Abstract
The coronavirus SARS-CoV-2 pandemic became a global health burden. We determined the susceptibility of SARS-CoV-2 to irradiation with ultraviolet light. The virus was highly susceptible to ultraviolet light. A viral stock with a high infectious titer of 5 × 106 TCID50/mL was completely inactivated by UVC irradiation after nine minutes of exposure. The UVC dose required for complete inactivation was 1,048 mJ/cm2. UVA exposure demonstrated only a weak effect on virus inactivation over 15 minutes. Hence, inactivation of SARS-CoV-2 by UVC irradiation constitutes a reliable method for disinfection purposes in health care facilities and for preparing SARS-CoV-2 material for research purpose.
Key Words: COVID-19, Ultraviolet light, Inactivation
Background
In December 2019, a novel coronavirus causing severe acute respiratory disease (SARS-CoV-2) was newly identified in the Hubei province, PR China, before becoming a global pandemic and causing tremendous health and socio-economic burdens.1 At the time of writing, more than 14.9 million cases and >618,000 deaths were reported worldwide (2020.07.23). The actual number of people infected with SARS-CoV-2 is most likely to be much higher since numerous infections, especially in younger people, are asymptomatic and frequently not captured by routine diagnostic methods.2 The symptoms of COVID-19 range from mild respiratory illness accompanied by cough, fever, myalgia, and fatigue, to severe, life-threatening pneumonia, and acute respiratory distress syndrome.3 Clearly, the prevention of the transmission of respiratory infections especially within hospitals or other institutions is of central importance. The disinfection of objects using UV-irradiation is an environmentally friendly method of killing bacteria, fungi and viruses without the use of harmful chemicals or heat. Consequently, UV light disinfection is becoming increasingly applied in healthcare facilities for disinfecting healthcare equipment, surfaces, and operating rooms.4 However, the efficacy of UV irradiation on the inactivation of SARS-CoV-2 in fluids has not been described thus far. In the present study, we investigated the susceptibility of high titer viral stocks of SARS-CoV-2 to combined or separate UVA and/or UVC irradiation.
Material and Methods
Isolation of SARS-CoV-2 from a nasopharyngeal swab
A clinical isolate of SARS-CoV-2 was isolated from a nasopharyngeal swab of a patient suffering from COVID-19 disease. The patient was hospitalized at the Department of Infectious Diseases of the University Hospital Essen. The swab was taken using a Virocult vial (Sigma, Germany). The Virocult medium was then incubated on Vero E6 cells cultured in DMEM containing 10% (v/v) fetal calf serum and supplemented with Penicillin (100 IU/mL), Streptomycin (100 µg/mL), Ciprofloxacin (10 µg/mL), and Amphotericin B (2.5 µg/mL). After 5 days of incubation, the supernatant was harvested and cell debris was removed by centrifugation. Afterwards, 100 µL of the clear supernatant was used for subsequent infection of a new Vero E6 cell culture flask. After 5 days of incubation, supernatants were found to be positive for SARS-CoV-2 by a conventional qualitative PCR. The virus suspension was harvested and cleared from cellular debris by centrifugation and stored at −80°C. Viral titers were determined by endpoint dilution assay and the 50% tissue culture infective dose (TCID50) was calculated.
Inactivation of SARS-CoV-2 by UV-irradiation
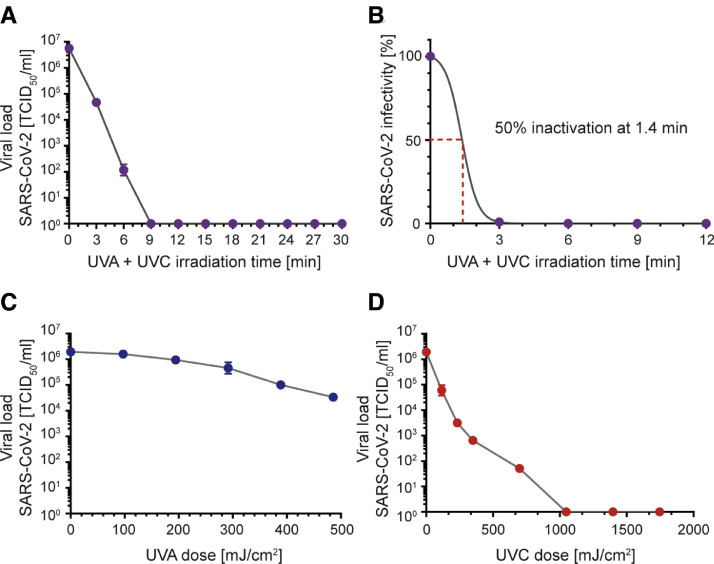
To determine the susceptibility of SARS-CoV-2 to UVA and/or UVC irradiation, a viral stock at a concentration of 5 × 106 TCID50/mL was irradiated with UV light for up to 30 minutes. UV exposure was performed by separate or combined irradiation with UVC (254 nm) and/or UVA (365 nm) of 600 µL virus stock in 24-well plates. The UV light source (UV-4 S/L, order no. 2950440, Herolab, Wiesloch, Germany) was placed at a distance of 3 cm above the bottom of the plate. The emitted light intensity was UVC (254 nm) = 1940 µW/cm2 and UVA (365 nm) = 540 µW/cm2 at a distance of 3 cm, as measured by radiometric analysis. This corresponds to an applied light dose of 1.94 mJ/cm2 per second for UVC and 0.54 mJ/cm2 per second for UVA, while µW = 10−6 J/s. The samples were taken after 0, 3, 6, 9, 12, 15, 18, 21, 24, 27, and 30 minutes of combined UVA and UVC irradiation. Samples irradiated with UVA were taken after 0, 3, 6, 9, 12, 15 minutes, and after UVC-irradiation after 0, 1, 2, 3, 6, 9, and 15 minutes. The TCID50/ml concentration of each sample was determined by endpoint dilution, respectively.
Results
SARS-CoV-2 showed high susceptibility to UV irradiation. Total inactivation of SARS-CoV-2 at a concentration of 5 × 106 TCID50/mL was achieved after 9 minutes of combined UVA and UVC exposure (Fig 1 A). As calculated by nonlinear regression, 50% of the virus could be inactivated after 1.4 minutes of UV-treatment (Fig 1B). UVA exposure alone was less effective on virus inactivation. After 9 minutes of irradiation and an emitted dose of 292 mJ/cm2, 1 log reduction of the viral load was observed. In contrast, complete virus inactivation was achieved after a 9 minute exposure to UVC and an emitted UVC dose of 1048 mJ/cm2. These data confirm former findings that UVC is more effective in inactivating viruses, and highlight UVC irradiation as an effective method for the inactivation of SARS-CoV-2.
Fig 1.
Inactivation of SARS-CoV-2 by UV irradiation. SARS-CoV-2 at a starting concentration of 5 × 106 TCID50/ml was irradiated with ultraviolet light (UV). UV treatment was performed by irradiation with UVC (254 nm) and/or UVA (365 nm) on 600 µl aliquots of virus in 24-well plates. The UV light source was placed at a distance of 3 cm above the bottom of the plate. Viral loads were determined by end point dilution after (A) combined UVA/UVC exposure at the indicated time points or separate exposure to (C) UVA light after 0, 3, 6, 9, 12, and 15 minutes or (D) UVC light after 0, 1, 2, 3, 6, 9, 12, and 15 minutes. (B) Nonlinear regression was conducted to calculate the duration of combined UVA and UVC irradiation sufficient to inactivate the virus by 50%. The emitted light dose was measured with = 1940 µW/cm2 for UVC (254 nm) and 540 µW/cm2 for UVA (365 nm) at a distance of 3 cm. This corresponds to an applied light dose of 1.94 mJ/cm2 per second for UVC and 0.54 mJ/cm2 per second for UVA (µW = 10−6 J/s). The experiments were performed in triplicates. Error bars represent the standard deviation of the mean.
Discussion
In the present study, we demonstrated that SARS-CoV-2 could effectively be inactivated by UVC irradiation, even at high viral titers, whereas UVA-irradiation was much less effective. These data are in line with previous reports where other coronaviruses eg, SARS-CoV-1 were shown to be susceptible to UVC irradiation.5, 6, 7 Viral stocks with titers of 1 × 106 TCID50/mL of SARS-CoV-1 could be almost completely inactivated after 6 minutes of UVC-irradiation, corresponding to a UVC dose of 1446 mJ/cm2.5 In our study, the emitted dose required for a complete inactivation of SARS-CoV-2 was 1048 mJ/cm2 after 9 minutes of exposure. A similar dose of 1 J/cm2 was also required to inactivate a viral load of 1 * 106 TCID50 H1N1 influenza virus.7 UV light disinfection is chemical free and thus a suitable method for applying in healthcare facilities to disinfect healthcare equipment.4 Most recently, a protocol for the disinfection of personal protective equipment including filtering face pieces from health care workers described the potential use of ultraviolet light to inactivate SARS-CoV-2.6 Taken together, we demonstrated that UV irradiation is a highly effective method to inactivate the new corona virus SARS-CoV-2, even at the higher viral load levels that are found in research laboratories eg, in cell-culture supernatants or in diagnostic material taken from the respiratory tract of COVID-19 patients.
Conclusion
We demonstrated that SARS-CoV-2, even at high viral titers, could be inactivated rapidly by UVC irradiation, revealing that this method is reliable not only for disinfection purposes in health care facilities but also for preparing inactivated SARS-CoV-2 material for research.
Acknowledgments
This study was supported by the Stiftung Universitätsmedizin Essen (awarded to K. Sutter, M. Trilling and A. Krawczyk) and the Rudolf Ackermann Foundation (awarded to O. Witzke). The authors thank Delia Cosgrove for the proofreading of the manuscript.
Footnotes
Conflicts of interest: None to report.
Author Contributions: C.S. Heilingloh, U. Dittmer, O. Witzke: study design, data collection, data interpretation; U.W. Aufderhorst, L. Schipper, M. Alt: experiments, data analysis, data interpretation; D. Yang, X. Zheng, K. Sutter, M. Trilling: data analysis, data interpretation; writing; C.S. Heilingloh, E. Steinmann, A. Krawczyk study design, writing, figure design.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casini B, Tuvo B, Cristina ML, et al. Evaluation of an ultraviolet C (UVC) light-emitting device for disinfection of high touch surfaces in hospital critical areas. Int J Environ Res Pub Health. 2019;16 doi: 10.3390/ijerph16193572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell ME, Subbarao K, Feinstone SM, Taylor DR. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson WA, Derraik JGB, Connelly EA, Anderson YC. Rapid evidence summary on SARS-CoV-2 survivorship and disinfection, and a reusable PPE protocol using a double-hit process. medRxiv. 6 Apr 2020 https://www.medrxiv.org/content/10.1101/2020.04.02.20051409v1 [Epub ahead of print]. Available at: [Google Scholar]
- 7.Brian Heimbuch DH. Applied Research Associates; 2019. Research to mitigate a shortage of respiratory protection devices during public health emergencies.https://www.ara.com/sites/default/files/ARAReviewN95FFRDecontamination.pdf Available at: [Google Scholar]