ABSTRACT
Aim:
Cigarette smoking has been recognized as an important risk factor in periodontal diseases. One of the suggested mechanisms behind this association is that nicotine alters the microcirculation and causes vasoconstriction and reduced blood flow through the periodontal tissues. Scarce information is currently available relative to the microvascular alterations associated with smoking and the distribution of capillaries through the various areas of the gingival tissues. The aims of this study were to assess, in human interproximal gingival biopsies, the number and diameter of gingival capillaries in periodontally affected smokers and nonsmokers using the CD34 immunohistochemical staining method. The pattern of distribution of vessels in the different areas of the gingival tissues was also assessed.
Materials and Methods:
Systemically healthy patients with moderate chronic periodontitis and ranging in age between 30 and 60 years were recruited for the study from the patient population attending the Periodontology Department of the Faculty of Dental Medicine at the Lebanese University of Beirut. The patients were selected to have a group of 10 patients (Group SP) of smokers (>10 cigarettes/day for the last 10 years) and a second group (Group NP) consisting of nonsmoking periodontally affected patients. Three to four weeks following initial preparation, one interproximal gingival biopsy was obtained from each patient. Immunohistochemical staining with CD34 mouse monoclonal antibody was used to identify the endothelial cells of the blood vessels within each sample. Twelve biopsy samples (five in Group NP and seven in Group SP) were chosen for the measurement of the number and diameter of vessels in three regions of the connective tissue of the biopsy under a blinded protocol.
Results:
In the two groups, the quantitative distribution of small, medium, and large vessels followed a similar trend with the number of small vessels being significantly greater than both medium and large vessels. Small vessels prevailed in the peripheral regions, whereas large vessels were more abundant in the deeper connective tissue areas. The total number of vessels seemed unaffected by chronic cigarette smoking in both groups in the entire biopsy area and in the separate connective tissue regions. Quantitative alteration in the total number of gingival capillaries was not observed in chronic smokers. A redistribution of small and large vessels in the superficial and deeper connective tissue areas of the gingival papilla was noted as a result of smoking in periodontal patients.
Conclusion:
The quantitative distribution of small, medium, and large vessels follows a similar trend with the content in small vessels being significantly more important than both medium and large vessels. Smoking and periodontitis result in a redistribution of small and large vessels in the superficial and deeper connective tissue areas of the gingival papilla compared to nonsmoking periodontal patients. The significance and clinical implications of such rearrangement of vasculature within the gingival tissue need to be further investigated.
KEYWORDS: Biopsy, blood vessel, capillary, gingival tissue, microcirculation, papilla, periodontitis, smoking
INTRODUCTION
Smoking has been clearly established as one of the most important risk factors in the development and progression of periodontal disease.[1,2,3] It has been associated with periodontal tissue destruction,[4,5,6,7,8,9] impaired wound healing following various surgical procedures,[10,11] negative outcome of periodontal therapy,[12,13,14,15,16,17] and impairment of gingival vasculature.[18,19]
The qualitative and quantitative changes in gingival vasculature are crucial in the understanding of the pathogenic mechanisms involved in the initiation and progression of periodontal diseases. In healthy gingival tissues, smoking is associated with altered gingival vasculature.[18,19]
Studies have shown that gingival vasculature in smokers shows morphologic alterations,[20] higher percentage of slender and smaller blood vessels,[21] tortuousness of capillary loops,[22] microaneurysm, and microhemorrhage.[23] In periodontally involved gingiva, vascular changes such as increased density and dilatation occur in the gingival tissues independently of the smoking status.[24] Limited data are currently available relative to the changes in gingival vasculature in smokers with chronic periodontal disease compared with nonsmokers with chronic periodontitis.[25]
Rezavandi et al.[25] suggested that the inflammatory response in smokers with periodontitis may not be accompanied by an equivalent increase in vascularity, and a significantly larger number of vessels were observed in inflamed tissues of nonsmokers than smokers. When gingival blood flow was compared between patients with different severity of periodontitis,[26] laser Doppler flowmetry showed that smoking decreased gingival blood flow in smokers with periodontitis compared to nonsmokers with periodontitis. In a cross-sectional study comparing the vascular changes in the gingival of smokers and nonsmokers with chronic periodontal disease, Kumar and Faizuddin[27] concluded that although smokers have less vascular density and reduced lumen area than nonsmokers, these differences did not reach statistical significance. This study was undertaken to determine in human interproximal gingival biopsies the number of gingival vessels in periodontitis-affected smokers and nonsmokers using the CD34 immunohistochemical staining method. Furthermore, the distribution of differently sized vessels in the different areas of the gingival connective tissue was evaluated.
MATERIALS AND METHODS
PATIENT POPULATION
Systemically healthy patients with moderate chronic periodontitis, according to the classification of the American Academy of Periodontology (AAP) in 1999,[28] ranging in age between 30 and 60 years were recruited for the study from the patient population attending the Periodontology Department (Faculty of Dental Medicine, Lebanese University, Beirut, Lebanon). The diagnosis of moderate periodontitis was based on radiographic findings (localized or generalized bone loss affecting 1/3 to 2/3 of the roots with pocket depths ranging between 6 and 8mm in at least two sites). The following patients were excluded from the study: (1) patients taking medications that could affect the general or gingival vasculature and vascular, (2) patients taking medications known to cause hyperplastic/hypertrophic gingival changes, and (3) patients having received previous periodontal surgical or nonsurgical treatment except for regular supragingival prophylaxis.
The patients were selected matched in age and sex to have two groups of 10 patients each according to the following:
-
-
In the smokers group (Group SP), according to the classification by Yun et al.,[29] we included patients smoking more than 10 cigarettes/day for the last 10 years. This standard was based on the premise that smoking and periodontitis are dependent on the dose and years of exposure to tobacco products;[30,31,32] the criteria for smoking was based on a self-reported history.
-
-
Group NP: nonsmokers.
In all smokers, the number of cigarettes per day and the duration of the smoking habit was recorded. All individuals were subjected to full mouth prophylaxis and oral hygiene instructions 3–4 weeks before the study. The study protocol was approved by the Ethics Committee in Research at the Lebanese University, Beirut, Lebanon (CUEMB No. 23). All patients were given detailed information about the study objectives and procedures, and their written informed consent was obtained.
SITE SELECTION AND CLINICAL MEASUREMENTS
One site per patient was selected for biopsy taken during surgery for periodontal treatment using open flap curettage or modified Widman flap. The selection of the biopsy site was based on the following criteria: (1) interdental buccal location in non-aesthetic areas of the mouth, (2) absence of tooth abnormalities, caries, or inadequate interproximal restorations, (3) probing depth of 6–8mm on the proximal surfaces of both adjacent teeth, and (4) bone loss in the interproximal area as ascertained on a periapical radiograph taken with the long cone paralleling technique. Teeth carriers of removable or fixed prostheses in the biopsy area were excluded.
Before the biopsy procedure, the following clinical parameters were recorded: (1) clinical attachment level (CAL) on the two proximal surfaces of the adjacent teeth, (2) gingival index (GI),[33] and (3) plaque index (PI)[34] measured as the mean of the two recordings on the adjacent teeth. All measurements were performed by the same operator previously calibrated.
BIOPSY PROCEDURE
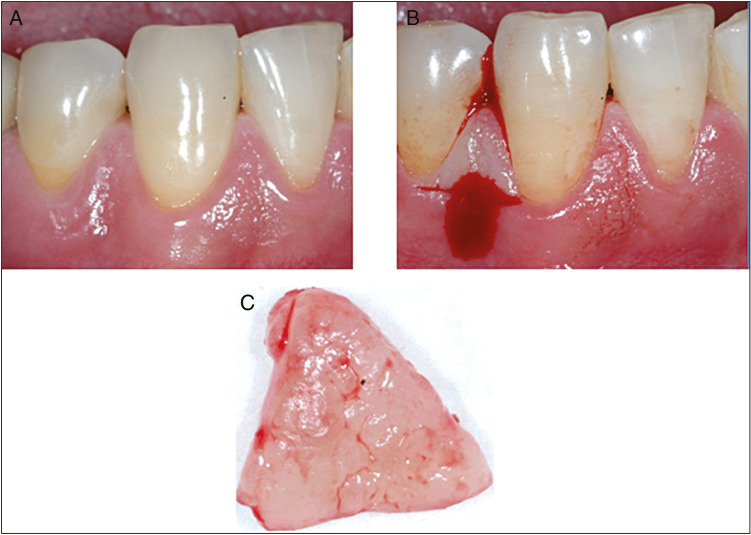
Before the biopsy procedure, all patients were asked to rinse with chlorhexidine 0.2% for 15s. The selected sites in the two groups received supragingival scaling using light ultrasonic instrumentation. Local anesthesia without vasoconstrictor (Scandicaine 3%, Mepivacaïne, Septodont, Saint-Maur-des–Fosses, France) was injected in the alveolar mucosa to circumvent gingival vessels alteration or vasoconstriction. Local infiltration in the surrounding gingiva was avoided. A standardized area, including the proximal papilla with a height of 5 mm and a depth of 3–4 mm, was resected. A horizontal mesiodistal incision located at 5 mm apically to the tip of the papilla, and extending in a lingual direction to a depth of 3–4 mm was supplemented by a vertical incision in the interproximal area [Figure 1].
Figure 1.
Buccal photographs showing the incisions used for biopsy harvesting in the interproximal area canine–first premolar in mandibular (A–C)
HISTOLOGICAL AND HISTOCHEMICAL PREPARATION
Immediately following biopsy procurement, all samples were fixed in 10% buffered formalin solution for at least 24h. As per the steps required for histological and histochemical preparation, the samples were dehydrated in five subsequent alcohol baths of ascending concentrations, cleaned in four successive xylol baths, and embedded in paraffin. The samples were subsequently sectioned along a plane perpendicular to the oral vestibular epithelium and the long axis of the tooth, starting from the coronal aspect of the papilla and proceeding apically. Two 5–6 micron sections were obtained at 600-μm intervals, resulting in sections showing both sulcular epithelia of the two adjacent teeth and the keratinized buccal oral epithelium. Each specimen yielded 4 or 5 levels of sections according to the size of the specimen. After staining with hematoxylin–eosin, the most representative section was selected according to the following criteria: (1) sections large enough to contain at least 1 mm of connective tissue subjacent to the sulcular epithelia of the adjacent teeth, and (2) no tissue folding in the area of interest. The section stained with hematoxylin–eosin was used for evaluation of tissue morphology, whereas the corresponding second section of the selected level was prepared for immunohistochemical staining.
IMMUNOHISTOCHEMICAL ANALYSIS
Vascular endothelium was labeled immunohistochemically using the anti-CD34 primary antibody directed against the human CD34 antigen expressed by endothelial cells (Novocastra clone, QBEnd/10, Novocastra Laboratories, Newcastle upon Tyne, UK). As most formalin-fixed tissues require an antigen retrieval step before immunohistochemical staining, the sections were heated in a microwave oven in two 5–10 min periods. Endogenous peroxidase was inhibited by incubation in phosphate-buffered saline (pH 7.2). Nonspecific binding of the antibody was blocked with 10% normal rabbit serum. The sections were then incubated with the primary antibody (diluted 1:100) for 60 min at room temperature. The binding antibody was visualized by means of a immunostaining kit; 3.3’-diamino-benzidine was used as a chromogen, and the sections were counterstained with Mayer’s hematoxylin.
SELECTION OF MEASURING FIELDS AND ASSESSMENT
Field selection in the specimens was performed in the following three different areas [Figure 2]:
Figure 2.
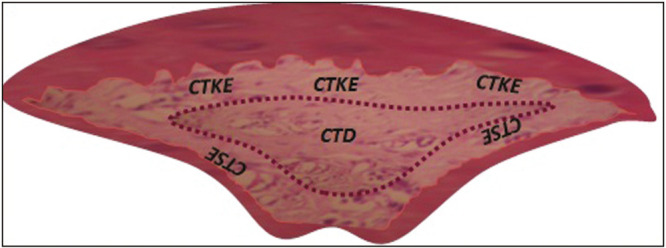
Histological section of a papilla with the schematic representation of the three regions selected for immunohistochemical evaluation of connective tissue vasculature. CTD refers to the deep connective tissue of the papilla, CTKE to the connective tissue subjacent to the oral epithelium, and CTSE to the connective tissue immediately subjacent to the sulcular non-keratinized epithelium
-
-
The deep connective tissue in the central portion of the biopsy (Region CTD)
-
-
The connective tissue portion of the biopsy immediately subjacent to the oral epithelium (Region CTKE)
-
-
The connective tissue portion of the biopsy immediately subjacent to the sulcular epithelium (Region CTSE)
Under a blinded protocol, the selected slides were numbered, coded, and subsequently analyzed by the same investigator.
The slides were observed at ×40 magnification under a light microscope equipped with an incorporated micrometer. One to three square fields measuring (250 × 250) μm2 were selected in each region according to the size of the specimen. The total number of capillaries (V) per unit area, the number of small vessels (internal circumference <50 μm) (S), medium vessels (internal circumference between 50 and 100 μm) (M), and larger vessels (internal circumference >100 μm) (L) were evaluated in all fields [Table 1]. In cases where more than one field was evaluated, the average of the fields was calculated for the region. A strict set of measurement criteria was used for consistent and reproducible recognition of blood vessels in each section: (1) structures were counted as blood vessels only if a definite lumen was present, and (2) structures were counted as blood vessels only if a closed circular or elliptical endothelial cell pattern was observed.
Table 1.
Intergroup and intragroup comparison of the number of small, medium, and large vessels in the three regions taken separately or as a whole
| SP | NP | |||||||
|---|---|---|---|---|---|---|---|---|
| CTD | CTKE | CTSE | CT | CTD | CTKE | CTSE | CT | |
| Small | 3.20 (±1.68) | 5.40 (±2.90) | 6.80 (±1.89) | 15.40 (±4.46) | 3.88 (±2.25) | 7.50 (±4.21) | 8.50 (±2.29) | 19.88 (± 5.48) |
| Medium | 2.60 (±1.64) | 2.80 (±2.49) | 2.80 (±1.48) | 8.20 (±4.01) | 2.25 (±0.83) | 1.50 (±1.00) | 2.30 (±1.79) | 6.05 (± 2.36) |
| Large | 2.20 (±1.15) | 0.60 (±1.34) | 0.50 (±0.50) | 3.30 (±2.68) | 2.00 (±1.22) | 0.30 (±0.45) | 0.60 (±1.34) | 2.90 (± 2.13) |
| Total | 8.00 (±3.82) | 8.80 (±3.17) | 10.10 (±0.74) | 26.90 (±6.20) | 8.13 (±4.16) | 9.30 (±4.16) | 11.40 (±2.97) | 28.83 (± 7.65) |
CTD = deep connective tissue in the central portion of the biopsy, CTKE = connective tissue portion of the biopsy immediately subjacent to the oral epithelium, CTSE = connective tissue portion of the biopsy immediately subjacent to the sulcular epithelium, CT = the whole connective tissue of the papilla, NP = nonsmoker patients with periodontitis, SP = smoker patients with periodontitis
STATISTICAL ANALYSIS
Mean values, ranges, and standard deviations were computed for the variables V, S, M, and L for each of the three regions, for single patients and for groups of patients. The Wilcoxon test was used to compare the mean of all variables within regions, in between regions in the same group of patients, and between patient groups. Data were analyzed using the SAS 9.1.3. Service Pack 4 (SAS Institute, Cary, North Carolina), and the level of statistical significance was set at P < 0.05.
RESULTS
Only 12 of the 20 biopsies procured yielded representative sections that were considered adequate for histological analysis. Mean age in the corresponding patient population was 42.75 ± 5.7 years. These representative biopsies were distributed as follows: seven in the SP group, and five in the NP group. Immediately before the biopsy procedures, all patients in the two groups had a full mouth plaque score (FMPS) <10%, a full mouth mean GI <1, and the biopsy sites were devoid of visual inflammatory signs. All biopsy sites healed uneventfully with total rebound of the interproximal gingival tissues within 2–3 months. No buccal or lingual recessions were observed in the short-term follow-up period up to 3 months. At the biopsy sites, the CALs averaged 6.65 ± 1.04 mm in the two groups. The mean total numbers of vessels, in addition to the mean number of small, medium, and large vessels in the various connective tissue regions of the two patient groups, are reported in Table 1.
INTERREGIONAL AND INTERGROUP COMPARISON OF THE TOTAL NUMBER OF VESSELS INCLUDING SMALL, MEDIUM, AND LARGE DIAMETER VASCULATURE
The mean total number of vessels (V) in the three regions taken separately in specific regions (CTD V versus CTKE V versus CTSE V) and as a whole were compared. The total number of vessels (S + M + L) in the entire connective tissue area of the biopsy was not statistically different between the two patient groups: SP and NP (P > 0.05). Furthermore, when the three regions of the biopsy were taken separately, no statistically significant differences could be detected between patient groups. In addition, when the number of vessels in each patient group was compared between the three connective tissue areas (CTD, CTKE, and CTSE), the differences were not statistically different.
INTERREGIONAL AND INTERGROUP COMPARISON OF THE SMALL-SIZED VESSELS
The differences between the total number of small vessels in the various regions of the two groups were compared. Overall, no statistically significant differences were observed between groups when comparing the number of small vessels in the three connective tissue areas. However, the number of small vessels (S) was significantly smaller in the CTD region when compared to the more superficial connective tissue area CTSE of the biopsy in both groups.
INTERREGIONAL AND INTERGROUP COMPARISON OF THE MEDIUM-SIZED VESSELS
Similar evaluations were carried out for medium size vessels. The results show no statistically significant differences between the two groups when considering the total number of medium vessels (M) or the fraction of medium vessels in the various regions of the connective tissue. Moreover, no significant difference was observed in the number of medium vessels (M) between the three regions (CTD, CTKE, and CTSE) in each of the two groups.
INTERREGIONAL AND INTERGROUP COMPARISON OF THE LARGE-SIZED VESSELS
When the total number of large vessels was assessed, no significant difference was found between the two groups. Interregional comparison showed that the number of large vessels in the CTD region was significantly higher than that in the CTKE region for the two groups. No such difference was evident between the CTKE and the CTSE connective tissue areas. Overall, the CTSE contained a less significant number of large vessels than CTD in the two groups.
DISCUSSION
This study was performed to investigate in periodontally affected subjects, the effect of smoking on the number of vessels and internal circumference of vessels stained with monoclonal antibody to CD34. This immunohistochemical staining technique has been reported to be a reproducible method for this purpose as the antibody to CD34 has been shown to be mostly expressed on microvessels with no staining of inflammatory cells and with little or no background staining.[30] Further objectives of this investigation were to evaluate the distribution of vessels per diameter and connective tissue region of the biopsy.
The results of this study showed that smoking and periodontitis do not significantly alter the total number of vessels in the entire connective tissue area of the biopsy (CT) compared to periodontitis alone. These conclusions are in agreement with the findings of Mirbod et al.,[21] which highlighted the absence of statistically significant differences in vascular density in the vestibular gingiva between smokers and nonsmokers treated for periodontal disease. Similarly, Sönmez et al.[35] indicated that smoking did not affect the number of vessels per millimeter square of stroma in the subepithelial connective tissue of gingival biopsies procured from periodontitis patients after initial periodontal therapy, in which signs of inflammation were minimized. In contrast, Rezavandi et al.[25] reported a significantly larger number of vessels in inflamed gingival tissue biopsies obtained from nonsmoking patients with periodontitis when compared to those harvested in periodontally affected smokers. The discrepancies observed between the aforementioned studies could be related to the unresolved inflammation in the untreated periodontal patient sample included in a study by Rezavandi et al.[25]
Vascular response to induced gingival inflammation in a primate model has been reported to result in a generalized increase in blood vessel numbers in the gingival connective tissues.[36] In humans, Penmetsa et al.[37] reported an increase in the number of vessels in gingival biopsies in fields adjacent to plaque irritants from patients with chronic gingivitis and chronic periodontitis. In this study, the specific effect of periodontitis compared to healthy gingiva was not evaluated. When considering the distribution of vessels per diameter and region, the two patient groups had similar total numbers of medium vessels and fractions of medium vessels in the three considered regions of the biopsies. The relevance of this finding and its hemodynamic significance are difficult to interpret. Conversely, smoking and periodontitis resulted in a significant redistribution of small and large vessels among regions compared to periodontitis alone. Although a similar distribution of small vessels was observed within the various regions, the small vessels seem to predominate in the peripheral regions in the two groups. This distribution is to be expected as it conforms with the physiological vascular distribution within tissues where small capillaries prevail peripherally.
Although small vessels prevailed in the peripheral regions of the gingival connective tissue in the two groups, large vessels were predominant in the deep areas. Chapple et al.[38] reported an increase in larger diameter vessels through an extensive remodeling of the vasculature in untreated chronic periodontal disease. This study showed that in smokers, periodontitis did not result in a significant change in the number of large or small vessels in the entire biopsy or in individual regions when compared to nonsmokers with periodontal involvement. A controversial conclusion was suggested by Mirbod et al.,[21] they indicated that smokers showed a higher proportion of small and lower proportion of large vessels regardless of the level of inflammation. The analysis of these findings is intricate as the significance of capillary diameter in inflammation and tobacco use is still unclear. In addition, variations in the site of biopsy harvesting (buccal versus interproximal), patients age,[39] the level of inflammation (treated versus chronic versus acute), and the method of inflammation evaluation render difficult the interpretation of results.
This study had certain limitations due to the inability of the immunohistochemical preparation to differentiate between functionally open and closed vessels. Therefore, the functional blood flow was not reflected in the measurements. The quantitative analysis evidenced certain trends, which were not always statistically significant due to the small number of specimens and large intragroup variations. Moreover, in both groups, the quantitative distribution of small, medium, and large vessels follows a similar trend with the content in small vessels being significantly more important than both medium and large vessels. This finding applies to the entire biopsy area and fractioned regions taken separately. Small vessels predominate in the peripheral regions, whereas large vessels are more abundant in the deeper connective tissue areas.
The total number of vessels in the interdental marginal gingiva is unaffected by chronic cigarette smoking in periodontally affected patients in the entire biopsy area and in the separate connective tissue regions; there is no effect of smoking on the number of medium vessels in the entire or fractioned connective tissue fields in periodontitis patients.
CONCLUSION
Smoking and periodontitis result in a redistribution of small and large vessels in the superficial and deeper connective tissue areas of the gingival papilla compared to nonsmoking periodontal patients. This vascular redistribution can be the result of vascular remodeling or angiogenesis. The significance of such rearrangement of vasculature within the gingival tissue remains to be elucidated.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
Ethical approval was obtained from the ethics committee of the Lebanese University (CUEMB No. 23) for this study.
DECLARATION OF PATIENT CONSENT
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
ACKNOWLEDGEMENT
We acknowledge the valuable help of Dr. Khaled Habib with the histological and histochemical preparation of the samples.
REFERENCES
- 1.Joseph B, Javali MA, Khader MA, Al Qahtani SM, Amanullah M. Salivary osteocalcin as potential diagnostic marker of periodontal bone destruction among smokers. Biomolecules. 2020;10:E380. doi: 10.3390/biom10030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Y, Zhou X, Cheng L, Li M. The impact of smoking on subgingival microflora: From periodontal health to disease. Front Microbiol. 2020;11:66. doi: 10.3389/fmicb.2020.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutton JD, Salas Martinez ML, Gerkovich MM. Environmental tobacco smoke and periodontitis in United States non-smokers, 2009 to 2012. J Periodontol. 2017;88:565–74. doi: 10.1902/jop.2017.160725. [DOI] [PubMed] [Google Scholar]
- 4.Baljoon M, Natto S, Bergström J. Long-term effect of smoking on vertical periodontal bone loss. J Clin Periodontol. 2005;32:789–97. doi: 10.1111/j.1600-051X.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 5.Bergström J. Influence of tobacco smoking on periodontal bone height. Long-term observations and a hypothesis. J Clin Periodontol. 2004;31:260–6. doi: 10.1111/j.1600-051X.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang CH, Han ML, Teng NC, Lee CY, Huang WT, Lin CT, et al. Cigarette smoking aggravates the activity of periodontal disease by disrupting redox homeostasis—An observational study. Sci Rep. 2018;8:11055. doi: 10.1038/s41598-018-29163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung WK, Ng DK, Jin L, Corbet EF. Tooth loss in treated periodontitis patients responsible for their supportive care arrangements. J Clin Periodontol. 2006;33:265–75. doi: 10.1111/j.1600-051X.2006.00903.x. [DOI] [PubMed] [Google Scholar]
- 8.Kubota M, Yanagita M, Mori K, Hasegawa S, Yamashita M, Yamada S, et al. The effects of cigarette smoke condensate and nicotine on periodontal tissue in a periodontitis model mouse. PLoS One. 2016;11:e0155594. doi: 10.1371/journal.pone.0155594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matuliene G, Pjetursson BE, Salvi GE, Schmidlin K, Brägger U, Zwahlen M, et al. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J Clin Periodontol. 2008;35:685–95. doi: 10.1111/j.1600-051X.2008.01245.x. [DOI] [PubMed] [Google Scholar]
- 10.Mosely LH, Finseth F, Goody M. Nicotine and its effect on wound healing. Plast Reconstr Surg. 1978;61:570–5. doi: 10.1097/00006534-197804000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Javed F, Al-Rasheed A, Almas K, Romanos GE, Al-Hezaimi K. Effect of cigarette smoking on the clinical outcomes of periodontal surgical procedures. Am J Med Sci. 2012;343:78–84. doi: 10.1097/MAJ.0b013e318228283b. [DOI] [PubMed] [Google Scholar]
- 12.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 13.Laxman VK, Annaji S. Tobacco use and its effects on the periodontium and periodontal therapy. J Contemp Dent Pract. 2008;9:97–107. [PubMed] [Google Scholar]
- 14.Şentürk RA, Sezgin Y, Bulut Ş, Özdemir BH. The effects of smoking on the expression of gelatinases in chronic periodontitis: A cross-sectional study. Braz Oral Res. 2018;32:e114. doi: 10.1590/1807-3107bor-2018.vol32.0114. [DOI] [PubMed] [Google Scholar]
- 15.Muniandy S. Knowledge on smoking and periodontal disease: A cross-sectional survey among targeted respondents. J Indian Soc Periodontol. 2019;23:275–80. doi: 10.4103/jisp.jisp_479_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan CP, Leung WK, Wong MC, Wong RM, Wan P, Lo EC, et al. Effects of smoking on healing response to non-surgical periodontal therapy: A multilevel modelling analysis. J Clin Periodontol. 2009;36:229–39. doi: 10.1111/j.1600-051X.2008.01371.x. [DOI] [PubMed] [Google Scholar]
- 17.Chambrone L, Chambrone D, Pustiglioni FE, Chambrone LA, Lima LA. The influence of tobacco smoking on the outcomes achieved by root-coverage procedures: A systematic review. J Am Dent Assoc. 2009;140:294–306. doi: 10.14219/jada.archive.2009.0158. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Wolff L, Aeppli D, Guo Z, Luan W, Baelum V, et al. Cigarette smoking, salivary/gingival crevicular fluid cotinine and periodontal status. A 10-year longitudinal study. J Clin Periodontol. 2001;28:331–9. doi: 10.1034/j.1600-051x.2001.028004331.x. [DOI] [PubMed] [Google Scholar]
- 19.McGuire JR, McQuade MJ, Rossmann JA, Garnick JJ, Sutherland DE, Scheidt MJ, et al. Cotinine in saliva and gingival crevicular fluid of smokers with periodontal disease. J Periodontol. 1989;60:176–81. doi: 10.1902/jop.1989.60.4.176. [DOI] [PubMed] [Google Scholar]
- 20.Johnson GK, Fung YK, Squier CA. Effects of systemic administration of nicotine on capillaries in rat oral mucosa. J Oral Pathol Med. 1989;18:230–2. doi: 10.1111/j.1600-0714.1989.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 21.Mirbod SM, Ahing SI, Pruthi VK. Immunohistochemical study of vestibular gingival blood vessel density and internal circumference in smokers and non-smokers. J Periodontol. 2001;72:1318–23. doi: 10.1902/jop.2001.72.10.1318. [DOI] [PubMed] [Google Scholar]
- 22.Scardina GA, Messina P. Morphologic changes in the microcirculation induced by chronic smoking habit: A videocapillaroscopic study on the human gingival mucosa. Am J Dent. 2005;18:301–4. [PubMed] [Google Scholar]
- 23.Lova RM, Miniati B, Macchi C, Gulisano M, Gheri G, Catini C, et al. Morphologic changes in the microcirculation induced by chronic smoking habit: A videocapillaroscopic study on the human labial mucosa. Am Heart J. 2002;143:658. doi: 10.1067/mhj.2002.121461. [DOI] [PubMed] [Google Scholar]
- 24.Bonakdar MP, Barber PM, Newman HN. The vasculature in chronic adult periodontitis: A qualitative and quantitative study. J Periodontol. 1997;68:50–8. doi: 10.1902/jop.1997.68.1.50. [DOI] [PubMed] [Google Scholar]
- 25.Rezavandi K, Palmer RM, Odell EW, Scott DA, Wilson RF. Expression of ICAM-1 and E-selectin in gingival tissues of smokers and non-smokers with periodontitis. J Oral Pathol Med. 2002;31:59–64. doi: 10.1046/j.0904-2512.2001.joptest.doc.x. [DOI] [PubMed] [Google Scholar]
- 26.Grudianov AI, Kemulariia IV. [Laser Doppler estimation of the influence of tobacco-smoking on the blood microcirculation in the periodont at the patients with the different stages of periodontal diseases] Stomatologiia (Mosk) 2010;89:10–4. [PubMed] [Google Scholar]
- 27.Kumar V, Faizuddin M. Effect of smoking on gingival microvasculature: A histological study. J Indian Soc Periodontol. 2011;15:344–8. doi: 10.4103/0972-124X.92566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geurs N, Iacono V, Krayer J, Mealey BL, Paquette D, Pearson B, et al. American Academy of Periodontology Task Force report on the update to the 1999 classification of periodontal diseases and conditions. J Periodontol. 2015;86:835–8. doi: 10.1902/jop.2015.157001. [DOI] [PubMed] [Google Scholar]
- 29.Yun WJ, Shin MH, Kweon SS, Ryu SY, Rhee JA. Association of smoking status, cumulative smoking, duration of smoking cessation, age of starting smoking, and depression in Korean adults. BMC Public Health. 2012;12:724. doi: 10.1186/1471-2458-12-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260–7. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 31.Alpagot T, Wolff LF, Smith QT, Tran SD. Risk indicators for periodontal disease in a racially diverse urban population. J Clin Periodontol. 1996;23:982–8. doi: 10.1111/j.1600-051x.1996.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 32.Norderyd O, Hugoson A. Risk of severe periodontal disease in a Swedish adult population. A cross-sectional study. J Clin Periodontol. 1998;25:1022–8. doi: 10.1111/j.1600-051x.1998.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 33.Löe H. The gingival index, the plaque index and the retention index system. J Periodontol. 1967;38:610–16. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 34.Silness J, Loe H. periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 35.Sönmez S, Canda T, Ozkara E, Ak D. Quantitative evaluation of the vasculature and fibronectin localization in gingival connective tissue of smokers and non-smokers. J Periodontol. 2003;74:822–30. doi: 10.1902/jop.2003.74.6.822. [DOI] [PubMed] [Google Scholar]
- 36.Nemeth E, Kulkarni GV, McCulloch CA. Proliferative responses of endothelial cell populations to experimentally induced inflammatory lesions of gingival connective tissues in the cynomolgus monkey (Macaca fascicularis) Anat Rec. 1994;239:9–17. doi: 10.1002/ar.1092390103. [DOI] [PubMed] [Google Scholar]
- 37.Penmetsa GS, Baddam S, Manyam R, Dwarakanath CD. Comparison of the number of gingival blood vessels between type 2 diabetes mellitus and chronic periodontitis patients: An immunohistological study. J Indian Soc Periodontol. 2015;19: 164–8. doi: 10.4103/0972-124X.152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapple CC, Kumar RK, Hunter N. Vascular remodelling in chronic inflammatory periodontal disease. J Oral Pathol Med. 2000;29:500–6. doi: 10.1034/j.1600-0714.2000.291004.x. [DOI] [PubMed] [Google Scholar]
- 39.Matheny JL, Johnson DT, Roth GI. Aging and microcirculatory dynamics in human gingiva. J Clin Periodontol. 1993;20:471–5. doi: 10.1111/j.1600-051x.1993.tb00393.x. [DOI] [PubMed] [Google Scholar]