Abstract
Antimalaria drugs such as chloroquine (CQ) and hydroxychloroquine (HCQ) have been administered to several inflammatory diseases including rheumatoid arthritis and systemic lupus erythematosus, and infectious diseases such as acquired immune deficiency syndrome and influenza. Recently, several patients infected with novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were given HCQ, and showed a discrepant response. HCQ inhibits SARS-CoV-2 cell entry, and inflammatory cascade by interfering with lysosomal and endosomal activities, and autophagy, impeding virus-membrane fusion, and inhibiting cytokine production resulted from inflammatory pathways activation. Despite ongoing administration of HCQ in a wide spectrum of disorders, there are some reports about several side effects, especially retinopathy in some patients treated with HCQ. Cytochrome P450 (CYP450) and its isoforms are the main metabolizers of HCQ and CQ. Pharmacokinetic properties of CYP enzymes are influenced by CYP polymorphism, non-coding RNAs, and epigenetic mechanisms such as DNA methylation, and histone acetylation. Accumulating evidence about side effects of HCQ in some patients raise the possibility that different response of patients to HCQ might be due to difference in their genome. Therefore, CYP450 genotyping especially for CYP2D6 might be helpful to refine HCQ dosage. Also, regular control of retina should be considered for patients under HCQ treatment. The major focus of the present review is to discuss about the pharmacokinetic and pharmacodynamic properties of CQ and HCQ that may be influenced by epigenetic mechanisms, and consequently cause several side effects especially retinopathy during SARS-CoV-2 therapy.
Keywords: Hydroxychloroquine, SARS-CoV-2, Cytochrome P450 (CYP), Pharmacogenetics, Epigenetics, Retinopathy
1. Introduction
Potential antimalarial drugs such as chloroquine (CQ) and hydroxychloroquine (HCQ) have been long used to prophylaxis and treatment of malaria (Warhurst et al., 2003). Although, administration of CQ and HCQ have extended to several inflammatory diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and against some viruses including human immunodeficiency virus (HIV), and influenza (Chiang et al., 1996; Ooi et al., 2006). The current pandemy of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused a high morbidity and mortality with 17, 189, 755 confirmed cases and 670,256 deaths until July 30, 2020 (worldometers, 2020). Recently several studies suggested that HCQ could be an effective agent to decrease the viral load, and ameliorate clinical manifestations of patients infected with SARS-CoV-2 (Chen et al., 2020a, Chen et al., 2020b; Gautret et al., 2020). Even though, clinical trial studies of HCQ in patients with SARS-CoV-2 showed inconsistent results about the effect of HCQ on decreasing patients' infection (Chen et al., 2020a, Chen et al., 2020b; Gautret et al., 2020). There is some evidence that administration of HCQ gives rise to retinopathy in some patients affected with autoimmune diseases such as RA and SLE (Kobak and Deveci, 2010; Lee et al., 2015). It's noteworthy that several risk factors including duration of use, exceeding of recommended dose (>5 mg/kg per day (400 mg in an 80 Kg person)), and pre-existing retinal disease have been identified for ocular toxicity in patients who were under HCQ therapy. Correspondingly, there is also evidence that prolonged use of HCQ increases the risk of ocular toxicity, and high doses could lead to rapid changes in vision and consequently retinopathy development. The much higher doses of HCQ used in combat against viral infections such as SARS-CoV-2 (600 or 1000 mg daily dose) in comparison with its recommended doses for treatment of RA and SLE (200–400 mg/day) have raised the possibility of more remarkable retinopathy progression in SARS-CoV-2 patients under HCQ therapy (Mahase, 2020; Gautret et al., 2020; Pereira, 2020, reference.medscape, 2020). Furthermore, growing body of evidence demonstrated that several factors including CYP450 single nucleotide polymorphisms (SNPs), and epigenetic molecules such as non-coding RNAs (ncRNAs), DNA methylation and histone acetylation influenced the expression levels of CYP450, and consequently might influence HCQ metabolism. (Li et al., 2015; Zeng et al., 2017; Wang et al., 2019). The major purpose of this review is to discuss the pharmacokinetic and pharmacodynamic characteristics of CQ and HCQ that may be influenced by epigenetic mechanisms including ncRNAs and CYP2D6 SNPs, and thereby cause several side effects such as cardiotoxicity, prolonged QT interval, gastrointestinal problems (like dyspepsia and abdominal cramps), central nervous system or skin disorders, and especially retinopathy. Also, we review the advantage of personalized medicine to determining HCQ dosage regarding the unique genotype of patients for SARS-CoV-2 treatment.
2. History and pharmacokinetic characterization of hydroxychloroquine and chloroquine
CQ and HCQ have been long recognized as antimalarial drugs (Ben-Zvi et al., 2012). Administration of these drugs rapidly developed in management of several disorders including RA and SLE due to their rapid absorption, low cost, and immunomodulatory properties (Rainsford et al., 2015). Both drugs are appropriately distributed to aqueous cellular and intercellular compartments after oral administration, and have a prolonged residence time (one month in average) (Augustijns et al., 1992; Schrezenmeier and Dörner, 2020). Table 1 summarizes the pharmacokinetic of these drugs. HCQ similar to CQ is absorbed by upper intestinal tract cells, and its renal clearance is 2.5 times less than CQ (HCQ: 21% versus CQ: 51%). The apparent volumes of distribution of HCQ are 47,257 L (calculated from plasma) and 5500 L (calculated from blood), respectively which would indicate that HCQ accumulates in other compartments. CQ has an apparent volume of distribution equal to 65,000 L (calculated from plasma), and 15,000 L (calculated from blood) (Cutler et al., 1988; Tett et al., 1990). Also, un-metabolized excretion of HCQ (62%) is approximately similar to CQ (58%) (Collins et al., 2018; Cutler et al., 1988). Accumulating evidence revealed that HCQ similar to CQ is excreted into breast milk, and moves across the placenta to exert its pharmacokinetic effects on fetus (Law et al., 2008; Motta et al., 2005). Recently, HCQ was mostly applied to treat a wide spectrum of disorders including connective tissue diseases, and autoimmune disorders in comparison with CQ due to lower toxicity, and side effects (Liu et al., 2020a, Liu et al., 2020b; Stokkermans and Trichonas, 2020). Previous studies on animal models revealed that HCQ may be distributed in a wide spectrum of tissues including adrenal, spleen, lung, liver, kidney, heart, retina-choroid, skin, pituitary, muscle, brain, fat, and bone (McChesney, 1983; McChesney et al., 1967).
Table 1.
Pharmacokinetic of hydroxychloroquine and chloroquine.
| Pharmacokinetic features | HCQ | CQ |
|---|---|---|
| Absorption Locations | Upper intestinal tract | Upper intestinal tract |
| Renal Clearance | 21% | 51% |
| Plasma (VD) | 47,257 L | 65,000 L |
| Blood (VD) | 5500 L | 15,000 L |
| Excretion | 62% | 58% |
| Breast milk Excretion | Yes | Yes |
| Transport from placenta | Yes | Yes |
HCQ: hydroxychloroquine; CQ: chloroquine.
3. Mechanism of action of hydroxychloroquine
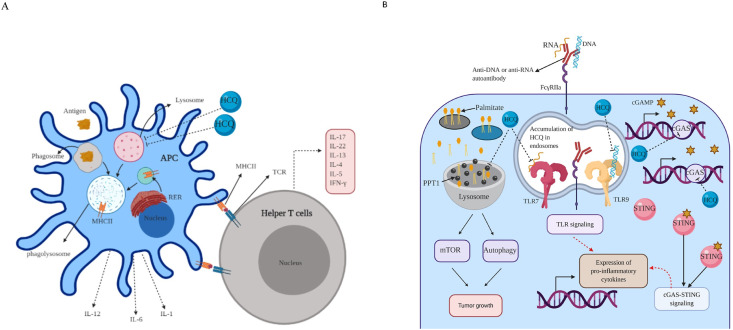
Several studies conducted on pharmacodynamic of HCQ proposed a multi-pathway model for HCQ effects (Fox, 1996; Kyburz et al., 2006). Obtained results showed that the major mechanism of HCQ action is modulation (elevation) of cellular pH (newsweek, 2020; Fox, 1996). HCQ properly passes through the lysosomal membrane, and inhibits several processes including protein degradation, antigen presentation, and toll- like receptor (TLR) signalling by increasing the pH of lysosome and endosome from 4 to 6 (Fox, 1996; Kyburz et al., 2006). Elevated levels of pH in lysosome lead to metabolites deposition, and impediment of antigen presentation mediated by major histocompatibility complex (MHC) class II through lysosomal acidic hydrolysis blockage (Fox, 1996; Schrezenmeier and Dörner, 2020). Inhibition of antigen presenting cells including dendritic cells, macrophages, and B cells prevents the process of T cells activation mediated by MHC (Fig. 1 A) (Ireland and Unanue, 2011). Several studies reported that lysosomal enzymes blockage prolongates the degradation of macromolecules in lysosome, and thereby progressively converts them into lipofuscin (Ding et al., 2016; Sundelin and andTerman, 2002). Lipofuscins are brown-yellow accumulated metabolites such as inclusions which appear gradually with age (Terman and Brunk, 2004). On the other hand, HCQ prevents from autoantigen presentation mediated by MHC II on antigen presenting cells due to obstruction of autoantigenic proteins degradation (Yoon et al., 2013). Furthermore, HCQ exerts its anti inflammatory effects via increasing the pH levels of endosome which is essential in TLR signaling in innate immunity (Thwaites et al., 2014). Also, HCQ could inhibit the innate immunity by binding to TLR ligands including DNA and single strand RNA (ssRNA) which interact with TLR9 and TLR7, respectively (Schrezenmeier and Dörner, 2020; Thwaites et al., 2014). Strinkingly, several recent studies suggested that HCQ acts as an anti inflammatory agent through disruption in cyclic GMP-AMP (cGAMP) synthase (cGAS) binding with its ligand (cytosolic DNA) (An et al, 2015, 2018). Activated cGAS by binding to cytosolic DNA leads to production and binding of cGAMP to stimulator of interferon genes (STING), and thereby production of several cytokines especially type I interferons (An et al., 2016; Zhang et al., 2014). Recent findings revealed that palmitoyl-protein thioesterase 1 (PPT1) that is localized in lysosome, is a central target of HCQ to modulating of pH (Rebecca et al., 2019). PPT1 enzyme cleaves and removes long-chain fatty acids called palmitate from proteins that are no longer required. PPT1 facilitates tumor growth via two downstream pathways including mTOR and autophagy (Rebecca et al., 2017). Increasing body of evidence illustrated that HCQ potentially prevents tumor growth and inflammatory pathways (e.g RA) via inhibiting PPT1 (Fig. 1B) (Cook et al., 2014; Schrezenmeier and Dörner, 2020). Recently, organic anion transporter family member 1A2 (OATP1A2) was identified as a novel target of HCQ (Xu et al., 2016). OATP1A2, is a solute carrier transporter, expressed in a wide spectrum of tissues such as brain, liver, breast, testis, lung, kidney, and in retinal pigment epithelial (RPE) cells to regulate the transport and uptake of multiple substrates especially drugs and xenobiotics (Lee et al., 2005). Collectively, HCQ by influencing multiple pathways supresses inflammation in several inflammatory diseases, infectious diseases, cancers, and recently SARS-CoV-2 infection.
Fig. 1.
Mechanism of hydroxychloquine action.
A: HCQ inhibits the innate and adaptive immune system via preventing T cells antigen presentation mediated by MHC II on APC; B: hydroxychloroquine (HCQ) enters and accumulates in lysosomes and endosomes, and causes an increase of their pH. HCQ potentially inhibits tumor growth by inhibiting PPT1 (resided in lysosome) and its downstream pathways. Also, HCQ prevents the expression of pro-inflammatory cytokines by inhibiting toll-like receptors (TLR) and cyclic GMP-AMP (cGAMP) synthase (cGAS)–STING signaling; IL: interleukin; TCR: T cell receptor; RER: rough endoplasmic reticulum; IFNγ: Interferon gamma; PPT1: palmitoyl-protein thioesterase 1; APCs: antigen presenting cells; MHC II: major histocompatibility complex II.
4. Role of hydroxychloroquine and chloroquine in treating SARS-CoV-2
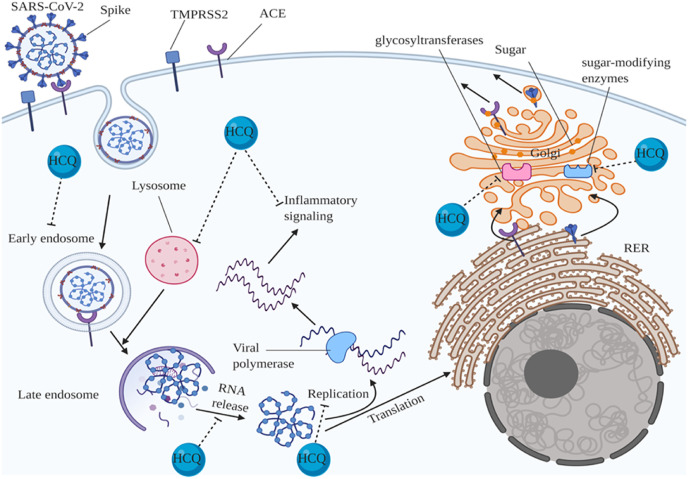
It is becoming increasingly evident that HCQ may probably be a potential agent to combat SARS-CoV-2 (Liu et al., 2020a, Liu et al., 2020b). Previously, several studies revealed the antiviral effects of HCQ against some viruses such as HIV and influenza (Chiang et al., 1996; Ooi et al., 2006). Recently, various studies demonstrated that HCQ prevents SARS-CoV-2 from cell entry via several processes (Fig. 2 ). HCQ interferes with enzymes involved in glycosylation and activation of angiotensin converting enzyme (ACE) including glycosyltransferases and sugar-modifying enzymes, and consequently blocks virus-membrane fusion. Also, HCQ interferes with spike (S) protein glycosylation, and disrupts endosome-mediated virus cell fusion. Moreover, HCQ impedes S protein cleavage which is a key point for cell fusion of SARS-CoV-2 by increasing lysosomal pH levels, and inhibiting its proteases (Gautret et al., 2020; Millet and Whittaker, 2015; Vincent et al., 2005; Yao et al., 2020). Also, recent in vitro study showed that HCQ and CQ could decrease the viral replication in a concentration-dependent manner. Moreover, HCQ showed a higher safety and anti-SARS-CoV-2 efficacy relative to CQ (Yao et al., 2020). HCQ may also prevent from SARS-CoV-2 spreading after cell entry through elevating ‘endosomal and lysosomal pH, and thereby preventing from activation of inflammation cascades (Srivatsan Padmanabhan, 2020; Vincent et al., 2005). Furthermore, pseudovirion colocalization with Niemann-Pick C1 (NPC1)-positive endolysosomes (LE/Lys) disclosed that HCQ could exert its antiviral effects through disturbance in SARS-CoV-2 replication (Srivatsan Padmanabhan, 2020). Intriguingly, a study performed on HCQ mechanism of action illustrated that HCQ inhibits viral genome release by changing the number, size, and morphology of early endosomes or endolysosomes (Liu et al., 2020a, Liu et al., 2020b; Mingo et al., 2015). Furthermore, HCQ could diminish inflammation responses through several pathways including inhibiting MHC II-mediated T cell activation, inhibiting the release of cytokines such as IL-1, IL-6, TNF-α induced by T-cell activation, blockage of TLR signaling, stimulation of interferon genes (the STING pathway) by cGAS and thereby inhibiting cytokines release including I interferon, IL-1 and TNF-α induced by TLR and STING signaling (Fig. 1A and B) (Fox, 1996; Schrezenmeier and Dörner, 2020; Ireland and Unanue, 2011; Thwaites et al., 2014; An et al., 2015). Therefore, anti-inflammatory mechanism of HCQ action created this hypothesis that it might be able to halt cytokine release syndrome, and reduce consequently organ damage especially lung, induced by COVID-19. There are some reports about the remarkable association between cytokine storm especially IL-6 and clinical and pathological manifestations of patients with SARS-CoV-2, serum SARS-CoV-2 viral load (RNAaemia), and mechanical ventilation requirement (Chen et al., 2020a, Chen et al., 2020b; Gao et al., 2020; Herold et al., 2020; Liu et al., 2020a, Liu et al., 2020b). Collectively, HCQ could exert its anti-COVID-19 effects via impeding virus' cell entry and post cell entry events such as viral replication, and inflammatory responses.
Fig. 2.
Hydroxychloroquine inhibits SARS-CoVs' cell entry.
HCQ inhibits cell entry of SARS-CoV-2 via several processes including blockage of glycosylation and of activation of angiotensin converting enzyme (ACE) and spike (S) protein mediated by glycosyltransferases and sugar-modifying enzymes, preventing endosome-mediated cell fusion of SARS-CoV-2, inhibiting S protein cleavage by disrupting lysosome acidic condition, preventing inflammatory signaling activation, impeding SARS-CoV-2 replication, and preventing viral genome release by interfering with early endosomes (EEs) or endolysosomes (ELs); TMPRSS2: transmembrane protease serine protease 2; RER: rough endoplasmic reticulum.
5. Cytochrome P450 is a key modulator in hydroxychloroquine metabolism: implication in drug-drug interaction
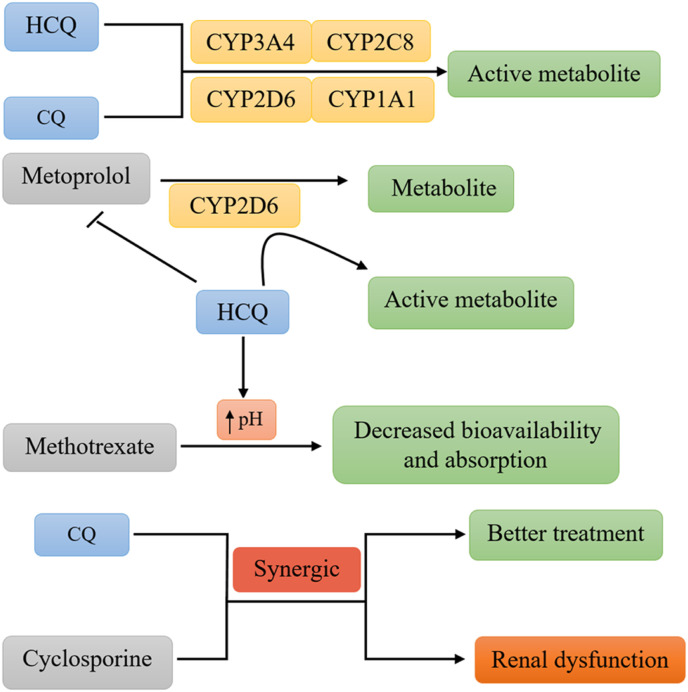
HCQ is structurally different from CQ only by a hydroxyl group in its N-ethyl side chain (Finbloom et al., 1985). CYP450 and its isoforms play a crucial role in HCQ and CQ metabolism (Stokkermans and Trichonas, 2020). Both of them undergo some changes especially dealkylation by CYP isomers, and become converted into active metabolites (Furst, 1996; Projean et al., 2003). Several investigations suggested that multiple CYP450 isoforms are involved in HCQ and CQ metabolism including CYP2C8, CYP3A4, CYP2D6, and CYP1A1 although the first three of them are more important relative to CYP1A1 (Fig. 3 ) (Li et al., 2003; Projean et al., 2003). There is some evidence that administration of HCQ could interfere with other CYP-metabolized drugs such as metoprolol (Somer et al., 2000). Metoprolol is a beta blocker drug metabolized through CYP2D6 that is administered to prevent angina (chest pain) and manage high blood pressure. During HCQ and metoprolol combination therapy, HCQ interferes with metoprolol metabolism by competing for CYP2D6 (Somer et al., 2000). Another drug-drug interaction that could take place is simultaneous administration of HCQ and methotrexate in management of RA. HCQ decreases the gastrointestinal absorption and bioavailability of methotrexate by modulating pH levels (Bannwarth et al., 1996). Concurrent administration of low dose of CQ with cyclosporine was shown to cause a better treatment in patients with RA via synergic effects with cyclosporine, although significant renal dysfunction was observed (Landewé et al., 1998). It's noteworthy to mention that HCQ could interfere with those drugs elevating gastric pH levels (e.g. H+/K+ ATPase pump inhibitors) including omeprazole, lansoprazole, pantoprazole, esomeprazole, and rabeprazole (Namazi, 2009). Therefore administration of HCQ in patients with COVID-19 that are simultaneously taking other mentioned drugs could lead to unwanted side effects.
Fig. 3.
Metabolism and drug-drug interactions of HCQ. CYP450 and its isoforms mediate dealkylation of HCQ. Co-administration of HCQ or CQ with other drugs leads to different pharmacokinetic effects.
6. Expression levels of CYP450 variants are influenced by epigenetic mechanisms
NcRNAs such as miRNAs and lncRNAs affect post-transcriptionally the expression levels of several CYP genes, and thereby influence the metabolism, and bioavailability of CYP450-mediated drugs (Jilek et al., 2017; Jin et al., 2016). Moreover, DNA methylation and histone acetylation could affect the CYP-metabolized drugs through modulating CYP expression. Table 2 summarizes known epigenetic modulators of CYP450 variants and their mechanism of action. However, more studies are needed to recognizing of other CYP modulators that could pave the way to refine HCQ dosage, and further reduce its side effects.
Table 2.
Epigenetic modulators of CYP450.
| Epigenetic modulator | Targeted CYP | Tissue or cell line | Effect on CYP | References | |
|---|---|---|---|---|---|
| MiRNAs | Hsa-miR-25-3p | CYP2B6 | Human embryonic kidney (HEK) 293T cells | Downregulation | Jin et al. (2016) |
| MiR-27b | CYP1B1 | Breast tumor and paired-normal tissue | Downregulation | Tsuchiya et al. (2006) | |
| MiR-101 and miR-128-2 | CYP2D6 | SH-SY5Y, and U251 cell lines | Downregulation | Li et al. (2015) | |
| Hsa-miR-370-3p | CYP2D6 | HepG2 cell line | Downregulation | Zeng et al. (2017) | |
|
MiR-142-3p |
CYP2D6 |
Mice bile acid |
Upregulation |
Pan et al. (2017) |
|
| LncRNAs | HNF1a-AS1 | CYP2C8, 2C9, 2C19, 2D6, 2E1, and 3A4 | Human liver tissues and, Huh7 cells | Upregulation | Wang et al. (2019) |
| Lnc-Lstr | CYP8b1 | Plasma of mice | Upregulation | Li et al. (2015) | |
|
Lnc-Hc |
CYP7A1 |
Rat hepatocyte |
Downregulation |
Lan et al. (2016) |
|
| DNA methylation and histone acetylation | Isoniazid | CYP1A1 and CYP1B1 | liver injury in rat | Downregulation of CYP1A1 and CYP1B1; Promotion of liver injury in rat | Li et al. (2018) |
| Trichostatin A | CYP2E1 | HepG2 cells | Induction of apoptosis and antitumor effects by acetylation of CYP2E1 promoter | Yang et al. (2010) | |
| IL-6 | CYP1B1 | CRC cell lines HCT116 and SW480 | Upregulation of CYP1B1; Promotion of colorectal cancer by increasing methylation of miR-27b promoter |
Patel et al. (2014) |
6.1. Regulation by miRNA
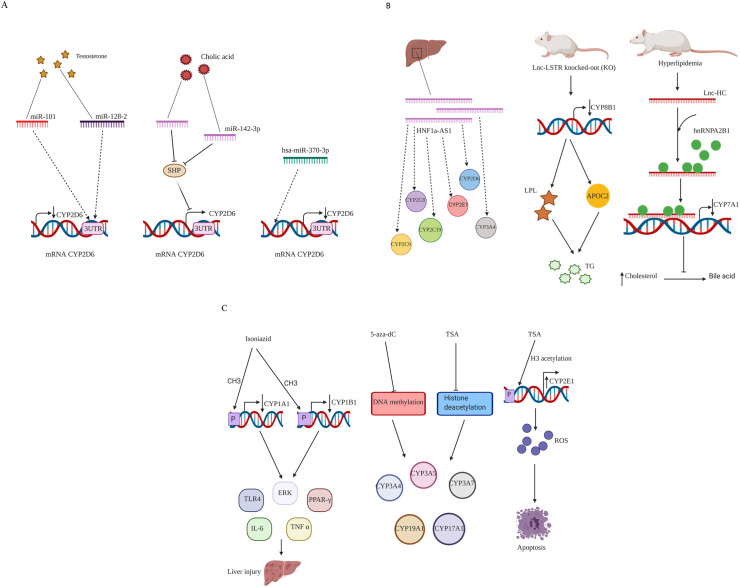
MiRNAs are a novel class of non-coding transcripts with 21–23 nucleotides length, and play a crucial role in a broad spectrum of disorders (Tiwari et al., 2018; Vishnoi and Rani, 2017). In silico and in vitro (human embryonic kidney (HEK) 293T cells) investigation on effect of hsa-miR-25–3p on expression levels of CYP2B6 showed that hsa-miR-25–3p suppresses CYP2B6 expression by binding to CYP2B6 mRNA 3’ UTR (Jin et al., 2016). Furthermore, a study carried out on 24 patients with breast cancer (breast tumor and paired-normal tissue) showed a negative association between expression levels of miR-27b and CYP1B1. Also, it showed that CYP1B1 contrary to miR-27b is highly expressed in cancerous tissues. CYP1B1 is expressed in estrogen-regulated tissues through catabolism of various procarcinogens, and 4-hydroxylation of 17β-estradiol (Tsuchiya et al., 2006). Accumulating evidence showed that the expression levels of CYP2D6 which have a fundamental role in HCQ metabolism is influenced by several miRNAs (Pan et al., 2017; Zeng et al., 2017). Surprisingly, a study performed on SH-SY5Y, and U251 cell lines demonstrated that testosterone could decrease CYP2D6 activity by increasing the level of miR-101 and miR-128-2 which bind to the 3′ UTR of CYP2D6 mRNA (Li et al., 2015). Investigation of HepG2 cell line transfection with hsa-miR-370–3p indicated that hsa-miR-370–3p decreases the expression of CYP2D6 via binding to coding regions of the CYP2D6 mRNA (Zeng et al., 2017). Astonishingly, examination of mice fed with cholic acid illustrated that it decreases the expression levels of small heterodimer partner (SHP) by targeting and inducing miR-142–3p. Decreasing of SHP, a transcriptional repressor of CYP2D6 expression, induced by cholic acid is significantly associated with CYP2D6 upregulation which emphasizes on the key role of bile acid levels in regulating CYP2D6 expression (Fig. 4 A) (Pan et al., 2017).
Fig. 4.
Epigenetic mechanism influenced expression of CYP2D6.
A: several miRNAs influence the expression levels of CYP2D6 by binding to 3′ UTR and coding regions of CYP2D6 mRNA; B: multiple lncRNAs including HNF1a-AS1, lnc-LSTR, and lnc-HC modulate several CYP enzymes by binding to CYP mRNAs; C: methylation and histone acetylation of CYP mRNAs promoter change the expression levels of multiple CYPs, and cause liver injury, apoptosis, and antitumor effects; SHP: small heterodimer partner; LPL: lipoprotein lipase; TG: triglyceride; APOC2: apolipoprotein C2; 5-aza-dC: 5-aza-2′-deoxycytidine; TSA: trichostatin A; ROS: reactive oxygen species; TLR: toll- like receptor; IL-6: interleukin 6; ERK: extracellular-signal-regulated kinase; TNF-α: tumor necrosis factor-α; PPAR-γ: peroxisome proliferator-activated receptor-γ.
6.2. Regulation by lncRNA
LncRNAs are other non-coding transcripts with longer than 200 nucleotides length, and have a pivotal role in promoting of wide spectrum disorders from cancer to neurodegenerative diseases. Recently, studies on human liver tissues and Huh7 cells demonstrated the positive correlation between expression levels of lncRNA hepatocyte nuclear factor 1 alpha antisense 1 (HNF1a-AS1), and several CYP enzymes including CYP2C8, 2C9, 2C19, 2D6, 2E1, and 3A4 (Wang et al., 2019). The liver-specific triglyceride regulator (lnc-Lstr) modulates the triglyceride plasma levels in mice by counteracting the repressor effects of TAR DNA binding protein 43 (TDP-43) on CYP8b1 promoter. Lnc-Lstr knocked-out mice led to repression of Cyp8b1 and reduction in triglyceride plasma levels through inducing apolipoprotein C2 and lipoprotein lipase which are involved in triglyceride plasma clearance (Li et al., 2015). The high levels of cholesterol in rat induce the expression and binding of Lnc-Hc, a hepatocyte-specific lncRNA, to hnRNPA2B1. Lnc-HC/hnRNPA2B1 complex inhibits cholesterol-bile acid conversion by decreasing CYP7A1 (Fig. 4B) (Lan et al., 2016).
6.3. DNA methylation and histone acetylation
DNA methylation and histone acetylation are the important epigenetic modifications that cells use to control gene expression of large number of genes (Verdone et al., 2006; Razin and Kantor, 2005). In this view, CYP might be influenced by other epigenetic mechanisms such as DNA methylation, and histone modification (Li et al., 2019a, Li et al., 2019b). Isoniazid induced promoter methylation of CYP1A1 and CYP1B1 resulted in their downregulation and liver injury promotion in rat. Indeed, liver injury occurs probably due to upregulation of TLR4, extracellular-signal-regulated kinase (ERK), peroxisome proliferator-activated receptor (PPAR)-γ, IL-6, and TNF-α, caused by decreasing levels of CYP1A1 and CYP1B1 (Li et al., 2018). Human hepatoma cell line HepG2 were treated with 5-aza-2′-deoxycytidine that inhibits DNA methylation, and trichostatin A that inhibits histone deacetylation caused expression changes in a wide spectrum of genes such as xenobiotic metabolism (CYP3A4, CYP3A5, and CYP3A7), and steroid biosynthesis (CYP17A1 and CYP19A1) genes (Dannenberg and Edenberg, 2006). Trichostatin A is an antitumor drug that was shown to induce apoptosis in HepG2 cells through histone H3 acetylation of CYP2E1 promoter. Trichostatin A-mediated CYP2E1 upregulation promotes apoptosis and antitumor effects by producing mitochondrial reactive oxygen species (Fig. 4C) (Yang et al., 2010). Astonishingly, the pro-inflammatory cytokine IL-6 that plays a fundamental role in promoting colorectal cancer, was shown to increase the expression levels of CYP1B1 in vitro and therefore mediates the metabolic activation of pro-carcinogens, which may lead to generation of reactive metabolites capable of damaging DNA by genome-wide methylation of miR-27b promoter (Patel et al., 2014). It seems that epigenetic mechanisms including ncRNAs, DNA methylation, and histone modification might play a vital role in regulating the expression and function of CYP2D6 in metabolizing several drugs such as metoprolol, methotrexate, cyclosporine, H+/K+ ATPase pump inhibitors, CQ, and HCQ. However, further studies are required to investigate the possible connection between epigenetic-dependent changes in cytochrome P450 expression and actual changes in response to HCQ (Ladda and Goralski, 2016; Li et al., 2019a, Li et al., 2019b; Tang and Chen, 2015).
7. Mechanism of retinopathy caused by hydroxychloroquine
7.1. Mechanism underlying vision: role of RPE
Light sensitivity is an essential process to intact vision (Garway-Heath et al., 2000). Permanent sensitivity to light is provided by ongoing generation and condensation of rhodopsin on the photoreceptor outer segment discs by rough endoplasmic reticulum and Golgi apparatus (Papermaster and Schneider, 1982; Ritter et al., 2008). Photoreceptor inner segment supports the outer segment by supplying necessary substances. Also, the photoreceptor inner segment facilitates the elongation of photoreceptor outer segment toward RPE by continuous synthesis of discs (Chen et al., 2009). A study conducted on retinal photoreceptors of multiple animal models including rhesus monkey, eastern gray squirrels, and California ground squirrels showed that photoreceptor outer segments are growing and extending continuously (Steinberg et al., 1980). RPE cells protect photoreceptor outer segments via phagocytosis of the outer segment redundancies of photoreceptors (Chen et al., 2009). RPE cells are playing a crucial role in maintenance of photoreceptors stability by transporting nutrients, electrolytes, ions, and water to the retina, and choroid capillaries. Deposition of debris in the extracellular space between RPE cells and photoreceptors were shown to be correlated with dystrophic retina. RPE cells prevent the accumulation of debris in photoreceptor outer segments by scavenging debris, and thereby causing rhodopsin cells renewal (Kevany and Palczewski, 2010). RPE cells transfection with mer tyrosine kinase (MERTK) siRNA resulted in phagocytosis inhibition, due to downregulation of MERTK which is involved in RPE phagocytosis activity (Strick et al., 2009). Correspondingly, Mertk mutated Royal College of Surgeons rats were diagnosed with autosomal recessive retinitis pigmentosa which emphasized on the fundamental role of phagocytosis activity of RPE cells in visual intact function (D' Cruz et al., 2000).
7.2. Hydroxychloroquine promotes retinopathy by influencing lysosomes, phagocytes, and melanin
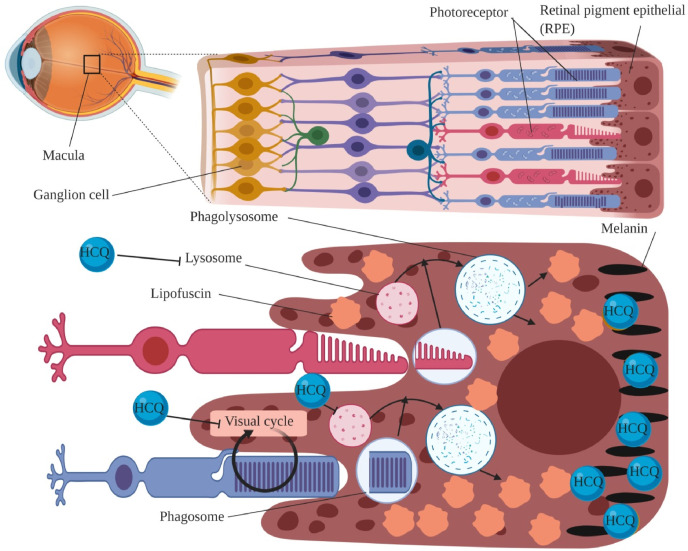
The 4-aminoquinoline derivatives such as HCQ and CQ might cause retinopathy by affecting the lysosomal function, digestion ability of phagocytes, and binding to the melanin (Browning, 2014; Lee et al., 2015). HCQ and CQ enter the lysosome and inactivate its enzymes including N-acetyl-β-glucosaminidase, and cathepsin D through alteration of its vital acidic condition (Toimela et al., 1995). Moreover, HCQ and CQ cause massive aggregation of lysosomal associated organelles, membranous cytoplasmic bodies, and lipofuscin in RPE neuron cells by reducing the lysosomal acidic potency (Mahon et al., 2004). Ongoing accumulation of lipofuscin in the RPE cells induces a positive feedback resulting in more accumulation of lipofuscin. Progressive aggregation of lipofuscin gradually damages RPE cells, and may cause several diseases such as age-related macular degeneration, and Stargardt disease (Dorey et al., 1989; Wolf, 2003). In addition, lipofuscins aggregation in RPE cells may reduce the normal supportive function of RPE cells by disrupting organelle's cytoplasmic trafficking (R Sparrrow et al., 2010; Terman and Brunk, 2004). Growing evidence suggest that HCQ promotes retinopathy by binding tightly to melanin in RPE. Furthermore, the strong binding of HCQ to melanin in other tissues including skin and ciliary bodies was shown to cause severe disorders such as skin hyperpigmentation (Fig. 5 ) (Ding et al., 2016; Tracy et al., 2013).
Fig. 5.
Mechanism of retinopathy caused by hydroxychloroquine. Macula is a central part of retina which have vital role in central, high-resolution, and color vision. Retina is a thin layer near the optic nerve. Retina is containing ganglion cells, and photoreceptors which conduct visual transduction by converting light into nerve impulses. Hydroxychloroquine (HCQ) promotes retinopathy through inactivating lysosomal enzymes, blocking phagocytes' digestion ability, binding to the melanin, and blocking organic anion transporter family member 1A2 OATP1A2. QATP1A2 is involved in visual cycle by transporting of all-trans-retinol (atROL) (vitamin A) from the interphotoreceptor matrix to the retinal pigment epithelia (RPE). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
7.3. Blocking of organic anion transporter family member 1A2 (OATP1A2) by hydroxychloroquine promotes retinopathy
Visual cycle is a vital process to visual perception that is conducted by engaging transporters and binding proteins (Thompson and Gal, 2003). Visual cycle exchanges vitamin A compounds (retinol) and facilitates signal transduction via providing substrates to the photoreceptors (Bok, 1993). Carrier organic anion transporter 1A2 (SLCO1A2) encodes OATP1A2 which participates in retinol circulation (Kalliokoski and Niemi, 2009). Increasing evidence suggest that OATPs have a fundamental role in the endogenous and exogenous substances influx into several organs including liver (OATP1B1), kidney, intestine, brain, and liver (OATP1A2) (Zaïr et al., 2008). OATP1B1 is the most important transporter expressed in hepatocytes and is localized in the sinusoidal membrane of hepatocytes. OATP1A2 is located in RPE apex, and mediates the transport of all-trans-retinol (atROL) from the interphotoreceptor matrix to the RPE (Chan et al., 2015). Interestingly, HCQ and CQ probably disrupt the activity of OATP1A2 in transporting of atROL influx by competing with atROL to occupation of OATP1A2. Consequently, atROL accumulates within the interphotoreceptor matrix, and results in deficiency of 11-cis-retinal (11cRAL) and rhodopsin, and thereby caused impaired vision (Xu et al., 2016).
8. Discussion
The current novel SARS-CoV-2 has been widely spread throughout the world (203 countries) with high mortality and morbidity ( worldometers, 2020). A global effort is underway to find a potential drug to counter SARS-CoV-2 (newsweek, 2020). Recently, growing body of evidence showed that HCQ might be a potential drug to counteract the novel coronavirus (Gautret et al., 2020; Liu et al., 2020a, Liu et al., 2020b; Yao et al., 2020). HCQ and CQ were administrated to patients affected with infectious diseases (HIV and influenza), and immune diseases (RA and SLE) (Chiang et al., 1996; Ooi et al., 2006; Rainsford et al., 2015). Several investigations have reported that HCQ has a higher safety and anti-viral activity to combat COVID-19 in comparison with CQ (Yao et al., 2020; Liu et al., 2020a, Liu et al., 2020b). In this context, an in vitro study carried out on African green monkey kidney Vero cells infected by SARS-CoV-2 and treated with HCQ and CQ showed that both of them decreased the viral replication in a concentration-dependent manner although HCQ showed a larger in vitro antiviral effects relative to CQ (Yao et al., 2020). An investigation conducted in Shanghai, China, on 30 confirmed cases with COVID-19 including 15 cases that were given conventional treatment and 15 cases that were given 400 mg per day HCQ along with conventional treatment for 5 days revealed the similar frequency of negative patients for nasopharyngeal swabs test of COVID-19 on day 7 after treatment between two groups. Also, the median time of normalization of body temperature was similar in both groups, whereas worsening computerized tomography findings of lung were seen in 5 patients (33.3%) of case group and 7 subjects (46.7%) of control group (Chen et al., 2020a, Chen et al., 2020b). A clinical trial study performed on 36 French confirmed SARS-CoV-2 cases (6 patients left the study) including 20 cases that were given HCQ (600 mg/day) (6 of them received azithromycin (500 mg on day 1 followed by 250 mg/day, the next four days) along with HCQ), and 16 cases receiving neither HCQ nor azithromycin illustrated a positive effect of combination therapy on ameliorating clinical manifestations. Surprisingly, their findings demonstrated that the nasopharyngeal PCR test at days 6 after treatment was negative for 100% of cases that underwent combined therapy whereas only 57% of patients that received HCQ only, and 12.5% of control group patients showed a negative nasopharyngeal PCR test (Gautret et al., 2020). The discrepancy between results of Shanghai and French study may be due to small sample size in both study or combination of HCQ and azithromycin in the French study in comparison with Shanghai study which used conventional and HCQ treatment. Strikingly, the achieved results from study performed on 11 patients affected with SARS-CoV-2 (7 men and 4 women) that 8 had significant comorbidities associated with poor outcomes (obesity: 2; solid cancer: 3; hematological cancer: 2; HIV-infection: 1) have shown an inconsistent finding about co-administration of azithromycin and HCQ (the dosage was similar to French study) in comparison with French study. It was revealed that one patient died, two were transferred to the ICU, and therapy was discontinued in one patient due to prolongation of the QT interval. Also, PCR test from nasopharyngeal swabs of 10 patients who were alive showed a positive SARS-CoV-2 finding in 8 of them on day 5–6 post treatment (Molina et al., 2020). Different response of patients affected with COVID-19 to HCQ supports the hypothesis that different CYP SNPs and epigenetic mechanisms might be responsible for different HCQ metabolism, and therefore different response. The CYP450 and its isoforms have a key role in metabolism and conversion of HCQ into pharmacologically active substances (Stokkermans and Trichonas, 2020). The effects of CYP SNPs on variable metabolism of HCQ and CQ among different individuals were investigated. Achieved results from a study conducted on 194 patients suffering from SLE that were given HCQ for more than 3 months showed the considerable effects of CYP2D6 SNPs on blood HCQ levels. Patients with GG genotype for rs1065852 showed a higher ratio of [DHCQ]:[HCQ] (DHCQ: N-desethyl HCQ is a product of HCQ metabolism) relative to patients with AA genotype that showed a lowest [DHCQ]:[HCQ] ratio. Correspondingly, patients with CC genotype for rs1135840 presented a higher [DHCQ]:[HCQ] ratio in comparison with patients with GG genotype for rs1135840 (Lee et al., 2016). Besides, several studies reported multiple drug-drug interactions for HCQ and other drugs including metoprolol, methotrexate, cyclosporine, and H+/K+ ATPase pump inhibitors (omeprazole, lansoprazole, pantoprazole, esomeprazole, and rabeprazole) (Bannwarth et al., 1996; Landewé et al., 1998; Namazi, 2009; Somer et al., 2000). Also, there are some evidence about the critical role of ncRNAs, DNA methylation, and histone modification in CYP expression regulation. Evidence of miRNAs and lncRNAs' role in regulating expression levels of CYP raise the possibility that other competing RNAs such as circular RNAs and pseudogenes may also play a crucial role in regulation of CYP expression. IL-6, is a proinflammatory cytokine that increases the expression levels of CYP1B1 by epigenetic mechanisms, and thereby promotes colorectal cancer. Given the higher expression levels of IL-6 in patients infected with SARS-CoV-2 in comparison with normal subjects, there is a possibility that IL-6 interferes with HCQ metabolism by affecting the expression of some nc-RNAs. Also, nc-RNAs could be a potential biomarker to prognosis, and diagnosis of pateints infected with SARS-CoV-2. Recent studies on nc-RNAs have shed some light on their potential role in therapeutic content. Beside the effectiveness of HCQ in counteracting SARS-CoV-2, there are some reports about the side effects of HCQ including cardiotoxicity, rhythm disorders (prolonged QT interval), gastrointestinal difficulties (dyspepsia, abdominal cramps, and dysgeusia), central nervous system disorders (headaches, dizziness, anxiety, and rarely psychosis and convulsions), skin disorders (rash and discoloration), and retinopathy (Ali and Jones, 2018; Joyce et al., 2013; Pelle and Callen, 2002). Administration of higher dosage of HCQ in patients with SARS-CoV-2 raised the possibility that HCQ induced-side effects may probably take place more rapidly. On the other hand, studies showed that retinopathy induced by HCQ could progress and become irreversible even after drug cessation in some patients (Pandya et al., 2015; Yusuf et al., 2017). Investigation on 218 RA patients under HCQ treatment via spectral domain optical coherence tomography, fundus autofluorescence, and visual fields demonstrated that 9 (4.1%) showed a toxicity. Eight out of 9 showed predominantly pericentral pattern of retinal change, and 1 showed the classic parafoveal distribution of retinal damage. Also, a significant progression of retinopathy was seen in 3 patients (Lee et al., 2015). Achieved results from a retrospective study (2005–2009) on 85 patients suffering from RA that were given HCQ or CQ indicated that 21 (24.7%) out of 85 developed retinopathy. Also, they revealed that patients that underwent CQ treatment were significantly more susceptible to retinopathy in comparison with patients who underwent HCQ treatment (P = 0.001) (Kobak and Deveci, 2010). Given the high morbidity and mortality rate from the current SARS-CoV-2 pandemy, global attempts are ongoing to find an effective drug to combat it. In recent weeks several clinical trials were conducted to clarify the effectiveness of HCQ in diminishing clinical manifestations of SARS-CoV-2 including body temperature, computerized tomography findings of the lung, viral load, and time of hospitalization. Administration of HCQ to manage patients infected with SARS-CoV-2 was challenged by several side effects especially retinopathy and different metabolism of HCQ among individuals with different CYP450 genotypes. Given the achieved results from study conducted by Lee et al. (2015) we speculated that SNPs may explain partly the different response of subjects to the equal dosage of HCQ in Shanghai and French study. It's noteworthy to emphasize on regular ophthalmologic screening to check the HCQ mediated-retinopathy. Also, CYP450 genotyping especially for CYP2D6 may help to determine the best HCQ dosage in the context of personalized medicine.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Authors declare no conflict of interest.
Acknowledgements:
Not applicable.
References
- Ali S.S., Jones H. 23. An adverse neuropsychiatric reaction following treatment with hydroxychloroquine: a case report. Rheumatol Adv Pract. 2018;2 doi: 10.1093/rap/rky033.014. [DOI] [Google Scholar]
- An J., Woodward J.J., Sasaki T., Minie M., Elkon K.B. Cutting edge: antimalarial drugs inhibit IFN-β production through blockade of cyclic GMP-AMP synthase–DNA interaction. J. Immunol. 2015;194(9):4089–4093. doi: 10.4049/jimmunol.1402793. [DOI] [PubMed] [Google Scholar]
- An J., Woodward J., Minie M., Sun X., Tanaka L., Peng Y., Elkon K.B. Novel anti‐malarial drug derivative inhibited type I interferon production and autoimmune inflammation through inhibition of cGAS‐STING pathway in trex1−/− mouse. Arthritis Rheum. 2016;68(Suppl 10) http://acrabstracts.org/abstract/novel-anti-malarial-drug-derivative-inhibited-type-i-interferon-production-and-autoimmune-inflammation-through-inhibition-of-cgas-sting-pathway-in-trex1-mouse/ URL. [Google Scholar]
- An J., Woodward J.J., Lai W., Minie M., Sun X., Tanaka L., Elkon K.B. Inhibition of cyclic GMP‐AMP synthase using a novel antimalarial drug derivative in trex1‐deficient mice. Arthritis Rheum. 2018;70(11):1807–1819. doi: 10.1002/art.40559. [DOI] [PubMed] [Google Scholar]
- Augustijns P., Geusens P., Verbeke N. Chloroquine levels in blood during chronic treatment of patients with rheumatoid arthritis. Eur. J. Clin. Pharmacol. 1992;42(4):429–433. doi: 10.1007/BF00280130. [DOI] [PubMed] [Google Scholar]
- Bannwarth B., Péhourcq F., Schaeverbeke T., Dehais J. Clinical pharmacokinetics of low-dose pulse methotrexate in rheumatoid arthritis. Clin. Pharmacokinet. 1996;30(3):194–210. doi: 10.2165/00003088-199630030-00002. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi I., Kivity S., Langevitz P., Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin. Rev. Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D. The retinal pigment epithelium: a versatile partner in vision. J. Cell Sci. Suppl. 1993;(17):189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- Browning D.J. In: Hydroxychloroquine and Chloroquine Retinopathy. Browning D.J., editor. Springer Science + Business Media; New York: 2014. Toxicology of hydroxychloroquine and chloroquine and the pathology of the retinopathy they cause; pp. 65–83. [Google Scholar]
- Chan T., Zhu L., Madigan M.C., Wang K., Shen W., Gillies M.C., Zhou F. Human organic anion transporting polypeptide 1A2 (OATP1A2) mediates cellular uptake of all-trans-retinol in human retinal pigmented epithelial cells. Br. J. Pharmacol. 2015;172(9):2343‐2353. doi: 10.1111/bph.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Lukas T.J., Du N., Suyeoka G., Neufeld A.H. Dysfunction of the retinal pigment epithelium with age: increased iron decreases phagocytosis and lysosomal activity. Invest. Ophthalmol. Vis. Sci. 2009;50(4):1895–1902. doi: 10.1167/iovs.08-2850. [DOI] [PubMed] [Google Scholar]
- Chen J., Lui D., Liu L., Lui P., Xu Q., Xia L., Lu H. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Yang B. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang G., Sassaroli M., Louie M., Chen H., Stecher V.J., Sperber K. Inhibition of HIV-1 replication by hydroxychloroquine: mechanism of action and comparison with zidovudine. Clin. Therapeut. 1996;18(6):1080–1092. doi: 10.1016/s0149-2918(96)80063-4. [DOI] [PubMed] [Google Scholar]
- Collins K.P., Jackson K.M., Gustafson D.L. Hydroxychloroquine: a physiologically-based pharmacokinetic model in the context of cancer-related autophagy modulation. J. Pharmacol. Exp. Therapeut. 2018;365(3):447–459. doi: 10.1124/jpet.117.245639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K.L., Wärri A., Soto-Pantoja D.R., Clarke P.A., Cruz M.I., Zwart A., Clarke R. Chloroquine inhibits autophagy to potentiate antiestrogen responsiveness in ER+ breast cancer. ClinCancer Res. 2014;20(12):3222–3232. doi: 10.1158/1078-0432.CCR-13-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler D., MacIntyre A., Tett S. Pharmacokinetics and cellular uptake of 4-aminoquinoline antimalarials. Agents Actions Suppl. 1988;(24):142–157. doi: 10.1007/978-3-0348-9160-8_13. [DOI] [PubMed] [Google Scholar]
- Dannenberg L.O., Edenberg H.J. Epigenetics of gene expression in human hepatoma cells: expression profiling the response to inhibition of DNA methylation and histone deacetylation. BMC Genom. 2006;7(1):181. doi: 10.1186/1471-2164-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H.J., Denniston A.K., Rao V.K., Gordon C. Hydroxychloroquine-related retinal toxicity. Rheumatology. 2016;55(6):957–967. doi: 10.1093/rheumatology/kev357. [DOI] [PubMed] [Google Scholar]
- Dorey C.K., Wu G., Ebenstein D., Garsd A., Weiter J. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest. Ophthalmol. Vis. Sci. 1989;30(8):1691–1699. [PubMed] [Google Scholar]
- D'Cruz P.M., Yasumura D., Weir J., Matthes M.T., Abderrahim H., LaVail M.M., Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000;9(4):645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Finbloom D., Silver K., Newsome D., Gunkel R. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J. Rheumatol. 1985;12(4):692–694. [PubMed] [Google Scholar]
- Fox R. Anti-malarial drugs: possible mechanisms of action in autoimmune disease and prospects for drug development. Lupus. 1996;5(1_Suppl. l):4–10. [PubMed] [Google Scholar]
- Furst D.E. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996;5(1_Suppl. l):11–15. [PubMed] [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garway-Heath D.F., Poinoosawmy D., Fitzke F.W., Hitchings R.A. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107(10):1809–1815. doi: 10.1016/s0161-6420(00)00284-0. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Herold T., Arnreich C., Hellmuth J., von Bergwelt-Baildon M., Klein M., Weinberger T. Google Scholar; 2020. Level of IL-6 Predicts Respiratory Failure in Hospitalized Symptomatic COVID-19 Patients. medRxiv 2020. [Google Scholar]
- Ireland J.M., Unanue E.R. Autophagy in antigen-presenting cells results in presentation of citrullinated peptides to CD4 T cells. J. Exp. Med. 2011;208(13):2625–2632. doi: 10.1084/jem.20110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek J.L., Tian Y., Yu A.-M. Effects of microRNA-34a on the pharmacokinetics of cytochrome P450 probe drugs in mice. Drug Metabol. Dispos. 2017;45(5):512–522. doi: 10.1124/dmd.116.074344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Yu D., Tolleson W.H., Knox B., Wang Y., Chen S., Ning B. MicroRNA hsa-miR-25-3p suppresses the expression and drug induction of CYP2B6 in human hepatocytes. Biochem. Pharmacol. 2016;113:88–96. doi: 10.1016/j.bcp.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce E., Fabre A., Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care. 2013;2(1):77–83. doi: 10.1177/2048872612471215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski A., Niemi M. Impact of OATP transporters on pharmacokinetics. Br. J. Pharmacol. 2009;158(3):693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany B.M., Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology. 2010;25(1):8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobak S., Deveci H. Retinopathy due to antimalarial drugs in patients with connective tissue diseases: are they so innocent? A single center retrospective study. Int J Rheum Dis. 2010;13(3):e11–e15. doi: 10.1111/j.1756-185X.2010.01478.x. [DOI] [PubMed] [Google Scholar]
- Kyburz D., Brentano F., Gay S. Mode of action of hydroxychloroquine in RA—evidence of an inhibitory effect on Toll-like receptor signaling. Nat. Clin. Pract. Rheumatol. 2006;2(9):458–459. doi: 10.1038/ncprheum0292. [DOI] [PubMed] [Google Scholar]
- Ladda M.A., Goralski K.B. The effects of CKD on cytochrome P450–mediated drug metabolism. Adv. Chron. Kidney Dis. 2016;23(2):67–75. doi: 10.1053/j.ackd.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Lan X., Yan J., Ren J., Zhong B., Li J., Li Y., Yang X. A novel long noncoding RNA Lnc‐HC binds hnRNPA2B1 to regulate expressions of Cyp7a1 and Abca1 in hepatocytic cholesterol metabolism. Hepatology. 2016;64(1):58–72. doi: 10.1002/hep.28391. [DOI] [PubMed] [Google Scholar]
- Landewé R., Rietveld J., Zwinderman A., Bruyn G., Breedveld F., Dijkmans B. Combination therapy in recent onset rheumatoid arthritis: a randomized double blind trial of the addition of low dose cyclosporine to patients treated with low dose chloroquine. J rRheumatol. 1998;25(8):1493–1498. [PubMed] [Google Scholar]
- Law I., Ilett K.F., Hackett L.P., Page‐Sharp M., Baiwog F., Gomorrai S., Davis T.M. Transfer of chloroquine and desethylchloroquine across the placenta and into milk in Melanesian mothers. Br. J. Clin. Pharmacol. 2008;65(5):674–679. doi: 10.1111/j.1365-2125.2008.03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Glaeser H., Smith L.H., Roberts R.L., Moeckel G.W., Gervasini G., Kim R.B. Polymorphisms in Human Organic Anion-transporting Polypeptide 1A2 (OATP1A2) implications for altered drug disposition and central nervous system drug entry. J. Biol. Chem. 2005;280(10):9610–9617. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- Lee D.H., Melles R.B., Joe S.G., Lee J.Y., Kim J.-G., Lee C.-K., Marmor M.F. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology. 2015;122(6):1252–1256. doi: 10.1016/j.ophtha.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Vinayagamoorthy N., Han K., Kwok S.K., Ju J.H., Park K.S., Park S.H. Association of polymorphisms of cytochrome P450 2D6 with blood hydroxychloroquine levels in patients with systemic lupus erythematosus. Arthritis Rheum. 2016;68(1):184–190. doi: 10.1002/art.39402. [DOI] [PubMed] [Google Scholar]
- Li X.-Q., Björkman A., Andersson T.B., Gustafsson L.L., Masimirembwa C.M. Identification of human cytochrome P 450 s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur. J. Clin. Pharmacol. 2003;59(5–6):429–442. doi: 10.1007/s00228-003-0636-9. [DOI] [PubMed] [Google Scholar]
- Li J., Xie M., Wang X., Ouyang X., Wan Y., Dong G., Yue J. Sex hormones regulate cerebral drug metabolism via brain miRNAs: down‐regulation of brain CYP 2 D by androgens reduces the analgesic effects of tramadol. Br. J. Pharmacol. 2015;172(19):4639–4654. doi: 10.1111/bph.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li Y., Zheng G., Zhu L., Wang J., Mu S., Feng F. Cytochrome P450 1A1 and 1B1 promoter CpG island methylation regulates rat liver injury induced by isoniazid. Mol. Med. Rep. 2018;17(1):753–762. doi: 10.3892/mmr.2017.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Tolleson W.H., Yu D., Chen S., Guo L., Xiao W., Ning B. Regulation of cytochrome P450 expression by microRNAs and long noncoding RNAs: epigenetic mechanisms in environmental toxicology and carcinogenesis. J Environ. Sci. Health, C. Environ. Carcinog Ecotoxicol. Rev. 2019;37(3):180–214. doi: 10.1080/10590501.2019.1639481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Meng Q., Yang M., Liu D., Hou X., Tang L., Liu K. Current trends in drug metabolism and pharmacokinetics. Acta Pharm. Sin. B. 2019;9(6):1113–1144. doi: 10.1016/j.apsb.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: six million doses of hydroxychloroquine donated to US despite lack of evidence. BMJ. 2020;368:m1166. doi: 10.1136/bmj.m1166. [DOI] [PubMed] [Google Scholar]
- Mahon G., Anderson H., Gardiner T., McFarlane S., Archer D., Stitt A. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr. Eye Res. 2004;28(4):277–284. doi: 10.1076/ceyr.28.4.277.27835. [DOI] [PubMed] [Google Scholar]
- McChesney E.W. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am. J. Med. 1983;75(1):11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- McChesney E.W., Shekosky J.M., Hernandez P.H. Metabolism of chloroquine-3-14C in the rhesus monkey. Biochem. Pharmacol. 1967;16(12):2444–2447. [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingo R.M., Simmons J.A., Shoemaker C.J., Nelson E.A., Schornberg K.L., D'Souza R.S., White J.M. Ebola virus and severe acute respiratory syndrome coronavirus display late cell entry kinetics: evidence that transport to NPC1+ endolysosomes is a rate-defining step. J. Virol. 2015;89(5):2931–2943. doi: 10.1128/JVI.03398-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Maladies Infect. 2020;50(4):384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M., Tincani A., Faden D., Zinzini E., Lojacono A., Marchesi A., Chirico G. Follow-up of infants exposed to hydroxychloroquine given to mothers during pregnancy and lactation. J. Perinatol. 2005;25(2):86–89. doi: 10.1038/sj.jp.7211208. [DOI] [PubMed] [Google Scholar]
- Namazi M. SAGE Publications Sage UK; London, England: 2009. The Potential Negative Impact of Proton Pump Inhibitors on the Immunopharmacologic Effects of Chloroquine and Hydroxychloroquine. [DOI] [PubMed] [Google Scholar]
- newsweek 2020. https://www.newsweek.com/fda-says-hydroxychloroquine-chloroquine-can-used-treat-coronavirus-1494925 Retrieved from.
- Ooi E.E., Chew J.S.W., Loh J.P., Chua R.C. In vitro inhibition of human influenza A virus replication by chloroquine. Virol. J. 2006;3(1):39. doi: 10.1186/1743-422X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Kent R., Won K.-J., Jeong H. Cholic acid feeding leads to increased CYP2D6 expression in CYP2D6-humanized mice. Drug Metab. Dispos. 2017;45(4):346–352. doi: 10.1124/dmd.116.074013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya H.K., Robinson M., Mandal N., Shah V.A. Hydroxychloroquine retinopathy: a review of imaging. Indian J. Ophthalmol. 2015;63(7):570–574. doi: 10.4103/0301-4738.167120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster D.S., Schneider B.G. Cell biology of the eye: Academic Press; New York: 1982. Biosynthesis and Morphogenesis of Outer Segment Membranes in Vertebrate Photoreceptor Cells; pp. 475–531. [Google Scholar]
- Patel S.A., Bhambra U., Charalambous M.P., David R.M., Edwards R.J., Lightfoot T., Gooderham N.J. Interleukin-6 mediated upregulation of CYP1B1 and CYP2E1 in colorectal cancer involves DNA methylation, miR27b and STAT3. Br. J. Canc. 2014;111(12):2287–2296. doi: 10.1038/bjc.2014.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelle M.T., Callen J.P. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch. Dermatol. 2002;138(9):1231–1233. doi: 10.1001/archderm.138.9.1231. [DOI] [PubMed] [Google Scholar]
- Pereira B.B. Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review. J. Toxicol. Environ. Health B Crit. Rev. 2020;23(4):177–181. doi: 10.1080/10937404.2020.1752340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projean D., Baune B., Farinotti R., Flinois J.-P., Beaune P., Taburet A.-M., Ducharme J. In vitro metabolism of chloroquine: identification of CYP2C8, CYP3A4, and CYP2D6 as the main isoforms catalyzing N-desethylchloroquine formation. Drug Metab. Dispos. 2003;31(6):748–754. doi: 10.1124/dmd.31.6.748. [DOI] [PubMed] [Google Scholar]
- R Sparrrow J., Hicks D., P Hamel C. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010;10(9):802–823. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainsford K., Parke A.L., Clifford-Rashotte M., Kean W. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- Razin A., Kantor B. DNA methylation in epigenetic control of gene expression. Epigenet. Chromatin. 2005:151–167. doi: 10.1007/3-540-27310-7_6. Springer. [DOI] [PubMed] [Google Scholar]
- Rebecca V.W., Nicastri M.C., McLaughlin N., Fennelly C., McAfee Q., Ronghe A., Chude C.I. A unified approach to targeting the lysosome's degradative and growth signaling roles. Canc. Discov. 2017;7(11):1266–1283. doi: 10.1158/2159-8290.CD-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecca V.W., Nicastri M.C., Fennelly C., Chude C.I., Barber-Rotenberg J.S., Ronghe A., Goldman A.R. PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. Canc. Discov. 2019;9(2):220–229. doi: 10.1158/2159-8290.CD-18-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- referencemedscape 2020. https://reference.medscape.com/drug/plaquenil-hydroxychloroquine-sulfate-343205
- Ritter E., Elgeti M., Bartl F.J. Activity switches of rhodopsin. Photochem. Photobiol. 2008;84(4):911–920. doi: 10.1111/j.1751-1097.2008.00324.x. [DOI] [PubMed] [Google Scholar]
- Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- Somer M., Kallio J., Pesonen U., Pyykkö K., Huupponen R., Scheinin M. Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br. J. Clin. Pharmacol. 2000;49(6):549–554. doi: 10.1046/j.1365-2125.2000.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsan Padmanabhan M. 2020. Potential Dual Therapeutic Approach against SARS-CoV-2/covid-19 with Nitazoxanide and Hydroxychloroquine. [DOI] [Google Scholar]
- Steinberg R.H., Fisher S.K., Anderson D.H. Disc morphogenesis in vertebrate photoreceptors. J. Comp. Neurol. 1980;190(3):501–518. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- Stokkermans T.J., Trichonas G. StatPearls Publishing; Treasure Island, FL: 2020. Chloroquine and Hydroxychloroquine Toxicity. 2019. StatPearls.https://www.ncbi.nlm.nih.gov/books/NBK537086/ [PubMed] [Google Scholar]
- Strick D.J., Feng W., Vollrath D. Mertk drives myosin II redistribution during retinal pigment epithelial phagocytosis. Invest. Ophthalmol. Vis. Sci. 2009;50(5):2427–2435. doi: 10.1167/iovs.08-3058. [DOI] [PubMed] [Google Scholar]
- Sundelin S.P., andTerman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. Apmis. 2002;110(6):481–489. doi: 10.1034/j.1600-0463.2002.100606.x. [DOI] [PubMed] [Google Scholar]
- Tang X., Chen S. Epigenetic regulation of cytochrome P450 enzymes and clinical implication. Curr. Drug Metabol. 2015;16(2):86–96. doi: 10.2174/138920021602150713114159. [DOI] [PubMed] [Google Scholar]
- Terman A., Brunk U.T. Lipofuscin. Int. J. Biochem. Cell Biol. 2004;36(8):1400–1404. doi: 10.1016/j.biocel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Tett S., Cutler D., Day R. Antimalarials in rheumatic diseases. Bailliere’s Clin. Rheumatol. 1990;4(3):467–489. doi: 10.1016/s0950-3579(05)80004-4. [DOI] [PubMed] [Google Scholar]
- Thompson D.A., Gal A. Vitamin A metabolism in the retinal pigment epithelium: genes, mutations, and diseases. Prog. Retin. Eye Res. 2003;22(5):683–703. doi: 10.1016/s1350-9462(03)00051-x. [DOI] [PubMed] [Google Scholar]
- Thwaites R., Chamberlain G., Sacre S. Emerging role of endosomal Toll-like receptors in rheumatoid arthritis. Front. Immunol. 2014;5:1. doi: 10.3389/fimmu.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Mukherjee B., Dixit M. MicroRNA key to angiogenesis regulation: miRNA biology and therapy. Curr. Cancer Drug Targets. 2018;18(3):266–277. doi: 10.2174/1568009617666170630142725. [DOI] [PubMed] [Google Scholar]
- Toimela T., Tähti H., Salminen L. Retinal pigment epithelium cell culture as a model for evaluation of the toxicity of tamoxifen and chloroquine. Ophthalmic Res. 1995;27(Suppl. 1):150–153. doi: 10.1159/000267861. [DOI] [PubMed] [Google Scholar]
- Tracy C.L., Blakey B., Parker G., Roebuck J. Hydroxychloroquine-induced hyperpigmentation. J. Clin. Rheumatol. 2013;19(5):292. doi: 10.1097/RHU.0b013e31829d547b. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y., Nakajima M., Takagi S., Taniya T., Yokoi T. MicroRNA regulates the expression of human cytochrome P450 1B1. Canc. Res. 2006;66(18):9090–9098. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- Verdone L., Agricola E., Caserta M., Di Mauro E. Histone acetylation in gene regulation. Briefings Funct. Genomics Proteomics. 2006;5(3):209–221. doi: 10.1093/bfgp/ell028. [DOI] [PubMed] [Google Scholar]
- Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2(1):69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnoi A., Rani S. MiRNA biogenesis and regulation of diseases: an overview. MicroRNA Profiling. 2017:1–10. doi: 10.1007/978-1-4939-6524-3_1. Springer. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yan L., Liu J., Chen S., Liu G., Nie Y., Zhong X. The HNF1α-regulated lncRNA HNF1α-AS1 is involved in the regulation of cytochrome P450 expression in human liver tissues and Huh7 cells. J. Pharmacol. Exp. Therapeut. 2019;368(3):353–362. doi: 10.1124/jpet.118.252940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst D.C., Steele J.C., Adagu I.S., Craig J.C., Cullander C. Hydroxychloroquine is much less active than chloroquine against chloroquine-resistant Plasmodium falciparum, in agreement with its physicochemical properties. Antimicrob. Chemother. 2003;52(2):188–193. doi: 10.1093/jac/dkg319. [DOI] [PubMed] [Google Scholar]
- Wolf G. Lipofuscin and macular degeneration. Nutr. Rev. 2003;61(10):342–346. doi: 10.1301/nr.2003.oct.342-346. [DOI] [PubMed] [Google Scholar]
- worldometers 2020. https://www.worldometers.info/coronavirus/coronavirus-cases/ Retrieved from.
- Xu C., Zhu L., Chan T., Lu X., Shen W., Madigan M.C., Zhou F. Chloroquine and hydroxychloroquine are novel inhibitors of human organic anion transporting polypeptide 1A2. J. Pharmacol. Sci. 2016;105(2):884–890. doi: 10.1002/jps.24663. [DOI] [PubMed] [Google Scholar]
- Yang H., Nie Y., Li Y., Wan Y.-J.Y. Histone modification-mediated CYP2E1 gene expression and apoptosis of HepG2 cells. Exp. Biol. Med. 2010;235(1):32–39. doi: 10.1258/ebm.2009.009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Song C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C.H., Ryu J.S., Kim M.K., Wee W.R. Effect of hydroxychloroquine on the inflammation in antigen presenting cells interacted with damaged corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2013;54(15) 2065-2065. [Google Scholar]
- Yusuf I., Sharma S., Luqmani R., Downes S. Hydroxychloroquine retinopathy. Eye. 2017;31(6):828–845. doi: 10.1038/eye.2016.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaïr Z.M., Eloranta J.J., Stieger B., Kullak-Ublick G.A. Pharmacogenetics of OATP (SLC21/SLCO), OAT and OCT (SLC22) and PEPT (SLC15) transporters in the intestine, liver and kidney. Pharmacogenomics. 2008;9(5):597–624. doi: 10.2217/14622416.9.5.597. [DOI] [PubMed] [Google Scholar]
- Zeng L., Chen Y., Wang Y., Yu L.-R., Knox B., Chen J., Guo L. MicroRNA hsa-miR-370-3p suppresses the expression and induction of CYP2D6 by facilitating mRNA degradation. Biochem. Pharmacol. 2017;140:139–149. doi: 10.1016/j.bcp.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wu J., Du F., Xu H., Sun L., Chen Z., Chen Z.J. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Rep. 2014;6(3):421–430. doi: 10.1016/j.celrep.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]