Abstract
BACKGROUND
Refractory hypertension (RfHTN), a phenotype of antihypertensive treatment failure, is defined as uncontrolled automated office blood pressure (AOBP) ≥130/80 mm Hg and awake ambulatory blood pressure (ABP) ≥130/80 mm Hg on ≥5 antihypertensive medications, including chlorthalidone and a mineralocorticoid receptor antagonist. Previous studies suggest that RfHTN is attributable to heightened sympathetic tone. The current study tested whether reserpine, a potent sympatholytic agent, lowers blood pressure (BP) in patients with RfHTN.
METHODS
Twenty-one out of 45 consecutive patients with suspected RfHTN were determined to be fully adherent with their antihypertensive regimen. Seven patients agreed to participate in the current clinical trial with reserpine and 6 patients completed the study. Other sympatholytic medications, such as clonidine or guanfacine, were tapered and discontinued before starting reserpine. Reserpine 0.1 mg daily was administered in an open-label fashion for 4 weeks. All patients were evaluated by AOBP and 24-hour ABP at baseline and after 4 weeks of treatment.
RESULTS
Reserpine lowered mean systolic and diastolic AOBP by 29.3 ± 22.2 and 22.0 ± 15.8 mm Hg, respectively. Mean 24-hour systolic and diastolic ABPs were reduced by 21.8 ± 13.4 and 15.3 ± 9.6 mm Hg, mean awake systolic and diastolic ABPs by 23.8 ± 11.8 and 17.8 ± 9.2 mm Hg, and mean asleep systolic and diastolic ABPs by 21.5 ± 11.4 and 13.7 ± 6.4 mm Hg, respectively.
CONCLUSIONS
Reserpine, a potent sympatholytic agent, lowers BP in patients whose BP remained uncontrolled on maximal antihypertensive therapy, lending support to the hypothesis that excess sympathetic output contributes importantly to the development of RfHTN.
Keywords: blood pressure, hypertension, refractory hypertension, reserpine, sympathetic activity
Refractory hypertension (RfHTN) is a phenotype of antihypertensive treatment failure defined as uncontrolled blood pressure (BP ≥130/80 mm Hg) despite treatment with maximally tolerated doses of 5 or more different classes of antihypertensive medications, including a long-acting thiazide-like diuretic (chlorthalidone) and a mineralocorticoid receptor antagonist (MRA) such as spironolactone or eplerenone.1,2 In contrast, resistant hypertension (RHTN) is defined as uncontrolled BP on 3 or more medications, including a diuretic, while BP controlled on 4 or more medications is referred to as controlled RHTN.3 Prior studies have indicated that RfHTN is rare, comprising only about 5% of patients referred to a hypertension specialty clinic for uncontrolled RHTN.1,4,5 Patients with RfHTN are more likely to be female, African American and have higher rates of cardiovascular complications, including stroke, left ventricular hypertrophy, and congestive heart failure compared with patients with controlled RHTN.1,4,5 In contrast to patients with RHTN, in whom the prevalence of white-coat effect, defined as uncontrolled BP in clinic but controlled out-of-clinic in treated hypertensive patients is high (37–49%),6–8 patients with RfHTN have a low prevalence of a white-coat effect (6.5%).9 Antihypertensive medication adherence, however, is similar in patients with RfHTN and RHTN, with approximately 50% of both patient types adherent with prescribed medical regimens.10–13
Many studies indicate that RHTN is attributable to excess fluid retention related in large part to high dietary sodium intake and aldosterone excess.3,14,15 In contrast, indirect assessments of sympathetic tone suggest that RfHTN is attributable to heightened sympathetic output and not by persistent excess fluid retention,1,16,17 To test the hypothesis that RfHTN is neurogenic in etiology, the current proof-of-concept study was carried out to assess the effect of reserpine, a potent sympatholytic agent, on BP in patients with RfHTN whose BP remained uncontrolled in spite of being adherent with at least 5 different class of antihypertensive, including optimal diuretic treatment with use of chlorthalidone and an MRA.
METHODS
Study population
Patients referred to the UAB Hypertension Clinic for uncontrolled RHTN were recruited between July 2015 and October 2018. Patients were evaluated for secondary causes of hypertension, including hyperaldosteronism, pheochromocytoma, and renal artery stenosis as clinically indicated. Patients were eligible for enrollment if their automated office blood pressure (AOBP) remained elevated ≥130/80 mm Hg after having been seen by a hypertension specialist for a minimum of 3 follow-up visits and had confirmation of full adherence with at least 5 antihypertensive agents from different classes, including chlorthalidone and an MRA. Exclusions included patients with chronic kidney disease stage 4 or 5 (estimated glomerular filteration rate <30 ml/min/1.73 m2) or pregnancy. The study was approved by the UAB Institutional Review Board and written informed consent was obtained from all participants.
Determine of medication adherence
Antihypertensive medication adherence was detected by high-performance liquid chromatography–tandem mass spectrometry to detect antihypertensive medications and metabolites.10
Withdrawal of centrally acting agents
Before addition of reserpine, central acting α1-agonists (i.e., clonidine or guanfacine) were tapered and withdrawn in 3 patients. Other medications were adjusted to maintain and prevent further increases in the BP.
Open-label reserpine
Participants were dispensed reserpine 0.1 mg to be taken orally once daily for 4 weeks in addition to their other antihypertensive medications. Reserpine is not commercially available in the United States as pills, but is available in bulk form. The bulk drug was obtained from the manufacturer and encapsulated by a local compounding pharmacy (Double Oak Mountain Pharmacy, Birmingham, AL). The reserpine 0.1mg capsules were verified to be within United States Pharmacopeia potency guidelines confirmed by high-performance liquid chromatography analysis of a random sample of capsules with 94.2% of expected drug by an independent laboratory (ARL Bio Pharma, Oklahoma City, OK).
Blood pressure measurement
Clinic AOBP measurement
AOBP was measured after at least 5 minutes of quiet rest in a sitting position with the back supported and the arm supported at heart level using the BpTRU device, which automatically obtains 6 serial BP readings, 1 minute apart, before displaying the average of the last 5 readings.18–20 All BpTRU assessments were unattended, i.e., unobserved in clinic.19,21–24 An appropriate sized cuff was used with a cuff bladder encircling at least 80% of the arm.19,25 A BP cutoff of ≥130/80 mm Hg for hypertension was used.2,20 AOBP was measured at baseline and at 4 weeks of the reserpine treatment.
Out-of-clinic 24-hour ambulatory blood pressure monitoring
An automated, noninvasive, oscillometric device (Oscar 2; Suntech Medical, Morrisville, NC) was used to perform 24-hour ambulatory blood pressure monitoring (ABPM).26,27 Recordings were made every 20 minutes during the awake (daytime) and every 30 minutes during the nighttime (asleep) phases of the 24-hour period. Awake and asleep times were determined by patient self-report.26,27 The 24-hour ABPM was determined to be valid if ≥80% of measurements were successful, including at least 20 awake (daytime) and 7 asleep (nighttime) valid BP measurements.20,28 Uncontrolled 24-hour ABPM was defined as mean 24-hour BP ≥125/75 mm Hg, mean awake (daytime) ambulatory blood pressure (ABP) ≥130/80 mm Hg and mean asleep (nighttime) ABP ≥115/75 mm Hg.2,20 All patients were counseled to take all antihypertensive medications during the 24-hour ABPM period. ABP was measured at baseline and at end of 4 weeks of reserpine treatment.
Biochemical analysis
Serum electrolytes, blood urea nitrogen, and creatinine were measured according to routine laboratory methods at baseline and at 4 weeks of reserpine treatment period.
Electrocardiogram
An electrocardiogram was done at baseline and at 4 weeks of the reserpine treatment to monitor patients for potential bradycardia and/or conduction abnormalities.
Statistical analysis
Descriptive analyses were performed to summarize the demographic and biochemical characteristics, as well as comorbidities and adherence to antihypertensive medication classes in RfHTN. BP and heart rate (HR) were assessed at baseline and after reserpine treatment by paired T-test. All analyses were performed using SPSS version 25.
RESULTS
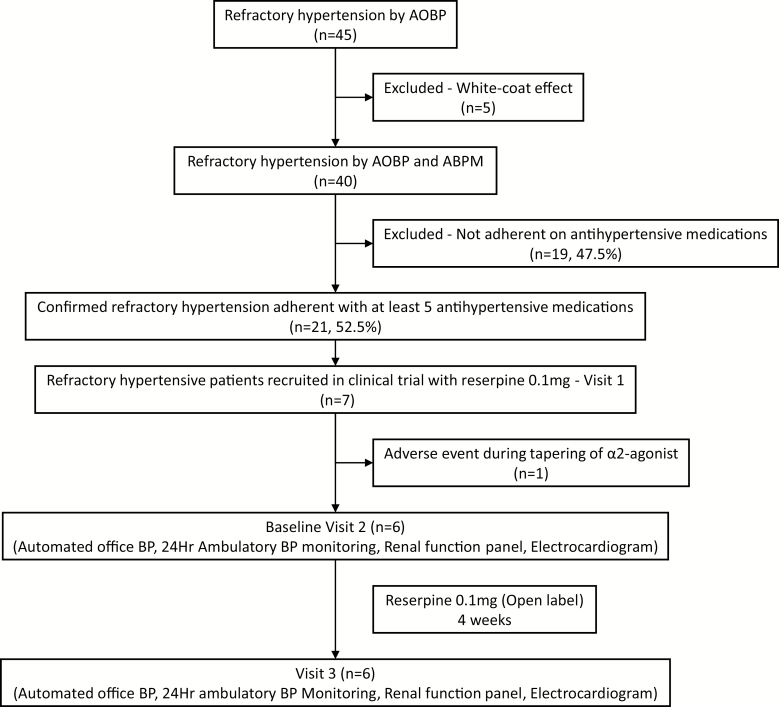
Out of 45 patients, 21 were confirmed to have true RfHTN based on AOBP, ABPM, and confirmation of full antihypertensive medication adherence by liquid chromatography–tandem mass spectrometry.10 Of these 21 patients, 7 agreed to participate in the current clinical trial. One patient had Non ST-elevation myocardial infarction during tapering of α2-agonist (clonidine) and the remaining 6 patients completed the study (Figure 1).
Figure 1.
Schematic of enrolled study participants. Abbreviations: AOBP, automated office blood pressure; BP, blood pressure.
Baseline characteristics
At baseline, the mean age of the study participants was 49.5 ± 9.4 years; 66.7% were female and 100.0% were African American. Overall, 50% of the study participants had a history of heart failure; 50% had a history of prior stroke or transient ischemic attack; 33.3% had a history diabetes; and 33.3% had dyslipidemia (Table 1).
Table 1.
Baseline characteristics in patients with refractory hypertension
| Demographics | |
| Age (y) | 49.5 ± 9.4 |
| Females | 4 (66.7%) |
| African Americans | 6 (100.0%) |
| Comorbidities | |
| Current smoker | 2 (33.3%) |
| Current alcohol | 1 (16.7%) |
| Dyslipidemia | 2 (33.3%) |
| Congestive heart failure | 3 (50.0%) |
| Coronary artery disease | 1 (16.7%) |
| Diabetes | 2 (33.3%) |
| Prior stroke/transient ischemic attack | 3 (50.0%) |
| Body mass index (kg/m2) | 31.1 ± 2.2 |
Baseline medication use
At the baseline visit, all patients were on 5 or more antihypertensive medications including an angiotensin-converting enzyme inhibitor (lisinopril or quinapril) or an angiotensin receptor blocker (losartan), a long-acting dihydropyridine calcium channel blocker (amlodipine), a long-acting thiazide or thiazide-like diuretic (chlorthalidone), and an MRA (spironolactone or eplerenone). The fifth or higher antihypertensive medications were a αβ-blocker (carvedilol or labetalol), and/or a vasodilator (hydralazine or minoxidil) and/or a α2-agonist (clonidine or guanfacine).
Blood pressure measurement
Clinic AOBP
The mean systolic and diastolic AOBPs at baseline were 161.5 ± 25.5/100.0 ± 16.2 mm Hg. The mean HR was 80.3 ± 12.3 beats/min.
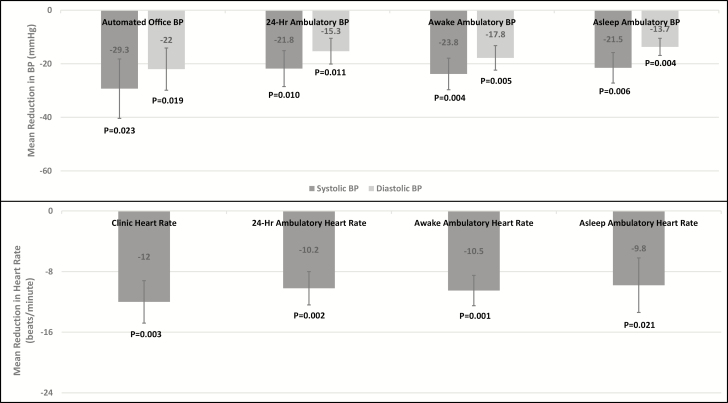
Four weeks of reserpine therapy reduced systolic AOBP by 29.3 ± 22.2 (P-value = 0.023) and diastolic AOBP by 22.0 ± 15.8 mm Hg (P-value = 0.019, Figure 2). HR was reduced by 12.0 ± 5.6 beats/min (P-value = 0.003, Figure 2).
Figure 2.
Mean reduction in automated office and ambulatory BP and heart rate after reserpine treatment. Abbreviation: BP, blood pressure.
Out-of-clinic 24-hour ABPM
At baseline, the mean 24-hour systolic and diastolic ABPs were 171.5 ± 21.3/101.8 ± 15.0 mm Hg. The mean awake (daytime) systolic and diastolic ABPs were 174.3 ± 20.1/105.8 ± 14.7 mm Hg. The mean asleep (nighttime) systolic and diastolic ABPs were 161.8 ± 22.8/90.8 ± 12.8 mm Hg. At baseline, the mean 24-hour HR was 77.3 ± 7.1 beats/min, mean awake (daytime) HR was 77.5 ± 6.9 beats/min, and asleep (nighttime) HR was 78.5 ± 7.4 beats/min (Table 2).
Table 2.
Blood pressure, heart rate and biochemistry in patients with refractory hypertension at baseline and after treatment with reserpine
| Variables | Baseline | After reserpine intervention |
|---|---|---|
| Automated office blood pressure | ||
| Systolic BP (mm Hg) | 161.5 ± 25.5 | 132.2 ± 18.9 |
| Diastolic BP (mm Hg) | 100.0 ± 16.2 | 78.0 ± 11.6 |
| Heart rate (beats/min) | 80.3 ± 12.3 | 68.3 ± 13.3 |
| 24-Hour ambulatory blood pressure | ||
| 24-Hour (overall) systolic BP (mm Hg) | 171.5 ± 21.3 | 149.7 ± 13.7 |
| 24-Hour (overall) diastolic BP (mm Hg) | 101.8 ± 15.0 | 86.5 ± 10.9 |
| 24-Hour (overall) mean arterial pressure (mm Hg) | 125.2 ± 16.3 | 107.8 ± 10.6 |
| 24-Hour (overall) heart rate (beats/min) | 77.3 ± 7.1 | 67.2 ± 5.5 |
| Awake (daytime) systolic BP (mm Hg) | 174.3 ± 20.1 | 150.5 ± 13.6 |
| Awake (daytime) diastolic BP (mm Hg) | 105.8 ± 14.7 | 88.0 ± 9.6 |
| Awake (daytime) mean arterial pressure (mm Hg) | 128.8 ± 15.4 | 108.8 ± 9.4 |
| Awake (daytime) heart rate (beats/min) | 77.5 ± 6.9 | 67.0 ± 5.9 |
| Asleep (nighttime) systolic BP (mm Hg) | 161.8 ± 22.8 | 140.3 ± 13.5 |
| Asleep (nighttime) diastolic BP (mm Hg) | 90.8 ± 12.8 | 77.2 ± 9.6 |
| Asleep (nighttime) mean arterial pressure (mm Hg) | 114.5 ± 15.2 | 98.3 ± 9.8 |
| Asleep (nighttime) heart rate (beats/min) | 78.5 ± 7.4 | 68.7 ± 6.0 |
| Biochemistry | ||
| Sodium (mmol/l) | 138.0 ± 4.7 | 138.6 ± 0.8 |
| Potassium (mmol/l) | 4.4 ± 0.4 | 4.3 ± 0.4 |
| Bicarbonate (mmol/l) | 24.5 ± 3.3 | 23.8 ± 1.7 |
| Blood urea nitrogen (mg/dl) | 17.6 ± 7.2 | 16.4 ± 10.2 |
| Creatinine (mg/dl) | 1.1 ± 0.2 | 1.1 ± 0.3 |
Abbreviation: BP, blood pressure.
Twenty-four hour systolic and diastolic ABPs were reduced by 21.8 ± 13.4 (P-value = 0.010) and 15.3 ± 9.6 mm Hg, respectively (P-value = 0.011). Awake (daytime) systolic and diastolic ABPs were reduced by 23.8 ± 11.8 (P-value = 0.004) and 17.8 ± 9.2 mm Hg, respectively (P-value = 0.005). Asleep (nighttime) systolic and diastolic ABPs were reduced by 21.5 ± 11.4 (P-value = 0.006) and 13.7 ± 6.4 mm Hg, respectively (P-value = 0.004, Figure 2). There was a significant reduction in 24-hour HR of 10.2 ± 4.4 beats/min (P-value = 0.002), awake HR of 10.5 ± 4.0 beats/min (P-value = 0.001), and asleep HR of 9.8 ± 7.2 beats/min (P-value = 0.021, Figure 2).
Adverse effects with reserpine
No participant reported any adverse effect with use of reserpine during the 4-week treatment period.
Biochemical analysis
There was no significant change in serum potassium, sodium, or creatinine with reserpine use (Table 2).
Electrocardiogram
There was no evidence of atrioventricular conduction delays or bradycardia during the reserpine treatment period.
DISCUSSION
RHTN is a well-characterized phenotype of difficult-to-treat hypertension that occur most commonly in older, or obese person, or African Americans, and those with chronic kidney disease, or diabetes. A large body literature, including the PATHWAY-2 main study and substudy, has shown that RHTN is largely attributable to excess intravascular fluid retention, i.e., is volume dependent, and that the inappropriate fluid retention is in large part secondary to high dietary sodium intake and aldosterone excess.29 Accordingly, after failure to control BP on a triple combination of an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker, a long-acting dihydropyridine calcium channel blocker, and a long-acting thiazide or thiazide-like diuretic, the most effective agent for treatment of RHTN has been shown to be an MRA, specifically, spironolactone. In contrast, RfHTN is an extreme phenotype of antihypertensive treatment failure, in which BP remains uncontrolled despite use of 5 or more antihypertensive agents of difficult classes, including a thiazide-like diuretic and an MRA. Risk factors for RfHTN overlap with those for RHTN, including obesity and African American race, with some studies reporting that patients with RfHTN tend to younger and more often female than patients with controlled RHTN.1,4,5 Patients with RfHTN are at higher cardiovascular risk than those with controlled or uncontrolled RHTN, with cross-sectional studies indicating higher rates of prevalent left ventricular hypertrophy, chronic kidney disease, congestive heart failure (especially with preserved left ventricular ejection fraction), and stroke.1,4,5
Importantly, previous studies have indicated that, unlike RHTN that is largely volume dependent in etiology, heightened sympathetic output may underlie the antihypertensive treatment failure that characterizes RfHTN.1,3,14,15,17 In the setting of treatment with chlorthalidone, an MRA and to at least 3 other classes of antihypertensive agents, indices of intravascular volume have been shown not to differ between patients with RfTHN vs. those with controlled RHTN.16 Specifically, indices of intravascular volume, including plasma renin activity, natriuretic peptide levels, intracardiac volumes, and thoracic impedance were the same or even lower in patients with RfHTN vs. patients with controlled RHTN.1,16 However, in these comparisons, patients with RfHTN had higher indices of sympathetic tone, including increased peripheral vascular resistance, HR variability, 24-hour urinary norepinephrine levels, and daytime and nighttime HR. Elevated HR values have been consistent finding in patients with RfHTN.1
The current study, in a proof-of-concept design, was conducted to directly test the hypothesis that patients with confirmed RfHTN have heightened sympathetic tone underlying their treatment failure. The enrolled patients were determined to be truly refractory to antihypertensive treatment based elevated office and ambulatory BP levels in spite of use of 5 classes of antihypertensive agents, including chlorthalidone and an MRA in maximally tolerated doses. Adherence to antihypertensive medications was confirmed by direct measurement of urinary drug and drug metabolite levels by liquid chromatography–tandem mass spectrometry.10 In this setting, reserpine 0.1 mg once daily substantially lowered both clinic and ambulatory BP. The mean reduction in 24-hour systolic and diastolic BP was 21.8 ± 13.4/15.3 ± 9.6 mm Hg. One patient had an extreme 24-hour systolic and diastolic ABP reduction of 47/33 mm Hg, but even after excluding her from the analysis, the mean reduction in the 24-hour ABP of the other 5 participants remained significant (mean reduction of 24-hour systolic ABP was 16.8 ± 5.7, P-value = 0.003 and diastolic ABP was 11.8 ± 4.4 mm Hg, P-value = 0.004).
The current results provide important preliminary evidence that antihypertensive treatment failure in the presence of appropriately dosed diuretic therapy, including chlorthalidone and an MRA is attributable, at least in part, to heightened sympathetic output. Failure of regimens that include effective diuretic therapy, i.e., a long-acting thiazide diuretic and an MRA is likely an important discriminating factor; by failing generally effective antihypertensive regimens, including specifically blocking aldosterone, volume dependent causes of treatment resistance have likely been overcome, leaving other undertreated etiologies of treatment resistance, which the current and prior findings suggest may be in large part sympathetic hyperactivity. If so, it is hypothesized that these patients may preferentially benefit from effective sympatholytic therapies, such as long-acting and well-tolerated medications or device-based treatments.
The current findings are clearly preliminary given the small cohort size and open-label, uncontrolled study design. Larger, blinded, and controlled trials will be needed to more rigorously test this hypothesis. Such assessments will likely need to be multicenter in design given the low prevalence of true RfHTN and the fact that such patients tend to present with serious comorbidities related to their uncontrolled and often severe hypertension that may preclude participation in a clinical trial.
Reserpine, a potent sympatholytic agent, substantially lowers BP in patients whose BP remained uncontrolled on maximal antihypertensive therapy, lending support to the hypothesis that excess sympathetic output contributes importantly to the development of RfHTN.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
Supplementary Figure 1. Automated office and ambulatory BP and heart rate at baseline and after reserpine treatment.
Supplementary Figure 2. Individual drop in automated office and ambulatory BP and heart rate after reserpine treatment.
FUNDING
The National Institutes of Health (NIH R01 HL113004 and 2T32HL007457-36A1) and the American Heart Association (AHA 15SFRN239002) supported this research.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Dudenbostel T, Acelajado MC, Pisoni R, Li P, Oparil S, Calhoun DA. Refractory hypertension: evidence of heightened sympathetic activity as a cause of antihypertensive treatment failure. Hypertension 2015; 66:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 3. Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council . Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension 2018; 72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Acelajado MC, Pisoni R, Dudenbostel T, Dell’Italia LJ, Cartmill F, Zhang B, Cofield SS, Oparil S, Calhoun DA. Refractory hypertension: definition, prevalence, and patient characteristics. J Clin Hypertens (Greenwich) 2012; 14:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calhoun DA, Booth JN 3rd, Oparil S, Irvin MR, Shimbo D, Lackland DT, Howard G, Safford MM, Muntner P. Refractory hypertension: determination of prevalence, risk factors, and comorbidities in a large, population-based cohort. Hypertension 2014; 63:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension 2011; 57(5):898–902. [DOI] [PubMed] [Google Scholar]
- 7. Muxfeldt ES, Bloch KV, Nogueira Ada R, Salles GF. True resistant hypertension: is it possible to be recognized in the office? Am J Hypertens 2005; 18:1534–1540. [DOI] [PubMed] [Google Scholar]
- 8. Modolo R, Ruggeri Barbaro N, de Faria AP, Rodrigues Sabbatini A, Paganelli MO, Fontana V, Moreno H. The white-coat effect is an independent predictor of myocardial ischemia in resistant hypertension. Blood Press 2014; 23:276–280. [DOI] [PubMed] [Google Scholar]
- 9. Siddiqui M, Judd EK, Oparil S, Calhoun DA. White-coat effect is uncommon in patients with refractory hypertension. Hypertension 2017; 70:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siddiqui M, Judd EK, Dudenbostel T, Gupta P, Tomaszewski M, Patel P, Oparil S, Calhoun DA. Antihypertensive medication adherence and confirmation of true refractory hypertension. Hypertension 2020; 75(2): 510– 515. doi: 10.1161/HYPERTENSIONAHA.119.14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grigoryan L, Pavlik VN, Hyman DJ. Characteristics, drug combinations and dosages of primary care patients with uncontrolled ambulatory blood pressure and high medication adherence. J Am Soc Hypertens 2013; 7:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, Toennes SW. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 2013; 31:766–774. [DOI] [PubMed] [Google Scholar]
- 13. Strauch B, Petrák O, Zelinka T, Rosa J, Somlóová Z, Indra T, Chytil L, Marešová V, Kurcová I, Holaj R, Wichterle D, Widimský J Jr. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. J Hypertens 2013; 31:2455–2461. [DOI] [PubMed] [Google Scholar]
- 14. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896. [DOI] [PubMed] [Google Scholar]
- 15. Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 2007; 131:453–459. [DOI] [PubMed] [Google Scholar]
- 16. Velasco A, Siddiqui M, Kreps E, Kolakalapudi P, Dudenbostel T, Arora G, Judd EK, Prabhu SD, Lloyd SG, Oparil S, Calhoun DA. Refractory hypertension is not attributable to intravascular fluid retention as determined by intracardiac volumes. Hypertension 2018; 72:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Navarro-Soriano C, Martínez-García MA, Torres G, Barbé F, Caballero-Eraso C, Lloberes P, Cambriles TD, Somoza M, Masa JF, González M, Mañas E, de la Peña M, García-Río F, Montserrat JM, Muriel A, Oscullo G, Olmos LF, García-Ortega A, Calhoun D, Campos-Rodriguez F; Spanish Sleep Network . Factors associated with the changes from a resistant to a refractory phenotype in hypertensive patients: a Pragmatic Longitudinal Study. Hypertens Res 2019; 42:1708–1715. [DOI] [PubMed] [Google Scholar]
- 18. Terént A, Breig-Asberg E. Epidemiological perspective of body position and arm level in blood pressure measurement. Blood Press 1994; 3:156–163. [DOI] [PubMed] [Google Scholar]
- 19. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716. [DOI] [PubMed] [Google Scholar]
- 20. Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB, Wright JT Jr. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension 2019; 73:e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord 2005; 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wright JM, Mattu GS, Perry TL Jr, Gelferc ME, Strange KD, Zorn A, Chen Y. Validation of a new algorithm for the BPM-100 electronic oscillometric office blood pressure monitor. Blood Press Monit 2001; 6:161–165. [DOI] [PubMed] [Google Scholar]
- 23. Mattu GS, Heran BS, Wright JM. Overall accuracy of the BpTRU—an automated electronic blood pressure device. Blood Press Monit 2004; 9:47–52. [DOI] [PubMed] [Google Scholar]
- 24. Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42. [DOI] [PubMed] [Google Scholar]
- 25. Manning DM, Kuchirka C, Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation 1983; 68:763–766. [DOI] [PubMed] [Google Scholar]
- 26. O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring . European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 27. Pickering T. Recommendations for the use of home (self) and ambulatory blood pressure monitoring. American Society of Hypertension Ad Hoc Panel. Am J Hypertens 1996; 9:1–11. [DOI] [PubMed] [Google Scholar]
- 28. de la Sierra A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, de la Cruz JJ, Sobrino J, Llisterri JL, Alonso J, Vinyoles E, Pallares V, Sarria A, Aranda P, Ruilope LM; Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry I . Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension 2009; 53:466–472. [DOI] [PubMed] [Google Scholar]
- 29. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society’s PATHWAY Studies Group . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.