Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder caused by the depletion of dopaminergic neurons in the basal ganglia, the movement center of the brain. Approximately 60,000 people are diagnosed with PD in the United States each year. Although the direct cause of PD can vary, accumulation of oxidative stress-induced neuronal damage due to increased production of reactive oxygen species (ROS) or impaired intracellular antioxidant defenses invariably occurs at the cellular levels. Pharmaceuticals such as dopaminergic prodrugs and agonists can alleviate some of the symptoms of PD. Currently, however, there is no treatment to halt the progression of PD pathology. Due to the nature of PD, a long and progressive neurodegenerative process, strategies to prevent or delay PD pathology may be well suited to lifestyle changes like dietary modification with antioxidant-rich foods to improve intracellular redox homeostasis. In this review, we discuss cellular and genetic factors that increase oxidative stress in PD. We also discuss neuroprotective roles of dietary antioxidants including vitamin C, vitamin E, carotenoids, selenium, and polyphenols along with their potential mechanisms to alleviate PD pathology.
Keywords: antioxidant, mitochondria, neurodegeneration, nutrient, apoptosis
1. Introduction
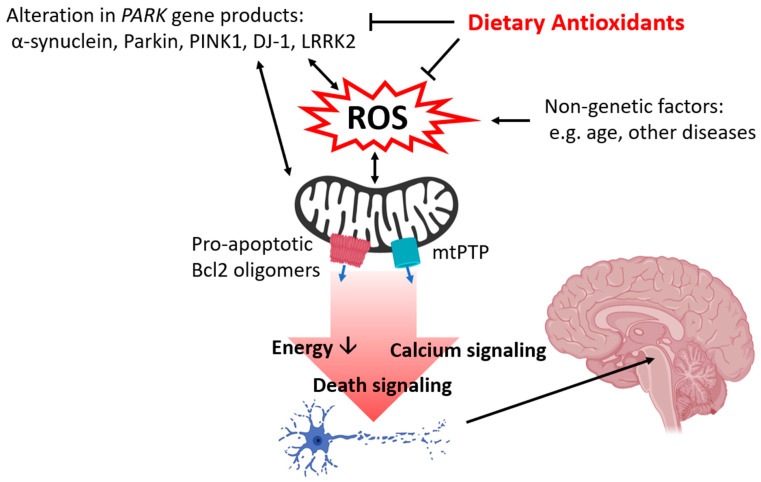
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by inadequate levels of dopamine that is caused by loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the basal ganglia. Dopamine also acts in other regions of the brain like the striatum, a substructure of the forebrain that regulates the motor system. Patients with PD exhibit motor symptoms including tremor, bradykinesia, rigidity, and speech difficulties, and also frequently suffer from nonmotor symptoms including depression and insomnia [1,2]. The incidence of sporadic PD is influenced by many factors including lifestyle, environment, age, and pre-existing conditions. Oxidative stress generated by many of these factors has been addressed as a major contributor to the development and progression of neurodegeneration at the cellular levels (Figure 1) [3,4,5]. In particular, mitochondrial dysfunction is a key finding in reactive oxygen species (ROS)-induced PD pathology [4,5,6,7]. Complex I, also known as NADH oxidoreductase of the electron transport chain (ETC) transfers electrons from NADH to ubiquinone and so plays a key role in oxidative phosphorylation. Complex I is vulnerable to oxidative damage, and its inhibition is also strongly associated with the generation of ROS such as superoxide and hydrogen peroxide presenting a positive feedback loop [8,9,10]. Currently, neurotoxins that target complex I like 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone are used to induce parkinsonism in both in vitro and in vivo models for research, and treatment with these drugs is known to induce oxidative stress [11,12]. In addition, imbalances in dopamine metabolism contribute to ROS generation, thus damaging dopaminergic neurons. Under normal physiological conditions, dopamine is synthesized from the amino acids tyrosine and tyramine. Hydroxylation and decarboxylation of tyrosine produce dopamine, and dopamine is further converted to norepinephrine and epinephrine or undergoes degradation. However, dopamine can also undergo metabolism by monoamine oxidase (MAO) producing the highly reactive metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL) [13], and dopamine itself can undergo oxidation. Accumulation of DOPAL and oxidized dopamine increases the production of ROS damaging mitochondria [14,15,16,17,18].
Figure 1.
Summary of the protective roles of dietary antioxidants in Parkinson’s disease (PD). Both genetic and nongenetic factors contribute to the accumulation of oxidative stress by enhancing ROS production and impairing cellular antioxidant defense systems. Oxidative stress damages intracellular organelles, most notably the mitochondria, impairing neuronal energy metabolism and thus hindering the energy-demanding process in the brain including neurotransmission and neuritogenesis. Mitochondrial dysfunction primes apoptosis, calcium release, and opening of mtPTP which leads to the death of neurons, including the specific dopaminergic population of the SNpc which produces the signs and symptoms of PD. Illustration by BioRender.
2. Oxidative Stress and PARK Genes
Approximately 5-10% of PD cases are associated with abnormalities of PARK genes [19,20,21]. The mutation of PARK genes increases oxidative stress in neurons by enhancing the production of ROS or impairing intracellular antioxidant defense systems. ROS predisposes PARK genes to abnormal protein production and vice versa (Figure 1). α-synuclein is a protein encoded by the SNCA (PARK1) gene. Although α-synuclein supports synaptogenesis and synaptic plasticity during normal physiology [22,23], α-synuclein aggregation-associated neuronal damage is a common finding in PD affected tissue. Application of oligomeric α-synuclein increases ROS production and lipid peroxidation [24,25]. α-synuclein is translocated to mitochondria and directly interacts with mitochondrial proteins including ATP synthase to lower mitochondrial inner membrane potential, thus altering neuronal energy metabolism and opening mitochondrial death channels [24,26]. Increased oxidative stress induced by treatment with hydrogen peroxide or depletion of antioxidant enzymes enhances post-translational modification and aggregation of α-synuclein and worsens the progression of PD [27,28,29].
Parkin, the ubiquitin E3 ligase encoded by the PARK2 gene, regulates mitochondrial quality control. Mutations of PARK2 are the most common cause of early-onset PD. Approximately 77% of early-onset familial PD in patients younger than 30 years old have Parkin mutations [30]. Parkin works in conjunction with the myocytes lacking PTEN-induced kinase 1 (PINK1), a key enzyme responsible for carrying out autophagy, encoded by the PARK6 gene. PINK1-mediated phosphorylation of ubiquitin activates Parkin, and this enhances the removal of unwanted mitochondria [31]. Additionally, Parkin-mediated ubiquitination also targets mitofusin and miro, key components of mitochondrial fusion and mitochondrial transport, respectively [32,33]. The deletion of Parkin or PINK1 impairs mitophagy, and failure to remove dysfunctional mitochondria increases ROS production [34]. PARK2 knockout transgenic mice treated with chronic ethanol, a stimulator of ROS, show abnormally high superoxide accumulation and glutathione depletion [35]. Application of the mitophagy inducer, autophagy and beclin 1 regulator 1 (AMBRA1), restores mitophagy in PINK1 knockout mice and suppresses ROS production [36]. Overall, the Parkin/PINK1 system plays a critical role in regulating intracellular ROS by mitochondrial quality control, removing inefficient and damaged mitochondria.
Protein deglycase DJ-1 is encoded by the PARK 7 gene. Although the function of DJ-1 is less studied than other PARK gene products, it is reported to play an important role in supporting mitochondrial function. DJ-1 binds directly to F1Fo ATP synthase and the antiapoptotic protein Bcl-xL, and this interaction promotes mitochondrial energy metabolism and survival of dopaminergic neurons [37]. The depletion of DJ-1 increases the vulnerability of mitochondria to neurotoxic insults which mimic PD pathology [38,39], whereas overexpression of DJ-1 improves intracellular antioxidants and protects neurons [38,40,41]. DJ-1 plays an important role in sensing intracellular redox status during oxidative stress [42,43,44]. Under oxidative stress, DJ-1 undergoes post-translational oxidation at its Cys106 residue to form cysteine-sulfonic acid and cysteine-sulfinic acid [42,45,46], and oxidative stress also enhances translocation of DJ-1 to mitochondria. Thus, it is possible that oxidation of Cys106 may act as the signal for DJ-1 to prevent mitochondrial dysfunction during ROS production in PD. In addition, DJ-1 regulates the expression of antioxidant genes by promoting nuclear translocation of Nrf2, a transcription factor that binds to genes containing an antioxidant response element (ARE) [41,47].
LRRK2, also known as dardarin, is a kinase with guanosine triphosphatase (GTPase) and scaffolding domains [48]. LRRK2 is found in the mitochondrial membrane and interacts with other PARK gene products including Parkin, PINK1, and DJ-1 [49,50]. LRRK2 is encoded by the LRRK2 (PARK8) gene. Mutation of LRRK2 is associated with the gain of kinase activity, and this is common among patients with late-onset autosomal-dominant PD [51]. Mutation of the kinase domain of LRRK2, G2019S, exacerbates ROS-induced dopaminergic neuronal death, and application of truncated LRRK2 reverses ROS accumulation and prevents morphological alteration of these neurons [52]. In the same way, the depletion of LRRK2 or application of LRRK2 inhibitors decreases ROS, restores mitochondrial function, prevents mitochondrial fragmentation, and blocks increases in proapoptotic proteins including caspase 3, Bax, and apoptotic-inducing factor [53,54,55].
3. Oxidative Stress and Mitochondrial Dysfunction
Oxidative stress and mitochondrial dysfunction eventually lead to neuronal apoptosis during PD. Neurotoxic stimulation and ROS exposure increase the abundance of proapoptotic Bcl-2 protein Bax and Bak in the mitochondrial membrane (Figure 1). Oligomerization of proapoptotic proteins increases the permeability of the mitochondrial membrane causing the release of cytochrome c. Cytochrome c forms apoptosomes and activates executor caspases like caspase 3. Antiapoptotic proteins Bcl-2 and Bcl-xL are reported to block apoptosis by directly binding proapoptotic Bcl-2 proteins. Upregulation of proapoptotic proteins such as Bax and Bim as well as of other mechanisms including caspase activation and cytoplasmic release of cytochrome c have been reported in various PD models [54,56,57,58,59,60]. Transgenic mice lacking Bax are resistant to MPTP-induced neuronal death in the SNpc [56], and application of microRNA (miR) including miR216a and miR7 targeting Bax are protective against MPTP treatment in an in vitro and in vivo PD models [61,62]. Bcl-xL is an antiapoptotic protein that binds to DJ-1 and regulates energy metabolism in dopaminergic neurons [37,63]. A recent study shows that Bcl-xL undergoes post-translational cleavage during oxidative stress, and the accumulation of truncated Bcl-xL leads to mitochondrial dysfunction [64]. Approaches that inhibit proteolytic cleavage of Bcl-xL are reported to be protective against neurotoxicity. Treatment with antioxidants prevents the accumulation of truncated Bcl-xL and rescues neurons from oxidative stress [64]. SH-SY5Y cells derived from human bone marrow that overexpress PINK1 show decreased proteolytic cleavage of Bcl-xL by enhancing phosphorylation of Bcl-xL [65]. Bcl-xL Cre-lox knockout mice show decreased tyrosine hydroxylase-positive cells indicating loss of dopaminergic neurons in the SNpc [66]; thus, maintaining functional Bcl-xL may be critical in preventing PD-associated neuronal death.
The association between neuronal death and opening of mitochondrial permeability transition pore (mtPTP), a large less-selective mitochondrial inner membrane death channel, has been documented in PD models [24,67,68]. The opening of mtPTP allows the passage of ions and small molecules less than 1.5KDa and depolarizes the mitochondrial inner membrane. mtPTP also enhances calcium release [24] which can trigger apoptosis (Figure 1) [69]. Loss of the mitochondrial electrochemical gradient impairs ATP production by the F1Fo ATP synthase and impairs neuronal energy metabolism [70,71,72]. The F1Fo ATP synthase plays a key role in ATP production and mPTP formation [72,73,74]. The F1Fo ATP synthase interacts with PARK gene products DJ-1 and α-synuclein [24,37]. Interaction between DJ-1 and F1Fo ATP synthase enhances neuronal energy metabolism and promotes elongation and arborization of dopaminergic neurons [37]. On the other hand, oligomeric α-synuclein co-localizes with the F1Fo ATP synthase and causes oxidative modification of its β subunit, the key subunit that interacts with ADP and ATP [24]. This oxidative modification increases the opening of mtPTP. Similarly, treatment with α-synuclein, known to form insoluble fibrils during PD pathology, favors mtPTP opening in both in vitro and in vivo models, and application of the mPTP inhibitor cyclosporin A reverses α-synuclein-induced mitochondrial dysfunction [60,75]. The depletion of PINK1 decreases mitochondrial inner membrane potential and increases the opening of mtPTP, and this leads to mitophagy and neuronal death [76,77].
4. Neuroprotective Dietary Antioxidants
Neurodegeneration at the cellular level develops years before patients exhibit clinical manifestations of PD. Therefore, finding strategies that can be applied over a lifetime seems of logical importance in fighting against PD. An increasing number of studies have addressed neuroprotective roles of nutrients and functional foods against neurodegeneration [78,79,80]. In particular, certain vitamins, minerals, and phytochemicals exhibit their antioxidant properties by directly scavenging ROS, binding to antioxidant enzymes as cofactors, and by regulating genes that control intracellular antioxidant systems (Figure 1). Advancing technologies in liquid chromatography and mass spectrometry such as LC/MS/MS and MALDI-TOF allow quantitative analysis of these nutrients and application of molecular approaches including sequencing, polymerase chain reaction, and electrophoresis to elucidate the association between PARK genes and dietary antioxidants. Here, we discuss dietary antioxidants that may potentially prevent or delay the progression of PD (Table 1).
Table 1.
List of studies investigating the roles of antioxidant nutrients in PD models.
| Vit C | Vit E | Vit A & Car | Se | GSH & NAC | Cur | Res | Cat | Ole | |
|---|---|---|---|---|---|---|---|---|---|
| α-synuclein | [81,82] | [83] | [84] | [85,86,87] | [88,89] | [90,91,92,93,94,95,96] | [97,98] | ||
| Oxidative stress and antioxidant | [99] | [99] | [100] | [101] | [102] | [87,103,104,105,106,107] | [108,109,110,111,112,113,114] | [93,115,116,117,118,119] | [97,98,120,121,122,123,124] |
| Electron transport chain | [125] | [103,126] | [109,110,113] | ||||||
| Neuronal death and apoptotic pathway | [127] | [128] | [83,100,129,130,131,132,133] | [102,112,134,135] | [87,103,104,107,126,136,137,138] | [88,89,108,111,113] | [95,116,118,119,139,140] | [97,121,122,123,141,142] | |
| Behavioral or motor function | [127] | [128] | [130] | [101] | [105,136,143,144,145,146] | [88,108,110,113,147] | [92,115,116,117,118,139,148] | [123] |
Vitamin C (Vit C), vitamin E (Vit E), vitamin A (Vit A), carotenoids (Car), selenium (Se), glutathione (GSH), N-acetylcysteine (NAC), curcumin (Cur), resveratrol (Res), catechin (Cat), oleuropein (Ole).
4.1. Vitamin C
Vitamin C, also called ascorbic acid or ascorbate, is abundant in fruits and vegetables. Although most mammals are able to synthesize vitamin C endogenously, humans lack the necessary enzyme L-gulonolactone oxidase, so humans must ingest this essential nutrient in food or supplements [149]. Vitamin C acts as an antioxidant by donating electrons to neutralize the toxic effect of free radicals. Depending on available in vivo concentration, at high doses (≥500 mg/d), vitamin C has been shown to exhibit prooxidant properties [150]. In addition to its role in regulating cellular redox status, vitamin C supports the actions of hydroxylases involved in neurotransmitter synthesis including dopamine β-hydroxylase. Neural tissue including the brain contains high levels of vitamin C relative to other tissues, and neuroprotective roles of vitamin C have been discussed in various neurodegenerative disease models [151]. Treatment with divalent metal cations like copper and iron augment oligomerization of α-synuclein during challenge with DOPAL, a neurotoxic byproduct of dopamine metabolism [81], and treatment with vitamin C prevents α-synuclein oligomerization by inhibiting the oxidation of DOPAL [81] or interaction with copper [82]. A Drosophila model of PD shows increased oxidative stress with subsequent loss of dopaminergic neurons and locomotor deficits; treatment with vitamin C increased antioxidant enzyme activity and alleviated the PD-associated phenotype [99,127,152,153]. This model of PD is based on depletion of ubiquitin c-terminal hydrolase (UCH), an antioxidant enzyme, that thus enhances aging-associated degeneration of dopaminergic neurons and decreases dopamine content in the brain. The application of vitamin C (0.5 mM) compensates for these effects of UCH knockdown in Drosophila [152]. Vitamin C activates ten-eleven-translocation 1-3 (Tet1-3) enzymes and Jumonji C-domain-containing histone demethylases (Jmjds) [154]. These enzymes catalyze the formation of 5-hydroxymethylcytosine in DNA [155] and demethylation of lysine residues in histone, respectively. Tets and JmJds are required during the early stages of dopaminergic neuron differentiation, and treatment with vitamin C advances the development of neural stem cells derived from the embryonic midbrain [154]. Despite the protective roles of vitamin C found in in vitro and animal models, the efficacy of vitamin C against PD in humans is still controversial. Blood samples collected from PD patients show increased lipid peroxidation coupled with significantly lower levels of vitamin C compared to healthy controls [156], but some studies have also reported negligible effects of vitamin C on PD in human subjects [157]. Despite controversial results in human subjects, vitamin C may improve the therapeutic capacity of levodopa by enhancing its bioavailability and alleviating its toxic side effects [158,159].
4.2. Vitamin E
Vitamin E encompasses the tocopherols and tocotrienols found in plant sources including grains, legumes, vegetables, and seeds. Both tocopherols and tocotrienols have a chromanol ring and a hydrocarbon chain. Tocopherols have a saturated chain whereas tocotrienols contain double bonds. Vitamin E exhibits strong antioxidant properties by acting as a ROS scavenger, attenuating mitochondrial dysfunction, and preventing neuronal apoptosis during neurotoxic insults that mimic neurodegenerative disease [64,160]. Both tocopherol and tocotrienol bind to α-tocopherol transfer protein (TTP), a critical regulator of vitamin E movement and metabolism. α-tocopherol has an 8.5-fold higher affinity for TTP than α-tocotrienol [161], thus α-tocopherol is generally considered to have better bioavailability. However, studies are increasingly demonstrating that tocopherols and tocotrienols have varying roles in different tissues and microenvironments. For example, tocotrienols exhibit a stronger antioxidant capacity in lipid-rich biological membranes [162], thus tocotrienols may be effective in protecting lipid-rich organs like the brain [64,163]. Long-term intraperitoneal injection of α-tocopherol and the water-soluble analog Trolox improved long-term potentiation (LTP) and long-term depression (LTD) in PINK1 knockout mice [164]. Martella et al. report that chronic treatment with low concentration rotenone does not alter ATP production or viability of dopaminergic neurons in heterozygous PINK1 knockout (PINK1 +/−) mice [165]. Despite this seemingly insignificant outcome, this treatment also completely impairs both LTP and LTD, and intraperitoneal injection of α-tocopherol (100 mg/kg) and Trolox (5 mg/kg) reverse this synaptic plasticity impairment [165]. DJ-1 mutant flies show altered redox homeostasis as evidenced by high levels of global ROS and hydrogen peroxide production and decreased activity of catalase and superoxide dismutase [99]. However, supplementation with α-tocopherol decreases global ROS levels in DJ-1 mutant flies [99]. In addition to tocopherols, tocotrienols protect neurons against oxidative stress-associated damage. Primary hippocampal neurons treated with α-tocotrienol show a decrease in total and mitochondrial ROS accumulation, and α-tocotrienol attenuates glutamate-induced post-translational cleavage of Bcl-xL to enhance the functions of antiapoptotic Bcl-xL [166,167]. In this study, α-tocotrienol was suggested to exert its effect by blocking the oligomerization of proapoptotic Bcl-2 proteins [167]. Oral administration of 100 μg/kg δ-tocotrienol prevents the loss of dopaminergic neurons in the SNpc and improves motor behavior in a mouse model of PD [128]. δ-tocotrienol binds to the estrogen receptor β and activates PI3K/Akt signaling pathways including phosphorylation of protein kinase B (PKB, Akt) and extracellular signal-regulated kinase (ERK) 1/2 [128,168]. Akt activates Nrf2 [169,170], and Nrf2-mediated upregulation of antioxidant and prosurvival genes is an important mechanism for the neuroprotective properties of many antioxidant nutrients [171,172,173]. Clinical studies with PD patients show that higher consumption of dietary vitamin E is inversely related to PD occurrence [157,164,174,175]. However, contrary reports have also been published on PD in human subjects [176,177]. Data from randomized controlled trials with vitamin E are limited. However, in a randomized double-blind placebo-controlled trial, Taghizadeh et al. reported significant improvement in clinical symptoms as assessed by the Unified Parkinson’s Disease Rating Scale (UPDRS) among PD patients who received 400 IU of vitamin E in combination with 1000 mg of omega-3 fatty acids [178]. These researchers also reported increases in circulating glutathione and total antioxidant capacity along with decreased high-sensitivity C-reactive protein with treatment compared to placebo. Although promising, further investigation into the specific roles of vitamin E subgroups will be important to clarify the efficacy of vitamin E in clinical disease.
4.3. Vitamin A and Carotenoids
Vitamin A is a fat-soluble vitamin found in both animal (e.g., liver) and plant sources and can also be produced from provitamin A carotenoids. Vitamin A exists as multiple forms: retinol (alcohol), retinal (aldehyde), retinoic acid (carboxylic acid), and retinyl ester (ester form). Retinal binds to opsin and activates rhodopsin, a G-protein coupled receptor that senses light in the eye. Retinoic acid binds to nuclear receptors including retinoic acid receptor (RAR) and retinoid X receptor (RXR) and regulates transcription of genes that control growth and differentiation [179]. In addition to these roles, vitamin A exhibits neuroprotective properties against neurodegeneration. Retinoic acid promotes differentiation of GABAergic neurons expressing dopamine receptors [132,133,179], and changes in PD include inhibition of retinoic acid-mediated neuronal differentiation [180]. Oral supplementation with retinoic acid upregulates the μ-type opioid receptor (MOR1), a G-protein-coupled receptor that mediates inhibitory signaling, in the dorsal striatum and attenuates repetitive dyskinetic movements in PD mice [181].
Carotenoids include the yellow, orange, and red pigments found in fruits and vegetables like carrots, tomatoes, watermelons, and pumpkins, and are also found in algae, salmon, and shrimp. Examples of carotenoids include carotene, lycopene, lutein, and astaxanthin. Serum α-carotene, β-carotene, and lycopene levels are significantly decreased in PD patients, and decreased serum carotenoid levels are also associated with poorer motor function [174,182]. However, a meta-analysis that examined the association between PD and vitamin A and carotenoids (lutein, α-carotene, β-carotene, lycopene, β-cryptoxanthin, zeaxanthin and canthaxanthin) concluded that the evidence was insufficient to make an epidemiological association between vitamin A/carotenoids and risk of developing PD [183]. In an in vivo animal model, oral administration of lycopene (5–20 mg/kg) attenuates oxidative stress induced by intraperitoneal injection of MPTP in mice, and lycopene also inhibits apoptosis by decreasing Bax and caspases while increasing Bcl-2 [129]. Treatment with lutein prevents MPTP-induced Bax and caspase increases, and lutein also improves motor function in MPTP challenged mice [130]. Astaxanthin lowers intracellular ROS and improves superoxide dismutase and catalase activity, and treatment with astaxanthin prevents apoptotic death in MPTP challenged SH-SY5Y cells [100]. Astaxanthin attenuates MPTP-induced neuronal injury via the downregulation of α-synuclein [83]. miR-7 directly binds to the 3′ UTR of α-synuclein mRNA and decreases the translation of α-synuclein [184]. Treatment with astaxanthin prevents the loss of miR-7 to lower the toxic effects of α-synuclein in SH-SY5Y cells [83]. Although clinical trials are lacking, oral supplementation with astaxanthin prevents loss of neurons in the SNpc and tyrosine hydroxylase-positive cells in the striatum from intraperitoneally injected MPTP in mice [131].
4.4. Selenium
Selenium is an essential trace mineral-rich in Brazil nuts, seafood, and organ meats and is also found in water and soil. The selenium content of plants is directly related to the selenium content of the soil [185]. Enzymes that regulate intracellular redox status likes glutathione peroxidase and thioredoxin reductase are selenoproteins that require selenium at their active sites, and mutations of the selenocysteine residues impair enzyme activity [186]. Microarray investigation reveals that rotenone treatment downregulates the SELENBP1 gene which encodes selenium binding protein 1, along with other genes that control apoptosis and mitochondrial function [187]. Neuroprotective functions of the selenium-containing quinoline derivative, 7-chloro-4-(phenylselanyl) quinoline, against the rotenone challenge highly correlates with selenium content in the brain of fruit flies [188]. Intraperitoneal delivery of selenium selenite (0.1, 0.2, and 0.3 mg/kg) increases glutathione peroxidase activity, alleviates lipid peroxidation, and improves motor function of the 6-hydroxydopamine challenged striatum in rats [101]. Interestingly, selenium treatment also shows dose-dependent protection of other antioxidant enzymes including glutathione reductase, glutathione transferase, and catalase [101]. Intraperitoneal injection of selenium partially improves dopamine metabolism during the MPTP challenge [189]. Analysis of soil samples from 4856 sites in the US demonstrates that higher selenium content inversely correlates with mortality from PD [190]. Human studies investigating selenium supplementation for PD are lacking. However, low plasma selenium concentrations are associated with decreased performance in neurological tests among older adults [191]. Conversely, increased levels of selenium in cerebrospinal fluid and plasma have been reported in PD patients [192,193]. Chronic exposure to selenium enhances oxidative stress in the brain and leads to cognitive impairment in animal models [194,195]. The underlying mechanism for these findings is unclear; however, evidence suggests that either a deficiency or excess of selenium may contribute to neurodegeneration or conversely PD pathology may impair mobilization of selenium in neurons. The Recommended Dietary Allowance for selenium is 55 mg/day, and the Institute of Medicine has established a Tolerable Upper Intake Level for selenium at 400 mg/day. Therefore, meeting the RDA without excess may be prudent [196].
4.5. Glutathione
Glutathione is a tripeptide of glycine, cysteine, and glutamate that is widely present in both plant and animal foods. In particular, avocados, asparagus, spinach, and amino acid-rich meat, fish and poultry are good sources of glutathione. Glutathione is a major intracellular antioxidant that reduces reactive oxygen species by being oxidized to glutathione disulfide. Glutathione is required by glutathione peroxidase during the conversion of hydrogen peroxide to water. The depletion of glutathione leads to oxidative stress-induced mitochondrial dysfunction and degeneration of dopaminergic neurons [125,134,197]. Interestingly, excess of glutathione also causes neuronal damage [134], and this may be due to the overproduction of glutathione disulfide, an oxidized form of glutathione responsible for mitochondrial dysfunction and neuronal death [198]. Strategies to support glutathione homeostasis by preventing loss of glutathione or facilitating clearance of glutathione disulfide protect the brain [163,198]. Treatment with glutathione’s precursor N-acetylcysteine (NAC) prevents oxidative stress and calcium overload and rescues neurons and other brain cells during PD-like stress [102,112,135]. Consistently, a protective effect of intravenous and oral delivery of NAC has been reported in PD patients [199,200,201]; NAC is naturally found in onions and garlic, and it is available in various dosages as an over-the-counter dietary supplement [202]. However, the best duration and concentration of supplementation to consistently show a therapeutic effect in humans has not been established [200,203]. Therefore, further investigation is required. Additionally, since oral glutathione is less bioavailable, finding nutrients that enhance the body’s ability to synthesize glutathione may also be of benefit.
5. Polyphenols
Polyphenols are characterized by the presence of multiple phenol groups and a six-membered hydrocarbon ring structure. Based on the arrangement of phenol groups, hydrocarbon chain and additional functional groups, polyphenols are further classified into subgroups including flavonoids, isoflavonoids, curcuminoids, tannins, and stilbenoids. There are estimated to be over 8000 different polyphenols present in nature [204]. We will describe four well-investigated polyphenols—curcumin, resveratrol, catechin, and oleuropein—and their role in PD models.
5.1. Curcumin
Curcumin, 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione is a polyphenol found in turmeric. Curcumin scavenges biological radicals including superoxide anion, hydrogen peroxide, 1,1-diphenyl-2-picryl-hydrazyl free radical, 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) radical, and N,N-dimethyl-p-phenylenediamine dihydrochloride radical [205]. In addition, treatment with curcumin (10 μM) decreases oxidation-associated protein modification including carbonylation and nitrotyrosine formation to rescue dopaminergic cells [106]. Curcumin effectively protects mitochondria from oxidative stress-associated damage [206]. Curcumin (2 μM) prevents loss of mitochondrial membrane potential and electron transfer system capacity in SH-SY5Y cells depleted with PINK1 [126]. Similarly, treatment with curcumin monoglucoside (0.25–5 μM) restores mitochondrial complex I and IV activity by decreasing the accumulation of hydroperoxides and increasing glutathione levels [103]. Curcumin exhibits antiapoptotic properties. Treatment with curcumin (5 μM) decreases ROS-induced calcium influx, lowering activation of caspase 3 and caspase 9 [104]. In addition, curcumin interferes with prodeath JNK signaling to prevent downstream apoptotic pathways including the release of cytochrome c and cleavage of procaspase 3 [103,138]. In vivo studies demonstrated antioxidant [105,144] and antiapoptotic [87,136] effects of curcumin to improve PD-associated neurobehavior [103,105,107,143,144,145,146]. Intraperitoneal injection of curcumin (200 mg/kg) attenuates rotenone-induced motor impairment in rats [143]. Male Wistar rat orally administered 5–20 mg/kg demethoxycurcumin, a derivative of curcumin, show concentration-dependent protection against rotenone challenge [105]. Demethoxycurcumin attenuates rotenone-induced oxidative stress and prevents loss of dopamine in the brain [105], and animals treated with demethoxycurcumin show improved motor function [105]. Dietary supplementation with 0.5% and 2% curcumin also show similar effects on MPTP-induced mouse PD models [137]. In addition to neuroprotection, curcumin may regulate cell differentiation and proliferation. C57BL mice transplanted with curcumin-activated mesenchymal stem cells have increased antiapoptotic Bcl-2, decreased proapoptotic Bax and caspases, and avoided the loss of dopaminergic neurons during MPTP challenge [136]. Curcumin prevents α-synuclein aggregation [87] and attenuates α-synuclein-induced cytotoxicity [85]. Curcumin derivative increases the nuclear translocation of transcription factor EB, a regulator of autophagy, potentially promoting degradation of α-synuclein [86].
5.2. Resveratrol
Resveratrol, 3,5,4′-trihydroxy-trans-stilbene is a nonflavonoid polyphenol with two aromatic ring structures. Resveratrol is found in grapes and berries, and it is also commonly consumed in red wine. Resveratrol promotes brain cell differentiation and proliferation during normal physiology [207], and it is well-described to attenuate oxidative stress-associated damage during the progression of PD pathology [108,109,110,111,112,114]. Intraperitoneally administered resveratrol (20 mg/kg) decreases lipid peroxidation, increases glutathione levels, and prevents deterioration of rat SNpc against 6-hydroxydopamine, an oxidant that causes degeneration of dopaminergic neurons [108]. Various research groups have shown that resveratrol effectively protects mitochondria by decreasing the accumulation of mitochondrial ROS, preventing mitochondrial inner membrane potential loss, restoring mitochondrial respiratory enzyme activity, regulating mitochondrial fission and fusion, and protecting mitochondrial DNA in PARK2 mutation [109,110,111,113]. Wang et al. showed that resveratrol treatment (25 μM) increases phosphorylation of Akt and prevents rotenone-induced death of PC12 cells [111]. Akt upregulates genes containing cAMP response element (CRE) including Bcl-2 [208,209], and it inactivates proapoptotic Bad and proteolytic caspases [210]. Thus resveratrol-mediated Akt phosphorylation may hinder apoptotic death during PD-like challenges. In addition, resveratrol may alleviate PARK gene-associated PD pathology. Male C57BL/6 mice subjected to intragastric gavage of 100 mg/kg resveratrol attenuate the loss of dopaminergic neurons and have improved motor behavior during the MPTP challenge [88]. This same study also shows that resveratrol significantly increases protein levels of LC3-II, a key protein found in the membrane of autophagosomes, and thereby facilitates degradation of α-synuclein [88]. Resveratrol also increases microRNA-214 which potentially inhibits translation of α-synuclein [89]. Fibroblasts isolated from patients with PARK2 mutations have increased production of whole-cell ROS and mitochondrial ROS, and treatment with resveratrol protects mitochondria and improves respiration and ATP production in these cells [109].
5.3. Catechin
Catechins are flavonoids containing two benzene rings and one dihydropyran heterocycle. Catechins are found in various herbs and fruits. Tea in particular is a good source of catechins. Four major catechins include (−)-epicatechin (EC), (−)-epicatechin-3-gallate (ECG), (−)-epigallocatechin (EGC), and (−)-epigallocatechin-3-gallate (EGCG) [211]. Catechins donate an electron from a phenolic hydroxyl group and to scavenge free radicals and thus exhibit direct antioxidant properties [212,213,214]. Catechins also improve intracellular redox status by preventing the loss of other antioxidants [116]. Treatment with 10 μM EGCG lowers the accumulation of ROS and prevents activation of caspases during hydrogen peroxide challenge and protects N27 dopaminergic cells from apoptotic death [119]. Koch et al. show that a longer brewing time tends to enhance antiradical activity in teas [215] indicating that catechins retain antioxidant properties after exposure to high temperature. Although further investigation is needed, orally supplemented catechins are shown to be delivered to the brain (0.5 nmol/g) in rats [216] and an in vitro blood–brain barrier system (BBB) shows that <10% of catechins are BBB permeable [217,218]. Various research groups have demonstrated that EGCG prevents neurotoxicity associated with α-synuclein [91,92,94,95]. EGCG chelates metal ions including Cu(II) and Fe(III) to inhibit fibrillation of α-synuclein [90,93]. EGCG (350μM) enhances the formation of stable oligomers (a less-toxic form) thus prevents the accumulation of pathological fibril [95] EGCG immobilizes α-synuclein and interferes with its oligomerization in biological membranes [96], thus EGCG helps to maintain membrane integrity [95,96]. EGCG suppresses fibrillation of γ-synuclein, a type of synuclein also found in Lewy bodies [91]. EGCG improves motor behavior in Drosophila by preventing mitochondrial dysfunction caused by abnormalities of LRRK2 and Parkin genes [148]. Chemically induced rodent PD models produced by injection with MPTP and 6-hydroxydopamine demonstrate PD-like symptoms like bradykinesia, and administration of 10-50 mg catechin (both oral and intraperitoneal injection) improves locomotor behavior in these animals [115,116,139]. Intraperitoneal injection of 10 or 30 mg/kg catechin restores glutathione levels and increases dopamine in the rat brain [116]. Oral supplementation with 25 mg EGCG reduces oxidative stress and preserves striatal dopamine in C57BL/6J mice challenged with MPTP [115]. C57BL/6J mice intraperitoneally injected with MPTP demonstrate PD-like symptoms including bradykinesia due to loss of SNpc dopaminergic neurons, and oral administration of EGCG (25 and 50 mg/kg) in these animals lowers proinflammatory cytokines, rescues dopaminergic neurons from death, and improves motor behavior [139]. In addition to catechins’ role inhibiting PD pathology, catechins may also support existing PD treatments. Orally administered EGCG (100 and 400 mg/kg) inhibits methylation of levodopa to improve bioavailability [219].
5.4. Oleuropein
Oleuropein contains hydroxytyrosol, elenolic acid, and glucose. It is a major phenolic compound found in olive oil. Although oleuropein is predominant, other oleuropein derivatives such as oleuropein aglycon and oleuroside are also found in olive oil [220]. Oleuropein acts as a scavenger of superoxide, nitric oxide, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid, and 2,2-diphenyl-1-picrylhydrazyl radicals [221,222]. Various research groups have demonstrated that treatment with oleuropein and its derivatives inhibit the accumulation of ROS and prevent the progression of PD pathology [97,98,120]. Palazzi et al. demonstrated that in vitro incubation with oleuropein aglycone stabilizes α-synuclein monomers to prevent pathological aggregation [97]. Similarly, Mohammad-Beigi et al. show that olive fruit extracts containing oleuropein and oleuropein aglycone inhibit α-synuclein fibril elongation, decreasing cytotoxicity caused by α-synuclein oligomers [98]. In addition, oleuropein activates redox-sensitive transcription factors like Nrf2 to potentially improve intracellular antioxidant capacity via the upregulation of antioxidant genes [120,223]. Oleuropein protects mitochondria by mitigating mitochondrial superoxide production [121]. PC12 cells treated with 1-50 μM oleuropein retain mitochondrial membrane potential during the 6-hydroxydopamine challenge, and oleuropein also alleviates endoplasmic reticulum stress to protect PC12 cells from apoptotic death [141]. Oleuropein increases mitochondrial antiapoptotic Bcl-2 and decreases proapoptotic Bax and apoptotic-inducing factor [121,142]. Furthermore, oleuropein regulates phosphorylation of dynamin-related protein 1 (Drp1) [142] and LC3-II [121], key proteins that control mitochondrial fission and mitophagy, respectively. Thus, oleuropein potentially supports an optimal mitochondrial population in cells. Oral supplementation with olive leaf extract (75–300 mg/kg) significantly increases antioxidant enzymes including superoxide dismutase and glutathione peroxidase in the rat brain [123]. Rats fed with olive leaf extract are protected from loss of dopaminergic neuron during rotenone-induced mitochondrial damage, and showed improved neurobehavior [123]. Similarly, rats supplemented with extra virgin olive oil extract show decreased lipid peroxidation and increased antioxidant enzyme activities [124]. Oral administration of oleuropein is distributed to the brain 2h after ingestion [224], so oleuropein may be a key component in olive leaf and olive oil-mediated neuroprotection.
6. Conclusions
Although increasing numbers of studies performed in vitro and using animal models demonstrate a potential role in dietary prevention of PD, the efficacy of nutritional intervention to do so in humans remains controversial. Epidemiological studies examining dietary intake of antioxidant micronutrients and the risk of developing PD have yielded equivocal results, and there is a paucity of data from randomized controlled trials among people with pre-existing PD. Dietary antioxidants exhibit multiple effects rather than targeting a single specific process. Vitamin C, vitamin E, and polyphenols directly interact with ROS and terminate oxidative chain reactions. Other minerals like selenium act as cofactors to support the activity of antioxidant enzymes. Many antioxidant nutrients are involved in signaling transduction and protect downstream targets of oxidative stress to alleviate the damage that promotes the development of PD. Nutrients also regulate genes that control the development, growth, and survival of dopaminergic neurons. Polyphenols like curcumin, resveratrol, catechin, and oleuropein inhibit the formation of Lewy bodies. In this review, we have described the complex cellular and molecular mechanisms of these dietary antioxidants as an important step in developing a therapeutic strategy against PD. Future clinical studies with data safety and monitoring are warranted to determine whether these antioxidant micronutrients may act individually or in synergy as a nonpharmacological means of prevention and treatment.
Author Contributions
Writing and editing: H.-A.P. and A.C.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Armstrong M.J., Okun M.S. Diagnosis and treatment of parkinson’s disease: A review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 2.Goldman J.G., Guerra C.M. Treatment of nonmotor symptoms associated with Parkinson’s disease. Neurol. Clin. 2020;38:269–292. doi: 10.1016/j.ncl.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Blesa J., Trigo D.I., Quiroga V.A., Jackson L.V.R. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dias V., Junn E., Mouradian M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puspita L., Chung S.Y., Shim J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain. 2017;10:53. doi: 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J.S., Davis R.L., Sue C.M. Mitochondrial dysfunction in parkinson’s disease: New mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. Rep. 2018;18:21. doi: 10.1007/s11910-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winklhofer K.F., Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. Biophys. Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Keeney P.M., Xie J., Capaldi R.A., Bennett J.P.J. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker W.D.J., Parks J.K., Swerdlow R.H. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res. 2008;1189:215–218. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdez L.B., Zaobornyj T., Bandez M.J., Lopez-Cepero J.M., Boveris A., Navarro A. Complex I syndrome in striatum and frontal cortex in a rat model of Parkinson’s disease. Free Radic. Biol. Med. 2019;135:274–282. doi: 10.1016/j.freeradbiomed.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Perier C., Bove J., Vila M., Przedborski S. The rotenone model of Parkinson’s disease. Trends Neurosci. 2003;26:345–346. doi: 10.1016/S0166-2236(03)00144-9. [DOI] [PubMed] [Google Scholar]
- 12.Sriram K., Pai K.S., Boyd M.R., Ravindranath V. Evidence for generation of oxidative stress in brain by MPTP: In vitro and in vivo studies in mice. Brain Res. 1997;749:44–52. doi: 10.1016/S0006-8993(96)01271-1. [DOI] [PubMed] [Google Scholar]
- 13.Meiser J., Weindl D., Hiller K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013;11:34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coelho E.-C., de Araujo C.C., Follmer C. Formation of large oligomers of DOPAL-modified alpha-synuclein is modulated by the oxidation of methionine residues located at C-terminal domain. Biochem. Biophys. Res. Commun. 2019;509:367–372. doi: 10.1016/j.bbrc.2018.12.128. [DOI] [PubMed] [Google Scholar]
- 15.Plotegher N., Berti G., Ferrari E., Tessari I., Zanetti M., Lunelli L. DOPAL derived alpha-synuclein oligomers impair synaptic vesicles physiological function. Sci. Rep. 2017;7:40699. doi: 10.1038/srep40699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarafian T.A., Yacoub A., Kunz A., Aranki B., Serobyan G., Cohn W. Enhanced mitochondrial inhibition by 3,4-dihydroxyphenyl-acetaldehyde (DOPAL)-oligomerized alpha-synuclein. J. Neurosci. Res. 2019;97:1689–1705. doi: 10.1002/jnr.24513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristal B.S., Conway A.D., Brown A.M., Jain J.C., Ulluci P.A., Li S.W. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic. Biol. Med. 2001;30:924–931. doi: 10.1016/S0891-5849(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 18.Burbulla L.F., Song P., Mazzulli J.R., Zampese E., Wong Y.C., Jeon S. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein C., Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas B., Beal M.F. Parkinson’s disease. Hum. Mol. Genet. 2007;16:R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 21.Lesage S., Brice A. Parkinson’s disease: From monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 22.Cheng F., Vivacqua G., Yu S. The role of alpha-synuclein in neurotransmission and synaptic plasticity. J. Chem. Neuroanat. 2011;42:242–248. doi: 10.1016/j.jchemneu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Hsu L.J., Mallory M., Xia Y., Veinbergs I., Hashimoto M., Yoshimoto M. Expression pattern of synucleins (non-Abeta component of Alzheimer’s disease amyloid precursor protein/alpha-synuclein) during murine brain development. J. Neurochem. 1998;71:338–344. doi: 10.1046/j.1471-4159.1998.71010338.x. [DOI] [PubMed] [Google Scholar]
- 24.Ludtmann M.H.R., Angelova P.R., Horrocks M.H., Choi M.L., Rodrigues M., Baev A.Y. Alpha-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat. Commun. 2018;9:2293. doi: 10.1038/s41467-018-04422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perni M., Galvagnion C., Maltsev A., Meisl G., Muller M.B., Challa P.K. A natural product inhibits the initiation of alpha-synuclein aggregation and suppresses its toxicity. Proc. Natl. Acad. Sci. USA. 2017;114:E1009–E1017. doi: 10.1073/pnas.1610586114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding H., Xiong Y., Sun J., Chen C., Gao J., Xu H. Asiatic acid prevents oxidative stress and apoptosis by inhibiting the translocation of alpha-synuclein into mitochondria. Front Neurosci. 2018;12:431. doi: 10.3389/fnins.2018.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scudamore O., Ciossek T. Increased oxidative stress exacerbates alpha-synuclein aggregation in vivo. J. Neuropathol. Exp. Neurol. 2018;77:443–453. doi: 10.1093/jnen/nly024. [DOI] [PubMed] [Google Scholar]
- 28.Kruger R., Kuhn W., Muller T., Woitalla D., Graeber M., Kosel S. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 29.Xiang W., Schlachetzki J.C., Helling S., Bussmann J.C., Berlinghof M., Schaffer T.E. Oxidative stress-induced posttranslational modifications of alpha-synuclein: Specific modification of alpha-synuclein by 4-hydroxy-2-nonenal increases dopaminergic toxicity. Mol. Cell Neurosci. 2013;54:71–83. doi: 10.1016/j.mcn.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Lucking C.B., Durr A., Bonifati V., Vaughan J., De Michele G., Gasser T. Association between early-onset Parkinson’s disease and mutations in the parkin gene. N. Engl. J. Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 31.Koyano F., Okatsu K., Kosako H., Tamura Y., Go E., Kimura M. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 32.Wang X., Winter D., Ashrafi G., Schlehe J., Wong Y.L., Selkoe D. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y., Dorn G.W., II PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barodia S.K., Creed R.B., Goldberg M.S. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res. Bull. 2017;133:51–59. doi: 10.1016/j.brainresbull.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang C.J., Kim Y.E., Son D.J., Park M.H., Choi D.Y., Park P.H. Parkin deficiency exacerbate ethanol-induced dopaminergic neurodegeneration by P38 pathway dependent inhibition of autophagy and mitochondrial function. Redox Biol. 2017;11:456–568. doi: 10.1016/j.redox.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rita D.A., D’Acunzo P., Simula L., Campello S., Strappazzon F., Cecconi F. AMBRA1-Mediated mitophagy counteracts oxidative stress and apoptosis induced by neurotoxicity in human neuroblastoma SH-SY5Y cells. Front Cell Neurosci. 2018;12:92. doi: 10.3389/fncel.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen R., Park H.A., Mnatsakanyan N., Niu Y., Licznerski P., Wu J. Parkinson’s disease protein DJ-1 regulates ATP synthase protein components to increase neuronal process outgrowth. Cell Death Dis. 2019;10:469. doi: 10.1038/s41419-019-1679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao L.Y., Giasson B.I., Bonini N.M. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc. Natl. Acad. Sci. USA. 2010;107:9747–9752. doi: 10.1073/pnas.0911175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsen N.J., Ambrosi G., Mullett S.J., Berman S.B., Hinkle D.A. DJ-1 knock-down impairs astrocyte mitochondrial function. Neuroscience. 2011;196:251–264. doi: 10.1016/j.neuroscience.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Miranda B.R., Rocha E.M., Bai Q., Ayadi E.A., Hinkle D., Burton E.A. Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson’s disease. Neurobiol. Dis. 2018;115:101–114. doi: 10.1016/j.nbd.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li R., Wang S., Li T., Wu L., Fang Y., Feng Y. Salidroside protects dopaminergic neurons by preserving complex I activity via DJ-1/Nrf2-Mediated antioxidant pathway. Parkinsons Dis. 2019;2019:6073496. doi: 10.1155/2019/6073496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canet-Aviles R.M., Wilson M.A., Miller D.W., Ahmad R., McLendon C., Bandyopadhyay S. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitsumoto A., Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic. Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- 44.Saito Y. Oxidized DJ-1 as a possible biomarker of Parkinson’s disease. J. Clin. Biochem. Nutr. 2014;54:138–144. doi: 10.3164/jcbn.13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinumi T., Kimata J., Taira T., Ariga H., Niki E. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- 46.Blackinton J., Lakshminarasimhan M., Thomas K.J., Ahmad R., Greggio E., Raza A.S. Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J. Biol. Chem. 2009;284:6476–6485. doi: 10.1074/jbc.M806599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narasimhan K.K.S., Jayakumar D., Velusamy P., Srinivasan A., Mohan T., Ravi D.B. Morinda citrifolia and its active principle scopoletin mitigate protein aggregation and neuronal apoptosis through augmenting the DJ-1/Nrf2/ARE signaling pathway. Oxid. Med. Cell Longev. 2019;2019:2761041. doi: 10.1155/2019/2761041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaleel M., Nichols R.J., Deak M., Campbell D.G., Gillardon F., Knebel A. LRRK2 phosphorylates moesin at threonine-558: Characterization of how Parkinson’s disease mutants affect kinase activity. Biochem. J. 2007;405:307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith W.W., Pei Z., Jiang H., Moore D.J., Liang Y., West A.B. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc. Natl. Acad. Sci. USA. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venderova K., Kabbach G., Abdel-Messih E., Zhang Y., Parks R.J., Imai Y. Leucine-Rich Repeat Kinase 2 interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson’s disease. Hum. Mol. Genet. 2009;18:4390–4404. doi: 10.1093/hmg/ddp394. [DOI] [PubMed] [Google Scholar]
- 51.Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Vermilyea S.C., Babinski A., Tran N., To S., Guthrie S., Kluss J.H. In vitro CRISPR/Cas9-Directed gene editing to model LRRK2 G2019S Parkinson’s disease in common marmosets. Sci. Rep. 2020;10:3447. doi: 10.1038/s41598-020-60273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J., Pajarillo E., Rizor A., Son D.S., Lee J., Aschner M. LRRK2 kinase plays a critical role in manganese-induced inflammation and apoptosis in microglia. PLoS ONE. 2019;14:e0210248. doi: 10.1371/journal.pone.0210248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendivil M.-P., Velez C.-P., Jimenez-Del-Rio M. Neuroprotective Effect of the LRRK2 kinase inhibitor PF-06447475 in human nerve-like differentiated cells exposed to oxidative stress stimuli: Implications for Parkinson’s disease. Neurochem. Res. 2016;41:2675–2692. doi: 10.1007/s11064-016-1982-1. [DOI] [PubMed] [Google Scholar]
- 55.Saez-Atienzar S., Bonet-Ponce L., da Casa C., Perez-Dolz L., Blesa J.R., Nava E. Bcl-xL-mediated antioxidant function abrogates the disruption of mitochondrial dynamics induced by LRRK2 inhibition. Biochim. Biophys. Acta. 2016;1862:20–31. doi: 10.1016/j.bbadis.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 56.Vila M., Jackson-Lewis V., Vukosavic S., Djaldetti R., Liberatore G., Offen D. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2001;98:2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perier C., Bove J., Wu D.C., Dehay B., Choi D.K., Jackson-Lewis V. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2007;104:8161–8166. doi: 10.1073/pnas.0609874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen Y.F., Zhu Z.Y., Qian S.X., Xu C.Y., Wang Y.P. MiR-30b protects nigrostriatal dopaminergic neurons from MPP(+)-induced neurotoxicity via SNCA. Brain Behav. 2020;10:e01567. doi: 10.1002/brb3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dionisio P.A., Oliveira S.R., Gaspar M.M., Gama M.J., Castro-Caldas M., Amaral J.D. Ablation of RIP3 protects from dopaminergic neurodegeneration in experimental Parkinson’s disease. Cell Death Dis. 2019;10:840. doi: 10.1038/s41419-019-2078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao G., Wang Z., Lu L., Duan C., Wang X., Yang H. Morphological analysis of mitochondria for evaluating the toxicity of alpha-synuclein in transgenic mice and isolated preparations by atomic force microscopy. Biomed. Pharmacother. 2017;96:1380–1388. doi: 10.1016/j.biopha.2017.11.057. [DOI] [PubMed] [Google Scholar]
- 61.Yang X., Zhang M., Wei M., Wang A., Deng Y., Cao H. MicroRNA-216a inhibits neuronal apoptosis in a cellular Parkinson’s disease model by targeting Bax. Metab. Brain Dis. 2020 doi: 10.1007/s11011-020-00546-x. [DOI] [PubMed] [Google Scholar]
- 62.Li S., Lv X., Zhai K., Xu R., Zhang Y., Zhao S. MicroRNA-7 inhibits neuronal apoptosis in a cellular Parkinson’s disease model by targeting Bax and Sirt2. Am. J. Transl. Res. 2016;8:993–1004. [PMC free article] [PubMed] [Google Scholar]
- 63.Alavian K.N., Li H., Collis L., Bonanni L., Zeng L., Sacchetti S. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat. Cell Biol. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park H.A., Mnatsakanyan N., Broman K., Davis A.U., May J., Licznerski P. Alpha-Tocotrienol prevents oxidative stress-mediated post-translational cleavage of Bcl-xL in primary hippocampal neurons. Int. J. Mol. Sci. 2019;21 doi: 10.3390/ijms21010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arena G., Gelmetti V., Torosantucci L., Vignone D., Lamorte G., De Rosa P. PINK1 protects against cell death induced by mitochondrial depolarization, by phosphorylating Bcl-xL and impairing its pro-apoptotic cleavage. Cell Death Differ. 2013;20:920–930. doi: 10.1038/cdd.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Savitt J.M., Jang S.S., Mu W., Dawson V.L., Dawson T.M. Bcl-x is required for proper development of the mouse substantia nigra. J. Neurosci. 2005;25:6721–6728. doi: 10.1523/JNEUROSCI.0760-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin L.J., Semenkow S., Hanaford A., Wong M. Mitochondrial permeability transition pore regulates Parkinson’s disease development in mutant alpha-synuclein transgenic mice. Neurobiol. Aging. 2014;35:1132–1152. doi: 10.1016/j.neurobiolaging.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rasheed M.Z., Tabassum H., Parvez S. Mitochondrial permeability transition pore: A promising target for the treatment of Parkinson’s disease. Protoplasma. 2017;254:33–42. doi: 10.1007/s00709-015-0930-2. [DOI] [PubMed] [Google Scholar]
- 69.Pivovarova N.B., Nguyen H.V., Winters C.A., Brantner C.A., Smith C.L., Andrews S.B. Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. J. Neurosci. 2004;24:5611–5622. doi: 10.1523/JNEUROSCI.0531-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jonas E., Porter G.A., Beutner G., Mnatsakanyan N., Park H.A., Mehta N. The mitochondrial permeability transition pore: Molecular structure and function in health and disease. In: Rostovtseva T.K., editor. Molecular Basis for Mitochondrial Signaling. Springer; Cham, Germany: 2017. pp. 69–105. [Google Scholar]
- 71.Jonas E., Sacchetti S., Park H.A., Lazrove E., Beutner G., Porter G.A. The C-Subunit of the ATP Synthase Forms the Pore of the PTP. Biophys. J. 2014;106:3a–4a. doi: 10.1016/j.bpj.2013.11.049. [DOI] [Google Scholar]
- 72.Alavian K.N., Beutner G., Lazrove E., Sacchetti S., Park H.A., Licznerski P. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. USA. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mnatsakanyan N., Llaguno M.C., Yang Y., Yan Y., Weber J., Sigworth F.J. A mitochondrial megachannel resides in monomeric F1FO ATP synthase. Nat. Commun. 2019;10:5823. doi: 10.1038/s41467-019-13766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonora M., Bononi A., De Marchi E., Giorgi C., Lebiedzinska M., Marchi S. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12:674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ganguly U., Banerjee A., Chakrabarti S.S., Kaur U., Sen O., Cappai R. Interaction of alpha-synuclein and Parkin in iron toxicity on SH-SY5Y cells: Implications in the pathogenesis of Parkinson’s disease. Biochem. J. 2020;477:1109–1122. doi: 10.1042/BCJ20190676. [DOI] [PubMed] [Google Scholar]
- 76.Gautier C.A., Giaime E., Caballero E., Nunez L., Song Z., Chan D. Regulation of mitochondrial permeability transition pore by PINK1. Mol. Neurodegener. 2012;7:22. doi: 10.1186/1750-1326-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui T., Fan C., Gu L., Gao H., Liu Q., Zhang T. Silencing of PINK1 induces mitophagy via mitochondrial permeability transition in dopaminergic MN9D cells. Brain Res. 2011;1394:1–13. doi: 10.1016/j.brainres.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 78.Park H.A., Broman K., Stumpf A., Kazyak S., Jonas E.A. Nutritional Regulators of Bcl-xL in the Brain. Molecules. 2018;23 doi: 10.3390/molecules23113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y.J., Gan R.Y., Li S., Zhou Y., Li A.N., Xu D.P. antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Virmani A., Pinto L., Binienda Z., Ali S. Food, nutrigenomics, and neurodegeneration–Neuroprotection by what you eat! Mol. Neurobiol. 2013;48:353–362. doi: 10.1007/s12035-013-8498-3. [DOI] [PubMed] [Google Scholar]
- 81.Jinsmaa Y., Sullivan P., Gross D., Cooney A., Sharabi Y., Goldstein D.S. Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein. Neurosci Lett. 2014;569:27–32. doi: 10.1016/j.neulet.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C., Liu L., Zhang L., Peng Y., Zhou F. Redox reactions of the alpha-synuclein-Cu (2+) complex and their effects on neuronal cell viability. Biochemistry. 2010;49:8134–8142. doi: 10.1021/bi1010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen D.F., Qi H.P., Ma C., Chang M.X., Zhang W.N., Song R.R. Astaxanthin suppresses endoplasmic reticulum stress and protects against neuron damage in Parkinson’s disease by regulating miR-7/SNCA axis. Neurosci. Res. 2020 doi: 10.1016/j.neures.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Wang R., Wang Y., Qu L., Chen B., Jiang H., Song N. Iron-induced oxidative stress contributes to alpha-synuclein phosphorylation and up-regulation via polo-like kinase 2 and casein kinase 2. Neurochem. Int. 2019;125:127–135. doi: 10.1016/j.neuint.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 85.Jha N.N., Ghosh D., Das S., Anoop A., Jacob R.S., Singh P.K. Effect of curcumin analogs onalpha-synuclein aggregation and cytotoxicity. Sci. Rep. 2016;6:28511. doi: 10.1038/srep28511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Z., Yang C., Liu J., Chun K., Tong B., Zhu Z., Malampati S. A curcumin derivative activates TFEB and protects against parkinsonian neurotoxicity in vitro. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21041515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma N., Nehru B. Curcumin affords neuroprotection and inhibits alpha-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology. 2018;26:349–360. doi: 10.1007/s10787-017-0402-8. [DOI] [PubMed] [Google Scholar]
- 88.Guo Y.J., Dong S.Y., Cui X.X., Feng Y., Liu T., Yin M. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of alpha-synuclein via SIRT1-deacetylated LC3. Mol. Nutr. Food Res. 2016;60:2161–2175. doi: 10.1002/mnfr.201600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z.H., Zhang J.L., Duan Y.L., Zhang Q.S., Li G.F., Zheng D.L. MicroRNA-214 participates in the neuroprotective effect of Resveratrol via inhibiting alpha-synuclein expression in MPTP-induced Parkinson’s disease mouse. Biomed. Pharmacother. 2015;74:252–256. doi: 10.1016/j.biopha.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 90.Teng Y., Zhao J., Ding L., Ding Y., Zhou P. Complex of EGCG with Cu(II) suppresses amyloid aggregation and Cu(II)-Induced cytotoxicity of alpha-Synuclein. Molecules. 2019;24 doi: 10.3390/molecules24162940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roy S., Bhat R. Suppression, disaggregation, and modulation of gamma-Synuclein fibrillation pathway by green tea polyphenol EGCG. Protein Sci. 2019;28:382–402. doi: 10.1002/pro.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y., Chen Z., Lu Z., Yang Q., Liu L., Jiang Z. “Cell-addictive” dual-target traceable nanodrug for Parkinson’s disease treatment via flotillins pathway. Theranostics. 2018;8:5469–5481. doi: 10.7150/thno.28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao J., Xu L., Liang Q., Sun Q., Chen C., Zhang Y. Metal chelator EGCG attenuates Fe(III)-induced conformational transition of alpha-synuclein and protects AS-PC12 cells against Fe(III)-induced death. J. Neurochem. 2017;143:136–146. doi: 10.1111/jnc.14142. [DOI] [PubMed] [Google Scholar]
- 94.Ponzini E., De Palma A., Cerboni L., Natalello A., Rossi R., Moons R. Methionine oxidation in alpha-synuclein inhibits its propensity for ordered secondary structure. J. Biol. Chem. 2019;294:5657–5665. doi: 10.1074/jbc.RA118.001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J.E., Rhoo K.Y., Lee S., Lee J.T., Park J.H., Bhak G. EGCG-mediated Protection of the Membrane Disruption and Cytotoxicity Caused by the ‘Active Oligomer’ of alpha-Synuclein. Sci. Rep. 2017;7:17945. doi: 10.1038/s41598-017-18349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lorenzen N., Nielsen S.B., Yoshimura Y., Vad B.S., Andersen C.B., Betzer C. How epigallocatechin gallate can inhibit alpha-synuclein oligomer toxicity in vitro. J. Biol. Chem. 2014;289:21299–21310. doi: 10.1074/jbc.M114.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Palazzi L., Bruzzone E., Bisello G., Leri M., Stefani M., Bucciantini M. Oleuropein aglycone stabilizes the monomeric alpha-synuclein and favours the growth of non-toxic aggregates. Sci. Rep. 2018;8:8337. doi: 10.1038/s41598-018-26645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mohammad H.-B., Aliakbari F., Sahin C., Lomax C., Tawfike A., Schafer N.P. Oleuropein derivatives from olive fruit extracts reduce alpha-synuclein fibrillation and oligomer toxicity. J. Biol. Chem. 2019;294:4215–4232. doi: 10.1074/jbc.RA118.005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Casani S., Gomez-Pastor R., Matallana E., Paricio N. Antioxidant compound supplementation prevents oxidative damage in a Drosophila model of Parkinson’s disease. Free Radic. Biol. Med. 2013;61:151–160. doi: 10.1016/j.freeradbiomed.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 100.Lee D.H., Kim C.S., Lee Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011;49:271–280. doi: 10.1016/j.fct.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zafar K.S., Siddiqui A., Sayeed I., Ahmad M., Salim S., Islam F. Dose-dependent protective effect of selenium in rat model of Parkinson’s disease: Neurobehavioral and neurochemical evidences. J. Neurochem. 2003;84:438–446. doi: 10.1046/j.1471-4159.2003.01531.x. [DOI] [PubMed] [Google Scholar]
- 102.Botsakis K., Theodoritsi S., Grintzalis K., Angelatou F., Antonopoulos I., Georgiou C.D. 17beta-Estradiol/N-acetylcysteine interaction enhances the neuroprotective effect on dopaminergic neurons in the weaver model of dopamine deficiency. Neuroscience. 2016;320:221–229. doi: 10.1016/j.neuroscience.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 103.Pandareesh M.D., Shrivash M.K., Naveen K.H.N., Misra K., Srinivas B.M.M. curcumin monoglucoside shows improved bioavailability and mitigates rotenone induced neurotoxicity in cell and drosophila models of Parkinson’s disease. Neurochem. Res. 2016;41:3113–3128. doi: 10.1007/s11064-016-2034-6. [DOI] [PubMed] [Google Scholar]
- 104.Oz A., Celik O. Curcumin inhibits oxidative stress-induced TRPM2 channel activation, calcium ion entry and apoptosis values in SH-SY5Y neuroblastoma cells: Involvement of transfection procedure. Mol. Membr. Biol. 2016;33:76–88. doi: 10.1080/09687688.2017.1318224. [DOI] [PubMed] [Google Scholar]
- 105.Ramkumar M., Rajasankar S., Gobi V.V., Janakiraman U., Manivasagam T., Thenmozhi A.J. Demethoxycurcumin, a natural derivative of curcumin abrogates rotenone-induced dopamine depletion and motor deficits by its antioxidative and anti-inflammatory properties in parkinsonian rats. Pharmacogn Mag. 2018;14:9–16. doi: 10.4103/pm.pm_113_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buratta S., Chiaradia E., Tognoloni A., Gambelunghe A., Meschini C., Palmieri L. Effect of Curcumin on Protein Damage Induced by Rotenone in Dopaminergic PC12 Cells. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21082761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y.L., Ju B., Zhang Y.Z., Yin H.L., Liu Y.J., Wang S.S. Protective effect of curcumin against oxidative stress-induced injury in rats with Parkinson’s disease through the Wnt/beta-catenin signaling pathway. Cell Physiol. Biochem. 2017;43:2226–2241. doi: 10.1159/000484302. [DOI] [PubMed] [Google Scholar]
- 108.Khan M.M., Ahmad A., Ishrat T., Khan M.B., Hoda M.N., Khuwaja G. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Res. 2010;1328:139–151. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 109.Ferretta A., Gaballo A., Tanzarella P., Piccoli C., Capitanio N., Nico B. Effect of resveratrol on mitochondrial function: Implications in parkin-associated familiar Parkinson’s disease. Biochim. Biophys. Acta. 2014;1842:902–915. doi: 10.1016/j.bbadis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 110.Palle S., Neerati P. Improved neuroprotective effect of resveratrol nanoparticles as evinced by abrogation of rotenone-induced behavioral deficits and oxidative and mitochondrial dysfunctions in rat model of Parkinson’s disease. Naunyn. Schmiedebergs Arch. Pharmacol. 2018;391:445–453. doi: 10.1007/s00210-018-1474-8. [DOI] [PubMed] [Google Scholar]
- 111.Wang H., Dong X., Liu Z., Zhu S., Liu H., Fan W. Resveratrol suppresses rotenone-induced neurotoxicity through activation of SIRT1/Akt1 signaling pathway. Anat. Rec. (Hoboken) 2018;301:1115–1125. doi: 10.1002/ar.23781. [DOI] [PubMed] [Google Scholar]
- 112.Sun Y., Sukumaran P., Selvaraj S., Cilz N.I., Schaar A., Lei S. TRPM2 promotes neurotoxin MPP(+)/MPTP-Induced cell death. Mol. Neurobiol. 2018;55:409–420. doi: 10.1007/s12035-016-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peng K., Tao Y., Zhang J., Wang J., Ye F., Dan G. Resveratrol regulates mitochondrial biogenesis and fission/fusion to attenuate rotenone-induced neurotoxicity. Oxid. Med. Cell Longev. 2016;2016:6705621. doi: 10.1155/2016/6705621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vergara D., Gaballo A., Signorile A., Ferretta A., Tanzarella P., Pacelli C. Resveratrol modulation of protein expression in parkin-mutant human skin fibroblasts: A proteomic approach. Oxid. Med. Cell Longev. 2017;2017:2198243. doi: 10.1155/2017/2198243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu Q., Langley M., Kanthasamy A.G., Reddy M.B. Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson’s disease. J. Nutr. 2017;147:1926–1931. doi: 10.3945/jn.117.255034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Teixeira M.D., Souza C.M., Menezes A.P., Carmo M.R., Fonteles A.A., Gurgel J.P. Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol. Biochem. Behav. 2013;110:1–7. doi: 10.1016/j.pbb.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 117.Martinez-Perez D.A., Jimenez-Del-Rio M., Velez-Pardo C. Epigallocatechin-3-Gallate protects and prevents paraquat-induced oxidative stress and neurodegeneration in knockdown dj-1-beta drosophila melanogaster. Neurotox. Res. 2018;34:401–416. doi: 10.1007/s12640-018-9899-x. [DOI] [PubMed] [Google Scholar]
- 118.Bitu P.N., da Silva A.B., Neves K.R., Silva A.H., Leal L.K., Viana G.S. Neuroprotective properties of the standardized extract from camellia sinensis (green tea) and its main bioactive components, epicatechin and epigallocatechin gallate, in the 6-OHDA model of Parkinson’s disease. Evid. Based Complement. Alternat. Med. 2015;2015:161092. doi: 10.1155/2015/161092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu Q., Kanthasamy A.G., Reddy M.B. Epigallocatechin gallate protects against TNFalpha- or H2O2- induced apoptosis by modulating iron related proteins in a cell culture model. Int. J. Vitam. Nutr. Res. 2018;88:158–165. doi: 10.1024/0300-9831/a000493. [DOI] [PubMed] [Google Scholar]
- 120.Lambert D.M.M., Courtel P., Sleno L., Abasq M.L., Ramassamy C. Synergistic properties of bioavailable phenolic compounds from olive oil: Electron transfer and neuroprotective properties. Nutr. Neurosci. 2019:1–14. doi: 10.1080/1028415X.2019.1666480. [DOI] [PubMed] [Google Scholar]
- 121.Achour I., Arel-Dubeau A.M., Renaud J., Legrand M., Attard E., Germain M. Oleuropein prevents neuronal death, mitigates mitochondrial superoxide production and modulates autophagy in a dopaminergic cellular model. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17081293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pasban-Aliabadi H., Esmaeili-Mahani S., Sheibani V., Abbasnejad M., Mehdizadeh A., Yaghoobi M.M. Inhibition of 6-hydroxydopamine-induced PC12 cell apoptosis by olive (Olea europaea L.) leaf extract is performed by its main component oleuropein. Rejuvenation Res. 2013;16:134–142. doi: 10.1089/rej.2012.1384. [DOI] [PubMed] [Google Scholar]
- 123.Sarbishegi M., Charkhat G.E.A., Khajavi O., Komeili G., Salimi S. The neuroprotective effects of hydro-alcoholic extract of olive (Olea europaea L.) leaf on rotenone-induced Parkinson’s disease in rat. Metab. Brain Dis. 2018;33:79–88. doi: 10.1007/s11011-017-0131-0. [DOI] [PubMed] [Google Scholar]
- 124.Amel N., Wafa T., Samia D., Yousra B., Issam C., Cheraif I. Extra virgin olive oil modulates brain docosahexaenoic acid level and oxidative damage caused by 2,4-Dichlorophenoxyacetic acid in rats. J. Food Sci. Technol. 2016;53:1454–1464. doi: 10.1007/s13197-015-2150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liang L.P., Kavanagh T.J., Patel M. Glutathione deficiency in Gclm null mice results in complex I inhibition and dopamine depletion following paraquat administration. Toxicol. Sci. 2013;134:366–373. doi: 10.1093/toxsci/kft112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van der Merwe C., van Dyk H.C., Engelbrecht L., van der Westhuizen F.H., Kinnear C., Loos B. Curcumin Rescues a PINK1 Knock Down SH-SY5Y Cellular Model of Parkinson’s Disease from Mitochondrial Dysfunction and Cell Death. Mol. Neurobiol. 2017;54:2752–2762. doi: 10.1007/s12035-016-9843-0. [DOI] [PubMed] [Google Scholar]
- 127.Man A.H., Linh D.M., My D.V., Phuong T., Thao D. Evaluating dose- and time-dependent effects of Vitamin C Treatment on a Parkinson’s disease fly model. Parkinsons Dis. 2019;2019:9720546. doi: 10.1155/2019/9720546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakaso K., Horikoshi Y., Takahashi T., Hanaki T., Nakasone M., Kitagawa Y. Estrogen receptor-mediated effect of delta-tocotrienol prevents neurotoxicity and motor deficit in the MPTP mouse model of Parkinson’s disease. Neurosci. Lett. 2016;610:117–122. doi: 10.1016/j.neulet.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 129.Prema A., Janakiraman U., Manivasagam T., Thenmozhi A.J. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015;599:12–19. doi: 10.1016/j.neulet.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 130.Nataraj J., Manivasagam T., Thenmozhi A.J., Essa M.M. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr. Neurosci. 2016;19:237–246. doi: 10.1179/1476830515Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 131.Grimmig B., Daly L., Subbarayan M., Hudson C., Williamson R., Nash K. Astaxanthin is neuroprotective in an aged mouse model of Parkinson’s disease. Oncotarget. 2018;9:10388–10401. doi: 10.18632/oncotarget.23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lopes F.M., da Motta L.L., De Bastiani M.A., Pfaffenseller B., Aguiar B.W., de Souza L.F. RA differentiation enhances dopaminergic features, changes redox parameters, and increases dopamine transporter dependency in 6-Hydroxydopamine-Induced neurotoxicity in SH-SY5Y cells. Neurotox. Res. 2017;31:545–559. doi: 10.1007/s12640-016-9699-0. [DOI] [PubMed] [Google Scholar]
- 133.Avola R., Graziano A.C.E., Pannuzzo G., Albouchi F., Cardile V. New insights on Parkinson’s disease from differentiation of SH-SY5Y into dopaminergic neurons: An involvement of aquaporin4 and 9. Mol. Cell Neurosci. 2018;88:212–221. doi: 10.1016/j.mcn.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 134.Garrido M., Tereshchenko Y., Zhevtsova Z., Taschenberger G., Bahr M., Kugler S. Glutathione depletion and overproduction both initiate degeneration of nigral dopaminergic neurons. Acta Neuropathol. 2011;121:475–485. doi: 10.1007/s00401-010-0791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gil-Martinez A.L., Cuenca L., Sanchez C., Estrada C., Fernandez-Villalba E., Herrero M.T. Effect of NAC treatment and physical activity on neuroinflammation in subchronic Parkinsonism; is physical activity essential? J. Neuroinflamm. 2018;15:328. doi: 10.1186/s12974-018-1357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y.L., Liu X.S., Wang S.S., Xue P., Zeng Z.L., Yang X.P. Curcumin-activated mesenchymal stem cells derived from human umbilical cord and their effects on mptp-mouse model of Parkinson’s disease: A new biological therapy for Parkinson’s disease. Stem. Cells Int. 2020;2020:4636397. doi: 10.1155/2020/4636397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.He X.J., Uchida K., Megumi C., Tsuge N., Nakayama H. Dietary curcumin supplementation attenuates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity in C57BL mice. J. Toxicol Pathol. 2015;28:197–206. doi: 10.1293/tox.2015-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pan J., Li H., Ma J.F., Tan Y.Y., Xiao Q., Ding J.Q. Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Transl. Neurodegener. 2012;1:16. doi: 10.1186/2047-9158-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou T., Zhu M., Liang Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018;17:4883–4888. doi: 10.3892/mmr.2018.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou W., Chen L., Hu X., Cao S., Yang J. Effects and mechanism of epigallocatechin-3-gallate on apoptosis and mTOR/AKT/GSK-3beta pathway in substantia nigra neurons in Parkinson’s rats. Neuroreport. 2019;30:60–65. doi: 10.1097/WNR.0000000000001149. [DOI] [PubMed] [Google Scholar]
- 141.Elmazoglu Z., Ergin V., Sahin E., Kayhan H., Karasu C. Oleuropein and rutin protect against 6-OHDA-induced neurotoxicity in PC12 cells through modulation of mitochondrial function and unfolded protein response. Interdiscip Toxicol. 2017;10:129–141. doi: 10.1515/intox-2017-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim M.H., Min J.S., Lee J.Y., Chae U., Yang E.J., Song K.S. Oleuropein isolated from Fraxinus rhynchophylla inhibits glutamate-induced neuronal cell death by attenuating mitochondrial dysfunction. Nutr. Neurosci. 2018;21:520–528. doi: 10.1080/1028415X.2017.1317449. [DOI] [PubMed] [Google Scholar]
- 143.Darbinyan L.V., Hambardzumyan L.E., Simonyan K.V., Chavushyan V.A., Manukyan L.P., Badalyan S.A. Protective effects of curcumin against rotenone-induced rat model of Parkinson’s disease: In vivo electrophysiological and behavioral study. Metab. Brain Dis. 2017;32:1791–1803. doi: 10.1007/s11011-017-0060-y. [DOI] [PubMed] [Google Scholar]
- 144.Song S., Nie Q., Li Z., Du G. Curcumin improves neurofunctions of 6-OHDA-induced parkinsonian rats. Pathol. Res. Pract. 2016;212:247–251. doi: 10.1016/j.prp.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 145.Abbaoui A., Chatoui H., El Hiba O., Gamrani H. Neuroprotective effect of curcumin-I in copper-induced dopaminergic neurotoxicity in rats: A possible link with Parkinson’s disease. Neurosci. Lett. 2017;660:103–108. doi: 10.1016/j.neulet.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 146.Laabbar W., Elgot A., Elhiba O., Gamrani H. Curcumin prevents the midbrain dopaminergic innervations and locomotor performance deficiencies resulting from chronic aluminum exposure in rat. J. Chem. Neuroanat. 2019;100:101654. doi: 10.1016/j.jchemneu.2019.101654. [DOI] [PubMed] [Google Scholar]
- 147.Sur M., Dey P., Sarkar A., Bar S., Banerjee D., Bhat S. Sarm1 induction and accompanying inflammatory response mediates age-dependent susceptibility to rotenone-induced neurotoxicity. Cell Death Discov. 2018;4:114. doi: 10.1038/s41420-018-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ng C.H., Guan M.S., Koh C., Ouyang X., Yu F., Tan E.K. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J. Neurosci. 2012;32:14311–14317. doi: 10.1523/JNEUROSCI.0499-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nishikimi M., Yagi K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am. J. Clin. Nutr. 1991;54:1203S–1208S. doi: 10.1093/ajcn/54.6.1203s. [DOI] [PubMed] [Google Scholar]
- 150.Podmore I.D., Griffiths H.R., Herbert K.E., Mistry N., Mistry P., Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392:559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 151.Kocot J., Luchowska-Kocot D., Kielczykowska M., Musik I., Kurzepa J. Does Vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients. 2017;9 doi: 10.3390/nu9070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tran H.H., Dang S.N.A., Nguyen T.T., Huynh A.M., Dao L.M., Kamei K. Drosophila ubiquitin C-terminal hydrolase knockdown model of Parkinson’s disease. Sci. Rep. 2018;8:4468. doi: 10.1038/s41598-018-22804-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khan S., Jyoti S., Naz F., Shakya B., Rahul A.M. Effect of L-ascorbic Acid on the climbing ability and protein levels in the brain of Drosophila model of Parkinson’s disease. Int. J. Neurosci. 2012;122:704–709. doi: 10.3109/00207454.2012.709893. [DOI] [PubMed] [Google Scholar]
- 154.He X.B., Kim M., Kim S.Y., Yi S.H., Rhee Y.H., Kim T. Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET1- and JMJD3-dependent epigenetic control manner. Stem. Cells. 2015;33:1320–1332. doi: 10.1002/stem.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shen L., Wu H., Diep D., Yamaguchi S., D’Alessio A.C., Fung H.L. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell. 2013;153:692–706. doi: 10.1016/j.cell.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Medeiros M.S., Schumacher S.A., Cardoso A.M., Bochi G.V., Baldissarelli J., Kegler A. Iron and oxidative stress in Parkinson’s disease: An observational study of injury biomarkers. PLoS ONE. 2016;11:e0146129. doi: 10.1371/journal.pone.0146129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Etminan M., Gill S.S., Samii A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson’s disease: A meta-analysis. Lancet Neurol. 2005;4:362–365. doi: 10.1016/S1474-4422(05)70097-1. [DOI] [PubMed] [Google Scholar]
- 158.Nagayama H., Hamamoto M., Ueda M., Nito C., Yamaguchi H., Katayama Y. The effect of ascorbic acid on the pharmacokinetics of levodopa in elderly patients with Parkinson’s disease. Clin. Neuropharmacol. 2004;27:270–273. doi: 10.1097/01.wnf.0000150865.21759.bc. [DOI] [PubMed] [Google Scholar]
- 159.Nikolova G., Karamalakova Y., Gadjeva V. Reducing oxidative toxicity of L-dopa in combination with two different antioxidants: An essential oil isolated from Rosa Damascena Mill., and vitamin C. Toxicol. Rep. 2019;6:267–271. doi: 10.1016/j.toxrep.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Numakawa Y., Numakawa T., Matsumoto T., Yagasaki Y., Kumamaru E., Kunugi H. Vitamin E protected cultured cortical neurons from oxidative stress-induced cell death through the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. J. Neurochem. 2006;97:1191–1202. doi: 10.1111/j.1471-4159.2006.03827.x. [DOI] [PubMed] [Google Scholar]
- 161.Hosomi A., Arita M., Sato Y., Kiyose C., Ueda T., Igarashi O. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997;409:105–108. doi: 10.1016/S0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 162.Suzuki Y.J., Tsuchiya M., Wassall S.R., Choo Y.M., Govil G., Kagan V.E. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry. 1993;32:10692–10699. doi: 10.1021/bi00091a020. [DOI] [PubMed] [Google Scholar]
- 163.Park H.A., Kubicki N., Gnyawali S., Chan Y.C., Roy S., Khanna S. Natural vitamin E alpha-tocotrienol protects against ischemic stroke by induction of multidrug resistance-associated protein 1. Stroke. 2011;42:2308–2314. doi: 10.1161/STROKEAHA.110.608547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schirinzi T., Martella G., Imbriani P., Di Lazzaro G., Franco D., Colona V.L. Dietary Vitamin E as a protective factor for Parkinson’s disease: Clinical and experimental evidence. Front. Neurol. 2019;10:148. doi: 10.3389/fneur.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martella G., Madeo G., Maltese M., Vanni V., Puglisi F., Ferraro E. Exposure to low-dose rotenone precipitates synaptic plasticity alterations in PINK1 heterozygous knockout mice. Neurobiol. Dis. 2016;91:21–36. doi: 10.1016/j.nbd.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 166.Park H.A., Jonas E.A. DeltaN-Bcl-xL, a therapeutic target for neuroprotection. Neural. Regen. Res. 2017;12:1791–1794. doi: 10.4103/1673-5374.219033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park H.A., Licznerski P., Mnatsakanyan N., Niu Y., Sacchetti S., Wu J. Inhibition of Bcl-xL prevents pro-death actions of DeltaN-Bcl-xL at the mitochondrial inner membrane during glutamate excitotoxicity. Cell Death Differ. 2017;24:1963–1974. doi: 10.1038/cdd.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakaso K., Tajima N., Horikoshi Y., Nakasone M., Hanaki T., Kamizaki K. The estrogen receptor beta-PI3K/Akt pathway mediates the cytoprotective effects of tocotrienol in a cellular Parkinson’s disease model. Biochim. Biophys. Acta. 2014;1842:1303–1312. doi: 10.1016/j.bbadis.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 169.Sotolongo K., Ghiso J., Rostagno A. Nrf2 activation through the PI3K/GSK-3 axis protects neuronal cells from Abeta-mediated oxidative and metabolic damage. Alzheimers Res. Ther. 2020;12:13. doi: 10.1186/s13195-019-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang L., Chen Y., Sternberg P., Cai J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest. Ophthalmol. Vis. Sci. 2008;49:1671–1678. doi: 10.1167/iovs.07-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Niture S.K., Jaiswal A.K. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic. Biol. Med. 2013;57:119–131. doi: 10.1016/j.freeradbiomed.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu Q., Jin Z., Xu Z., Yang H., Li L., Li G. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones. 2019;24:441–452. doi: 10.1007/s12192-019-00977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hu L., Chen W., Tian F., Yuan C., Wang H., Yue H. Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomed. Pharmacother. 2018;106:1484–1789. doi: 10.1016/j.biopha.2018.07.088. [DOI] [PubMed] [Google Scholar]
- 174.Yang F., Wolk A., Hakansson N., Pedersen N.L., Wirdefeldt K. Dietary antioxidants and risk of Parkinson’s disease in two population-based cohorts. Mov. Disord. 2017;32:1631–1636. doi: 10.1002/mds.27120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ghani H., Stevens D., Weiss J., Rosenbaum R. Vitamins and the risk for Parkinson’s disease. Neurology. 2002;59:E8–E9. doi: 10.1212/WNL.59.8.E8. [DOI] [PubMed] [Google Scholar]
- 176.Hughes K.C., Gao X., Kim I.Y., Rimm E.B., Wang M., Weisskopf M.G. Intake of antioxidant vitamins and risk of Parkinson’s disease. Mov. Disord. 2016;31:1909–1914. doi: 10.1002/mds.26819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.King D., Playfer J.R., Roberts N.B. Concentrations of vitamins A, C and E in elderly patients with Parkinson’s disease. Postgrad. Med. J. 1992;68:634–637. doi: 10.1136/pgmj.68.802.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Taghizadeh M., Tamtaji O.R., Dadgostar E., Daneshvar K.R., Bahmani F., Abolhassani J. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017;108:183–189. doi: 10.1016/j.neuint.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 179.Podlesny A.-D., Sobska J., de Lera A.R., Golembiowska K., Kaminska K., Dolle P. Distinct retinoic acid receptor (RAR) isotypes control differentiation of embryonal carcinoma cells to dopaminergic or striatopallidal medium spiny neurons. Sci. Rep. 2017;7:13671. doi: 10.1038/s41598-017-13826-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim S., Lim J., Bang Y., Moon J., Kwon M.S., Hong J.T. Alpha-Synuclein suppresses retinoic Acid-Induced neuronal differentiation by targeting the glycogen synthase Kinase-3beta/beta-Catenin signaling pathway. Mol. Neurobiol. 2018;55:1607–1619. doi: 10.1007/s12035-016-0370-9. [DOI] [PubMed] [Google Scholar]
- 181.Pan J., Yu J., Sun L., Xie C., Chang L., Wu J. ALDH1A1 regulates postsynaptic mu-opioid receptor expression in dorsal striatal projection neurons and mitigates dyskinesia through transsynaptic retinoic acid signaling. Sci. Rep. 2019;9:3602. doi: 10.1038/s41598-019-40326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim J.H., Hwang J., Shim E., Chung E.J., Jang S.H., Koh S.B. Association of serum carotenoid, retinol, and tocopherol concentrations with the progression of Parkinson’s Disease. Nutr. Res. Pract. 2017;11:114–120. doi: 10.4162/nrp.2017.11.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Takeda A., Nyssen O.P., Syed A., Jansen E., Bueno-de-Mesquita B., Gallo V. Vitamin A and carotenoids and the risk of Parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology. 2014;42:25–38. doi: 10.1159/000355849. [DOI] [PubMed] [Google Scholar]
- 184.Junn E., Lee K.W., Jeong B.S., Chan T.W., Im J.Y., Mouradian M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao C., Ren J., Xue C., Lin E. Study on the relationship between soil selenium and plant selenium uptake. Plant Soil. 2005;277:197–206. doi: 10.1007/s11104-005-7011-9. [DOI] [Google Scholar]
- 186.Zhong L., Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J. Biol. Chem. 2000;275:18121–18128. doi: 10.1074/jbc.M000690200. [DOI] [PubMed] [Google Scholar]
- 187.Cabeza Y.-A., Schiestl R.H. Transcriptome analysis of a rotenone model of parkinsonism reveals complex I-tied and -untied toxicity mechanisms common to neurodegenerative diseases. PLoS ONE. 2012;7:e44700. doi: 10.1371/journal.pone.0044700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.de Freitas C.S., Araujo S.M., Bortolotto V.C., Poetini M.R., Pinheiro F.C., Santos M.E.A. 7-chloro-4-(phenylselanyl) quinoline prevents dopamine depletion in a Drosophila melanogaster model of Parkinson’s-like disease. J. Trace Elem. Med. Biol. 2019;54:232–243. doi: 10.1016/j.jtemb.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 189.Khan H.A. Selenium partially reverses the depletion of striatal dopamine and its metabolites in MPTP-treated C57BL mice. Neurochem. Int. 2010;57:489–491. doi: 10.1016/j.neuint.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 190.Sun H. Association of soil selenium, strontium, and magnesium concentrations with Parkinson’s disease mortality rates in the USA. Environ. Geochem. Health. 2018;40:349–357. doi: 10.1007/s10653-017-9915-8. [DOI] [PubMed] [Google Scholar]
- 191.Shahar A., Patel K.V., Semba R.D., Bandinelli S., Shahar D.R., Ferrucci L. Plasma selenium is positively related to performance in neurological tasks assessing coordination and motor speed. Mov. Disord. 2010;25:1909–1915. doi: 10.1002/mds.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Maass F., Michalke B., Leha A., Boerger M., Zerr I., Koch J.C. Elemental fingerprint as a cerebrospinal fluid biomarker for the diagnosis of Parkinson’s disease. J. Neurochem. 2018;145:342–351. doi: 10.1111/jnc.14316. [DOI] [PubMed] [Google Scholar]
- 193.Zhao H.W., Lin J., Wang X.B., Cheng X., Wang J.Y., Hu B.L. Assessing plasma levels of selenium, copper, iron and zinc in patients of Parkinson’s disease. PLoS ONE. 2013;8:e83060. doi: 10.1371/journal.pone.0083060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Naderi M., Salahinejad A., Jamwal A., Chivers D.P., Niyogi S. Chronic Dietary Selenomethionine Exposure Induces Oxidative Stress, Dopaminergic Dysfunction, and Cognitive Impairment in Adult Zebrafish (Danio rerio) Environ. Sci. Technol. 2017;51:12879–12888. doi: 10.1021/acs.est.7b03937. [DOI] [PubMed] [Google Scholar]
- 195.Naderi M., Salahinejad A., Ferrari M.C.O., Niyogi S., Chivers D.P. Dopaminergic dysregulation and impaired associative learning behavior in zebrafish during chronic dietary exposure to selenium. Environ. Pollut. 2018;237:174–185. doi: 10.1016/j.envpol.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 196.Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Institute of Medicine; Washington, DC, USA: 2000. [PubMed] [Google Scholar]
- 197.Chinta S.J., Andersen J.K. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: Implications for Parkinson’s disease. Free Radic. Biol. Med. 2006;41:1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 198.Park H.A., Khanna S., Rink C., Gnyawali S., Roy S., Sen C.K. Glutathione disulfide induces neural cell death via a 12-lipoxygenase pathway. Cell Death Differ. 2009;16:1167–1179. doi: 10.1038/cdd.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Monti D.A., Zabrecky G., Kremens D., Liang T.W., Wintering N.A., Bazzan A.J. N-Acetyl cysteine is associated with dopaminergic improvement in Parkinson’s disease. Clin. Pharmacol. Ther. 2019;106:884–890. doi: 10.1002/cpt.1548. [DOI] [PubMed] [Google Scholar]
- 200.Hauser R.A., Lyons K.E., McClain T., Carter S., Perlmutter D. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson’s disease. Mov. Disord. 2009;24:979–983. doi: 10.1002/mds.22401. [DOI] [PubMed] [Google Scholar]
- 201.Monti D.A., Zabrecky G., Kremens D., Liang T.W., Wintering N.A., Cai J. N-Acetyl cysteine may support dopamine neurons in Parkinson’s disease: Preliminary clinical and cell line data. PLoS ONE. 2016;11:e0157602. doi: 10.1371/journal.pone.0157602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Salamon S., Kramar B., Marolt T.P., Poljsak B., Milisav I. Medical and dietary uses of N-Acetylcysteine. Antioxidants. 2019;8 doi: 10.3390/antiox8050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Coles L.D., Tuite P.J., Oz G., Mishra U.R., Kartha R.V., Sullivan K.M. Repeated-Dose oral N-Acetylcysteine in Parkinson’s disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharmacol. 2018;58:158–167. doi: 10.1002/jcph.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ak T., Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 206.Zhu Y.G., Chen X.C., Chen Z.Z., Zeng Y.Q., Shi G.B., Su Y.H. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol. Sin. 2004;25:1606–1612. [PubMed] [Google Scholar]
- 207.Namsi A., Nury T., Hamdouni H., Yammine A., Vejux A., Vervandier-Fasseur D. Induction of neuronal differentiation of murine n2a cells by two polyphenols present in the mediterranean diet mimicking neurotrophins activities: Resveratrol and apigenin. Diseases. 2018;6 doi: 10.3390/diseases6030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Almeida A., Heales S.J., Bolanos J.P., Medina J.M. Glutamate neurotoxicity is associated with nitric oxide-mediated mitochondrial dysfunction and glutathione depletion. Brain Res. 1998;790:209–216. doi: 10.1016/S0006-8993(98)00064-X. [DOI] [PubMed] [Google Scholar]
- 209.Pugazhenthi S., Nesterova A., Sable C., Heidenreich K.A., Boxer L.M., Heasley L.E. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 210.Zhou H., Li X.M., Meinkoth J., Pittman R.N. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 2000;151:483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reygaert W.C. Green tea catechins: Their use in treating and preventing infectious diseases. Biomed. Res. Int. 2018;2018:9105261. doi: 10.1155/2018/9105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Grzesik M., Naparlo K., Bartosz G., Sadowska-Bartosz I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018;241:480–492. doi: 10.1016/j.foodchem.2017.08.117. [DOI] [PubMed] [Google Scholar]
- 213.Bors W., Heller W., Michel C., Saran M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990;186:343–355. doi: 10.1016/0076-6879(90)86128-I. [DOI] [PubMed] [Google Scholar]
- 214.Bernatoniene J., Kopustinskiene D.M. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23 doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Koch W., Kukula-Koch W., Glowniak K. Catechin composition and antioxidant activity of black teas in relation to brewing time. J. AOAC Int. 2017;100:1694–1699. doi: 10.5740/jaoacint.17-0235. [DOI] [PubMed] [Google Scholar]
- 216.Nakagawa K., Miyazawa T. Absorption and distribution of tea catechin, (-)-epigallocatechin-3-gallate, in the rat. J. Nutr. Sci. Vitaminol. (Tokyo) 1997;43:679–684. doi: 10.3177/jnsv.43.679. [DOI] [PubMed] [Google Scholar]
- 217.Unno K., Pervin M., Nakagawa A., Iguchi K., Hara A., Takagaki A. Blood-Brain barrier permeability of green tea catechin metabolites and their neuritogenic activity in human neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700294. [DOI] [PubMed] [Google Scholar]
- 218.Pervin M., Unno K., Takagaki A., Isemura M., Nakamura Y. Function of green tea catechins in the brain: Epigallocatechin gallate and its metabolites. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20153630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kang K.S., Wen Y., Yamabe N., Fukui M., Bishop S.C., Zhu B.T. Dual beneficial effects of (-)-epigallocatechin-3-gallate on levodopa methylation and hippocampal neurodegeneration: In vitro and in vivo studies. PLoS ONE. 2010;5:e11951. doi: 10.1371/journal.pone.0011951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Omar S.H. Oleuropein in olive and its pharmacological effects. Sci Pharm. 2010;78:133–154. doi: 10.3797/scipharm.0912-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Visioli F., Bellomo G., Galli C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998;247:60–64. doi: 10.1006/bbrc.1998.8735. [DOI] [PubMed] [Google Scholar]
- 222.Lins P.G., Marina P.P.S., Scatolini A.M., de Melo M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon. 2018;4:e00805. doi: 10.1016/j.heliyon.2018.e00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sun W., Wang X., Hou C., Yang L., Li H., Guo J. Oleuropein improves mitochondrial function to attenuate oxidative stress by activating the Nrf2 pathway in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Neuropharmacology. 2017;113:556–566. doi: 10.1016/j.neuropharm.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 224.Serra A., Rubio L., Borras X., Macia A., Romero M.P., Motilva M.J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012;56:486–496. doi: 10.1002/mnfr.201100436. [DOI] [PubMed] [Google Scholar]