Highlights
-
•
The pathophysiology of SARS-CoV-2 infection may be attributed to cytokine release syndrome.
-
•
In this syndrome, interleukin 6 is released after the activation of the inflammatory cascade.
-
•
The mortality rate in the current study was 16%.
-
•
Tocilizumab may be a promising agent to decrease the mortality rate in severe or critical SARS-CoV-2 infection.
Keywords: Coronavirus, COVID-19, Interleukin 6, SARS-CoV-2, Tocilizumab
Abstract
Background
The clinical presentation of SARS-CoV-2 infection ranges from mild symptoms to severe complications, including acute respiratory distress syndrome. In this syndrome, inflammatory cytokines are released after activation of the inflammatory cascade, with the predominant role of interleukin (IL)-6. The aim of this study was to evaluate the effects of tocilizumab, as an IL-6 antagonist, in patients with severe or critical SARS-CoV-2 infection.
Methods
In this prospective clinical trial, 76 patients with severe or critical SARS-CoV-2 infection were evaluated for eligibility, and ultimately, 42 patients were included. Tocilizumab was administered at a dose of 400 mg as a single dose via intravenous infusion. Primary outcomes included changes in oxygenation support, need for invasive mechanical ventilation, and death. Secondary outcomes included radiological changes in the lungs, IL-6 plasma levels, C-reactive protein levels, and adverse drug reactions. The data were analyzed using SPSS software.
Results
Of the 42 included patients, 20 (48%) patients presented the severe infection stage and 22 (52%) were in the critical stage. The median age of patients was 56 years, and the median IL-6 level was 28.55 pg/mL. After tocilizumab administration, only 6 patients (14%) required invasive ventilation. Additionally, 35 patients (83.33%) showed clinical improvement. By day 28, a total of 7 patients died (6 patients in the critical stage and 1 patient in the severe stage). Neurological adverse effects were observed in 3 patients.
Conclusions
Based on the current results, tocilizumab may be a promising agent for patients with severe or critical SARS-CoV-2 infection, if promptly initiated during the severe stage.
1. Introduction
The novel coronavirus disease, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is rapidly spreading worldwide. The clinical presentation of SARS-CoV-2 infection ranges from mild symptoms to severe complications, such as acute respiratory distress syndrome [1]. As of July 18, 2020, a total of 14,243,592 cases were confirmed in 213 countries, with 600,496 individuals dead from the disease. The pathophysiology of severe SARS-CoV-2 infection is attributed to cytokine release syndrome (CRS). In this syndrome, inflammatory cytokines are released after the activation of the inflammatory cascade [2], [3]. During this stage, patients experience severe symptoms, including cardiovascular collapse, multiple organ failure, and death [4]. Therefore, early diagnosis and treatment of CRS are essential. Earlier studies have reported the predominant role of interleukin-6 (IL-6) in CRS [5]. Therefore, in patients with SARS-CoV-2 infection and elevated IL-6 levels, tocilizumab may be beneficial. Tocilizumab is a recombinant humanized monoclonal antibody of the immunoglobulin G1κ (gamma 1, kappa) subclass that binds to soluble IL-6 receptors and membrane-bound IL-6 receptors followed by inhibiting signal transduction cascade eliciting IL-6 biological activity. [6]. Tocilizumab was first approved in Japan in 2005 as an orphan drug for the treatment of Castleman’s disease. Additionally, it has been indicated for the treatment of diseases such as Crohn's disease, systemic lupus erythematosus, Takayasu arteritis, giant cell arteritis, CRS, and polymyalgia rheumatic [7], [8]. The aim of this study was to evaluate the clinical efficacy of tocilizumab in patients with SARS-CoV-2 infection, especially in those in the inflammatory phase of the disease.
2. Methods
2.1. Setting
This study was a prospective, non-controlled trial performed in March 2020 at the Dr. Masih Daneshvari Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. This hospital was the main referral center for SARS-CoV-2 infection in Iran.
2.2. Patients
Physicians sent tocilizumab prescription requests to the hospital’s clinical trial office. The patients were then evaluated for inclusion in this study. The inclusion and exclusion criteria are listed in Table 1 .
Table 1.
The inclusion and exclusion criteria of the study.
| Inclusion criteria | Age > 18 years; positive RT-PCR for SARS-CoV-2; IL-6 > 10 pg/mL; severe SARS-CoV-2 infection (defined as: SpO2 < 90, RR > 30, or bilateral progressive lung infiltration); critical SARS-CoV-2 infection (defined as: need for ICU or need for mechanical ventilation); no improvement despite receiving 72 h of standard care; signed informed consent |
|---|---|
| Exclusion criteria | Pregnancy or breastfeeding; mild SARS-CoV-2 infection (defined as: SpO2 > 90 or upper respiratory infection); active infection including tuberculosis or bacterial infections; allergy to tocilizumab or its ingredients; chronic kidney disease (eGFR < 30 mL/min); chronic liver disease (Child-Pugh C and D); patients who did not receive 72 h of standard care; neutropenia (ANC less than 1500/mm3); thrombocytopenia (platelet count less than 150,000/µL), dyslipidemia (total cholesterol and triglycerides more than 200 mg/dL and 150 mg/dL, respectively); receiving any anti-inflammatory agent |
RT-PCR, Reverse transcription polymerase chain reaction; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; ANC, absolute neutrophil count.
2.3. Study registration
This study was approved by the ethics committees of Shahid Beheshti University of Medical Sciences and the National Research Institute of Tuberculosis and Lung Diseases (IR.SBMU.NRITLD.REC.1399.002). The study protocol was also registered in the Iranian Registry of Clinical Trials (IRCT20151227025726N13). All enrolled patients provided a completed and signed informed consent form.
2.4. Interventions
The patients received tocilizumab (Actemra, Hoffmann–La Roche holding company, France) at a dose of 400 mg as a single dose via intravenous (IV) infusion over 2 h. All patients received only one dose of 400 mg of tocilizumab based on the limitation of tocilizumab resources and the patients’ response. For all patients, oxygenation was considered via nasal cannula, face mask, noninvasive strategies, or mechanical ventilation. The standard of care regimen consisted of 2 tablets of lopinavir/ritonavir (Kaletra, Abbott Laboratories, 200/50 mg per tablet) twice a day, which was considered for all patients for up to 5 days.
2.5. Outcomes
For all patients, demographic data, including age, sex, past medical history, and baseline laboratory results, were reported.
2.6. Primary outcomes
The primary outcome was the frequency of requiring invasive mechanical ventilation. This was evaluated daily for 28 days or until discharge. Additionally, clinical improvement, defined either as weaning from oxygen support or discharge from hospital, was reported. Mortality rate was reported at the end of day 28.
2.7. Secondary outcomes
IL-6 levels, intensive care unit (ICU) stay, hospital stay, and any adverse drug reactions were recorded. Lung images were assessed for up to 28 days. Chest X-ray and computed tomography (CT) imaging comparisons were performed at baseline (patient’s admission) and day 14.
2.8. Statistical analysis
The quantitative data were described as the mean ± standard deviation or as the median (interquartile range). The frequency rates were described by the number of cases (proportion or percent). The survival rate was measured using the Kaplan–Meier estimator. Results were summarized and analyzed using the Statistical Package for the Social Sciences (SPSS) v.24.0 software (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Patients and baseline characteristics
In this study, tocilizumab administration was assessed in 76 patients. Overall, 15 patients were excluded owing to the low IL-6 levels (<10 pg/mL), 9 were excluded owing to chronic kidney disease, 6 were excluded owing to latent tuberculosis, and 4 refused to sign the informed consent form. Therefore, 42 patients were included in the study.
In total, 27 (64%) patients were males, and the median age was 56 years (interquartile range, 44–61 years). At baseline, 31 patients (74%) were receiving oxygen via a face mask, whereas the rest received oxygen via nasal cannula. Underlying diseases were present in 33 patients (78%), and hypertension was the most prevalent disease (38%). After 72 h of standard care, 20 patients were in the severe stage of SARS-CoV-2 infection and 22 were in the critical stage. The median IL-6 level was 28.55 pg/mL (interquartile range, 19.0–47.5) when tocilizumab was administered. The baseline characteristics are shown in Table 2 .
Table 2.
Baseline demographic and clinical characteristics of the patients.
| Characteristic | Severe N = 20 (48%) |
Critical N = 22 (52%) |
Total N = 42 | ||
|---|---|---|---|---|---|
| Survivors N = 19 (95%) | Non-survivors N = 1 (5%) | Survivors N = 16 (73%) | Non-survivors N = 6 (27%) | ||
| Median age (IQR) – years | 56 (42–61) | 70 | 51 (43–60) | 52 (45–75) | 56 (44–61) |
| Age category - no. (%) | |||||
| <50 yrs | 7 (35) | 0 | 8 (36) | 3 (14) | 18 (43) |
| 50 to <70 yrs | 12 (60) | 0 | 6 (27) | 1 (4) | 19 (45) |
| ≥70 yrs | 0 | 1 (5) | 2 (9) | 2 (9) | 5 (12) |
| Male sex - no. (%) | 11 (55) | 1 (5) | 12 (54) | 3 (14) | 27 (64) |
| Oxygen support group - no. (%) | |||||
| Via nasal cannula | 5 (25) | 0 | 5 (23) | 1 (4) | 11 (26) |
| Via face mask | 14 (70) | 1 (5) | 11 (50) | 5 (5) | 31 (74) |
| Coexisting conditions - no. (%) | |||||
| Any condition | 11 (55) | 1 (5) | 17 (77) | 4 (18) | 33 (78) |
| Hypertension | 5 (25) | 1 (5) | 7 (32) | 3 (14) | 16 (38) |
| Diabetes | 3 (15) | 0 | 5 (23) | 1 (4) | 9 (21) |
| Chronic obstructive pulmonary disease | 2 (10) | 0 | 0 | 0 | 2 (5) |
| Bronchiectasis | 0 | 0 | 1 (4) | 0 | 1 (2) |
| Multiple sclerosis | 0 | 0 | 3 (14) | 0 | 3 (7) |
| Rheumatoid arthritis | 1 (5) | 0 | 0 | 0 | 1 (2) |
| Malignancy | 0 | 0 | 1 (4) | 0 | 1 (2) |
| Median laboratory values (IQR) | |||||
| White blood cells × 109 cells/L | 6.2 (5.4–8.4) | 6.3 | 8.34 (7.2–15.10) | 10.14 (7.35–15.17) | 7.70 (5.81–14.14) |
| Neutrophil-to-lymphocyte ratio | 22.58 (15.40–70.20) | 49.25 | 126.34 (22.54–275.24) | 94.80 (35.53–321.52) | 51.22 (16.74–226.93) |
| Creatinine (mg/dL) | 1.0 (0.9–1.1) | 1.2 | 0.95 (0.9–1.15) | 1 (0.9–1.1) | 1.0 (0.9–1.1) |
| ALT (IU/L) | 56 (32–90) | 34 | 43 (26–73) | 38 (28–40) | 48 (31–79) |
| AST (IU/L) | 58 (47–77) | 38 | 44 (31–57) | 42 (38–54) | 54 (36–65) |
| Triglyceride (mg/dL) | 121 (98–145) | 205 | 143 (118–169) | 84 (73–99) | 127 (98–156) |
| Ferritin (ng/mL) | 1071 (885–1497) | 1073 | 1312 (1106–1832) | 1117 (539–1700) | 1177 (885–1700) |
| CRP | 38 (25–55) | 71 | 42 (27–59) | 40 (30–58) | 39.5 (26–58) |
| Interleukin 6 (pg/mL) | 29 (20–47) | 36 | 29.5 (18.7–51.7) | 21.5 (11.7–23.9) | 28.55 (19.0–47.5) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; IQR, interquartile range.
3.2. Primary outcomes
At the time of tocilizumab administration, 10 patients were on noninvasive mechanical ventilation and 2 were on invasive mechanical ventilation. The remaining 30 patients received oxygen via a face mask. After tocilizumab administration, 6 patients (14%) required invasive ventilation (including 3 cases of extracorporeal membrane oxygenation (ECMO) and 3 cases of invasive mechanical ventilation), with deaths subsequently reported in all these patients (median, 7 days). One patient on noninvasive mechanical ventilation before tocilizumab administration died after four days. The results of the changes in oxygenation support are shown in Table 3 .
Table 3.
Changes in oxygenation before and after tocilizumab administration.
 |
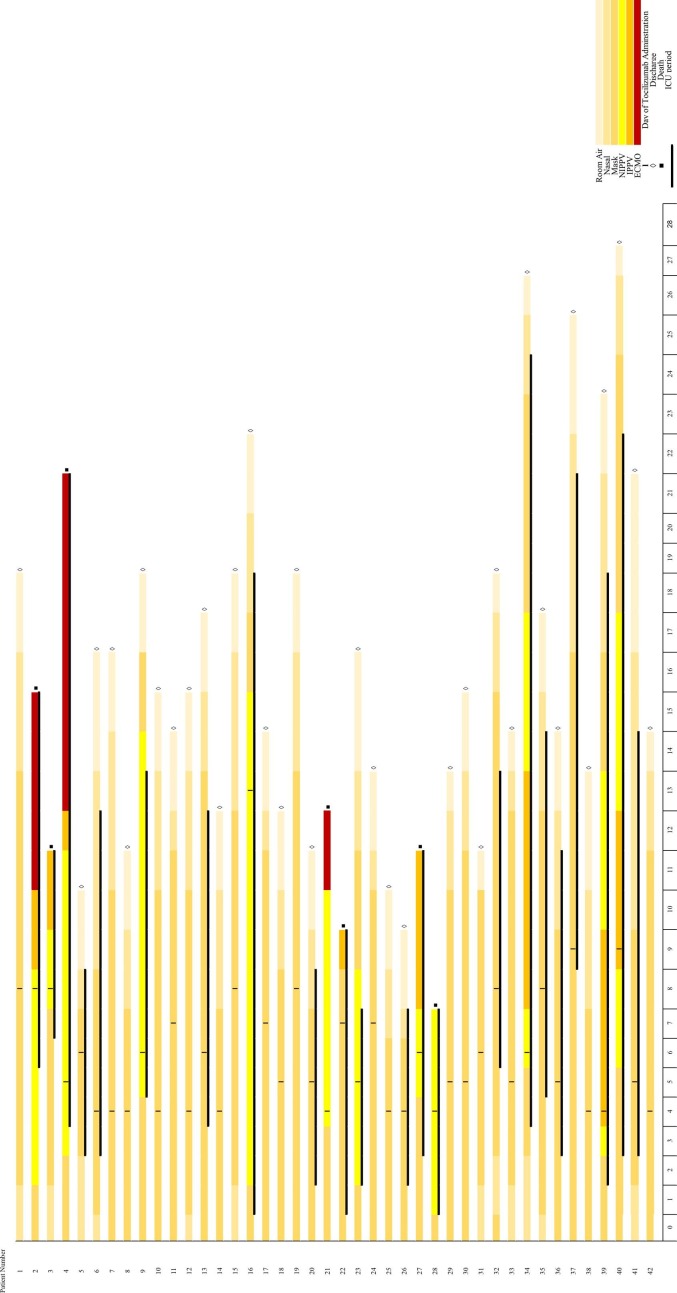
The results demonstrated that in the 20 severe cases, only 1 patient (5%) died, and among the 22 critical cases, 6 patients (27%) died. In total, 35 patients (83.33%) showed clinical improvement (83.33%). The study timeline is summarized in Fig. 1 .
Fig. 1.
The study timeline.
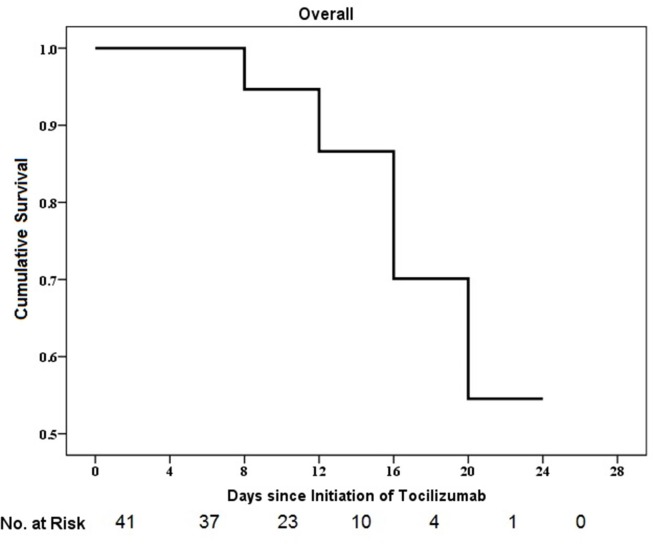
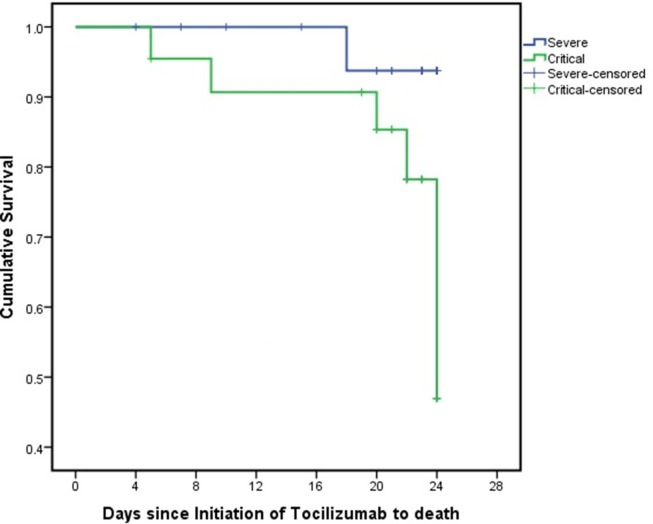
A Kaplan-Meier plot for the survival time of the whole study group was shown in the Fig. 2 . In addition, the Kaplan-Meier plot by severity group (severe or critical) is presented in the Fig. 3 . Although, by using a Log Rank (Mantel-Cox) test, no statistically significant difference was shown between time-to-death of patients with a severe condition compared to mortality rate of those with a critical condition (p = 0.06, 95% CI: 21.50–24.09). However, as it is illustrated in Fig. 3, cumulative survival rate was higher in the severe group compared to that of critical group. Survival rate of severe group was 100% for 17 days, and reduced to 93.8% at day18 and maintained at this level until the end of study (day 24). In contrary, the survival rates of the critical group on day 17 was slightly more than 90.0% and dropped to 85.3%, 78.2% and 46.9% for 20, 22 and 24 days after starting tocilizumab therapy, respectively. This could be in favor of the fact that the earlier initiation of tocilizumab, before clinical deterioration, may lead to the better survival results.
Fig. 2.
The cumulative incidence of death from baseline to day 28.
Fig. 3.
The cumulative incidence of death from baseline to day 28 stratified according to clinical condition. Survival rate of severe group was 100% for 17 days, and reduced to 93.8% at day18 and maintained at this level until the end of study (day 24). In contrary, the survival rates of the critical group on day 17 was slightly more than 90.0% and dropped to 85.3%, 78.2% and 46.9% for 20, 22 and 24 days after starting tocilizumab therapy, respectively. Fall in lines shows the death event and small vertical lines show the improvement event. These censored cases (improvement event) are listed below: [In the severe group]: Days 4 (1 case), 7 (1 case), 10 (1 case), 15 (1 case), 20 (2 cases), 21 (2 cases), 23 (5 cases) and 24 (6 cases). [In the critical group]: Days 5 (1 case), 19 (2 cases), 20 (2 cases), 21 (2 case), 22 (3 cases), 23 (3 cases) and 24 (3 cases). Severe, patients with SpO2 < 90, RR > 30 or bilateral progressive lung infiltration; critical, patients who need ICU admission or need for mechanical ventilation; severe-censored, patients in the severe group who had dropped out the study; critical-censored, patients in the critical group who had dropped out the study.
3.3. Secondary outcomes
The median IL-6 level was 28.55 pg/mL (interquartile range, 19–47.5 pg/mL) at baseline and decreased to 21.25 pg/mL (interquartile range, 11.5–32.7 pg/mL) and 8.5 pg/mL (interquartile range, 6–14.9 pg/mL) after four and nine days of tocilizumab initiation, respectively (P < 0.001). The median days of hospital stay and ICU stay was 15 days and 5 days, respectively. Three patients experienced significant adverse effects, including two patients who experienced transient diplopia and one patient experienced Bell's palsy.
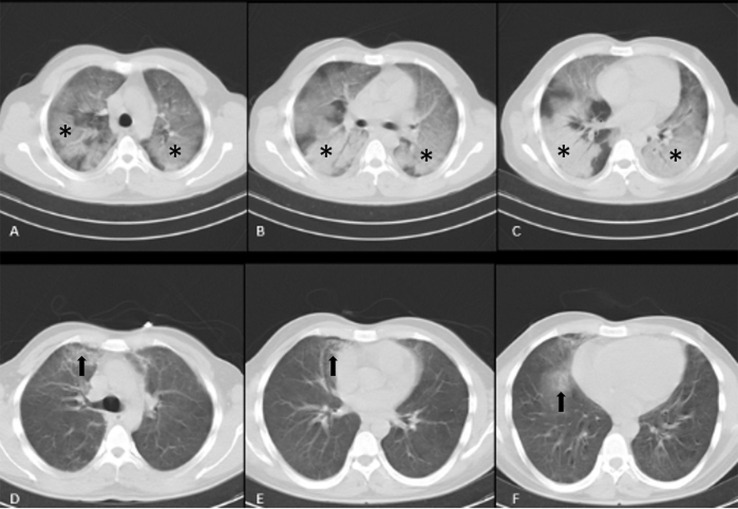
Lung CT and chest X-ray tests were performed at baseline and throughout the study. Lung imaging on the day of tocilizumab initiation was compared with the last imaging. The results showed that 7 patients presented worsening imaging, 7 patients failed to show significant changes in imaging, and 28 patients demonstrated significant changes in imaging. As seen in Fig. 4 and Fig. 5 , recovery occurred after tocilizumab administration.
Fig. 4.
Initial non-contrast enhanced CT scan (A, B, and C) of a 26-year-old man with respiratory distress and positive PCR for SARS-CoV-2 showed bilateral multilobar ground glass opacities and consolidations with air-bronchogram with no pleural effusion (*), subsequent CT scan (D, E, and F) after injection of tocilizumab, revealed significant improvement with near complete resolution of parenchymal infiltration just with few remaining parenchymal infiltrations (arrows). CT, computed tomography.
Fig. 5.
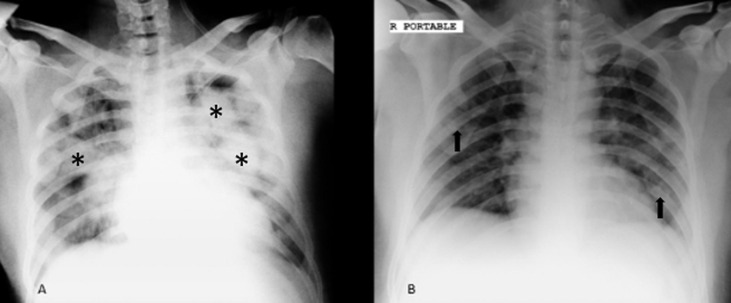
Portable CXR of 42-year-old man who was admitted to the intensive care unit after developing respiratory distress secondary to COVID induced pneumonia; initial CXR revealed bilateral confluent consolidations (*) after tocilizumab therapy, significant improvement of previous parenchymal consolidations was found with few remaining faintly visualized parenchymal infiltrations (arrows). (A, initial CXR, B, CXR after injection of tocilizumab). CXR, chest X-ray.
4. Discussion
SARS-CoV-2 infection is an emergent infectious disease with no established therapeutic strategy [9]. Here, we reported the results of tocilizumab administration in patients with severe or critical SARS-CoV-2 infection. Tocilizumab can potently antagonize IL-6 receptors, and thus, interfere with the inflammatory cascade [10], [11]. IL-6 can promote T-cell proliferation and activation, B-cell differentiation, regulate acute-phase responses, and affect the hormone-like properties of vascular diseases [12]. The mortality rate among patients receiving tocilizumab with severe or critical SARS-CoV-2 infection was reported 16% in the current study.
The mortality rate was reported 49% in the patients who admitted to the ICU. In earlier studies, predominantly performed in China, mortality rates of 17%-78% have been reported in COVID-19 cases [13], [14]. Among 201 patients hospitalized in Wuhan, China, the overall mortality was 22%, and 66% of patients required invasive mechanical ventilation (44 of 67 patients) [15]. According to Meng et al., 50% of patients who admitted to the ICU required invasive mechanical ventilation [16]. However, we found that tocilizumab administration could decrease this rate to 14%.
In a study that assessed the efficacy of remdesivir, a mortality rate of 13% has been reported. The study had included patients in different stages of SARS-CoV-2 infection [17]. In another study by Wang et al., this rate was reported 14%. In a recent randomized controlled trial of lopinavir/ritonavir in patients hospitalized for SARS-CoV-2 infection, the mortality rate was reported as 22% [18].
Currently, a small clinical trial in China (Clinical Trial Registry ID: ChiCTR2000029765) has reported promising efficacy with tocilizumab, including 21 patients with severe or critical SARS-CoV-2 infection. Their study showed that 75% of patients required reduced oxygen support, and 1 patient did not require supportive oxygen [19]. Clinical improvement defined either as weaning from oxygen support or discharge from hospital was seen in 83.33% of patients in our study.
In another study, tocilizumab and methylprednisolone were co-administered in 15 cases of SARS-CoV-2 infection, with 2 patients (13.3%) being moderately ill, 6 (40.0%) seriously ill, and 7 (46.7%) critically ill. Various doses of tocilizumab were administered to these patients (range, 80–600 mg each time) and observed that multiple tocilizumab administrations increase IL-6 levels [20]. Binding of tocilizumab to IL‐6 receptors inhibits the receptor‐mediated clearance of IL‐6, resulting in accumulation in the serum [21]. In contrast, we found that IL-6 levels decreased significantly during the 10 days of tocilizumab administration.
According to the study by Bhatraju et al., the median length of hospital and ICU stay were 17 and 10 days, respectively among critical cases of COVID-19 [22]. We found that tocilizumab administration could decrease the median hospital and the ICU length of stay to 15 and 5 days, respectively.
Cai et al., evaluated the efficacy of favipiravir in patients with COVID-19 and they found no significant difference in the improvement of lung infiltration in comparison with the control group before 14 days [23]. Dramatic radiological responses were observed following tocilizumab administration in our study. However, exacerbated lung infiltration was observed in patients who died.
Guarald et al., administered tocilizumab for 544 severe COVID-19 cases at dose of 8 mg/kg (intravenously) in two infusions with 12 h interval or at dose of 162 mg (subcutaneously) in two simultaneous doses. They found that tocilizumab administration is associated with lower risk of invasive mechanical ventilation or death. The mortality rates of their study were 7% in patients who received intravenous tocilizumab and 8% in patients who received subcutaneous tocilizumab. Also, 13% of their patients showed a new infection after tocilizumab therapy [24].
Campochiaro et al., reported the clinical results of tocilizumab administration in 32 cases with COVID-19 who had hyper-inflammatory condition. They administered a fix dose of tocilizumab at dose of 400 mg intravenously. A second dose of 400 mg was also administered in case of respiratory worsening. The morality rate in their study was 15% and the adverse effects were seen in 25% of patients [25].
Alattar et al., studied the effects of tocilizumab administration in 25 cases of severe COVID-19 cases. They administered tocilizumab at a median dose of 5.7 mg/kg and the mortality rate was 12%. Interestingly, they found that 92% of the patients faced at least one adverse effect while only 4% of them experienced a new infection [26].
Toniati et al., also administered tocilizumab at dose of 8 mg/kg (maximum 800 mg) by two repeated intravenous infusions with 12 h interval. A third infusion, was determined an optional choice based on the clinical response. From 100 included patients, 20% died. In point of adverse effects, 2% of their patients developed septic shock and died and 1% needed urgent surgery due to gastrointestinal perforation [27].
In the current study, neurological adverse effects were detected in 3 patients. The occurrence of these adverse effects may not be attributed to tocilizumab. These adverse effects may be due to the influence of SARS-CoV-2 infection on the neurological system [28], [29]. However, neurological squeal should be considered in cases of tocilizumab administration. Furthermore, reactivation of tuberculosis is an important challenge. Although none of our included patients demonstrated a positive QuantiFERON test, it should be considered in all patients who are eligible for tocilizumab administration [7].
Interpretation of the results of this study is limited by the small size and the lack of a randomized control group. Ongoing randomized, placebo-controlled trials can help to demonstrate the exact role of tocilizumab in COVID-19. Also, it is suggested to design the studies to compare the fix doses of tocilizumab with the weight-based regimen in order to determine the optimal dosing regimen. According to the previous studies, the morality rate may be lower with weight-based regimen, while the occurrence of the adverse effects may be a big concern in this situation.
It is concluded that there may be an ideal time point for initiating tocilizumab therapy, and all efforts should be made to administer it during the early stages of SARS-CoV-2 infection before deterioration of clinical conditions. If the inflammatory cascade progresses, the beneficial effects of tocilizumab will decrease. Based on the current results, tocilizumab may be a promising agent for patients with severe or critical SARS-CoV-2 infection, if promptly initiated during the severe stage.
Funding
No funding
CRediT authorship contribution statement
Farzaneh Dastan: Conceptualization, Methodology, Investigation, Visualization, Supervision, Writing - review & editing. Ali Saffaei: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. Sara Haseli: Data curation, Investigation, Visualization, Writing - review & editing. Majid Marjani: Data curation, Investigation, Writing - review & editing. Afshin Moniri: Data curation, Investigation, Writing - review & editing. Zahra Abtahian: Data curation, Investigation, Writing - review & editing. Atefeh Abedini: Data curation, Investigation, Writing - review & editing. Arda Kiani: Data curation, Investigation, Writing - review & editing. Sharareh Seifi: Data curation, Investigation, Writing - review & editing. Hamidreza Jammati: Data curation, Visualization, Writing - review & editing. Seyed Mohammad Reza Hashemian: Data curation, Writing - review & editing. Mihan Poorabdollah: Validation, Writing - review & editing. Alireza Eslaminejad: Data curation, Writing - review & editing. Jalal Heshmatnia: Data curation, Writing - review & editing. Mohsen Sadeghi: Data curation, Writing - review & editing. Seyed Alireza Nadji: Validation, Data curation, Writing - review & editing. Alireza Dastan: Formal analysis, Writing - review & editing. Parvaneh Baghaei Shiva: Methodology, Writing - review & editing. Mohammad Varahram: Software, Data curation, Writing - review & editing. Sahar Yousefian: Software, Data curation, Writing - review & editing. Jamshid Salamzadeh: Formal analysis, Software, Investigation, Validation, Writing - review & editing. Payam Tabarsi: Conceptualization, Methodology, Investigation, Data curation, Project administration, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are thankful to the Red Crescent Organization of Republic of China for their support. Also we are thankful to the Nursing Staff and Pharmaceutical Care Department of Masih Daneshvari Hospital for their support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.106869.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dastan F., Saffaei A., Mortazavi S.M., Jamaati H., Adnani N., Roudi S.S. Continues renal replacement therapy (CRRT) with disposable hemoperfusion cartridge: a promising option for severe COVID-19. J. Glob. Antimicrob. Resist. 2020;21:340–341. doi: 10.1016/j.jgar.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol.. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheppard M., Laskou F., Stapleton P.P., Hadavi S., Dasgupta B. Tocilizumab (Actemra) Hum. Vaccines. Immunother. 2017;13:1972–1988. doi: 10.1080/21645515.2017.1316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones G., Ding C. Tocilizumab: a review of its safety and efficacy in rheumatoid arthritis. Clin. Med. Insights. Arthritis. Musculoskelet. Disord. 2010;3:81–89. doi: 10.4137/CMAMD.S4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones G., Panova E. New insights and long-term safety of tocilizumab in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018;10:195–199. doi: 10.1177/1759720X18798462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamaati H., Dastan F., Tabarsi P., Marjani M., Saffaei A., Hashemian S.M. A fourteen-day experience with coronavirus disease 2019 (COVID-19) induced acute respiratory distress syndrome (ARDS): an Iranian treatment protocol. Iran. J. Pharm. Sci. 2019;19(2020):31–36. doi: 10.22037/ijpr.2020.113337.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald J.C., Weiss S.L., Maude S.L., Barrett D.M., Lacey S.F., Melenhorst J.J. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic Leukemia. Crit. Care. Med. 2017;45:e124–e131. doi: 10.1097/ccm.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dastan F., Nadji S.A., Saffaei A., Tabarsi P. Tocilizumab administration in a refractory case of COVID-19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.106043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khafaie M.A., Rahim F. Cross-country comparison of case fatality rates of COVID-19/SARS-COV-2. Osong. Public. Health. Res. perspect. 2020;11:74–80. doi: 10.24171/j.phrp.2020.11.2.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spychalski P., Błażyńska-Spychalska A., Kobiela J. Estimating case fatality rates of COVID-19. Lancet. Infect. Dis. 2020;20:774–775. doi: 10.1016/S1473-3099(20)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C., Chen X., Cai Y., Xia J.A., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;80:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng L., Qiu H., Wan L., Ai Y., Xue Z., Guo Q. Intubation and ventilation amid the COVID-19 outbreak: Wuhan’s experience. Anesthesiology. 2020;132:1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020;382:2327–2333. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shabani M., Shokouhi S., Moradi O., Saffaei A., Sahraei Z. Tocilizumab administration in patients with SARS-CoV-2 infection: subcutaneous injection vs intravenous infusion. J. Med. Virol. 2020 doi: 10.1002/jmv.26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region - case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet. Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alattar R., Ibrahim T.B.H., Shaar S.H., Abdalla S., Shukri K., Daghfal J.N. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. 2020:1–8. doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J. Neurol. Sci. 2020;413 doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baig A.M. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS. Neurosci. Ther. 2020;26:499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.