Abstract
BACKGROUND:
The United States is in the midst of an opioid epidemic, and opioid use disorder often begins with a prescription for acute pain. The perioperative period represents an important opportunity to prevent chronic opioid use, and recently there has been a paradigm shift toward implementation of enhanced recovery after surgery (ERAS) protocols that promote opioid-free and multimodal analgesia. The objective of this study was to assess the impact of an ERAS intervention for colorectal surgery on discharge opioid prescribing practices.
METHODS:
We conducted a historical-prospective quality improvement study of an ERAS protocol implemented for patients undergoing colorectal surgery with a focus on the opioid-free and multimodal analgesia components of the pathway. We compared patients undergoing colorectal surgery 1 year before implementation (June 15, 2015, to June 14, 2016) and 1 year after implementation (June 15, 2016, to June 14, 2017).
RESULTS:
Before the ERAS intervention, opioids at discharge were not significantly increasing (1% per month; 95% confidence interval [CI], −1% to 3%; P = .199). Immediately after the ERAS intervention, opioid prescriptions were not significantly lower (13%; 95% CI, −30% to 3%; P = .110). After the intervention, the rate of opioid prescriptions at discharge did not decrease significantly 1% (95% CI, −3% to 1%) compared to the pre-period rate (P = .399). Subgroup analysis showed that in patients with a combination of low discharge pain scores, no preoperative opioid use, and low morphine milligram equivalents consumption before discharge, the rate of discharge opioid prescription was 72% (95% CI, 61%–83%).
CONCLUSIONS:
This study is the first to report discharge opioid prescribing practices in an ERAS setting. Although an ERAS intervention for colorectal surgery led to an increase in opioid free anesthesia and multimodal analgesia, we did not observe an impact on discharge opioid prescribing practices. The majority of patients were discharged with an opioid prescription, including those with a combination of low discharge pain scores, no preoperative opioid use, and low morphine milligram equivalents consumption before discharge. This observation in the setting of an ERAS pathway that promotes multimodal analgesia suggests that our findings are very likely to also be observed in non-ERAS settings and offers an opportunity to modify opioid prescribing practices on discharge after surgery. For opioid-free anesthesia and multimodal analgesia to influence the opioid epidemic, the dose and quantity of the opioids prescribed should be modified based on the information gathered by in-hospital pain scores and opioid use as well as pain history before admission.
The United States is in the midst of an opioid epidemic largely driven by the misuse and abuse of physician-prescribed opioid medications.1,2 Nearly 2 million Americans are dependent on opioids, and >4 million Americans use prescription opioids nonmedically.3 Chronic opioid use often begins with a prescription for acute pain, either in the inpatient or ambulatory care setting. Even short courses of opioids can have long-term consequences, and research suggests that patients with higher opioid consumption during an inpatient stay are more likely to report higher use of opioids after discharge4 and patients leaving the hospital with an prescription order for opioids present with an increased likelihood of long-term opioid use.2,5
Recently, there has been a paradigm shift toward enhanced recovery after surgery (ERAS) protocols under the greater auspices of the perioperative surgical home to decrease practice variability, reduce morbidity, and shorten length of stay by mitigating the stress response after surgery.6,7 Opioid-free and multimodal analgesia are key elements of ERAS programs and aim to target different pain receptors and pain transmission pathways both peripherally and centrally.7,8 The concurrent use of primarily nonopioid analgesics can have synergistic effects that optimize analgesia while simultaneously preventing adverse effects of opioid medications (nausea, vomiting, sedation, ileus, pruritus, and respiratory depression) and facilitating the achievement of important ERAS milestones such as early mobilization and return of bowel function.9,10 The overarching aim is to avoid exposure to and limit the use of opioids in the perioperative setting.
Opioid-free and multimodal analgesia techniques promoted in ERAS pathways are thus especially important in the context of the opioid epidemic.10 The purpose of this study was to assess the impact of an ERAS protocol implementation for patients undergoing colorectal surgery at a tertiary academic medical center on the incidence of opioid prescription on hospital discharge. This study presents historical prospective, comparative effectiveness data on the effect of the ERAS intervention up to 1 year after its implementation. We hypothesized that patients undergoing colorectal surgery and receiving care under the ERAS protocol and opioid-free and multimodal analgesia would be less likely to receive a discharge opioid prescription than patients undergoing similar surgeries 1 year before the intervention. Additionally, we hypothesized that patients with a combination of low discharge pain scores, no preoperative opioid use, and low morphine equivalents consumption before discharge would be less likely to receive a discharge opioid prescription.
METHODS
This study was approved by the institutional review board of the University of California, Los Angles (UCLA) (IRB#17–000160; “Enhanced recovery after surgery [ERAS] implementation in colorectal surgery and its effect on intraoperative, postoperative and long-term opioid use and postoperative complication rates”). As this was a quality improvement initiative, patient consent requirements were waived, and it is reported following the Standards for Quality Improvement Reporting Excellence guidelines.11,12 It is presented as a historical-prospective, comparative effectiveness study following the Good Research for Comparative Effectiveness principles and checklist.13,14
From June 15, 2016, to June 15, 2017, all consecutive patients undergoing colectomy (Current Procedural Terminology [CPT] codes 44140, 44150, 44160, 44204, 44205, 44207, 44210, 44211, 44212, and 45402), proctectomy (CPT codes 45119, 45395, and 45397), enterectomy (CPT codes 44120 and 44202), exploratory laparotomy and laparoscopy (CPT codes 49000 and 44238), enterostomy (CPT codes 44310, 44320, 44187, and 44188), and enterostomy closure (CPT codes 44620, 44625, 44626, and 44227) with 3 colorectal surgeons were considered targets for this study. Anesthesia providers in the Department of Anesthesiology and Perioperative Medicine at UCLA (10 core ERAS attending anesthesiologists, 1 certified nurse anesthetist ERAS champion, in addition to several noncore ERAS attending anesthesiologist and residents) participated in this study. Patients 24 hours before surgery were excluded.
Designing the Intervention
From March 2016 to June 2016, a multidisciplinary team of anesthesiologists, surgeons, perioperative nurses, and pharmacist team leaders convened to develop an ERAS protocol for colorectal surgery at the UCLA Medical Center. The group met on a weekly and monthly basis to design the clinical protocols of care and operational pathways based on the consensus guidelines for ERAS programs to ensure successful implementation of the clinical protocols.9,15–17 Pathways were developed for euglycemia, goal-directed fluid therapy, opioidfree analgesia, and lung-protective ventilation in the preoperative, intraoperative, and postoperative phases of surgical care (Figure 1). In this manuscript, we focus on the pain management and opioid use and, therefore, primarily provide details on the processes and outcomes related to these processes.
Figure 1.
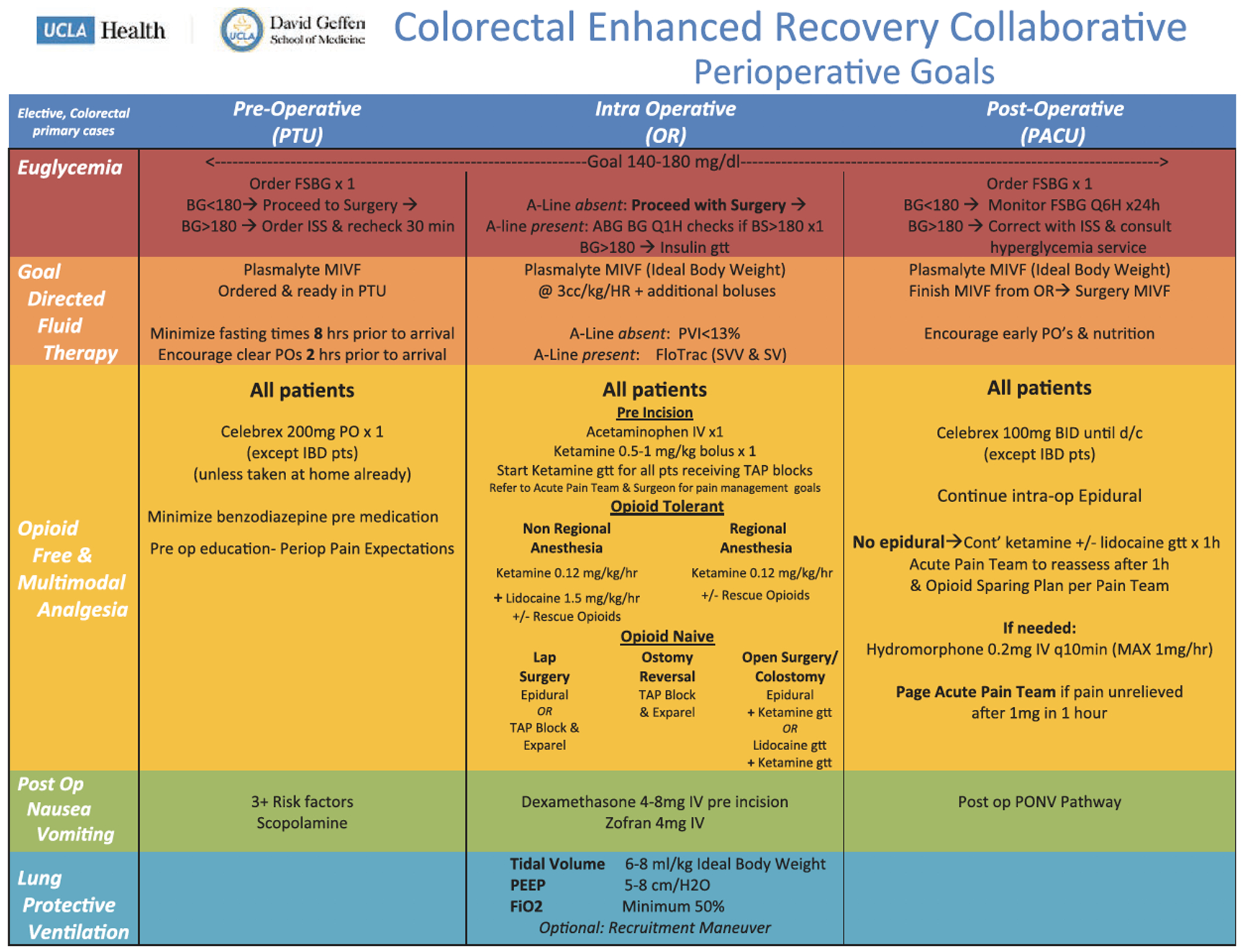
UCLA enhanced recovery after surgery anesthesia protocol. ABG indicates arterial blood gas; BG, blood glucose; BS, blood sugar; d/c, discontinue; Fio2, fraction of inspired oxygen; FSBG, fingerstick blood glucose; gtt, glucose tolerance test; HR, heart rate; IBD, inflamma- t bowel disease; ISS, Injury Severity Score; IV, intravenous; MIVF, maintenance intravenous fluids; OR, operating room; PACU, postanesthesia recovery unit; PEEP positive end-expiratory pressure; PO, oral; PONV, postoperative nausea and vomiting; PTU, procedural treatment unit; PVI, pleth variability index; Q1H, every hour; Q6H, every 6 hours; SV, stroke volume; SW, stroke volume variability; TAptransversus abdominis plane; UCLA, University of California, Los Angles.
On a weekly basis, all patients eligible for the ERAS protocol are identified and the list is distributed to the core ERAS anesthesiologists, colorectal surgery team, acute pain team, and postanesthesia recovery unit (PACU) nursing. In the preoperative period, all patients receive education regarding pain expectations with a focus on the impact of opioids on bowel function and potential for longer postoperative stay in the hospital when opioid is administered in excess. A dedicated clinical nurse ERAS project coordinator (C.L.) provides this education and also conducts patient rounding and follow-up throughout all phases of perioperative care. All patients, except patients with inflammatory bowel disease and/or renal insufficiency, receive oral celecoxib before surgery, and the use of benzodiazepine premedication is minimized at the discretion of the attending anesthesiologist.
The intraoperative opioid-free and multimodal analgesia component of the pathway was developed to discourage the use of opioid medications and promote the use of alternative therapies to optimize analgesia, minimize side effects of opioids and facilitate achievement of early mobilization, return of bowel function, and other key ERAS milestones9 (Figure 1). The Acute Pain Team plays a crucial role in the ERAS pathway and fosters a multidisciplinary collaboration between anesthesiologists and surgeons. The team consists of an attending anesthesiologist, 2 anesthesiology residents, and 2 nurse practitioners. Preoperatively, the Acute Pain Team contacts both the attending surgeon and the operating room anesthesiology team to formulate a perioperative pain management plan, which may include neuraxial block (thoracic epidural), abdominal wall block (transversus abdominis plane block), ketamine infusion, and/or lidocaine infusion.
The Acute Pain Team places all epidural catheters preoperatively and all abdominal wall blocks intraoperatively. The Acute Pain Team follows all patients in the immediate postoperative recovery period and throughout hospitalization until adequate pain control is achieved. In addition to managing epidural and intravenous (IV) infusions, the Acute Pain Team functions to promote multimodal analgesia including acetaminophen (IV in the first 24 postoperative hours followed by a transition to around-the-clock oral or rectal acetaminophen) and nonsteroidal anti-inflammatory drugs (celecoxib) are recommended for all patients without contraindications to therapy.
The Acute Pain Team assesses patients who receive a thoracic epidural catheter for primary surgical pain control on arrival to the PACU. The epidural infusion consists of bupivacaine and hydromorphone and is titrated and adjusted on at least a daily basis to avoid and minimize the need for rescue IV opioids and/or oral opioid medications. When the patient is able to tolerate an oral diet, the epidural infusion and catheter are discontinued, and patients are started on rescue opioid medications only if necessary. Patients are followed by the acute pain team until at least 1 day after epidural catheter discontinuation or until satisfactory pain control is achieved. Patients who receive an abdominal wall block and patients with a history of preoperative opioid use concomitantly receive an intraoperative low-dose ketamine infusion. Subsequently, they are evaluated in the PACU by the Acute Pain Team, and the low-dose ketamine infusion is continued postoperatively to minimize opioid consumption, if necessary. Patients who receive neither an epidural catheter nor an abdominal wall block receive an intraoperative lidocaine infusion, which is continued in the PACU and on the floor until adequate pain control is achieved.
The ERAS protocol at our institution did not provide guidelines for prescribing discharge pain medications and opioids. The choice of discharge pain medications was left to the surgical providers who ultimately discharged the patient home and who were all involved in the development of the ERAS pathway. This was the only part of the pain management that was not protocolized as part of the ERAS pathway.
Launch Period
On June 15, 2016, the ERAS program for colorectal surgery was officially launched and clinicians were expected to apply the protocol to all eligible patients undergoing colorectal surgery.
Comparison Groups
To study the impact of the opioid-free analgesia component of the ERAS intervention on postoperative outcomes, we compared patients undergoing the selected colorectal surgeries 1 year before ERAS protocol implementation (June 15, 2015, to June 14, 2016) and 1 year after implementation (June 15, 2016, to June 14, 2017).
Outcome Measurement
The Department of Anesthesiology and Perioperative Medicine at UCLA has developed and maintains a large perioperative data warehouse (PDW) that contains all clinical data entered as part of patient care from the electronic medical record (EMR; EPIC Systems, Verona, WI). We have described the development of the PDW previously.18 The PDW is a structured reporting schema that contains all the relevant clinical data entered into the EMR via the use of Clarity, the relational database created by EPIC for data analytics and reporting. An attempt was made to collect all data via the PDW, and manual collection from the EMR was utilized where acquisition from the PDW failed (Table 1).
Table 1.
Process Measures, Acronym, Definition, and Data Sources
| Data | Acronym | Definition | Source |
|---|---|---|---|
| Process measures | |||
| Preoperative oral celexocib | Celecoxib | Oral celecoxib administered in the 24 h before surgery | PDW |
| Opioid-free anesthesia | OFA | No opioid administered intraoperatively | PDW |
| Multimodal analgesia | Patient received 1 or more of the below | PDW | |
| IV ketamine infusion | Ketamine | Yes/no patient received an IV ketamine infusion | PDW |
| IV lidocaine infusion | Lidocaine | Yes/no patient received an IV lidocaine infusion | PDW |
| IV acetaminophen | Acetaminophen | Yes/no patient received IV acetaminophen | PDW |
| Thoracic epidural analgesia | Epidural | Yes/no patient received thoracic epidural analgesia | PDW |
| Transversus abdominis plane block | TAP block | Yes/no patient received a TAP block | PDW |
| Primary outcome | |||
| Discharge opioid prescription | Patient discharged from the hospital with a prescription for an opioid medication | Manual chart review | |
| Secondary outcomes | |||
| First PACU pain score (0–10) | First pain score reported in the PACU | PDW | |
| Last PACU pain score (0–10) | Last pain score reported in the PACU | PDW | |
| Highest PACU pain score (0–10) | Highest pain score reported in the PACU | PDW | |
| Postoperative methadone | Methadone | Yes/no patient received methadone after surgery | PDW |
| Patient-controlled analgesia | PCA | Patient received patient-controlled analgesia | PDW |
| Length of stay | LOS | No. of nights in the hospital after surgery | PDW |
| Highest discharge pain score | Highest pain 24h | Highest pain score in the 24 h before discharge | PDW |
| Lowest discharge pain score | Lowest pain 24h | Lowest pain score in the 24 h before discharge | PDW |
| Total morphine equivalents (mg) | Total morphine equivalents consumed IV and PO from day of surgery until hospital discharge | PDW | |
| Pre-discharge morphine equivalents (mg) | Total morphine equivalents consumed in the 24 h before discharge | PDW | |
| Postoperative methadone | Methadone | Yes/no patient received methadone after surgery | PDW |
Abbreviations: IV, intravenous; PACU, post-anesthesia care unit; PDW, perioperative data warehouse; PO, oral.
Process Measures
Several process measures were selected to assess adherence to the opioid-free analgesia component of the ERAS protocol (Table 1). These measures were selected to capture adherence in the preoperative, intraoperative, and postoperative phases of the perioperative period and included administration of preoperative oral celecoxib, opioid-free anesthesia (defined as no opioid administered intraoperatively), and utilization of multimodal analgesia intraoperatively and/or postoperatively (defined as IV ketamine and/ or IV lidocaine and/or IV acetaminophen and/or epidural analgesia and/or transversus abdominis plane block).
Outcome Measures
The primary outcome measure was presence of an opioid prescription at hospital discharge (Table 1). Secondary outcome measures included highest and lowest PACU pain scores, total morphine equivalents consumed from day of surgery until discharge from the hospital, total morphine equivalents consumed in the 24 hours before discharge, postoperative methadone consumption (yes/no), and highest and lowest pain scores in the 24 hours before discharge.
Statistical Analysis
Patient characteristics were summarized pre-/post-ERAS using means (standard deviation) for continuous variables and frequencies (%) for categorical variables and compared formally using the t test or χ2 test, respectively. Ordinal variables (eg, pain scores 0–10) were summarized using medians (quartile 1, quartile 3) and compared between groups using the Wilcoxon test. Summary statistics were reported with 95% confidence intervals (CIs) in parentheses unless otherwise noted. Forest plot thresholds for our subgroup analysis were dichotomized as follows: high pain (0–4 mild/5–10 high), preoperative use of opioids (yes/no), and high morphine equivalents consumption in the 24 hours before discharge (0–4, below median or >4, above median). Each patient was classified into one of these 8 subgroups and then the rate of opioids at discharge for each was plotted along with 95% CIs. For our primary outcome of assessing the change in opioid prescriptions as a result of ERAS, we used interrupted time series analysis (also known as segmented regression analysis) as described by Wagner et al.19 Our models included terms for the baseline trend, level change after intervention, and trend change after intervention, as well as 3 prespecified risk factors for opioid prescription including highest pain score 24 hours before discharge, preoperative opioid use, and total morphine equivalents 24 hours before discharge. Statistical summaries and figures of these models are presented. We also ran the same model for our secondary outcome of intraoperative morphine equivalent. Statistical analyses were performed using SPSS V24 (Armonk, NY) and SAS V9.4 (Cary, NC). P values <.05 were considered statistically significant.
A formal power calculation was not conducted before data collection, as we simply aimed to assess the effectiveness of the intervention 1 year before/after launch. However, given around 200 patients pre/post, and an 85% rate of opioid prescription pre-ERAS, we were adequately powered (80%) to detect a decrease of 13% (a change from 85% pre to 72% post using a χ2 test).
RESULTS
Nature of the Setting and Improvement Intervention
In the pre-implementation period, 194 patients were included from June 15, 2015, to June 14, 2016. In the post-implementation period, 189 patients were included from June 15, 2016, to June 14, 2017. Demographics of patients in the pre-implementation and post-implementation periods are presented in Table 2. Baseline characteristics of the pre-ERAS and ERAS groups were similar and not statistically significantly different. The majority of patients in both groups (56% pre-ERAS and 55% ERAS) were American Society of Anesthesiology (ASA) physical status II, undergoing primarily laparoscopic surgery (62% pre-ERAS and 62% ERAS) for a diagnosis of cancer (60% pre-ERAS and 57% ERAS). Sixty-seven percent of patients in the pre-ERAS group reported preoperative opioid use compared to 60% of ERAS patients, but the difference was not statistically significant (P = .174)
Table 2.
Patients Demographic in the Pre- and Postintervention Phases
| Pre-ERAS (194) | ERAS (189) | P-Value | Overall | Difference | 95% Confidence Intervals | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Age (y) | 54 | 16% | 54.3 | 16% | 0.858 | 54.1 | 16% | −0.3 | −3.5% to 2.9% |
| Male | 98 | 51% | 104 | 55% | 0.377 | 202 | 53% | −4% | −14% to 5% |
| ASA physical status | 0.375 | ||||||||
| I | 0 | 0% | 3 | 2% | 3 | 1% | |||
| II | 109 | 56% | 104 | 55% | 213 | 56% | |||
| III | 84 | 43% | 81 | 43% | 165 | 43% | |||
| IV | 1 | 1% | 1 | 1% | 2 | 1% | |||
| Diagnosis | 0.077 | ||||||||
| Cancer | 116 | 60% | 108 | 57% | 224 | 59% | |||
| IBD | 51 | 26% | 66 | 35% | 117 | 31% | |||
| Other | 26 | 14% | 15 | 8% | 41 | 11% | |||
| Preoperative opioid use | 129 | 67% | 113 | 60% | 0.174 | 242 | 63% | 7% | −3% to −16% |
| Laparoscopic | 120 | 62% | 117 | 62% | 0.992 | 237 | 62% | 0% | −10% to 10% |
Note: t test and 95% t interval computed for age. χ2 test and Wilson 95% confidence interval computed for categorical variables.
Abbreviations: ASA, American Society of Anesthesiologists; CI, confidence interval; ERAS, enhanced recovery after surgery; IBD, inflammatory bowel disease.
Changes in Process of Care and Patient Outcomes Associated With the Intervention
Process of Care. Although not the primary outcome, we found the utilization of opioid-free anesthesia (no opioid administered intraoperatively) increased from 17% in the pre-ERAS group to 58% in the post-ERAS group (P < .001), but this may have started increasing before the ERAS intervention took place. Therefore, we conducted an interrupted time series analysis of intraoperative opioid utilization that showed a sharp decline in intraoperative opioid use after ERAS implementation (Figure 2) as well as a significant decreasing slope during the pre- period. Significant increases in multimodal analgesia after implementation were observed for ketamine infusion (9% pre-ERAS and 57% ERAS; P < .001), IV acetaminophen (72% pre-ERAS and 87% ERAS; P < .001), neuraxial analgesia (77% pre-ERAS and 92% ERAS; P < .001), and preoperative oral celecoxib (0% pre-ERAS and 40% ERAS; P < .001) (Table 3).
Figure 2.
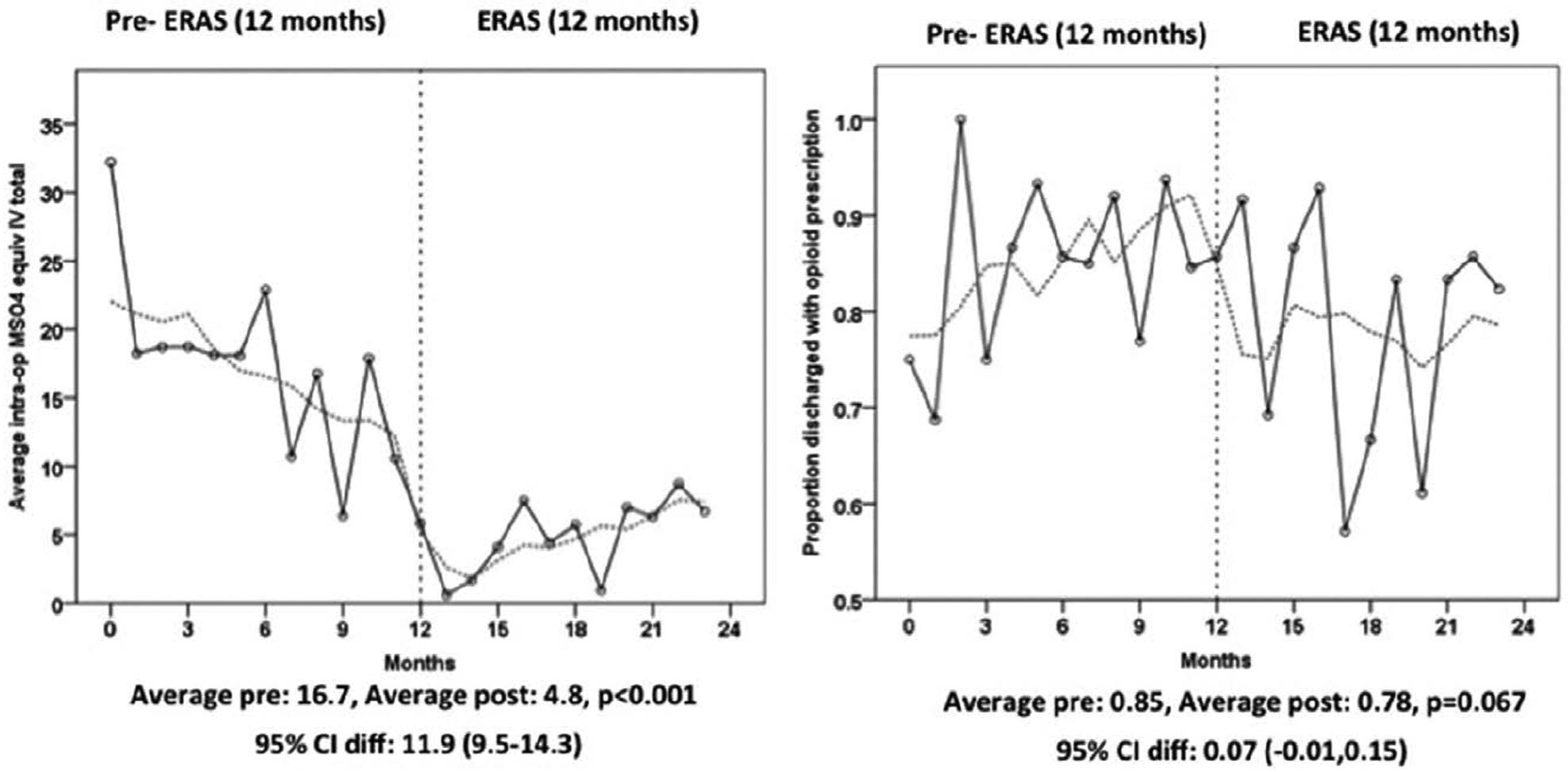
Interrupted time series plots of morphine equivalent intraoperatively and rate of opioid prescription at discharge. Cl indicates confidence interval; ERAS, enhanced recovery after surgery; IV, intravenous; MS04, morphine equivalent in milligram.
Table 3.
Process of Care for Pain Management in the Pre- and Postintervention Phases
| Pre-ERAS (194) | ERAS (189) | Difference | (95% Confidence Intervals) | P Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| OFA | 33 | 17% | 110 | 58% | −41% | (−49% to −32%) | <.001 |
| Multimodal analgesia | 188 | 97% | 189 | 100% | −3% | (−7% to −1%) | 0.03 |
| Ketamine | 17 | 9% | 107 | 57% | −48% | (−55% to −39%) | <.001 |
| Lidocaine | 1 | 1% | 3 | 2% | −1% | (−4% to 2%) | 0.367 |
| Acetaminophen | 140 | 72% | 164 | 87% | −15% | (−22% to −7%) | <.001 |
| Regional anesthesia | 150 | 77% | 173 | 92% | −14% | (−21% to −7%) | <.001 |
| Celecoxib | 0 | 0% | 75 | 40% | −40% | (−47% to −33% | <.001 |
Note: Fisher exact test and Wilson 95% CI computed.
Abbreviations: CI, confidence interval; ERAS, enhanced recovery after surgery; OFA, opioid-free analgesia.
Outcomes
The incidence of opioid prescription at hospital discharge was 82% over the entire study period and was estimated to be 85% in the pre-implementation period to 78% after ERAS implementation (difference of −7%; 95% CI, −15% to 1%). However, this decline was not statistically significant (P = .067; Table 4). Before the ERAS intervention, the monthly rate of opioids at discharge was not significantly increasing (estimated at 1% per month; 95% CI, −1% to 3%; P = .199). Immediately after the ERAS intervention, opioid prescriptions did not go down significantly 13% (95% CI, −30% to 3%; P = .110). After the intervention, the rate of opioid prescriptions did not change and the estimated difference in slopes was −1% (95% CI,−3% to 1%; P = .399) compared to the pre- period. Before the ERAS intervention, intraoperative morphine equivalents were decreasing on average by 0.95 mg per month (95% CI, −1.44 to −0.46; P < .001). Immediately after the ERAS intervention, intraoperative morphine equivalent went down an average of 11.4 units (95% CI, 9.5–14.3; P < .001). After the intervention, the rate of intraoperative morphine equivalent increased by 1.59 units compared to the pre-period (P < .001). The time series plot of presence of opioid prescription at hospital discharge for each patient is shown in Figure 2 and Table 5. Pre-ERAS patients were more likely to receive postoperative patient-controlled analgesia (21% pre-ERAS and 1% post-ERAS; P < .001). There were no differences between the groups in total postoperative morphine equivalents consumption and morphine equivalents consumption in the 24 hours before discharge. Discharge pain scores and length of stay were also similar between the pre-ERAS and ERAS groups.
Table 4.
Outcome Measure in the Pre- and Postintervention Phases
| Pre-ERAS (194) | ERAS (189) | Difference | (95% Confidence Intervals) | P Value | |||
|---|---|---|---|---|---|---|---|
| Primary outcome | N | % or range | N | % or range | |||
| Discharge opioid prescription | 165 | 85% | 147 | 78% | 7% | (−1% to 15%) | 0.067 |
| Secondary outcome | |||||||
| First PACU pain score | 0 | 0–7 | 3 | 0–7 | −0.69 | (−1.46 to 0.08) | 0.078 |
| Methadone | 2 | 1% | 2 | 1% | 0% | (−3% to 3%) | 0.979 |
| PCA | 41 | 21% | 1 | 1% | 21% | (15%–27%) | <.001 |
| Epidural | 116 | 60% | 108 | 57% | 3% | (−7% to 12%) | 0.599 |
| Total morphine equivalents (mg) | 40 | 20–81 | 27 | 10–68 | 13 | (2.4–22.0) | 0.009 |
| Pre-discharge morphine equivalents (mg) | 6 | 0–16.4 | 4 | 0–12 | 3.31 | (−1.46 to 0.08) | 0.17 |
| Highest discharge pain score | 4 | 2–6 | 4 | 2–6 | 0.03 | (−0.52 to 0.59) | 0.884 |
| Lowest discharge pain score | 0 | 0–0 | 0 | 0–0 | −0.05 | (−0.23 to 0.14) | 0.497 |
Abbreviations: CI, confidence interval; ERAS, enhanced recovery after surgery; PACU, post-anesthesia care unit; PCA, patient-controlled analgesia.
Table 5.
Interpretation of Model Terms After Controlling for Preoperative Opioid Use, Highest Pain, and MOE 24 Hours Before Discharge
| Model Terms (Discharge Opioids) | Coefficient | 95% CI | P Value |
|---|---|---|---|
| Intercept | 0.56 | (0.33–0.79) | <.001 |
| Baseline trend (per month) | 0.01 | (−0.01 to 0.03) | 0.199 |
| Level change after intervention (rate of opioid change) | −0.13 | (−0.30 to 0.03) | 0.110 |
| Trend change after intervention (monthly rate difference) | −0.01 | (−0.03 to 0.01) | 0.399 |
| Covariates included/controlled for | |||
| Preoperative use | 0.02 | (−0.06 to 0.11) | 0.555 |
| Highest pain 24h | 0.03 | (0.01–0.04) | 0.001 |
| MOE 24h prior | 0.00 | (0.00–0.00) | 0.766 |
| Model Terms (Intraoperative MOE) | Coefficient | 95% CI | P Value |
| Intercept | 9.59 | (2.63 – 16.55) | 0.007 |
| Baseline trend (per month) | −0.95 | (−1.44 to −0.46) | <.001 |
| Level change after intervention (mg MOE change) | −11.40 | (−16.39 to −6.42) | <.001 |
| Trend change after intervention (monthly MOE difference, mg) | 1.59 | (0.87–2.30) | <.001 |
| Covariates included/controlled for | |||
| Preoperative usage | 1.06 | (−1.43 to 3.54) | 0.403 |
| Highest pain 24 h | 0.21 | (−0.26 to 0.68) | 0.386 |
| MOE 24h prior | 0.06 | (0.01 – 0.12) | 0.026 |
Baseline trend: before the ERAS intervention, the rate of opioids at discharge was estimated at a rate of 0.01 (95% CI, −0.01 to 0.03) per month (P = .199).
Level change after intervention: immediately after the ERAS intervention, opioid prescriptions were estimated at a nonsignificant change of −0.13 (95% CI, −0.30 to 0.03; P = .110). No difference was found between the rate of opioid prescriptions between the pre- and postintervention periods, with estimated difference in slopes of −0.01 (−0.03 to 0.01) (P = 0.399). Baseline trend: before the ERAS intervention, intraoperative morphine equivalents were decreasing on average by 0.95 (95% CI, 0.46–1.44) mg per month (P < .001). Level change after intervention: immediately after the ERAS intervention, intraoperative morphine equivalents went down an average of 11.4 (95% CI, 6.4–16.4) mg (P < .001). Trend change after intervention: after the intervention, the rate of intraoperative morphine equivalents increased by 1.59 (95% CI, 0.87–2.30) mg compared to the pre- period rate (P < 0.001).
Abbreviations: CI, confidence interval; ERAS, enhanced recovery after surgery; MOE, morphine equivalents.
Subgroup Analysis
Subgroup analysis was then conducted and showed high rates of opioid prescription across all subgroups ranging from 75% (69%–81%) in patients with low morphine equivalents consumption in the 24 hours before discharge to 88% (84%–93%) in patients with high morphine equivalents consumption in the 24 hours before discharge. Subgroup analysis was further stratified and showed that the highest rate of discharge opioid prescription was 90% (84%–96%) in patients with a combination of high discharge pain scores, preoperative opioid use, and high morphine equivalents consumption in the 24 hours before discharge (Figure 3). In patients with a combination of low discharge pain scores, no preoperative opioid use, and low morphine equivalents consumption before discharge, the rate of discharge opioid prescription was 72% (61%–83%).
Figure 3.
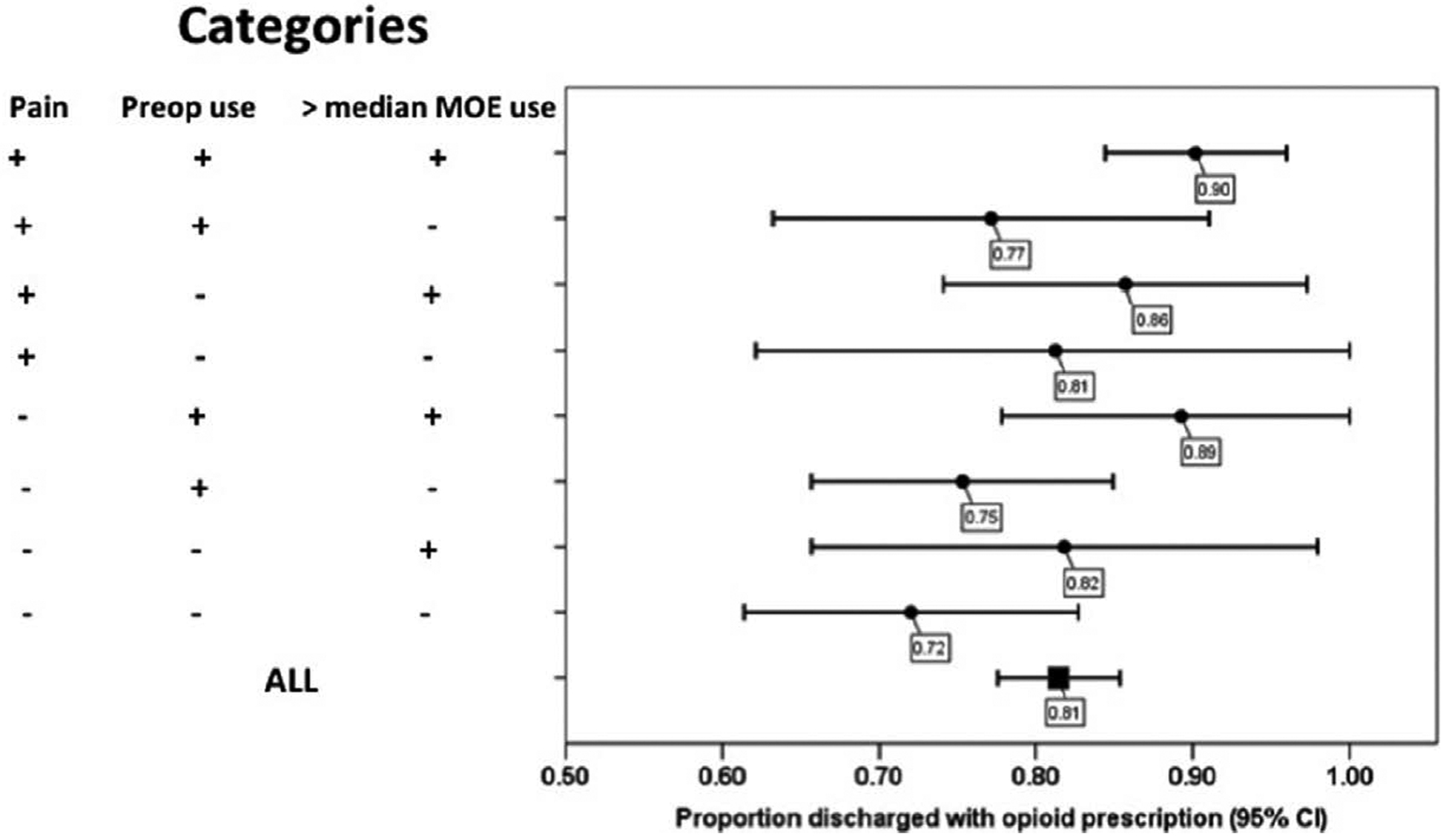
Forest plot of subgroup analysis for the incidence of opioid prescription at discharge from the hospital. Cl indicates confidence interval; MOE, morphine equivalents.
DISCUSSION
This is the first study to report discharge opioid prescribing practices in an ERAS setting. Our historical-prospective, comparative effectiveness study found that although an ERAS intervention for colorectal surgery led to an increase in opioid-free anesthesia and multimodal analgesia, there was no impact on discharge opioid prescribing practices. Over 80% of all patients in the study population were discharged with an opioid. Strikingly, 70% of patients with a combination of low discharge pain scores, no preoperative opioid use, and low morphine equivalents consumption before discharge were discharged with an opioid. These patients who should arguably never receive an opioid prescription were instead prescribed opioids at an alarmingly high rate. This presents an opportunity for altering prescription practices, as physician behavior, rather than the condition of the patient, may be the primary determinant of opioid prescribing practices in our study.
Ultimately, the opioid-free and multimodal analgesic techniques promoted in ERAS pathways will be rendered moot and unable to significantly impact the opioid epidemic if physician behavior is not modified for opioid prescription at discharge. Despite the fact that our institution’s ERAS pathway successfully protocolizes in-hospital pain management, it fails to address the crucial period immediately surrounding discharge and opioid prescriptions. We suspect that this also applies to many ERAS protocols nationally, and our observations in the setting of an ERAS environment that promotes multimodal analgesia are very likely to translate to non-ERAS environments. Physician behavior should be modified and updated to incorporate more objective patient data and practice guidelines into the clinical decision-making process of opioid prescription at discharge from the hospital after surgery. These objective data include in-hospital pain scores and morphine milligram equivalents consumption. Future studies should investigate the impact of these interventions on discharge opioid prescribing practices as well as chronic opioid use.
In the context of the national opioid epidemic, the perioperative period represents an important opportunity to prevent chronic opioid use, especially in opioid-naive patients.5 Long-term opioid use often begins with the treatment of acute pain, and having an opioid prescription increases the likelihood of long-term use, abuse, dependence, and overdose. 2,5 Patients receiving opioids within 7 days of surgery are 44% more likely to become long-term opioids users than those who do not receive opioids on discharge.20 Moreover, approximately 1 in 7 patients whose first episode of consumption is for >8 days continue to use opioids up to 1 year, and the rate of long-term use increases to 30% for patients who have a first episode of opioid use of 31 days or more.21
The strikingly high rate of opioid discharge prescriptions in this study suggests an opportunity to address the perioperative factors that may eventually contribute to chronic opioid use. Female gender and moderate-to-severe pain scores before discharge were the only 2 significant predictors of a discharge opioid prescription in this “real-life” implementation of an ERAS protocol for colorectal surgery. Prior studies have investigated the influence of sex on pain perception, and the majority have shown that women are more sensitive to experimentally induced pain as evidenced by higher ratings of pain and lower pain tolerance.22 Indeed, women in this study were also more likely to report moderate- to-severe pain than their male counterparts. Although pain scores have been validated in the literature, they require careful interpretation by health care providers.23,24 Physicians may be reluctant to not prescribe opioids even in the group of patients who likely do not require them (patients with a combination of low discharge pain scores, no preoperative opioid use, and low morphine equivalents consumption before discharge).
Physician behavior may be driving opioid prescribing practices in our study, and there are other similar examples in the literature of physician behavior profoundly impacting patient care. For example, regression analysis in a retrospective observational study of intraoperative fluid administration at 2 academic hospitals revealed that the most important predictor of fluid administration during abdominal surgery was the anesthesia provider and surgeon and that fluid administration was largely dependent on the individual provider’s habit.25,26 Similarly, a study of emergency department patients with musculoskeletal pain found that practice variation by providers was the only significant determinant of the disparities in opioid prescribing practices.27 Physicians are often accustomed to utilizing discharge order sets that make it easy to prescribe opioids to almost every patient, while there is a scarcity of order sets that can help guide physicians though discharging a patient without opioids. Finally, physicians may be concerned that the condition of the patient will change after discharge to require an opioid prescription and may feel that it is prudent to provide all patients with an opioid prescription. Further studies should impact protocolized opioid orders at discharge from the hospital on opioid prescription practices and long-term opioid use.
Limitations
The primary limitation of this study is that it is not a blinded, randomized controlled trial. In quality improvement studies, it is practically impossible to randomize and control the intervention. Moreover, it would be unethical to conduct a randomized trial and withhold ERAS treatment given the recent findings in the literature that support improved patient outcomes with ERAS protocols.9,28 In our study, there were no significant baseline differences between the control and intervention patient groups, minimizing the possibility of confounding. We also utilized multivariate regression analyses to strengthen our conclusions. Another limitation of our study is that we do not directly measure compliance to our institution’s ERAS protocol, and it is possible that effect sizes are underestimated due to decreased variability in practices between the control and intervention groups. Finally, we did not investigate rates of long-term opioid use. Although having a discharge opioid prescription has been shown to increase the likelihood of long-term opioid use,2,5 we do not report whether these prescriptions are ultimately filled by patients after discharge and if they are filled, the amount and duration of opioid consumption.
CONCLUSIONS
Although an ERAS intervention for colorectal surgery led to an increase in opioid-free anesthesia and multimodal analgesia, there was no impact on discharge opioid prescribing practices, and the majority of patients were discharged with an opioid prescription. This indicates that physician behavior, rather than the condition of the patient, is the primary determinant of opioid prescribing practices in our study and should be modified for opioid-free anesthesia and multimodal analgesia to impact the opioid epidemic.
Footnotes
DISCLOSURES
Name: Delara Brandal, MD.
Contribution: This author helped edit the manuscript and implement the study.
Conflicts of Interest: None.
Name: Michelle S. Keller, MPH.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Carol Lee, RN-BC.
Contribution: This author helped edit the manuscript, create the ERAS pathway, and implement the study.
Conflicts of Interest: None.
Name: Tristan Grogan, MS.
Contribution: This author helped edit the manuscript and analyze the statistical data.
Conflicts of Interest: None.
Name: Yohei Fujimoto, MD, PhD.
Contribution: This author helped edit the manuscript, implement the study, and analyze the data.
Conflicts of Interest: None.
Name: Yann Gricourt, MD.
Contribution: This author helped edit the manuscript, implement the study, and analyze the data.
Conflicts of Interest: None.
Name: Takashige Yamada, MD, PhD.
Contribution: This author helped edit the manuscript, implement the study, and analyze the data.
Conflicts of Interest: None.
Name: Siamak Rahman, MD.
Contribution: This author helped edit the manuscript, create the ERAS pathway, and implement the study.
Conflicts of Interest: None.
Name: Ira Hofer, MD.
Contribution: This author helped edit the manuscript, create the ERAS pathway, implement the study, and collect the data.
Conflicts of Interest: None.
Name: Kevork Kazanjian, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Jonathan Sack, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Aman Mahajan, MD, PhD.
Contribution: This author helped edit the manuscript and advise on the project.
Conflicts of Interest: None.
Name: Anne Lin, MD.
Contribution: This author helped edit the manuscript, lead the project, and create the ERAS pathway.
Conflicts of Interest: None.
Name: Maxime Cannesson, MD, PhD.
Contribution: This author helped edit the manuscript, lead the project, and create the ERAS pathway.
Conflicts of Interest: Maxime Cannesson is an editor for the Technology, Computing, and Simulation section of Anesthesia & Analgesia.
REFERENCES
- 1.Bedard NA, Pugely AJ, Westermann RW, Duchman KR, Glass NA, Callaghan JJ. Opioid use after total knee arthroplasty: trends and risk factors for prolonged use. J Arthroplasty. 2017;32:2390–2394. [DOI] [PubMed] [Google Scholar]
- 2.Kelly MA. Addressing the opioid epidemic with multimodal pain management. Am J Orthop (Belle Mead NJ). 2016;45:S6–S8. [PubMed] [Google Scholar]
- 3.Hedden S Behavioral Health Trends in the United States: Results From the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15–4927, NSDUH Series H-50). Washington, DC: Substance Abuse and Mental Health Services Administration, Department of Health and Human Services; 2015. [Google Scholar]
- 4.Bartels K, Mayes LM, Dingmann C, Bullard KJ, Hopfer CJ, Binswanger IA. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One. 2016;11:e0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naïve patients following common hand surgery procedures. J Hand Surg Am. 2016;41:947–957.e3. [DOI] [PubMed] [Google Scholar]
- 6.Sarin A, Litonius ES, Naidu R, Yost CS, Varma MG, Chen LL. Successful implementation of an enhanced recovery after surgery program shortens length of stay and improves postoperative pain, and bowel and bladder function after colorectal surgery. BMC Anesthesiol. 2016;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beverly A, Kaye AD, Ljungqvist O, Urman RD. Essential elements of multimodal analgesia in enhanced recovery after surgery (ERAS) guidelines. Anesthesiol Clin. 2017;35:e115–e143. [DOI] [PubMed] [Google Scholar]
- 8.Kehlet H Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. [DOI] [PubMed] [Google Scholar]
- 9.Feldheiser A, Aziz O, Baldini G, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60:289–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152:691–697. [DOI] [PubMed] [Google Scholar]
- 11.Davidoff F, Batalden P, Stevens D, Ogrinc G, Mooney SE; SQUIRE development group. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. BMJ. 2009;338:a3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13–i32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreyer NA. Using observational studies for comparative effectiveness: finding quality with GRACE. J Comp Eff Res. 2013;2:413–418. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer NA, Schneeweiss S, McNeil BJ, et al. ; GRACE Initiative. GRACE principles: recognizing high-quality observational studies of comparative effectiveness. Am J Manag Care. 2010;16:467–471. [PubMed] [Google Scholar]
- 15.Scott MJ, Baldini G, Fearon KC, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand. 2015;59:1212–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafsson UO, Scott MJ, Schwenk W, et al. ; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg. 2013;37:259–284. [DOI] [PubMed] [Google Scholar]
- 17.Nygren J, Thacker J, Carli F, et al. ; Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:801–816. [DOI] [PubMed] [Google Scholar]
- 18.Hofer IS, Gabel E, Pfeffer M, Mahbouba M, Mahajan A. A systematic approach to creation of a perioperative data warehouse. Anesth Analg. 2016;122:1880–1884. [DOI] [PubMed] [Google Scholar]
- 19.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- 20.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172:425–430. [DOI] [PubMed] [Google Scholar]
- 21.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use— United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain. 2006;7:151–160. [DOI] [PubMed] [Google Scholar]
- 23.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152:2399–2404. [DOI] [PubMed] [Google Scholar]
- 25.Lilot M, Ehrenfeld JM, Lee C, Harrington B, Cannesson M, Rinehart J. Variability in practice and factors predictive of total crystalloid administration during abdominal surgery: retrospective two-centre analysis. Br J Anaesth. 2015;114:767–776. [DOI] [PubMed] [Google Scholar]
- 26.Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114:717–721. [DOI] [PubMed] [Google Scholar]
- 27.Heins JK, Heins A, Grammas M, Costello M, Huang K, Mishra S. Disparities in analgesia and opioid prescribing practices for patients with musculoskeletal pain in the emergency department. J Emerg Nurs. 2006;32:219–224. [DOI] [PubMed] [Google Scholar]
- 28.Miller TE, Thacker JK, White WD, et al. ; Enhanced Recovery Study Group. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118:1052–1061. [DOI] [PubMed] [Google Scholar]


