes, without affecting cell viability [147]. It also enhanced the percutaneous penetration of quercetin and modulated the NF-kB/COX-2 signaling pathway, ameliorating skin edema in C57BL/6 mice exposed to UVB irradiation [147].
Based on the findings of these studies, it has been suggested that quercetin has the potential to be used in dermatological or cosmetological approaches to attenuate oxidative stress and inflammation of the skin due to exposure, although its activity may not be sufficient to exert anticarcinogenic effects.
9. Synthesis of Melanin
Melanin is synthesized through a series of oxidative reactions inside specialized organelles called melanosomes [148,149]. Proopiomelanocortin-derived peptide hormones, such as α-melanocyte stimulating hormone (MSH), β-MSH, and adrenocorticotrophic hormone, stimulate skin pigmentation in response to UV and/or inflammatory stimuli [53,150].
The binding of an agonist to the melanocortin 1 receptor (MC1R), a G protein-coupled receptor, initiates a series of signaling events. The activation of adenylate cyclase produces cAMP, which in turn activates protein kinase A (PKA), which phosphorylates and activates cAMP-responsive element-binding protein (CREB); the CREB transcription factor then induces microphthalmia-associated transcription factor (MITF) gene expression and activation [151]. In addition to the α-MSH/MC1R pathway, the stem cell factor (SCF)/tyrosine kinase receptor c-Kit/MAPK pathway and the Wnt/Frizzled/glycogen synthase kinase (GSK) 3β/β-catenin pathway can activate MITF [152,153]. Other intracellular signaling pathways, such as phospholipase C (PLC)/diacyl glycerol (DAG)/protein kinase C (PKC) β cascade and nitric oxide (NO)/cGMP/protein kinase G (PKG) cascade, are also involved in the regulation of melanogenesis [154,155].
MITF controls the biogenesis of melanosomes as well as the gene expression of TYR, tyrosinase-related protein 1 (TYRP1), and dopachrome tautomerase (DCT) in melanocytic cells [44,156]. TYR catalyzes the initial steps of melanin synthesis by converting L-tyrosine and L-dihydroxyphenylalanine (DOPA) to L-dopaquinone [157]. These reactions are followed by subsequent reactions, which may involve thiol conjugations, leading to the synthesis of reddish-yellow pheomelanin or brownish black eumelanin [158].
Mature melanosomes that contain melanin pigments are delivered from a single melanocyte to several tens of epidermal keratinocytes in close proximity, spreading melanin pigments throughout the epidermis [159].
10. Use of MC1R Agonists to Stimulate Melanin Synthesis
Previous studies have investigated a strategy for melanoma prevention through the enhancement of eumelanin synthesis using α-MSH analogs that function as MC1R agonists [160]. [Nle4-D-Phe7]-α-MSH is the first synthetic analog of α-MSH [161]. It is a linear 13 amino acid peptide and its amino sequence, Ac-Ser-Tyr-Ser-Nle-Glu-His-D-Phe-Arg-Trp-Gly-Lys Pro-Val-NH2, is similar to that of α-MSH. The methionine at the 4th position and L-phenylalanine at the 7th position of α-MSH are replaced by norleucine and D-phenylalanine, respectively, to enhance resistance to enzymatic degradation. [Nle4-D-Phe7]-α-MSH alone or in combination with UV radiation induces human skin tanning [162]. Phase II trials found that treatment with [Nle4-D-Phe7]-α-significantly increased melanin density and tolerance to artificial light [163].
Abdel-Malek et al. showed that tetrapeptide analogs of α-MSH, such as Ac-His-D-Phe-Arg-Trp-NH2, n-Pentadecanoyl-His-D-Phe-Arg-Trp-NH2, and 4-Phenylbutyryl-His-D-Phe-Arg-Trp-NH2, enhanced melanin synthesis and promoted human melanocyte survival under conditions of UV irradiation [164]. Jackson et al. identified tetrapeptide analogs of α-MSH that function as highly selective MC1R agonists [165]. They showed that the pentapeptides Bz-Gly-His-D-Phe-AAB-AAA-NR1R2 exhibited potency similar to that of [Nle4-D-Phe7]-α-MSH. In an ex vivo experiment on human skin tissue culture, Bz-Gly-His-D-Phe-D-Arg-D-Trp-N(CH2CH2CH3)2 induced the protein expression of MITF, TYR, and TYRP-1 and enhanced the activation of Nrf2 after UVA-irradiation. Further studies are needed to determine the in vivo efficacy of these melanogenic peptides.
11. Plant-Derived Materials that Stimulate Melanin Synthesis
The induction of melanin synthesis is an attractive strategy to alleviate UV-induced damage in the skin [166]. Table 4 shows selected studies that investigated botanical extracts and their constituents that were found to promote melanin synthesis in cells.
Table 4.
Induction of melanogenesis by plant-derived materials.
| Models | Materials | Key Findings | Literature |
|---|---|---|---|
| C57BL/6 mice | Forskolin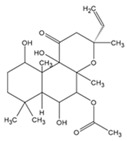
|
Forskolin-induced pigmentation was protective against UV-induced cutaneous DNA damage and tumorigenesis. | [168] |
| B16F10 mouse melanoma cells | Pratol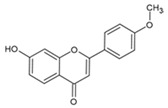
|
The compound induced melanogenesis via upregulation of phospho-p38 and phospho-JNK. | [169] |
Umbelliferone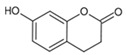
|
The compound stimulated melanogenesis and increased glutathione levels in cells. | [172] | |
Apigenin-7-butylene glucoside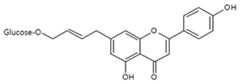
|
The compound induced melanogenesis by increasing tyrosinase activity in cells. | [170] | |
| Gynostemma pentaphyllum | Its saponins induced melanogenesis and activated the cAMP/PKA and Wnt/β-catenin signaling pathways. | [173] | |
| Argania Spinosa | Its fruit shell extract induced melanogenesis via activation of the cAMP signaling pathway. | [176] | |
| B16F10 mouse melanoma cells, Human melanoma cell lines (HMVII) | Liquiritin and liquiritigenin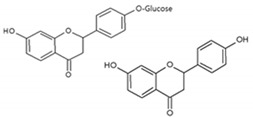
|
The compounds induced melanogenesis via enhancement of the p38 and PKA signaling pathways. | [171] |
| B16F10 mouse melanoma cells, Human epidermal melanocytes | Melia azedarach | Its ethanolic extract induced melanogenesis through the cAMP/PKA/CREB signaling pathway. | [175] |
| Cistanche deserticola | Its polysaccharides induced melanogenesis via activation of MAPK signaling pathway and upregulation of MITF. | [174] |
Forskolin is a diterpene compound isolated from the roots of Coleusforskohlii forskohlii, and it is a potent activator of adenylate cyclase [167]. Increased melanin levels, as a result of forskolin treatment, prevented the incidence of skin cancer in mice exposed to UV irradiation [168]. The promotion of melanin synthesis could alleviate UV-induced damage that causes skin photoaging and photocarcinogenesis.
Flavonoids, such as pratol [169], apigenin-7-butylene glucoside [170], liquiritin, and liquiritigenin [171], and coumarins, such as umbelliferone [172], promoted melanin synthesis in B16 F10 mouse melanoma cells. Some of these compounds activated the p38, JNK, and PKA signaling pathways. However, it is uncertain whether the rest of the compounds have similar mechanisms of action.
Gynostemma pentaphyllum saponins induced melanogenesis and activated the cAMP/PKA and Wnt/β-catenin signaling pathways [173], and Cistanche deserticola polysaccharides induced melanogenesis via activation of the MAPK signaling pathway and upregulation of MITF [174]. The extracts of Melia azedarach [175] and Argania Spinosa [174] induced melanin synthesis via activation of the cAMP signaling pathways in independent studies.
To date, most of the studies that have been conducted have shown that certain botanical products promote melanin production at the cellular level. Further studies are needed to examine whether increased melanin levels improve the resistance of cells to oxidative damage due to UV exposure. The identification of active constituents of these plant extracts and the exact mechanisms of action remains to be elucidated.
12. Plant-Derived Materials that Attenuate Extrinsic Skin Aging
A number of plant extracts and constituents have been shown to suppress the gene expression of MMPs in dermal fibroblasts and epidermal keratinocytes exposed to environmental factors including UV radiation and airborne particulate matters (PM), indicating their potential skin antiaging effects. As mentioned above, Bambusae caulis in Taeniam extract and p-coumaric acid, and Gardenia jasminoides extract attenuated the expression of MMP-1 in UVB-exposed HaCaT human keratinocytes [41,42]. Additionally, Quercus glauca extract and rutin (quercetin-3-O-rutinoside) inhibited the UVB-induced expression of MMP-1 in human dermal fibroblasts [177]. Geniposide was shown to attenuate UV-B-induced photooxidative stress and MMP-2 expression in human dermal fibroblasts [178].
In human epidermal keratinocytes exposed to PM, (−)-epigallocatechin gallate and punicalagin lowered the mRNA expression of MMP-1 [179]. (−)-Epigallocatechin gallate also decreased the expression of MMP-1, -2, -8, -9, and -13 in human dermal fibroblasts exposed to PM [180]. Camellia japonica flower extract inhibited urban dust-induced MMP-1 expression in cultured human dermal fibroblasts and in human skin explants [181]. Therefore, certain plant-derived materials can attenuate the extrinsic skin ageing process by suppressing the expression of MMPs involved in collagen degradation. For additional studies on the antiaging effects of plant-derived compounds, please see other review articles [182,183,184].
13. Conclusions
The hypothesis that plant-derived materials with UV-absorbing, antioxidant, anti-inflammatory, and melanin synthesis-promoting properties will alleviate UV-induced toxicity is supported by a variety of experimental evidence from in vitro and in vivo studies. Various plant-derived compounds, such as phenyl propanoids, flavonoids, and carotenoids, can absorb UV rays and release energy in the form of heat. These compounds can also act as antioxidants by directly scavenging various types of free radicals or by enhancing the intrinsic antioxidant capacity through the Nrf2-dependent pathway. Certain plant-derived compounds, such as quercetin, can also suppress the amplification of UV toxicity by inhibiting target enzymes involved in inflammation.
Melanocytes can be damaged by UV toxicity; however, these cells have the unique function of synthesizing melanin, which can assist in the protection of all types of skin cells, including keratinocytes, fibroblasts and melanocytes themselves. If melanocytes die or their ability to synthesize melanin decreases, the skin’s UV defense capability weakens. In other words, restoring the survival of melanocytes exposed to oxidative stress or promoting melanin synthesis will improve the overall UV tolerance capacity of the skin. Various flavonoids, iridoids, and terpenoids have been shown to alleviate oxidative stress and apoptosis of melanocytes exposed to hydrogen peroxide. Furthermore, various natural products, such as flavonoids, coumarins, polysaccharides, and saponins, have been shown to promote melanin synthesis in melanocytes.
Upon UV exposure, melanin synthesis in melanocytes acts as a defensive measure for all skin cells. After enough melanin is produced, the melanin acts as a filter or shield against UV light. However, during the period of melanin synthesis, when the melanin levels are not high enough for protection, the skin still has a high risk of UV-induced damage. This means that UV-assisted tanning can harm the skin and that “sunless tanning” is a better choice [160,168]. Natural products that can preserve melanocyte viability under conditions of oxidative stress and can induce melanin synthesis in the absence of UV radiation will provide a preemptive defense against UV exposure.
The regulation of melanin metabolism in the skin is important not only for skin health but also for cosmetic purposes. A substance that rescues the viability of melanocytes and stimulates melanin synthesis will be also useful in the prevention and treatment of hypopigmentation diseases, such as vitiligo [185]. Conversely, these substances may not be preferable for those who want to have a clean and light skin tone, as the promotion of melanin synthesis can lead to hyperpigmentation.
Plant-derived ingredients can be misunderstood to be safe because they are natural. However, certain plant-derived compounds can act as prooxidants rather than antioxidants depending on the situation [186]. In addition, they can induce cytotoxicity, cell death, inflammation, metabolic disturbance, and carcinogenesis in certain circumstances [187]. Therefore, every plant-derived component must be sufficiently examined from a toxicological aspect before use.
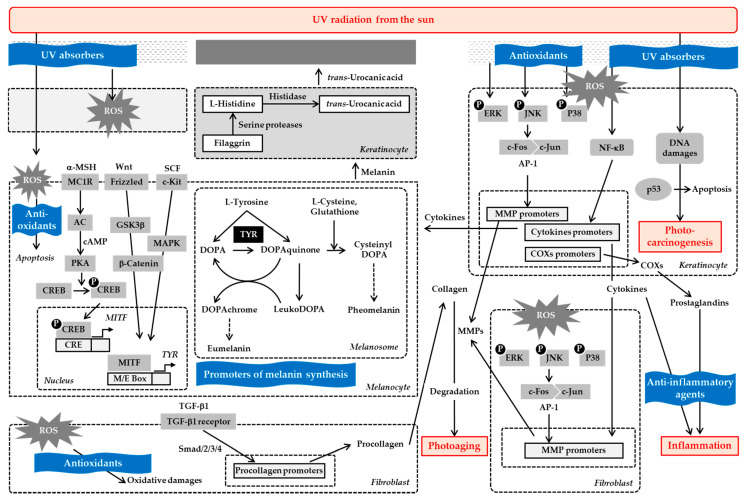
This review introduced emerging strategies for reducing UV toxicity using plant-derived materials (Figure 3). There are various substances that have been demonstrated to prevent UV-induced oxidative damage in epidermal keratinocytes or dermal fibroblasts, attenuate the death of epidermal melanocytes under oxidative stress conditions, and promote “sunless” melanin synthesis in melanocytes. Although future studies are required to verify the in vivo and clinical efficacy of these substances, emerging strategies using these plant-derived materials are expected to open new possibilities for the prevention of skin photoaging and photocarcinogenesis.
Figure 3.
Emerging strategies using plant-derived materials to protect the skin from ultraviolet (UV)-induced damage. Overexposure of the skin to UV radiation induces production of reactive oxygen species (ROS), gene expression of matrx metalloproteinases (MMPs), cytokines, and cyclooxygenases (COXs), p53 activation, and oxidative damages in DNA and other biomolecules. These can lead to photoaging, inflammation, apoptosis, and/or photocarcinogenesis. Melanin and trans-urocanic acid provide UV-defensive measures. Research has shown that various plant-derived materials, such as botanical extracts, flavonoids, phenylpropanoids, carotenoids, coumarins, iridoids, terpenoids, polysaccharides, and saponins, can help mitigate UV-induced toxicity. Plant-derived materials can act as UV absorbers, antioxidants, anti-inflammatory agents, and/or promoters of melanin synthesis. Future clinical studies are needed to determine whether these UV defense strategies using plant-derived materials can reduce the incidence or progression of photoaging, inflammation, and photocarcinogenesis in humans.
Abbreviations
| AP-1 | activator protein-1 |
| COX | cyclooxygenase |
| CREB | cAMP-responsive element-binding protein |
| DAG | diacyl glycerol |
| DOPA | dihydroxyphenylalanine |
| DCT | dopachrome tautomerase |
| ERK | extracellular signal-regulated kinase |
| FITC | fluorescein isothiocyanate |
| GSK3β | glycogen synthase kinase 3β |
| IL | interleukin |
| JNK | c-Jun-N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| MC1R | melanocortin 1 receptor |
| MITF | microphthalmia-associated transcription factor |
| MSH | melanocyte stimulating hormone |
| NO | nitric oxide |
| NF-κB | nuclear factor-κB |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PLGA | poly(D,L-lactide-co-glycolide) |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PKG | protein kinase G |
| PM | particulate matter |
| PTEN | phosphatase and tensin homolog deleted on chromosome 10 |
| ROS | reactive oxygen species |
| SCF | stem cell factor |
| siRNA | small interfering RNA |
| SPF | sun protection factor |
| TNF-α | tumor necrosis factor-α |
| TPGS | tocopheryl polyethylene glycol 1000 succinate |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| TYR | tyrosinase |
| TYRP1 | tyrosinase-related protein 1 |
| UV | ultraviolet |
| UVA-PF | UVA-protection factor |
Author Contributions
Investigation, writing, and funding acquisition, Y.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HP20C0004).
Conflicts of Interest
The author declares no conflicts of interest.
References
- 1.Rapf R.J., Vaida V. Sunlight as an energetic driver in the synthesis of molecules necessary for life. Phys. Chem. Chem. Phys. 2016;18:20067–20084. doi: 10.1039/C6CP00980H. [DOI] [PubMed] [Google Scholar]
- 2.Lucas R.M., Yazar S., Young A.R., Norval M., de Gruijl F.R., Takizawa Y., Rhodes L.E., Sinclair C.A., Neale R.E. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem. Photobiol. Sci. 2019;18:641–680. doi: 10.1039/C8PP90060D. [DOI] [PubMed] [Google Scholar]
- 3.Bernhard G.H., Neale R.E., Barnes P.W., Neale P.J., Zepp R.G., Wilson S.R., Andrady A.L., Bais A.F., McKenzie R.L., Aucamp P.J., et al. Environmental effects of stratospheric ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2019. Photochem. Photobiol. Sci. 2020;19:542–584. doi: 10.1039/D0PP90011G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K., Yamaguchi Y., Hoashi T., Hearing V.J. Melanin content and DNA damage in normal human skin in response to chronic ultraviolet radiation. J. Investig. Dermatol. 2005;124:A134. [Google Scholar]
- 5.Stremnitzer C., Barresi C., Mlitz V., Kezic S., Kammeyer A., Ghannadan M., Posa-Markaryan K., Selden C., Tschachler E., Eckhart L. Endogenous and exogenous urocanic acid protects against ultraviolet B-induced DNA damage. J. Investig. Dermatol. 2010;130:S136. doi: 10.1038/jid.2010.231. [DOI] [PubMed] [Google Scholar]
- 6.Markova N., Yarosh D., Smiles K., Karaman-Jurukovska N. The natural antioxidant L-ergothioneine is integral to the skin’s defense against ultraviolet-induced oxidative damage. J. Am. Acad. Dermatol. 2009;60:Ab156. [Google Scholar]
- 7.Hammiller B., Karuturi B.V.K., Miller C., Holmes M., Labhasetwar V., Madsen G., Hansen L.A. Delivery of antioxidant enzymes for prevention of ultraviolet irradiation-induced epidermal damage. J. Dermatol. Sci. 2017;88:373–375. doi: 10.1016/j.jdermsci.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Mohania D., Chandel S., Kumar P., Verma V., Digvijay K., Tripathi D., Choudhury K., Mitten S.K., Shah D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017;996:71–87. doi: 10.1007/978-3-319-56017-5_7. [DOI] [PubMed] [Google Scholar]
- 9.Kozma B., Eide M.J. Photocarcinogenesis: An epidemiologic perspective on ultraviolet light and skin cancer. Dermatol. Clin. 2014;32:301–313. doi: 10.1016/j.det.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Epstein J.H. Photocarcinogenesis, skin cancer, and aging. J. Am. Acad. Dermatol. 1983;9:487–502. doi: 10.1016/S0190-9622(83)70160-X. [DOI] [PubMed] [Google Scholar]
- 11.Afaq F., Mukhtar H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006;15:678–684. doi: 10.1111/j.1600-0625.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 12.Costin G.E., Hearing V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 13.Soter N.A. Acute effects of ultraviolet radiation on the skin. Semin. Dermatol. 1990;9:11–15. [PubMed] [Google Scholar]
- 14.Afaq F., Mukhtar H. Effects of solar radiation on cutaneous detoxification pathways. J. Photochem. Photobiol. B. 2001;63:61–69. doi: 10.1016/S1011-1344(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 15.Aitken G.R., Henderson J.R., Chang S.C., McNeil C.J., Birch-Machin M.A. Direct monitoring of UV-induced free radical generation in HaCaT keratinocytes. Clin. Exp. Dermatol. 2007;32:722–727. doi: 10.1111/j.1365-2230.2007.02474.x. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler A., Jonason A.S., Leffell D.J., Simon J.A., Sharma H.W., Kimmelman J., Remington L., Jacks T., Brash D.E. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 17.Van Laethem A., Claerhout S., Garmyn M., Agostinis P. The sunburn cell: Regulation of death and survival of the keratinocyte. Int. J. Biochem. Cell Biol. 2005;37:1547–1553. doi: 10.1016/j.biocel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 18.D’Agostini F., Balansky R.M., Camoirano A., De Flora S. Modulation of light-induced skin tumors by N-acetylcysteine and/or ascorbic acid in hairless mice. Carcinogenesis. 2005;26:657–664. doi: 10.1093/carcin/bgi008. [DOI] [PubMed] [Google Scholar]
- 19.Rabe J.H., Mamelak A.J., McElgunn P.J., Morison W.L., Sauder D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006;55:1–19. doi: 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Antoniou C., Kosmadaki M.G., Stratigos A.J., Katsambas A.D. Photoaging: Prevention and topical treatments. Am. J. Clin. Dermatol. 2010;11:95–102. doi: 10.2165/11530210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Scharffetter-Kochanek K., Brenneisen P., Wenk J., Herrmann G., Ma W., Kuhr L., Meewes C., Wlaschek M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000;35:307–316. doi: 10.1016/S0531-5565(00)00098-X. [DOI] [PubMed] [Google Scholar]
- 22.Kahari V.M., Saarialho-Kere U. Matrix metalloproteinases in skin. Exp. Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 23.Bode W., Fernandez-Catalan C., Tschesche H., Grams F., Nagase H., Maskos K. Structural properties of matrix metalloproteinases. Cell. Mol. Life Sci. 1999;55:639–652. doi: 10.1007/s000180050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park K.H., Lee M.W. Anti-oxidative, anti-inflammatory and whitening effects of phenolic compounds from Bambusae caulis in Liquamen. Nat. Prod. Res. 2012;26:1687–1691. doi: 10.1080/14786419.2011.593517. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y.M., Kim Y.K., Kim K.H., Park S.J., Kim S.J., Chung J.H. A novel role for the TRPV1 channel in UV-induced matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J. Cell. Physiol. 2009;219:766–775. doi: 10.1002/jcp.21729. [DOI] [PubMed] [Google Scholar]
- 26.Scharffetter K., Wlaschek M., Hogg A., Bolsen K., Schothorst A., Goerz G., Krieg T., Plewig G. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch. Dermatol. Res. 1991;283:506–511. doi: 10.1007/BF00371923. [DOI] [PubMed] [Google Scholar]
- 27.Brenneisen P., Oh J., Wlaschek M., Wenk J., Briviba K., Hommel C., Herrmann G., Sies H., Scharffetter-Kochanek K. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts. Photochem. Photobiol. 1996;64:649–657. doi: 10.1111/j.1751-1097.1996.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 28.Fisher G.J., Kang S., Varani J., Bata-Csorgo Z., Wan Y., Datta S., Voorhees J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 29.Fisher G.J., Talwar H.S., Lin J., Lin P., McPhillips F., Wang Z., Li X., Wan Y., Kang S., Voorhees J.J. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J. Clin. Investig. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenneisen P., Sies H., Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann. N. Y. Acad. Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 31.Westermarck J., Kahari V.M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. doi: 10.1096/fasebj.13.8.781. [DOI] [PubMed] [Google Scholar]
- 32.Dong K.K., Damaghi N., Picart S.D., Markova N.G., Obayashi K., Okano Y., Masaki H., Grether-Beck S., Krutmann J., Smiles K.A., et al. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp. Dermatol. 2008;17:1037–1044. doi: 10.1111/j.1600-0625.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- 33.Nickoloff B.J., Turka L.A. Keratinocytes: Key immunocytes of the integument. Am. J. Pathol. 1993;143:325–331. [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshizumi M., Nakamura T., Kato M., Ishioka T., Kozawa K., Wakamatsu K., Kimura H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008;32:1405–1411. doi: 10.1016/j.cellbi.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Ohguchi K., Itoh T., Akao Y., Inoue H., Nozawa Y., Ito M. SIRT1 modulates expression of matrix metalloproteinases in human dermal fibroblasts. Br. J. Dermatol. 2010;163:689–694. doi: 10.1111/j.1365-2133.2010.09825.x. [DOI] [PubMed] [Google Scholar]
- 36.Fagot D., Asselineau D., Bernerd F. Direct role of human dermal fibroblasts and indirect participation of epidermal keratinocytes in MMP-1 production after UV-B irradiation. Arch. Dermatol. Res. 2002;293:576–583. doi: 10.1007/s00403-001-0271-1. [DOI] [PubMed] [Google Scholar]
- 37.Ghahary A., Karimi-Busheri F., Marcoux Y., Li Y., Tredget E.E., Taghi Kilani R., Li L., Zheng J., Karami A., Keller B.O., et al. Keratinocyte-releasable stratifin functions as a potent collagenase-stimulating factor in fibroblasts. J. Investig. Dermatol. 2004;122:1188–1197. doi: 10.1111/j.0022-202X.2004.22519.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee C.H., Wu S.B., Hong C.H., Yu H.S., Wei Y.H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013;14:6414–6435. doi: 10.3390/ijms14036414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pustisek N., Situm M. UV-radiation, apoptosis and skin. Coll Antropol. 2011;35(Suppl. 2):339–341. [PubMed] [Google Scholar]
- 40.Sitailo L.A., Tibudan S.S., Denning M.F. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J. Biol. Chem. 2002;277:19346–19352. doi: 10.1074/jbc.M200401200. [DOI] [PubMed] [Google Scholar]
- 41.Park J., Seok J.K., Suh H.J., Boo Y.C. Gardenia jasminoides extract attenuates the UVB-induced expressions of cytokines in keratinocytes and indirectly Inhibits matrix metalloproteinase-1 expression in human dermal fibroblasts. Evid. Based Complement. Altern. Med. 2014;2014:429246. doi: 10.1155/2014/429246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seok J.K., Kwak J.Y., Seo H.H., Suh H.J., Boo Y.C. Effects of Bambusae caulis in Taeniam extract on UVB-induced cell death, oxidative stress and matrix metalloproteinase 1 expression in keratinocytes. J. Soc. Cosmet. Sci. Korea. 2015;41:9–20. [Google Scholar]
- 43.Seok J.K., Boo Y.C. p-Coumaric Acid Attenuates UVB-Induced Release of Stratifin from Keratinocytes and Indirectly Regulates Matrix Metalloproteinase 1 Release from Fibroblasts. Korean J. Physiol. Pharm. 2015;19:241–247. doi: 10.4196/kjpp.2015.19.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiaffino M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell. Biol. 2010;42:1094–1104. doi: 10.1016/j.biocel.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slominski A.T., Zmijewski M.A., Skobowiat C., Zbytek B., Slominski R.M., Steketee J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell. Biol. 2012;212:1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwata M., Corn T., Iwata S., Everett M.A., Fuller B.B. The relationship between tyrosinase activity and skin color in human foreskins. J. Investig. Dermatol. 1990;95:9–15. doi: 10.1111/1523-1747.ep12872677. [DOI] [PubMed] [Google Scholar]
- 47.Iozumi K., Hoganson G.E., Pennella R., Everett M.A., Fuller B.B. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J. Investig. Dermatol. 1993;100:806–811. doi: 10.1111/1523-1747.ep12476630. [DOI] [PubMed] [Google Scholar]
- 48.Tadokoro T., Yamaguchi Y., Batzer J., Coelho S.G., Zmudzka B.Z., Miller S.A., Wolber R., Beer J.Z., Hearing V.J. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J. Investig. Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- 49.Haltaufderhyde K.D., Oancea E. Genome-wide transcriptome analysis of human epidermal melanocytes. Genomics. 2014;104:482–489. doi: 10.1016/j.ygeno.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soejima M., Koda Y. Population differences of two coding SNPs in pigmentation-related genes SLC24A5 and SLC45A2. Int. J. Leg. Med. 2007;121:36–39. doi: 10.1007/s00414-006-0112-z. [DOI] [PubMed] [Google Scholar]
- 51.Ginger R.S., Askew S.E., Ogborne R.M., Wilson S., Ferdinando D., Dadd T., Smith A.M., Kazi S., Szerencsei R.T., Winkfein R.J., et al. SLC24A5 encodes a trans-Golgi network protein with potassium-dependent sodium-calcium exchange activity that regulates human epidermal melanogenesis. J. Biol. Chem. 2008;283:5486–5495. doi: 10.1074/jbc.M707521200. [DOI] [PubMed] [Google Scholar]
- 52.Cook A.L., Chen W., Thurber A.E., Smit D.J., Smith A.G., Bladen T.G., Brown D.L., Duffy D.L., Pastorino L., Bianchi-Scarra G., et al. Analysis of cultured human melanocytes based on polymorphisms within the SLC45A2/MATP, SLC24A5/NCKX5, and OCA2/P loci. J. Investig. Dermatol. 2009;129:392–405. doi: 10.1038/jid.2008.211. [DOI] [PubMed] [Google Scholar]
- 53.Slominski A., Tobin D.J., Shibahara S., Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 54.Fistarol S.K., Itin P.H. Disorders of pigmentation. J. Dtsch. Dermatol. Ges. 2010;8:187–201. doi: 10.1111/j.1610-0387.2009.07137.x. [DOI] [PubMed] [Google Scholar]
- 55.Rose P.T. Pigmentary disorders. Med. Clin. N. Am. 2009;93:1225–1239. doi: 10.1016/j.mcna.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Callender V.D., St Surin-Lord S., Davis E.C., Maclin M. Postinflammatory hyperpigmentation: Etiologic and therapeutic considerations. Am. J. Clin. Dermatol. 2011;12:87–99. doi: 10.2165/11536930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 57.Ganju P., Nagpal S., Mohammed M.H., Nishal Kumar P., Pandey R., Natarajan V.T., Mande S.S., Gokhale R.S. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci. Rep. 2016;6:18761. doi: 10.1038/srep18761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spritz R.A., Andersen G.H. Genetics of Vitiligo. Dermatol. Clin. 2017;35:245–255. doi: 10.1016/j.det.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slominski A., Kim T.K., Brozyna A.A., Janjetovic Z., Brooks D.L., Schwab L.P., Skobowiat C., Jozwicki W., Seagroves T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1alpha expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014;563:79–93. doi: 10.1016/j.abb.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slominski R.M., Zmijewski M.A., Slominski A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015;24:258–259. doi: 10.1111/exd.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.An S.M., Koh J.S., Boo Y.C. Inhibition of melanogenesis by tyrosinase siRNA in human melanocytes. BMB Rep. 2009;42:178–183. doi: 10.5483/BMBRep.2009.42.3.178. [DOI] [PubMed] [Google Scholar]
- 62.Yamaguchi Y., Beer J.Z., Hearing V.J. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: Factors influencing the incidence of skin cancer. Arch. Dermatol. Res. 2008;300(Suppl. 1):43–50. doi: 10.1007/s00403-007-0807-0. [DOI] [PubMed] [Google Scholar]
- 63.Tabachnick J. Urocanic acid, the major acid-soluble, ultraviolet-absorbing compound in guinea pig epidermis. Arch. Biochem. Biophys. 1957;70:295–298. doi: 10.1016/0003-9861(57)90107-8. [DOI] [PubMed] [Google Scholar]
- 64.Gibbs N.K., Norval M., Traynor N.J., Wolf M., Johnson B.E., Crosby J. Action spectra for the trans to cis photoisomerisation of urocanic acid in vitro and in mouse skin. Photochem. Photobiol. 1993;57:584–590. doi: 10.1111/j.1751-1097.1993.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 65.Zou M.H., Hou X.Y., Shi C.M., Nagata D., Walsh K., Cohen R.A. Modulation by peroxynitrite of AKt- and AMP-activated kinase-dependent serine phosphorylation of endothelial nitric oxide synthase. J. Biol. Chem. 2002;277:32552–32557. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]
- 66.Brookman J., Chacon J.N., Sinclair R.S. Some photophysical studies of cis- and trans-urocanic acid. Photochem. Photobiol. Sci. 2002;1:327–332. doi: 10.1039/b201621d. [DOI] [PubMed] [Google Scholar]
- 67.Barresi C., Stremnitzer C., Mlitz V., Kezic S., Kammeyer A., Ghannadan M., Posa-Markaryan K., Selden C., Tschachler E., Eckhart L. Increased sensitivity of histidinemic mice to UVB radiation suggests a crucial role of endogenous urocanic acid in photoprotection. J. Investig. Dermatol. 2011;131:188–194. doi: 10.1038/jid.2010.231. [DOI] [PubMed] [Google Scholar]
- 68.Gibbs N.K., Norval M. Urocanic acid in the skin: A mixed blessing? J. Investig. Dermatol. 2011;131:14–17. doi: 10.1038/jid.2010.276. [DOI] [PubMed] [Google Scholar]
- 69.De Fabo E.C., Noonan F.P. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J. Exp. Med. 1983;158:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reeve V.E., Greenoak G.E., Canfield P.J., Boehm-Wilcox C., Gallagher C.H. Topical urocanic acid enhances UV-induced tumour yield and malignancy in the hairless mouse. Photochem. Photobiol. 1989;49:459–464. doi: 10.1111/j.1751-1097.1989.tb09195.x. [DOI] [PubMed] [Google Scholar]
- 71.Iannacone M.R., Hughes M.C., Green A.C. Effects of sunscreen on skin cancer and photoaging. Photodermatol. Photoimmunol. Photomed. 2014;30:55–61. doi: 10.1111/phpp.12109. [DOI] [PubMed] [Google Scholar]
- 72.Nash J.F., Tanner P.R. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol. Photoimmunol. Photomed. 2014;30:88–95. doi: 10.1111/phpp.12113. [DOI] [PubMed] [Google Scholar]
- 73.Kim S., Choi K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ. Int. 2014;70:143–157. doi: 10.1016/j.envint.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Svobodova A., Psotova J., Walterova D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. Med. FAC Univ. Palacky Olomouc Czech. Repub. 2003;147:137–145. doi: 10.5507/bp.2003.019. [DOI] [PubMed] [Google Scholar]
- 75.Katiyar S.K., Korman N.J., Mukhtar H., Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 76.An S.M., Lee S.I., Choi S.W., Moon S.W., Boo Y.C. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by alpha-melanocyte stimulating hormone. Br. J. Dermatol. 2008;159:292–299. doi: 10.1111/j.1365-2133.2008.08653.x. [DOI] [PubMed] [Google Scholar]
- 77.Cole N., Sou P.W., Ngo A., Tsang K.H., Severino J.A., Arun S.J., Duke C.C., Reeve V.E. Topical ‘Sydney’ propolis protects against UV-radiation-induced inflammation, lipid peroxidation and immune suppression in mouse skin. Int. Arch. Allergy Immunol. 2010;152:87–97. doi: 10.1159/000265530. [DOI] [PubMed] [Google Scholar]
- 78.Dinkova-Kostova A.T., Fahey J.W., Benedict A.L., Jenkins S.N., Ye L., Wehage S.L., Talalay P. Dietary glucoraphanin-rich broccoli sprout extracts protect against UV radiation-induced skin carcinogenesis in SKH-1 hairless mice. Photochem. Photobiol. Sci. 2010;9:597–600. doi: 10.1039/b9pp00130a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murapa P., Dai J., Chung M., Mumper R.J., D’Orazio J. Anthocyanin-rich fractions of blackberry extracts reduce UV-induced free radicals and oxidative damage in keratinocytes. Phytother. Res. 2012;26:106–112. doi: 10.1002/ptr.3510. [DOI] [PubMed] [Google Scholar]
- 80.Lee S., Kim K.H., Park C., Lee J.S., Kim Y.H. Portulaca oleracea extracts protect human keratinocytes and fibroblasts from UV-induced apoptosis. Exp. Dermatol. 2014;23(Suppl. 1):13–17. doi: 10.1111/exd.12396. [DOI] [PubMed] [Google Scholar]
- 81.Perez-Sanchez A., Barrajon-Catalan E., Caturla N., Castillo J., Benavente-Garcia O., Alcaraz M., Micol V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol. B. 2014;136:12–18. doi: 10.1016/j.jphotobiol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Seok J.K., Kwak J.Y., Choi G.W., An S.M., Kwak J.H., Seo H.H., Suh H.J., Boo Y.C. Scutellaria radix Extract as a Natural UV Protectant for Human Skin. Phytother. Res. 2016;30:374–379. doi: 10.1002/ptr.5534. [DOI] [PubMed] [Google Scholar]
- 83.Karapetsas A., Voulgaridou G.P., Konialis M., Tsochantaridis I., Kynigopoulos S., Lambropoulou M., Stavropoulou M.I., Stathopoulou K., Aligiannis N., Bozidis P., et al. Propolis Extracts Inhibit UV-Induced Photodamage in Human Experimental In Vitro Skin Models. Antioxidants. 2019;8:125. doi: 10.3390/antiox8050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.An S.M., Lee S.J., Koh J.S., Park K., Boo Y.C. Effects of plant extract-containing creams on UVB radiation-induced inflammatory responses in mice. J. Soc. Cosmet. Sci. Korea. 2010;36:271–280. [Google Scholar]
- 85.Lee M.J., Kim M.J., Song Y.S., Song Y.O., Moon G.S. Bamboo culm extract supplementation elevates HDL-cholesterol and ameliorates oxidative stress in C57BL/6 mice fed atherogenic diet. J. Med. Food. 2008;11:69–77. doi: 10.1089/jmf.2007.009. [DOI] [PubMed] [Google Scholar]
- 86.Jiao J., Zhang Y., Lou D., Wu X. Antihyperlipidemic and antihypertensive effect of a triterpenoid-rich extract from bamboo shavings and vasodilator effect of friedelin on phenylephrine-induced vasoconstriction in thoracic aortas of rats. Phytother. Res. 2007;21:1135–1141. doi: 10.1002/ptr.2223. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y., Yao X., Bao B. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam) Phytother. Res. 2006;20:872–876. doi: 10.1002/ptr.1965. [DOI] [PubMed] [Google Scholar]
- 88.Sun J., Yu J., Zhang P.C., Tang F., Yue Y.D., Yang Y.N., Feng Z.M., Guo X.F. Isolation and identification of lignans from Caulis bambusae in Taenia with antioxidant properties. J. Agric. Food Chem. 2013;61:4556–4562. doi: 10.1021/jf4003686. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe T., Terabe S. Analysis of natural food pigments by capillary electrophoresis. J. Chromatogr. A. 2000;880:311–322. doi: 10.1016/S0021-9673(00)00209-0. [DOI] [PubMed] [Google Scholar]
- 90.Hsu J.D., Chou F.P., Lee M.J., Chiang H.C., Lin Y.L., Shiow S.J., Wang C.J. Suppression of the TPA-induced expression of nuclear-protooncogenes in mouse epidermis by crocetin via antioxidant activity. Anticancer Res. 1999;19:4221–4227. [PubMed] [Google Scholar]
- 91.Pham T.Q., Cormier F., Farnworth E., Tong V.H., Van Calsteren M.R. Antioxidant properties of crocin from Gardenia jasminoides Ellis and study of the reactions of crocin with linoleic acid and crocin with oxygen. J. Agric. Food Chem. 2000;48:1455–1461. doi: 10.1021/jf991263j. [DOI] [PubMed] [Google Scholar]
- 92.Jianjun J., Huiru D. Preparation of high-purity baicalein from Scutellaria baicalensis Georgi. Nat. Prod. Res. 2008;22:1410–1412. doi: 10.1080/14786410701823967. [DOI] [PubMed] [Google Scholar]
- 93.Yoon S.B., Lee Y.J., Park S.K., Kim H.C., Bae H., Kim H.M., Ko S.G., Choi H.Y., Oh M.S., Park W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009;125:286–290. doi: 10.1016/j.jep.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 94.Zhang X.W., Li W.F., Li W.W., Ren K.H., Fan C.M., Chen Y.Y., Shen Y.L. Protective effects of the aqueous extract of Scutellaria baicalensis against acrolein-induced oxidative stress in cultured human umbilical vein endothelial cells. Pharm. Biol. 2011;49:256–261. doi: 10.3109/13880209.2010.501803. [DOI] [PubMed] [Google Scholar]
- 95.Choi W., No R.H., Kwon H.S., Lee H.Y. Enhancement of skin anti-inflammatory activities of Scutellaria baicalensis extract using a nanoencapsulation process. J. Cosmet. Laser. 2014;16:271–278. doi: 10.3109/14764172.2014.946051. [DOI] [PubMed] [Google Scholar]
- 96.Min W., Lin X.F., Miao X., Wang B.T., Yang Z.L., Luo D. Inhibitory effects of Baicalin on ultraviolet B-induced photo-damage in keratinocyte cell line. Am. J. Chin. Med. 2008;36:745–760. doi: 10.1142/S0192415X0800620X. [DOI] [PubMed] [Google Scholar]
- 97.Wang S.C., Chen S.F., Lee Y.M., Chuang C.L., Bau D.T., Lin S.S. Baicalin scavenges reactive oxygen species and protects human keratinocytes against UVC-induced cytotoxicity. In Vivo. 2013;27:707–714. [PubMed] [Google Scholar]
- 98.Kimura Y., Sumiyoshi M. Effects of baicalein and wogonin isolated from Scutellaria baicalensis roots on skin damage in acute UVB-irradiated hairless mice. Eur. J. Pharm. 2011;661:124–132. doi: 10.1016/j.ejphar.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 99.Silva-Carvalho R., Baltazar F., Almeida-Aguiar C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid. Based Complement. Altern. Med. 2015;2015:206439. doi: 10.1155/2015/206439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kocot J., Kielczykowska M., Luchowska-Kocot D., Kurzepa J., Musik I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018;2018:7074209. doi: 10.1155/2018/7074209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sforcin J.M. Propolis and the immune system: A review. J. Ethnopharmacol. 2007;113:1–14. doi: 10.1016/j.jep.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 102.Kitamura H. Effects of Propolis Extract and Propolis-Derived Compounds on Obesity and Diabetes: Knowledge from Cellular and Animal Models. Molecules. 2019;24:4394. doi: 10.3390/molecules24234394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi A.J., Km C.J., Cho Y.J., Kim Y., Cha J.Y., Hwang J.K., Kim I.H., Kim C.T. Characterization of polysaccharides obtained from purslane (Portulaca olerace L.) using different solvents and enzymes. Food Sci. Biotechnol. 2007;16:928–934. [Google Scholar]
- 104.Denat L., Kadekaro A.L., Marrot L., Leachman S.A., Abdel-Malek Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014;134:1512–1518. doi: 10.1038/jid.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cotter M.A., Cassidy P., Grossman D. NAC protects melanocytes against oxidative stress/damage and delays onset of UV-induced melanoma in mice. J. Investig. Dermatol. 2007;127:S151. doi: 10.1158/1078-0432.CCR-07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y., Li S., Li C. Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity. Med. Sci. Monit. 2019;25:1017–1023. doi: 10.12659/MSM.914898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeong Y.M., Choi Y.G., Kim D.S., Park S.H., Yoon J.A., Kwon S.B., Park E.S., Park K.C. Cytoprotective effect of green tea extract and quercetin against hydrogen peroxide-induced oxidative stress. Arch. Pharm. Res. 2005;28:1251–1256. doi: 10.1007/BF02978208. [DOI] [PubMed] [Google Scholar]
- 108.Guan C., Xu W., Hong W., Zhou M., Lin F., Fu L., Liu D., Xu A. Quercetin attenuates the effects of H2O2 on endoplasmic reticulum morphology and tyrosinase export from the endoplasmic reticulum in melanocytes. Mol. Med. Rep. 2015;11:4285–4290. doi: 10.3892/mmr.2015.3242. [DOI] [PubMed] [Google Scholar]
- 109.Lin M., Lu S.S., Wang A.X., Qi X.Y., Zhao D., Wang Z.H., Man M.Q., Tu C.X. Apigenin attenuates dopamine-induced apoptosis in melanocytes via oxidative stress-related p38, c-Jun NH2-terminal kinase and Akt signaling. J. Dermatol. Sci. 2011;63:10–16. doi: 10.1016/j.jdermsci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 110.Yang B., Yang Q., Yang X., Yan H.B., Lu Q.P. Hyperoside protects human primary melanocytes against H2O2-induced oxidative damage. Mol. Med. Rep. 2016;13:4613–4619. doi: 10.3892/mmr.2016.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ning W., Wang S., Liu D., Fu L., Jin R., Xu A. Potent effects of peracetylated (−)-epigallocatechin-3-gallate against hydrogen peroxide-induced damage in human epidermal melanocytes via attenuation of oxidative stress and apoptosis. Clin. Exp. Dermatol. 2016;41:616–624. doi: 10.1111/ced.12855. [DOI] [PubMed] [Google Scholar]
- 112.Jung E., Kim J.H., Kim M.O., Lee S.Y., Lee J. Melanocyte-protective effect of afzelin is mediated by the Nrf2-ARE signalling pathway via GSK-3beta inactivation. Exp. Dermatol. 2017;26:764–770. doi: 10.1111/exd.13277. [DOI] [PubMed] [Google Scholar]
- 113.Ma J., Li S., Zhu L., Guo S., Yi X., Cui T., He Y., Chang Y., Liu B., Li C., et al. Baicalein protects human vitiligo melanocytes from oxidative stress through activation of NF-E2-related factor2 (Nrf2) signaling pathway. Free Radic. Biol. Med. 2018;129:492–503. doi: 10.1016/j.freeradbiomed.2018.10.421. [DOI] [PubMed] [Google Scholar]
- 114.Lu W., Zhao Y., Kong Y., Zhang W., Ma W., Li W., Wang K. Geniposide prevents H2O2-induced oxidative damage in melanocytes by activating the PI3K-Akt signalling pathway. Clin. Exp. Dermatol. 2018;43:667–674. doi: 10.1111/ced.13409. [DOI] [PubMed] [Google Scholar]
- 115.Lu L., Wang S., Fu L., Liu D., Zhu Y., Xu A. Bilobalide protection of normal human melanocytes from hydrogen peroxide-induced oxidative damage via promotion of antioxidase expression and inhibition of endoplasmic reticulum stress. Clin. Exp. Dermatol. 2016;41:64–73. doi: 10.1111/ced.12664. [DOI] [PubMed] [Google Scholar]
- 116.Mildner M., Jin J., Eckhart L., Kezic S., Gruber F., Barresi C., Stremnitzer C., Buchberger M., Mlitz V., Ballaun C., et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J. Investig. Dermatol. 2010;130:2286–2294. doi: 10.1038/jid.2010.115. [DOI] [PubMed] [Google Scholar]
- 117.Fritz R.R., Hodgins D.S., Abell C.W. Phenylalanine ammonia-lyase. Induction and purification from yeast and clearance in mammals. J. Biol. Chem. 1976;251:4646–4650. [PubMed] [Google Scholar]
- 118.Turner N.J. Ammonia lyases and aminomutases as biocatalysts for the synthesis of alpha-amino and beta-amino acids. Curr. Opin. Chem. Biol. 2011;15:234–240. doi: 10.1016/j.cbpa.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 119.Lee S.J., Mun G.I., An S.M., Boo Y.C. Evidence for the association of peroxidases with the antioxidant effect of p-coumaric acid in endothelial cells exposed to high glucose plus arachidonic acid. BMB Rep. 2009;42:561–567. doi: 10.5483/BMBRep.2009.42.9.561. [DOI] [PubMed] [Google Scholar]
- 120.Zang L.Y., Cosma G., Gardner H., Shi X., Castranova V., Vallyathan V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol. Cell. Physiol. 2000;279:C954–C960. doi: 10.1152/ajpcell.2000.279.4.C954. [DOI] [PubMed] [Google Scholar]
- 121.Lee S.I., An S.M., Mun G.I., Lee S.J., Park K.M., Park S.H., Boo Y.C. Protective effect of Sasa quelpaertensis and p-coumaric acid on ethanol-induced hepatotoxicity in mice. J. Appl. Biol. Chem. 2008;51:148–154. doi: 10.3839/jabc.2008.026. [DOI] [Google Scholar]
- 122.An S.M., Koh J.S., Boo Y.C. P-coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother. Res. 2010;24:1175–1180. doi: 10.1002/ptr.3095. [DOI] [PubMed] [Google Scholar]
- 123.Song K., Boo Y.C. UVB shielding Effects of para-Coumaric acid. J. Soc. Cosmet. Sci. Korea. 2012;38:263–273. [Google Scholar]
- 124.Song K., An S.M., Kim M., Koh J.S., Boo Y.C. Comparison of the antimelanogenic effects of p-coumaric acid and its methyl ester and their skin permeabilities. J. Dermatol. Sci. 2011;63:17–22. doi: 10.1016/j.jdermsci.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 125.Seo Y.K., Kim S.J., Boo Y.C., Baek J.H., Lee S.H., Koh J.S. Effects of p-coumaric acid on erythema and pigmentation of human skin exposed to ultraviolet radiation. Clin. Exp. Dermatol. 2011;36:260–266. doi: 10.1111/j.1365-2230.2010.03983.x. [DOI] [PubMed] [Google Scholar]
- 126.Fagot D., Asselineau D., Bernerd F. Matrix metalloproteinase-1 production observed after solar-simulated radiation exposure is assumed by dermal fibroblasts but involves a paracrine activation through epidermal keratinocytes. Photochem. Photobiol. 2004;79:499–505. doi: 10.1562/YG-03-11-R1.1. [DOI] [PubMed] [Google Scholar]
- 127.Ghahary A., Marcoux Y., Karimi-Busheri F., Li Y., Tredget E.E., Kilani R.T., Lam E., Weinfeld M. Differentiated keratinocyte-releasable stratifin (14-3-3 sigma) stimulates MMP-1 expression in dermal fibroblasts. J. Investig. Dermatol. 2005;124:170–177. doi: 10.1111/j.0022-202X.2004.23521.x. [DOI] [PubMed] [Google Scholar]
- 128.Lam E., Kilani R.T., Li Y., Tredget E.E., Ghahary A. Stratifin-induced matrix metalloproteinase-1 in fibroblast is mediated by c-fos and p38 mitogen-activated protein kinase activation. J. Investig. Dermatol. 2005;125:230–238. doi: 10.1111/j.0022-202X.2005.23765.x. [DOI] [PubMed] [Google Scholar]
- 129.Hseu Y.C., Korivi M., Lin F.Y., Li M.L., Lin R.W., Wu J.J., Yang H.L. Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of Nrf2-mediated antioxidant genes in human skin fibroblasts. J. Dermatol. Sci. 2018;90:123–134. doi: 10.1016/j.jdermsci.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 130.Staniforth V., Chiu L.T., Yang N.S. Caffeic acid suppresses UVB radiation-induced expression of interleukin-10 and activation of mitogen-activated protein kinases in mouse. Carcinogenesis. 2006;27:1803–1811. doi: 10.1093/carcin/bgl006. [DOI] [PubMed] [Google Scholar]
- 131.Yang G., Fu Y., Malakhova M., Kurinov I., Zhu F., Yao K., Li H., Chen H., Li W., Lim D.Y., et al. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer Prev. Res. 2014;7:1056–1066. doi: 10.1158/1940-6207.CAPR-14-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Balupillai A., Nagarajan R.P., Ramasamy K., Govindasamy K., Muthusamy G. Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signaling in human dermal fibroblasts and mouse skin. Toxicol. Appl. Pharm. 2018;352:87–96. doi: 10.1016/j.taap.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 133.Staniforth V., Huang W.C., Aravindaram K., Yang N.S. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J. Nutr. Biochem. 2012;23:443–451. doi: 10.1016/j.jnutbio.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 134.Ambothi K., Prasad N.R., Balupillai A. Ferulic acid inhibits UVB-radiation induced photocarcinogenesis through modulating inflammatory and apoptotic signaling in Swiss albino mice. Food Chem. Toxicol. 2015;82:72–78. doi: 10.1016/j.fct.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 135.Peres D.D., Sarruf F.D., de Oliveira C.A., Velasco M.V.R., Baby A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B. 2018;185:46–49. doi: 10.1016/j.jphotobiol.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 136.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. ScientificWorldJournal. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cazarolli L.H., Zanatta L., Alberton E.H., Figueiredo M.S., Folador P., Damazio R.G., Pizzolatti M.G., Silva F.R. Flavonoids: Prospective drug candidates. Mini Rev. Med. Chem. 2008;8:1429–1440. doi: 10.2174/138955708786369564. [DOI] [PubMed] [Google Scholar]
- 138.Terahara N. Flavonoids in foods: A review. Nat. Prod. Commun. 2015;10:521–528. doi: 10.1177/1934578X1501000334. [DOI] [PubMed] [Google Scholar]
- 139.Steerenberg P.A., Garssen J., Dortant P.M., van der Vliet H., Geerse E., Verlaan A.P., Goettsch W.G., Sontag Y., Bueno-de-Mesquita H.B., Van Loveren H. The effect of oral quercetin on UVB-induced tumor growth and local immunosuppression in SKH-1. Cancer Lett. 1997;114:187–189. doi: 10.1016/S0304-3835(97)04659-4. [DOI] [PubMed] [Google Scholar]
- 140.Steerenberg P.A., Garssen J., Dortant P., van de Vliet H., Geerse L., Verlaan A.P., Goettsch W., Sontag Y., Norval M., Gibbs N.K., et al. Quercetin prevents UV-induced local immunosuppression, but does not affect UV-induced tumor growth in SKH-1 hairless mice. Photochem. Photobiol. 1997;65:736–744. doi: 10.1111/j.1751-1097.1997.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 141.Erden Inal M., Kahraman A., Koken T. Beneficial effects of quercetin on oxidative stress induced by ultraviolet A. Clin. Exp. Dermatol. 2001;26:536–539. doi: 10.1046/j.1365-2230.2001.00884.x. [DOI] [PubMed] [Google Scholar]
- 142.Casagrande R., Georgetti S.R., Verri W.A., Jr., Dorta D.J., dos Santos A.C., Fonseca M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B. 2006;84:21–27. doi: 10.1016/j.jphotobiol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 143.Fahlman B.M., Krol E.S. Inhibition of UVA and UVB radiation-induced lipid oxidation by quercetin. J. Agric. Food Chem. 2009;57:5301–5305. doi: 10.1021/jf900344d. [DOI] [PubMed] [Google Scholar]
- 144.Vicentini F.T., He T., Shao Y., Fonseca M.J., Verri W.A., Jr., Fisher G.J., Xu Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kappaB pathway. J. Dermatol. Sci. 2011;61:162–168. doi: 10.1016/j.jdermsci.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 145.Zhu X.B., Li N., Wang Y.L., Ding L., Chen H.J., Yu Y.H., Shi X.J. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017;37:209–218. doi: 10.3892/or.2016.5217. [DOI] [PubMed] [Google Scholar]
- 146.Zhu X., Zeng X., Zhang X., Cao W., Wang Y., Chen H., Wang T., Tsai H.I., Zhang R., Chang D., et al. The effects of quercetin-loaded PLGA-TPGS nanoparticles on ultraviolet B-induced skin damages in vivo. Nanomedicine. 2016;12:623–632. doi: 10.1016/j.nano.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 147.Nan W.H., Ding L., Chen H.J., Khan F.U., Yu L., Sui X.B., Shi X.J. Topical Use of Quercetin-Loaded Chitosan Nanoparticles Against Ultraviolet B Radiation. Front. Pharmacol. 2018;9:826. doi: 10.3389/fphar.2018.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sulaimon S.S., Kitchell B.E. The biology of melanocytes. Vet. Dermatol. 2003;14:57–65. doi: 10.1046/j.1365-3164.2003.00327.x. [DOI] [PubMed] [Google Scholar]
- 149.Slominski A. Coming of age of melanogenesis-related proteins. Arch. Pathol. Lab. Med. 2002;126:775–777. doi: 10.5858/2002-126-0775-COAOMR. [DOI] [PubMed] [Google Scholar]
- 150.Steinhoff M., Stander S., Seeliger S., Ansel J.C., Schmelz M., Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch. Dermatol. 2003;139:1479–1488. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- 151.Busca R., Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 152.Flaherty K.T., Hodi F.S., Fisher D.E. From genes to drugs: Targeted strategies for melanoma. Nat. Rev. Cancer. 2012;12:349–361. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- 153.Serre C., Busuttil V., Botto J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018;40:328–347. doi: 10.1111/ics.12466. [DOI] [PubMed] [Google Scholar]
- 154.Rzepka Z., Buszman E., Beberok A., Wrzesniok D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. (Online) 2016;70:695–708. doi: 10.5604/17322693.1208033. [DOI] [PubMed] [Google Scholar]
- 155.D’Mello S.A., Finlay G.J., Baguley B.C., Askarian-Amiri M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016;17:1144. doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Simon J.D., Peles D., Wakamatsu K., Ito S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009;22:563–579. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 157.Cooksey C.J., Garratt P.J., Land E.J., Pavel S., Ramsden C.A., Riley P.A., Smit N.P. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 1997;272:26226–26235. doi: 10.1074/jbc.272.42.26226. [DOI] [PubMed] [Google Scholar]
- 158.Olivares C., Solano F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009;22:750–760. doi: 10.1111/j.1755-148X.2009.00636.x. [DOI] [PubMed] [Google Scholar]
- 159.Cardinali G., Ceccarelli S., Kovacs D., Aspite N., Lotti L.V., Torrisi M.R., Picardo M. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J. Investig. Dermatol. 2005;125:1190–1199. doi: 10.1111/j.0022-202X.2005.23929.x. [DOI] [PubMed] [Google Scholar]
- 160.Abu Ubeid A., Hantash B.M. Minireview: Peptide Analogs and Short Sequence Oligopeptides as Modulators of Skin Pigmentation. Curr. Top. Med. Chem. 2014;14:1418–1424. doi: 10.2174/1568026614666140601221519. [DOI] [PubMed] [Google Scholar]
- 161.Sawyer T.K., Sanfilippo P.J., Hruby V.J., Engel M.H., Heward C.B., Burnett J.B., Hadley M.E. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: A highly potent alpha-melanotropin with ultralong biological activity. Proc. Natl. Acad. Sci. USA. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dorr R.T., Ertl G., Levine N., Brooks C., Bangert J.L., Powell M.B., Humphrey S., Alberts D.S. Effects of a superpotent melanotropic peptide in combination with solar UV radiation on tanning of the skin in human volunteers. Arch. Dermatol. 2004;140:827–835. doi: 10.1001/archderm.140.7.827. [DOI] [PubMed] [Google Scholar]
- 163.Kreim S., Lautenschlager S., Minder E. Safety and efficacy of an agonistic alpha-melanocyte stimulating hormone analogue, afamelanotide (Scenesse (R)), in treating patients with erythropoietic protoporphyria for 2.5 consecutive years. Br. J. Dermatol. 2011;164:1149. [Google Scholar]
- 164.Abdel-Malek Z.A., Kadekaro A.L., Kavanagh R.J., Todorovic A., Koikov L.N., McNulty J.C., Jackson P.J., Millhauser G.L., Schwemberger S., Babcock G., et al. Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. FASEB J. 2006;20:1561. doi: 10.1096/fj.05-5655fje. [DOI] [PubMed] [Google Scholar]
- 165.Jackson E., Heidl M., Imfeld D., Meeus L., Schuetz R., Campiche R. Discovery of a Highly Selective MC1R Agonists Pentapeptide to Be Used as a Skin Pigmentation Enhancer and with Potential Anti-Aging Properties. Int. J. Mol. Sci. 2019;20:6143. doi: 10.3390/ijms20246143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fajuyigbe D., Lwin S.M., Diffey B.L., Baker R., Tobin D.J., Sarkany R.P.E., Young A.R. Melanin distribution in human epidermis affords localized protection against DNA photodamage and concurs with skin cancer incidence difference in extreme phototypes. FASEB J. 2018;32:3700–3706. doi: 10.1096/fj.201701472R. [DOI] [PubMed] [Google Scholar]
- 167.Seamon K.B., Padgett W., Daly J.W. Forskolin: Unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.D’Orazio J.A., Nobuhisa T., Cui R., Arya M., Spry M., Wakamatsu K., Igras V., Kunisada T., Granter S.R., Nishimura E.K., et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- 169.Chung Y.C., Kim S., Kim J.H., Lee G.S., Lee J.N., Lee N.H., Hyun C.G. Pratol, an O-Methylated Flavone, Induces Melanogenesis in B16F10 Melanoma Cells via p-p38 and p-JNK Upregulation. Molecules. 2017;22:1704. doi: 10.3390/molecules22101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xin X.J., Zou J.H., Zou T., Shang H.L., Sun L.Y. A Newly Authenticated Compound from Traditional Chinese Medicine Decoction Induces Melanogenesis in B16-F10 Cells by Increasing Tyrosinase Activity. Evid. Based Complement. Altern. Med. 2018;2018:5198594. doi: 10.1155/2018/8485670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Uto T., Ohta T., Yamashita A., Fujii S., Shoyama Y. Liquiritin and Liquiritigenin Induce Melanogenesis via Enhancement of p38 and PKA Signaling Pathways. Medicines. 2019;6:68. doi: 10.3390/medicines6020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee Y., Ku B., Kim D., Choi E.-M. Umbelliferone stimulated melanogenesis and increased glutathione level in B16F10 cells. Toxicol. Environ. Health Sci. 2017;9:152–160. doi: 10.1007/s13530-017-0316-2. [DOI] [Google Scholar]
- 173.Tsang T.F., Chan B., Tai W.C., Huang G., Wang J., Li X., Jiang Z.H., Hsiao W.L.W. Gynostemma pentaphyllum saponins induce melanogenesis and activate cAMP/PKA and Wnt/beta-catenin signaling pathways. Phytomedicine. 2019;60:153008. doi: 10.1016/j.phymed.2019.153008. [DOI] [PubMed] [Google Scholar]
- 174.Hu Y.B., Huang J.H., Li Y.X., Jiang L., Ouyang Y.J., Li Y.M., Yang L., Zhao X.J., Huang L.H., Xiang H., et al. Cistanche deserticola polysaccharide induces melanogenesis in melanocytes and reduces oxidative stress via activating NRF2/HO-1 pathway. J. Cell. Mol. Med. 2020;24:4023–4035. doi: 10.1111/jcmm.15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim M.O., Park S.J., Park S.H., Oh S.W., Lee S.E., Yoo J.A., Kwon K., Kim J., Kim M.H., Cho J.Y., et al. Ethanolic extract of Melia azedarach L. induces melanogenesis through the cAMP-PKA-CREB signaling pathway. Mol. Cell. Toxicol. 2019;15:75–83. doi: 10.1007/s13273-019-0009-9. [DOI] [Google Scholar]
- 176.Makbal R., Villareal M.O., Gadhi C., Hafidi A., Isoda H. Argania spinosa Fruit Shell Extract-Induced Melanogenesis via cAMP Signaling Pathway Activation. Int. J. Mol. Sci. 2020;21:2539. doi: 10.3390/ijms21072539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee S., Koh J.-S., Ha B.-J., Boo Y. Quercus glauca extract and rutin inhibit the UVB-induced expression of matrix metalloproteinase-1 in human dermalfibroblasts. J. Korean Soc. Appl. Biol. Chem. 2010;53:677–684. doi: 10.3839/jksabc.2010.103. [DOI] [Google Scholar]
- 178.Shin D., Lee S., Huang Y.H., Lim H.W., Lee Y., Jang K., Cho Y., Park S.J., Kim D.D., Lim C.J. Protective properties of geniposide against UV-B-induced photooxidative stress in human dermal fibroblasts. Pharm. Biol. 2018;56:176–182. doi: 10.1080/13880209.2018.1446029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Seok J.K., Lee J.W., Kim Y.M., Boo Y.C. Punicalagin and (−)-Epigallocatechin-3-Gallate Rescue Cell Viability and Attenuate Inflammatory Responses of Human Epidermal Keratinocytes Exposed to Airborne Particulate Matter PM10. Ski. Pharm. Physiol. 2018;31:134–143. doi: 10.1159/000487400. [DOI] [PubMed] [Google Scholar]
- 180.Wang L., Lee W., Cui Y.R., Ahn G., Jeon Y.J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-kappaB, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019;252:1318–1324. doi: 10.1016/j.envpol.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 181.Kim M., Son D., Shin S., Park D., Byun S., Jung E. Protective effects of Camellia japonica flower extract against urban air pollutants. BMC Complement. Altern. Med. 2019;19:30. doi: 10.1186/s12906-018-2405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Correa R.C.G., Peralta R.M., Haminiuk C.W.I., Maciel G.M., Bracht A., Ferreira I.C.F.R. New phytochemicals as potential human anti-aging compounds: Reality, promise, and challenges. Crit. Rev. Food Sci. Nutr. 2018;58:942–957. doi: 10.1080/10408398.2016.1233860. [DOI] [PubMed] [Google Scholar]
- 183.Petruk G., Del Giudice R., Rigano M.M., Monti D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxidative Med. Cell. Longev. 2018;2018:1454939. doi: 10.1155/2018/1454936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boo Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants. 2019;8:379. doi: 10.3390/antiox8090379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Niu C., Aisa H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules. 2017;22:1303. doi: 10.3390/molecules22081303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Eghbaliferiz S., Iranshahi M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016;30:1379–1391. doi: 10.1002/ptr.5643. [DOI] [PubMed] [Google Scholar]
- 187.Kyselova Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 2011;4:173. doi: 10.2478/v10102-011-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]