Abstract
Searching for the mechanisms of the polycystic ovary syndrome (PCOS) pathophysiology has become a crucial aspect of research performed in the last decades. However, the pathogenesis of this complex and heterogeneous endocrinopathy remains unknown. Thus, there is a need to investigate the metabolic pathways, which could be involved in the pathophysiology of PCOS and to find the metabolic markers of this disorder. The application of metabolomics gives a promising insight into the research on PCOS. It is a valuable and rapidly expanding tool, enabling the discovery of novel metabolites, which may be the potential biomarkers of several metabolic and endocrine disorders. The utilization of this approach could also improve the process of diagnosis and therefore, make treatment more effective. This review article aims to summarize actual and meaningful metabolomic studies in PCOS and point to the potential biomarkers detected in serum, urine, and follicular fluid of the affected women.
Keywords: metabolomics, polycystic ovary syndrome (PCOS), metabolites, biomarkers, mass spectrometry
1. Introduction
1.1. Polycystic Ovary Syndrome
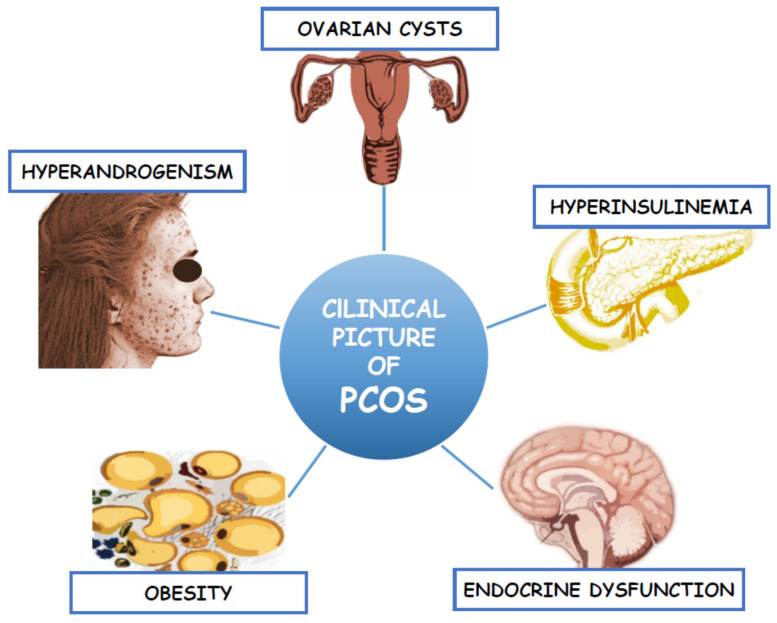
Polycystic ovary syndrome (PCOS) is a complex endocrinopathy, which affects more than 10% of women of reproductive age [1]. It is the main cause of female infertility due to oligo- or anovulation. Despite such a high incidence, the pathogenesis of PCOS is still unexplained. Some studies suggest that it is due to the genetic factors associated with ovarian steroidogenesis [2]. According to the Androgen Excess and PCOS Society (AE&PCOS), the diagnosis of PCOS should be based on the presence of clinical and/or biochemical hyperandrogenism (HA) and the ovarian dysfunction defined as menstrual abnormalities (anovulatory oligomenorrhea (AnO)) or/and the presence of the polycystic ovary morphology (PCOM) in the transvaginal ultrasound (TV-US) [3]. These criteria yield three separate PCOS phenotypes: A, B, and C. Phenotype A includes all the three features (HA, AnO, and PCOM) whereas phenotype B and C only two (HA and AnO or HA and PCOM, respectively). However, regarding Rotterdam criteria, the fourth phenotype (D) was separated to comprise AnO and PCOM presence. The clinical symptoms of hyperandrogenism include hirsutism (present in 60% of women), androgenic alopecia, and acne, which negatively affect women’s psyche, their femininity and lead to low self-esteem and depression [4]. In addition to the reproductive and endocrine dysfunction, PCOS is characterized by intrinsic insulin resistance (IR), which lead to the development of the metabolic syndrome (MetS) and its consequences such as disturbed carbohydrate metabolism and type 2 diabetes mellitus (T2DM). Most common clinical manifestation in PCOS is abdominal obesity, which is involved in the development of dyslipidemia, arterial hypertension (AH), as well as non-alcoholic fatty liver disease (NAFLD) [5,6,7,8]. These in turn lead to the development of cardiovascular disease (CVD), which still remains the main cause of death among women [9]. The clinical picture of this complex endocrinopathy was presented in Figure 1. Therefore, the treatment of PCOS focuses not only on the symptoms of hyperandrogenism and infertility, but also on improving IR and its metabolic consequences [10]. Thus, there is a need for a better understanding of the pathomechanisms of this complex disorder through the identification of potential biomarkers with the use of new, non-invasive and specific methods. In recent years, one of the developing scientific approaches is metabolomics [11].
Figure 1.
Clinical picture of Polycystic Ovary Syndrome.
1.2. Metabolomic Approach in Studying the Pathogenesis of Polycystic Ovary Syndrome
Among “omics” techniques, metabolomics plays an important role in studying the potential mechanisms responsible for the development of PCOS. Metabolomics allows to identify and quantify small molecules, which occur in all living organisms [12]. The set of all human metabolites that have been identified so far is stored in the Human Metabolome Database (HMDB). Each year, the number of identified metabolites grows. Few years ago, about 41,000 metabolites were found, but now this database contains over 114,190 compounds. Among them, the following groups can be found: amino acids, lipids, peptides, vitamins, organic acids and both endo- and exogenous carbohydrates. Therefore, metabolomics serves as a valuable source of information. The metabolome indicates not only a genetically determined phenotype, but also points to the differences determined by other factors, such as age, diet, or physical activity. The application of metabolomics enables monitoring of the state of an organism and provides information on the compounds formed as a result of many biochemical processes. Any disturbances occurring in a living organism cause changes to the qualitative and quantitative profile of the metabolites. The metabolome describes both the physiological and pathological state of the organism. For this reason, it is known to be an attractive approach, compared to genomics and proteomics, which only suggest the presence of metabolic derangements that occur in the organism [13,14,15,16]. Due to this fact, the use of metabolomics in studying the pathophysiology of PCOS allows to monitor even the smallest biochemical changes in this endocrinopathy and therefore, may help in its diagnosis [17].
Among the many analytical techniques, chromatography coupled with mass spectrometry (MS) seems to be the “gold technique”. While chromatography allows for the separation of metabolites present in complex, biological samples, mass spectrometry provides specific information about the chemical structure of the compounds, such as characteristic fragmentation ions, accurate mass, and isotope distribution pattern utilized for the identification of metabolites. MS characterizes very high selectivity and sensitivity that allows to detect and measure trace amounts of metabolites [18]. The combination of MS with gas chromatography (GC-MS) and liquid chromatography (LC-MS) enables to analyse complex biological samples broadly used in metabolomics. GC-MS is suitable for volatile and non-volatile compounds, which require a derivatization step, but first of all thermally stable analytes. The LC-MS technique is widely used for targeted and non-targeted metabolomic analysis and allows to qualify and identify more polar compounds [19]. Nuclear magnetic resonance (NMR), despite its lower sensitivity than MS, allows to analyse metabolites that are difficult to ionize or require derivative reaction for MS and identify compounds with the same masses [20]. A combination of these complementary techniques enables to analyse a broader array of metabolites and offers more certain results than their separate use.
1.3. Matrices for Metabolomic Studies
The application of metabolomics allows the use of several matrices such as tissue and body fluids (i.e., plasma, serum, saliva, follicular fluid, semen). The choice of the matrices is associated with the aim of the conducted study as well as the characteristics of the studied disorder. Ovarian tissue can also be used; however, sampling is invasive and problematic. It is usually obtained during laparoscopic wedge resection surgery. For this reason, the use of ovarian tissue in studying the pathophysiology of PCOS is not very common. The matrices widely used in metabolomic studies associated with PCOS are plasma and urine. Serum and urine samples are more common, because they are easily collectible and simple to prepare. On comparing the significantly altered metabolites, it can be observed that the results obtained for both matrices do not completely overlap. The new alternative matrix is follicular fluid, which is innovative in case of PCOS research, especially in terms of oocytes maturation and their quality [21,22,23].
2. Metabolic Alterations in PCOS
PCOS includes a number of abnormalities, which influence several metabolic pathways. It is especially characterized by disturbed metabolism of the steroid hormones, amino acids, carbohydrates, lipids, purines, and the citric acid cycle. Searching for these pathological changes is possible through the metabolomic analyses of biological samples such as serum or plasma, urine, and follicular fluid. Most studies focus on serum and plasma analysis; however, other biological samples also provide substantial information on the existing biochemical derangements. In this review paper, we concentrated on metabolomic studies that were performed in the period of 2014 to 2020 and analysed different biological samples. The PubMed database was searched using the terms “polycystic ovary syndrome” or “PCOS” and “metabolomics”. The most important and actual studies using plasma, serum, urine, or FF samples were then analysed.
2.1. Metabolomic Profile Plasma and Serum Samples
Murri et al. (2014) published a valuable review where they compared few studies based on the analyses of plasma obtained from PCOS women and healthy controls [11,24,25,26,27]. In this paper, we quoted this publication and also presented the results of new studies in this field published since 2014 [28,29,30,31,32,33,34,35,36,37]. A set of metabolites found as the most characteristic for PCOS is presented in Table 1. Additionally, information about the applied technique as well as the trend of regulation is included. As can be observed, three metabolic pathways seem to characterise PCOS. Among them, metabolites connected with lipid, amino acid, as well as energy metabolism such as citric acid cycle seem to be the most common. In the case of PCOS, down-regulation of glycerophospholipid metabolism and up-regulation of glucose metabolism was observed. The results published by Zhao et al. (2012) show that all of the determined fatty acids are up-regulated in PCOS compared to the control subjects [25]. A contrary phenomenon occurs in the case of phosphatidylcholine (PC), phosphatidylethanolamine (PE) and its derivatives lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE). Metabolites including PC, PE, LPE, and LPC are decreased. In turn, Fan et al. (2019) observed a decreased level of compounds involved in the metabolism of lecithin [36]. In several studies, androgen metabolism was also taken into account. Three major metabolites connected with elevated androgen metabolism were found, namely dehydroepiandrosterone sulphate (DHEAS), dihydrotestosterone sulphate (DHTS), and androsterone sulphate (ANDS) (Table 1). Amino acids (AAs) are the next group of endogenous compounds determined in samples collected from women with PCOS. According to the presented database, there is no homogenous pattern in AAs’ regulation. For instance, the levels of arginine, choline, citrulline, glutamate, glycine, and histidine were found to be decreased. In turn, Zhao et al. (2012) reported increased levels of endogenous AAs and glucogenic AAs [25].
Table 1.
The most significant changes in metabolites measured in plasma and serum samples in women with PCOS in comparison with control subjects.
| Metabolites | PCOS vs. Control | Metabolic Pathways | Studies | Techniques |
|---|---|---|---|---|
| Cholesterol | ↓ | Lipid metabolism | Zhao et al., 2012 | GC-MS GC-MS |
| ↓ | Escobar-Morreale et al., 2012 | |||
| ↓ | Buszewska-Forajta et al., 2019 | |||
| Alpha-Tocopherol | ↓ | Lipid metabolism | Escobar-Morreale et al., 2012 | GC-MS |
| HDL | ↓ | Lipid metabolism | Zhao et al., 2012 | NMR |
| Phosphatidylcholine | ↓ | Lipid metabolism | Zhao et al., 2012 | NMR |
| ↓ | Sun et al., 2012 | NMR | ||
| Linoleic acid | ↑ | Lipid metabolism | Zhao et al., 2012 | GC-MS |
| ↑ | Dong et al., 2015 | LC-MS | ||
| Lipoprotein | ↑ | Lipid metabolism | Zhao et al., 2012 | NMR |
| Palmitic acid | ↑ | Lipid metabolism | Zhao et al., 2012 | GC-MS |
| C18:0 stearic acid | ↑ | Lipid metabolism | Zhao et al., 2012 | GC-MS |
| ↓ | Szczuko et al., 2017 | GC-MS | ||
| Unsaturated fatty acid | ↑ | Lipid metabolism | Zhao et al., 2012 | NMR |
| VLDL/LDL | ↑ | Lipid metabolism | Zhao et al., 2012 | NMR |
| Lipid-CH2CH2CO | ↓ | Lipid metabolism | Atiomo et al., 2012 | NMR |
| ↑ | Zhao et al., 2012 | NMR | ||
| FFA 16:1 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 16:2 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 18:1 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 18:3 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 20:1 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 20:2 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 20:3 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 20:4 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 20:5 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 20:6 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 22:5 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 22:6 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| FFA 24:2 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| MG 18:1 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| MG 20:3 | ↑ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| LPC (16:1) | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| LPC (16:0) | ↓ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| LPC (18:0) | ↓ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| LPC (18:1) | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| ↓ | Haoula et al., 2015 | LC-MS | ||
| LPC (18:2) | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| ↓ | Dong et al., 2015 | LC-MS | ||
| ↓ | Jia et al., 2019 | LC-MS | ||
| ↑ | Buszewska-Forajta et al., 2019 | LC-MS | ||
| ↓ | Haoula et al., 2015 | LC-MS | ||
| LPC 18:3 | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| ↓ | Dong et al., 2015 | LC-MS | ||
| LPC 20:5 | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| LPC 22:5 | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| LPE 16:0 | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| LPE 18:1 | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| LPE 18:2 | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| LPE 20:4 | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| LPE 22:5 | ↓ | Lipid metabolism | Zhao et al., 2014 | LC-MS |
| ↓ | Dong et al., 2015 | LC-MS | ||
| ↓ | Jia et al., 2019 | LC-MS | ||
| PC (18:1/18:4) | ↓ | Lipid metabolism | Vonica et al., 2019 | LC-MS |
| PC (18:3/18:2) | ↓ | Lipid metabolism | Vonica et al., 2019 | LC-MS |
| PC (32:4) | ↓ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| PC (30:0) | ↓ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| PE (42:1) | ↓ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| PE (34:0) | ↓ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| SM (d18:0/20:2) | ↑ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| SM (d18:0/18:0) | ↑ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| Triglycerides | ↑ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| DG (36:2) | ↑ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| DG (36:3) | ↑ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| Plasmalogen (30:0) | ↓ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| Plasmalogen (40:7) | ↑ | Lipid metabolism | Haoula et al., 2015 | LC-MS |
| Azelaic acid | ↑ | Lipid metabolism | Dong et al., 2015 | LC-MS |
| N-undecanoylglycine | ↑ | Lipid metabolism | Dong et al., 2015 | LC-MS |
| Chenodeoxycholic acid | ↑ | Lipid metabolism | Fan et al., 2019 | LC-MS |
| Cholic acid | ↓ | Lipid metabolism | Fan et al., 2019 | LC-MS |
| Clupanodonylcarnitine | ↑ | Lipid metabolism | Fan et al., 2019 | LC-MS |
| 2-Hydroxylauroylcarnitine | ↑ | Lipid metabolism | Vonica et al., 2019 | LC-MS |
| Trans-2-dodecenoylcarnitine | ↑ | Lipid metabolism | Vonica et al., 2019 | LC-MS |
| Cholestane-3β | ↑ | Sterol lipid metabolism | Vonica et al., 2019 | LC-MS |
| Cholestane-5α (18:0/0:0) | ↑ | Sterol lipid metabolism | Vonica et al., 2019 | LC-MS |
| Cholestane-6β-triol | ↑ | Sterol lipid metabolism | Vonica et al., 2019 | LC-MS |
| Cholestane (18:1/0:0) | ↑ | Sterol lipid metabolism | Vonica et al., 2019 | LC-MS |
| Androsterone sulphate | ↑ | Lipid transport and metabolism | Fan et al., 2019 | LC-MS |
| 11′-Carboxy-α-chromanol | ↑ | Lipid transport and metabolism | Fan et al., 2019 | LC-MS |
| (9-cis,9′-cis)-7,7′,8,8′-Tetrahydro-y,y-Carotene | ↑ | Lipid transport and metabolism | Fan et al., 2019 | LC-MS |
| Sphinganine | ↓ | Sphingolipid metabolism | Dong et al., 2015 | LC-MS |
| ↓ | Jia et al., 2019 | LC-MS | ||
| ↑ | Buszewska-Forajta et al., 2019 | LC-MS | ||
| Phytosphingosine | ↓ | Sphingolipid metabolism | Dong et al., 2015 | LC-MS |
| Palmitoylsphingomyelin | ↑ | Sphingomyelin metabolism | Fan et al., 2019 | LC-MS |
| SM (d18:1/16:0) | ↑ | Sphingomyelin metabolism | Fan et al., 2019 | LC-MS |
| LysoPC (O-18:0) | ↓ | Lecithin metabolism | Fan et al., 2019 | LC-MS |
| LysoPC (16:0) | ↓ | Lecithin metabolism | Fan et al., 2019 | LC-MS |
| LysoPC [20:2(11Z,14Z)] | ↓ | Lecithin metabolism | Fan et al., 2019 | LC-MS |
| Glyceric acid | ↑ | Glycerolipid metabolism | Dong et al., 2015 | LC-MS |
| LPC (20:2) | ↓ | Glycerophospholipid metabolism | Dong et al., 2015 | LC-MS |
| 2-Arachidonoyl | ↑ | Glycerophospholipid metabolism | Fan et al., 2019 | LC-MS |
| glycerophosphocholine | ||||
| PG [18:1(9Z)/16:0] | ↓ | Glycerophospholipid metabolism | Fan et al., 2019 | LC-MS |
| PE [O-18:1(1Z)/20:4 (5Z,8Z,11Z,14Z)] |
↓ | Glycerophospholipid metabolism | Fan et al., 2019 | LC-MS |
| LysoPE [0:0/22:1(13Z)] | ↓ | Glycerophospholipid metabolism | Fan et al., 2019 | LC-MS |
| PE [O-16:1(1Z)/22:6 (4Z,7Z,10Z,13Z,16Z,19Z)] |
↓ | Glycerophospholipid metabolism | Fan et al., 2019 | LC-MS |
| PE [22:4(7Z,10Z,13Z,16Z)/16:0] | ↓ | Glycerophospholipid metabolism | Fan et al., 2019 | LC-MS |
| PC [16:1(9Z)/22:2(13Z,16Z)] | ↓ | Glycerophospholipid metabolism | Fan et al., 2019 | LC-MS |
| PG (18:0/16:0) | ↑ | Glycerophospholipid metabolism | Fan et al., 2019 | LC-MS |
| PG (18:1(9Z)/18:0) | ↓ | Glycerophospholipid metabolism | Fan et al., 2019 | LC-MS |
| DG (18:1n9/0:0/20:4n3) | ↑ | Diacyloglycerol metabolism | Fan et al., 2019 | LC-MS |
| TG (18:2/18:2/0-18:0) | ↑ | Diacyloglycerol metabolism | Vonica et al., 2019 | LC-MS |
| DG (22:2/0:0/22:4) | ↓ | Diacyloglycerol metabolism | Vonica et al., 2019 | LC-MS |
| Arginine | ↓ | Amino acids metabolism | Atiomo et al., 2012 | NMR |
| ↓ | Sun et al., 2012 | NMR | ||
| Choline | ↓ | Amino acids metabolism | Sun et al., 2012 | NMR |
| Citruline | ↓ | Amino acids metabolism | Atiomo et al., 2012 | NMR |
| Glutamate | ↓ | Amino acids metabolism | Atiomo et al., 2012 | NMR |
| Glycerophosphocholine/phosphocholine | ↓ | Amino acids metabolism | Sun et al., 2012 | NMR |
| Glycine | ↓ | Amino acids metabolism | Zhao et al., 2012 | GC-MS |
| Histidine | ↓ | Amino acids metabolism | Atiomo et al., 2012 | NMR |
| ↓ | RoyChoudhury et al., 2016 | |||
| AAA | ↑ | Amino acids metabolism | Zhao et al., 2012 | GC-MS |
| BCAA | ↑ | Amino acids metabolism | Zhao et al., 2012 | GC-MS |
| BCAA/AAA | ↓ | Amino acids metabolism | Zhao et al., 2012 | GC-MS |
| Aspartate | ↑ | Amino acids metabolism | Zhao et al., 2012 | GC-MS |
| Endogenous AAs | ↑ | Amino acids metabolism | Zhao et al., 2012 | GC-MS |
| Gluconeogenic AAs | ↑ | Amino acids metabolism | Zhao et al., 2012 | GC-MS |
| Serine | ↑ | Amino acids metabolism | Zhao et al., 2012 | GC-MS |
| 2-Aminobutyrate | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| 2-Hydroxybutyrate | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| 2-Hyroxyisovalerate | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| 2-Oxocaproate | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| 2-Oxoisocaproate | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| 3-Hydroxybutyrate | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| 3-Methyl-2-oxovalerate | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| Betadine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| Creatinine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| ↓ | Sun et al., 2012 | NMR | ||
| Dimethylamine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| Lysine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| ↑ | Zhao et al., 2012 | GC-MS | ||
| ↓ | Atiomo et al., 2012 | NMR | ||
| Methionine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| ↓ | Sun et al., 2012 | NMR | ||
| Ornithine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| ↑ | Zhao et al., 2012 | GC-MS | ||
| ↓ | Atiomo et al., 2012 | NMR | ||
| Sarcosine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| Taurine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| Tryptophan | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| ↑ | Zhao et al., 2012 | GC-MS | ||
| ↑ | Buszewska-Forajta et al., 2019 | GC/LC-MS | ||
| Tyrosine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| ↑ | Zhao et al., 2012 | GC-MS | ||
| ↑ | Buszewska-Forajta et al., 2019 | GC-MS | ||
| Glutamate | ↓ | Amino acids metabolism | RoyChoudhury et al., 2016 | NMR |
| ↑ | Whigham et al., 2014 | NMR | ||
| Glutamine | ↓ | Amino acids metabolism | RoyChoudhury et al., 2016 | NMR |
| ↑ | Whigham et al., 2014 | NMR | ||
| ↓ | Sun et al., 2012 | NMR | ||
| Proline | ↓ | Amino acids metabolism | Atiomo et al., 2012 | NMR |
| ↓ | Zhao et al., 2012 | GC-MS | ||
| ↓ | RoyChoudhury et al., 2016 | NMR | ||
| ↑ | Whigham et al., 2014 | NMR | ||
| Alanine | ↑ | Amino acids metabolism | RoyChoudhury et al., 2016 | NMR |
| ↑ | Whigham et al., 2014 | NMR | ||
| ↑ | Zhao et al., 2012 | NMR | ||
| ↓ | Escobar-Morreale et al., 2012 | GC-MS | ||
| Leucine | ↑ | Amino acids metabolism | RoyChoudhury et al., 2016 | NMR |
| ↑ | Whigham et al., 2014 | NMR | ||
| ↓ | Sun et al., 2012 | NMR | ||
| ↑ | Zhao et al., 2012 | GC-MS | ||
| Isoleucine | ↑ | Amino acids metabolism | Whigham et al., 2014 | NMR |
| ↓ | Zhao et al., 2012 | GC-MS | ||
| Valine | ↑ | Amino acids metabolism | RoyChoudhury et al., 2016 | NMR |
| ↑ | Whigham et al., 2014 | NMR | ||
| ↑ | Zhao et al., 2012 | GC-MS | ||
| Threonine | ↑ | Amino acids metabolism | RoyChoudhury et al., 2016 | NMR |
| ↑ | Whigham et al., 2014 | NMR | ||
| ↑ | Zhao et al., 2012 | GC-MS | ||
| ↑ | Buszewska-Forajta et al., 2019 | GC-MS | ||
| Cysteine-S-sulphate | ↑ | Amino acid metabolism | Fan et al., 2019 | LC-MS |
| Glu-Glu | ↑ | Amino acid metabolism | Dong et al., 2015 | LC-MS |
| Asparagine | ↑ | Amino acid metabolism | Whigham et al., 2014 | NMR |
| Ketoleucine | ↓ | Valine, leucine, and isoleucine degradation | Dong et al., 2015 | LC-MS |
| Glutamic acidc | ↑ | Glutamate metabolism, amino sugar metabolism | Dong et al., 2015 | LC-MS |
| Phenylpyruvic acid | ↑ | Phenylalanine and tyrosine metabolism | Dong et al., 2015 | LC-MS |
| Gly.Phe | ↑ | Phenylalanine and tyrosine metabolism | Zhao et al., 2014 | LC-MS |
| Phenylalanine | ↑ | Phenylalanine and tyrosine metabolism | Zhao et al., 2014 | LC-MS |
| ↑ | Whigham et al., 2014 | NMR | ||
| ↑ | Zhao et al., 2012 | GC-MS | ||
| ↑ | Buszewska-Forajta et al., 2019 | GC-MS | ||
| Phe−Phe | ↑ | Phenylalanine and tyrosine metabolism | Zhao et al., 2014 | LC-MS |
| Kynurenine | ↓ | Tryptophan metabolism | Zhao et al., 2014 | LC-MS |
| 5-Hydroxyindoleacetic acid | ↓ | Tryptophan metabolism | Dong et al., 2015 | LC-MS |
| Homoserine | ↓ | Methionine metabolism | Zhao et al., 2014 | LC-MS |
| ↑ | Whigham et al., 2014 | NMR | ||
| S-Adenosylmethionine | ↓ | Thiol amino acid metabolic cycle | Fan et al., 2019 | LC-MS |
| Pyroglutamic acid | ↑ | Glutathione metabolism | Dong et al., 2015 | LC-MS |
| Lysyl-albumin | ↓ | Protein metabolism | Zhao et al., 2012 | NMR |
| Trimethylamine N-oxide | ↓ | Protein metabolism | Sun et al., 2012 | NMR |
| 2-Ketoisocaproic acid | ↓ | Protein metabolism | Escobar-Morreale et al., 2012 | GC-MS |
| Dimethylamine | ↑ | Protein metabolism | Sun et al., 2012 | NMR |
| N-acetylglycoprotein | ↓ | Protein metabolism | Zhao et al., 2012 | NMR |
| ↑ | Sun et al., 2012 | NMR | ||
| Hypoxanthine | ↑ | Purine metabolism | Zhao et al., 2014 | LC-MS |
| Inosine | ↑ | Purine metabolism | Zhao et al., 2014 | LC-MS |
| Allantoic acid | ↑ | Purine metabolism | Dong et al., 2015 | LC-MS |
| Uric acid | ↑ | Purine metabolism | Zhao et al., 2012 | GC-MS |
| ↑ | Buszewska-Forajta et al., 2019 | GC/LC-MS | ||
| Cyclic GMP | ↑ | Purine metabolism | Fan et al., 2019 | LC-MS |
| Uridine | ↓ | Pyrimidine metabolism | Zhao et al., 2014 | LC-MS |
| ↓ | Dong et al., 2015 | LC-MS | ||
| 5,6-Dihydrouridine | ↑ | Pyrimidine metabolic cycle | Fan et al., 2019 | LC-MS |
| DHEAS | ↑ | Androgen metabolism | Zhao et al., 2014 | LC-MS |
| ↑ | Dong et al., 2015 | LC-MS | ||
| ↑ | Buszewska-Forajta et al., 2019 | LC-MS | ||
| ↑ | Jia et al., 2019 | LC-MS | ||
| ↑ | Fan et al., 2019 | LC-MS | ||
| ANDS | ↑ | Androgen metabolism | Zhao et al., 2014 | LC-MS |
| DHTS | ↑ | Androgen metabolism | Zhao et al., 2014 | LC-MS |
| Pregnenolone sulphate | ↓ | Steroid hormone biosynthesis | Dong et al., 2015 | LC-MS |
| 19-Oxotestosterone | ↑ | Steroid hormone biosynthesis | Dong et al., 2015 | LC-MS |
| C10:0 lauric acid | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C15:0 pentadecanoic acid | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C15:1 cis-10-pentadecanoic acid | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C17:0 heptadecanoic acid | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C20:0 arachidic acid | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C20:1 cis-11-eicosanoic acid | ↑ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C22:5 EPA | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C22:0 behenic acid | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C23:0 tricosanoic acid | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C22:4n6 docosatetraenic acid | ↑ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C24:0 lignoceric acid | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| C24:1 nervonic acid | ↑ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| 9-HODE/13-HODE | ↑ | Fatty acid metabolism | Dong et al., 2015 | LC-MS |
| α-Linolenic acid | ↑ | Fatty acid metabolism | Dong et al., 2015 | LC-MS |
| C18:2n6c linoleic acid | ↓ | Fatty acid metabolism | Szczuko et al., 2017 | GC-MS |
| Vaccenic acid | ↑ | Fatty acid metabolism | Dong et al., 2015 | LC-MS |
| Docosatrienoic acid | ↑ | Fatty acid metabolism | Dong et al., 2015 | LC-MS |
| Eicosapentaenoic acid | ↑ | Fatty acid metabolism | Dong et al., 2015 | LC-MS |
| Galbanic acid | ↑ | Fatty acid metabolism | Fan et al., 2019 | LC-MS |
| C14:0 myristic acid | ↑ | Fatty acid biosynthesis | Dong et al., 2015 | LC-MS |
| ↓ | Szczuko et al., 2017 | GC-MS | ||
| Palmitoleic acid | ↑ | Fatty acid biosynthesis | Dong et al., 2015 | LC-MS |
| Palmitoleoylethanolamide | ↑ | Fatty acid amide metabolism | Dong et al., 2015 | LC-MS |
| Oleamide | ↑ | Fatty acid amide metabolism | Zhao et al., 2014 | LC-MS |
| ↑ | Dong et al., 2015 | LC-MS | ||
| Palmitic amide | ↑ | Fatty acid amide metabolism | Zhao et al., 2014 | LC-MS |
| ↑ | Dong et al., 2015 | LC-MS | ||
| PEA | ↑ | Fatty acid amide metabolism | Zhao et al., 2014 | LC-MS |
| AEA | ↑ | Fatty acid amide metabolism | Zhao et al., 2014 | LC-MS |
| Carnitine C2:0 | ↑ | Beta oxidation of fatty acids | Zhao et al., 2014 | LC-MS |
| Carnitine C6:0 | ↑ | Beta oxidation of fatty acids | Zhao et al., 2014 | LC-MS |
| Carnitine C18 | ↑ | Beta oxidation of fatty acids | Zhao et al., 2014 | LC-MS |
| Carnitine | ↓ | Oxidation of fatty acids | Dong et al., 2015 | LC-MS |
| ↓ | Jia et al., 2019 | LC-MS | ||
| Glycocholic acid | ↓ | Bile acid metabolism | Zhao et al., 2014 | LC-MS |
| ↓ | Jia et al., 2019 | LC-MS | ||
| 3,7-Dihydroxy-5-cholestenoic acid | ↑ | Bile acid metabolism | Fan et al., 2019 | LC-MS |
| 3-β-Hydroxy-4-β-methyl-5-α-cholest-7-ene-4-α-carboxylate | ↑ | Bile acid metabolism | Fan et al., 2019 | LC-MS |
| Formate | ↑ | Pyruvate metabolism | Whigham et al., 2014 | NMR |
| Fructose | ↑ | Pyruvate metabolism | Whigham et al., 2014 | NMR |
| Mannose | ↑ | Pyruvate metabolism | Whigham et al., 2014 | NMR |
| Citrate | ↓ | TCA cycle metabolism | Whigham et al., 2014 | NMR |
| ↓ | Atiomo et al., 2012 | NMR | ||
| ↓ | Sun et al., 2012 | NMR | ||
| Acetate | ↑ | TCA cycle metabolism | RoyChoudhury et al., 2016 | NMR |
| ↑ | Whigham et al., 2014 | NMR | ||
| 4a-Methylzymosterol-4-carboxylic acid | ↑ | TCA cycle metabolism | Fan et al., 2019 | LC-MS |
| Lactate | ↑ | Gluconeogenesis/Glycolysis | RoyChoudhury et al., 2016 | NMR |
| ↑ | Whigham et al., 2014 | NMR | ||
| ↑ | Zhao et al., 2012 | GC-MS/ | ||
| NMR | ||||
| Lactic acid | ↑ | Gluconeogenesis/Glycolysis | Buszewska-Forajta et al., 2019 | GC-MS |
| Gluconolactone | ↑ | Pentose phosphate pathway | Dong et al., 2015 | LC-MS |
| 3-Hydroxybutyric acid | ↓ | Energy metabolism | RoyChoudhury et al., 2016 | NMR |
| Glucose | ↓ | Energy metabolism | RoyChoudhury et al., 2016 | NMR |
| ↓ | Zhao et al., 2012 | GC-MS | ||
| ↑ | Whigham et al., 2014 | /NMR | ||
| NMR | ||||
| Glyceraldehyde 3-phosphate | ↑ | ATP metabolism | Fan et al., 2019 | LC-MS |
| Glycerol | ↓ | Glucose metabolism | Whigham et al., 2014 | NMR |
| Acetoacetate | ↑ | Glucose metabolism | Whigham et al., 2014 | NMR |
| Pyruvate | ↑ | Glucose metabolism | Whigham et al., 2014 | NMR |
| Acetone | ↑ | Glucose metabolism | Whigham et al., 2014 | NMR |
| ↓ | Atiomo et al., 2012 | NMR | ||
| Fructose 6-phosphate | ↓ | Amino sugar metabolism | Dong et al., 2015 | LC-MS |
| Aspartic acid | ↑ | Aspartate metabolism | Zhao et al., 2014 | LC-MS |
| Thyroxine sulphate | ↓ | ATP metabolism | Fan et al., 2019 | LC-MS |
| Pantothenic acid | ↑ | Pantothenate and CoA biosynthesis | Dong et al., 2015 | LC-MS |
| Prostaglandin F2a | ↑ | Arachidonic acid metabolism | Dong et al., 2015 | LC-MS |
| ↑ | Vonica et al., 2019 | LC-MS | ||
| 25-Methyl-1-hexacosanol | ↓ | Fatty alcohols | Fan et al., 2019 | LC-MS |
| S-(PGJ2)—glutathione | ↑ | Immune modulation | Fan et al., 2019 | LC-MS |
| Oryzanol A | ↓ | Endocrine modulation | Fan et al., 2019 | LC-MS |
HDL = high-density lipoproteins, VLDL/LDL = very-low density lipoproteins/ low density lipoproteins, FFA = free fatty acid, PC = phosphatidylcholine, PE = phosphatidylethanolamine, LPC (LysoPC) = lysophosphatidylcholine, LPE = lysophospha-tidylethanolamine, SM = sphingomyelin, DG = diglyceride, TG = trigliceride, PG = phosphatidylglycerol, AAA = aromatic amino acids, BCAA = branched-chain amino acid, AAs = amino acid, GMP = guanosine monophosphate, DHEAS = dehydro-epiandrosterone sulphate, ANDS = androsterone sulphate, DHTS = dihydrotestosterone sulphate, EPA = eicosapentaenoic acid, HODE = hydroxyoctadecadienoic acid, PEA = palmitoylethanolamide, AEA = N-arachidonoylethanolamine; ↑ up-regulation; ↓ down-regulation.
2.2. Metabolomic Profile of the Urine Samples
There are relatively few metabolomic studies on PCOS where urine is used as the biological matrix. Urine samples are a convenient study material due to non-invasive sampling as well as easy sample preparation because of the lower content of protein compared to serum and plasma [38]. This matrix is also rich in metabolites of the metabolic pathways, which may be deranged in PCOS. Metabolites, which were down- and up-regulated in women with PCOS compared to healthy controls, are presented in Table 2 [39,40,41]. Zou et al. (2018) reported that some carbohydrates and fatty acids metabolites are up-regulated in PCOS in comparison with the control subjects [40]. A contrary phenomenon occurs in the case of glycerolipids, where levels of 5 out of 7 compounds are decreased, while up-regulation of triglyceride (TG) and DG (16:1(9Z)/14:0/0:0) was reported. Dhayat et al. (2018) focused on the determination of androgens in PCOS, which were all elevated [41]. A similar trend is reported for AAs, glucocorticoids, and peptides.
Table 2.
The most significant changes in urinary metabolites in women with PCOS in comparison with the control subjects.
| Metabolites | PCOS vs. Control | Metabolic Pathways | Studies | Techniques |
|---|---|---|---|---|
| Lactose | ↑ | Carbohydrate metabolism | Zou et al., 2018 | GC-MS |
| Gluconic acid | ↑ | Carbohydrate metabolism | Zou et al., 2018 | GC-MS |
| 3-hydroxypropionic acid | ↑ | Carbohydrate metabolism | Zou et al., 2018 | GC-MS |
| Arabinitol | ↑ | Carbohydrate metabolism | Zou et al., 2018 | GC-MS |
| Fucose | ↑ | Carbohydrate metabolism | Zou et al., 2018 | GC-MS |
| Oxalic acid | ↑ | Carbohydrate metabolism | Zou et al., 2018 | GC-MS |
| Arabic candy | ↑ | Lipid metabolism | Zou et al., 2018 | GC-MS |
| Stearic acid | ↑ | Lipid metabolism | Zou et al., 2018 | GC-MS |
| Palmitic acid | ↑ | Lipid metabolism | Zou et al., 2018 | GC-MS |
| Phosphoethanolamine | ↑ | Lipid metabolism | Zou et al., 2018 | GC-MS |
| 2-(14,15-Epoxyeicosatrienoyl) | ↓ | Glycerolipids | Wang et al., 2015 | LC-MS |
| TG (14:1(9Z)/14:0/22:2(13Z,16Z)) | ↓ | Glycerolipids | Wang et al., 2015 | LC-MS |
| TG (14:0/24:1(15Z)/14:1(9Z)) | ↓ | Glycerolipids | Wang et al., 2015 | LC-MS |
| TG(16:0/14:0/18:0) | ↓ | Glycerolipids | Wang et al., 2015 | LC-MS |
| TG (16:0/14:1(9Z)/20:1(11Z)) | ↓ | Glycerolipids | Wang et al., 2015 | LC-MS |
| TG | ↑ | Glycerolipids | Wang et al., 2015 | LC-MS |
| DG (16:1(9Z)/14:0/0:0) | ↑ | Glycerolipids | Wang et al., 2015 | LC-MS |
| PC (22:2(13Z,16Z)/18:1(9Z)) | ↓ | Glycerophospholipids | Wang et al., 2015 | LC-MS |
| PC (14:1(9Z)/14:1(9Z)) | ↓ | Glycerophospholipids | Wang et al., 2015 | LC-MS |
| LPA (16:0/0:0) | ↓ | Glycerophospholipids | Wang et al., 2015 | LC-MS |
| PE (14:1(9Z)/14:1(9Z)) | ↑ | Glycerophospholipids | Wang et al., 2015 | LC-MS |
| LysoPC (18:1(9Z)) | ↑ | Glycerophospholipids | Wang et al., 2015 | LC-MS |
| Cer (d18:0/20:0) | ↓ | Sphingolipids | Wang et al., 2015 | LC-MS |
| Phytosphingosine | ↓ | Sphingolipids | Wang et al., 2015 | LC-MS |
| Glycocholic acid | ↓ | Steroids | Wang et al., 2015 | LC-MS |
| Chenodeoxycholic acid 3-sulphate | ↓ | Steroids | Wang et al., 2015 | LC-MS |
| 3-Oxo-4,6-choladienoic acid | ↓ | Steroids | Wang et al., 2015 | LC-MS |
| Cortolone-3-glucuronide | ↑ | Steroids | Wang et al., 2015 | LC-MS |
| 11α-Hydroxyprogesterone | ↑ | Steroids | Wang et al., 2015 | LC-MS |
| Testosterone glucuronide | ↑ | Steroids | Wang et al., 2015 | LC-MS |
| Tetrahydroaldosterone-3-glucuronide | ↑ | Steroids | Wang et al., 2015 | LC-MS |
| Dehydroepiandrosterone | ↑ | Androgen metabolism | Dhayat et al., 2018 | GC-MS |
| 16α-OH-dehydroepiandrosterone | ↑ | Androgen metabolism | Dhayat et al., 2018 | GC-MS |
| Androstenediol | ↑ | Androgen metabolism | Dhayat et al., 2018 | GC-MS |
| Testosterone | ↑ | Androgen metabolism | Dhayat et al., 2018 | GC-MS |
| 5α-DH-testosterone | ↑ | Androgen metabolism | Dhayat et al., 2018 | GC-MS |
| Androstanediol | ↑ | Androgen metabolism | Dhayat et al., 2018 | GC-MS |
| Androsterone | ↑ | Androgen metabolism | Dhayat et al., 2018 | GC-MS |
| 11β-OH-androsterone | ↑ | Androgen metabolism | Dhayat et al., 2018 | GC-MS |
| Etiocholanolone | ↑ | Androgen metabolism | Dhayat et al., 2018 | GC-MS |
| Estriol | ↓ | Estrogen metabolism | Dhayat et al., 2018 | GC-MS |
| Suberic acid | ↑ | Fatty acid metabolism | Zou et al., 2018 | GC-MS |
| 3,4,5-hydroxyvaleric acid | ↑ | Fatty acid metabolism | Zou et al., 2018 | GC-MS |
| (R)-3-Hydroxy-hexadecanoic acid | ↓ | Fatty acid metabolism | Wang et al., 2015 | LC-MS |
| 6-Keto-decanoylcarnitine | ↓ | Fatty acid esters | Wang et al., 2015 | LC-MS |
| Tiglylcarnitine | ↑ | Fatty acid esters | Wang et al., 2015 | LC-MS |
| Butyrylcarnitine | ↑ | Fatty acid esters | Wang et al., 2015 | LC-MS |
| 4-hydroxyphenylacetic acid | ↑ | Tyrosine metabolism | Zou et al., 2018 | GC-MS |
| Capryloylglycine | ↓ | Amino acid metabolism | Wang et al., 2015 | LC-MS |
| N-(7-Isocucurbinoyl)isoleucine | ↑ | Amino acid metabolism | Wang et al., 2015 | LC-MS |
| Aspartylglycosamine | ↑ | Amino acid metabolism | Wang et al., 2015 | LC-MS |
| α-ketoglutarate | ↑ | Amino acid metabolism | Zou et al., 2018 | GC-MS |
| Threonine | ↑ | Amino acid metabolism | Zou et al., 2018 | GC-MS |
| Serine | ↑ | Amino acid metabolism | Zou et al., 2018 | GC-MS |
| Glycine | ↑ | Amino acid metabolism | Zou et al., 2018 | GC-MS |
| 5-Oxoproline | ↑ | Amino acid metabolism | Zou et al., 2018 | GC-MS |
| Benzoylglycine | ↑ | Amino acid metabolism | Zou et al., 2018 | GC-MS |
| Indoleacetyl glutamine | ↓ | Aromatic Amino acids | Wang et al., 2015 | LC-MS |
| Flazine methyl ether | ↓ | Aromatic Amino acids | Wang et al., 2015 | LC-MS |
| Succinyladenosine | ↑ | Aromatic Amino acids | Wang et al., 2015 | LC-MS |
| Thyronine | ↑ | Aromatic Amino acids | Wang et al., 2015 | LC-MS |
| Gamma-glutamyl-leucine | ↓ | Peptides | Wang et al., 2015 | LC-MS |
| Tryptophyl-proline | ↓ | Peptides | Wang et al., 2015 | LC-MS |
| Methionyl-phenylalanine | ↑ | Peptides | Wang et al., 2015 | LC-MS |
| Phenylalanyl-histidine | ↑ | Peptides | Wang et al., 2015 | LC-MS |
| Arginyl-valiney | ↑ | Peptides | Wang et al., 2015 | LC-MS |
| Threoninyl-lysine | ↑ | Peptides | Wang et al., 2015 | LC-MS |
| Tryptophyl-arginine | ↑ | Peptides | Wang et al., 2015 | LC-MS |
| Tyrosyl-leucine | ↑ | Peptides | Wang et al., 2015 | LC-MS |
| Tryptophyl-valine | ↑ | Peptides | Wang et al., 2015 | LC-MS |
| Cis-aconitic acid | ↑ | CTA metabolism | Zou et al., 2018 | GC-MS |
| 3-Hydroxy-3-Methylglutaric acid | ↑ | Energy metabolism | Zou et al., 2018 | GC-MS |
| 2-Hydroxyglutaric acid | ↑ | Energy metabolism | Zou et al., 2018 | GC-MS |
| Threonic acid | ↑ | Sugar acids metabolism | Zou et al., 2018 | GC-MS |
| Inosine | ↑ | Purine metabolism | Zou et al., 2018 | GC-MS |
| 2,3,4-Hydroxybutyric acid | ↑ | Energy metabolism | Zou et al., 2018 | GC-MS |
| 3,4-Hydroxybutyric acid | ↑ | Energy metabolism | Zou et al., 2018 | GC-MS |
| 4-Hydroxybutyric acid | ↑ | Energy metabolism | Zou et al., 2018 | GC-MS |
| 2-Hydroxyisobutyric acid | ↑ | Energy metabolism | Zou et al., 2018 | GC-MS |
| Uracil | ↑ | Pyrimidine metabolism | Zou et al., 2018 | GC-MS |
| Glyceryl acid | ↑ | Hydroxy acid metabolism | Zou et al., 2018 | GC-MS |
| Glycolic acid | ↑ | Hydroxy acid metabolism | Zou et al., 2018 | GC-MS |
| 2-Hydroxyisobutyric acid | ↑ | Energy metabolism | Zou et al., 2018 | GC-MS |
| Succinic acid | ↓ | Glucose metabolism | Zou et al., 2018 | GC-MS |
| Benzophenone | ↑ | Acetophenones | Wang et al., 2015 | LC-MS |
| 5′-Carboxy-γ-chromanol | ↓ | Benzopyrans | Wang et al., 2015 | LC-MS |
| 5′-Carboxy-α-chromanol | ↓ | Benzopyrans | Wang et al., 2015 | LC-MS |
| 9′-Carboxy-α-chromanol | ↓ | Benzopyrans | Wang et al., 2015 | LC-MS |
| 11′-Carboxy-α-tocotrienol | ↓ | Benzopyrans | Wang et al., 2015 | LC-MS |
| FMNH2 | ↑ | Pteridines | Wang et al., 2015 | LC-MS |
| Urobilin | ↓ | Tetrapyrroles | Wang et al., 2015 | LC-MS |
| Mesobilirubinogen | ↓ | Tetrapyrroles | Wang et al., 2015 | LC-MS |
| Harderoporphyrinogen | ↓ | Tetrapyrroles | Wang et al., 2015 | LC-MS |
| MG (18:4(6Z,9Z,12Z,15Z)/0:0/0:0) | ↑ | Lineolic acids | Wang et al., 2015 | LC-MS |
| Hydroxyvalerylcarnitine | ↑ | Alkylamines | Wang et al., 2015 | LC-MS |
| Labadoside | ↑ | Glycosides | Wang et al., 2015 | LC-MS |
| Dihydrocaffeic acid 3-O-glucuronide | ↓ | Sugar acids | Wang et al., 2015 | LC-MS |
| Dihydroferulic acid 4-O-glucuronide | ↓ | Sugar acids | Wang et al., 2015 | LC-MS |
| 5-Hydroxy-6-methoxyindole glucuronide | ↑ | Sugar acids | Wang et al., 2015 | LC-MS |
| p-Cresol glucuronide | ↓ | Sugar acids | Wang et al., 2015 | LC-MS |
| 6β-OH-cortisol | ↑ | Glucocorticoid metabolism | Dhayat et al., 2018 | GC-MS |
| 18-OH-cortisol | ↑ | Glucocorticoid metabolism | Dhayat et al., 2018 | GC-MS |
| TH-cortisol | ↑ | Glucocorticoid metabolism | Dhayat et al., 2018 | GC-MS |
| 11β-OH-etiocholanolone | ↑ | Glucocorticoid metabolism | Dhayat et al., 2018 | GC-MS |
| TH-cortisone | ↑ | Glucocorticoid metabolism | Dhayat et al., 2018 | GC-MS |
TG = triglyceride, DG = diglyceride, PC = phosphatidylcholine, (LysoPC) = lysophosphatidylcholine, LPA = lysophosphatidic acid, FMNH2 = reduced flavin mononucleotide; ↑ up-regulation; ↓ down-regulation.
2.3. Metabolomic Profile of Follicular Fluid Samples
Follicular fluid (FF) is an alternative and a useful biological matrix to study the potential mechanism of PCOS pathophysiology. FF is the product of plasma modified by the secretory activity of the granulosa and theca cells [42]. This matrix is collected from women with PCOS undergoing in vitro fertilization. FF contains metabolites essential for oocyte growth and maturation. The analysis of the metabolic derangements in FF samples from women with PCOS allows to understand pathological changes and also disclose the metabolites that could potentially disturb normal oocyte growth [43].
We reviewed a few metabolomic studies that analysed FF from women with PCOS and compared them with healthy controls [44,45,46,47,48]. They were performed with the use of different metabolomic techniques. As can be observed, the concentration of metabolites involved in the TCA cycle, as well as α-keto acids, are relatively higher in samples obtained from women with PCOS in comparison with the control subjects. The contrary trend was shown for acylcarnitines. For all of the determined metabolites belonging to acylcarnitines, the decreased level was determined for samples obtained from PCOS patients. However, there are a few metabolic pathways where the trend is not similar among the pathway, but is specific for individual subgroups of compounds. For example, as was reported by Liu et al. (2018), the decreased level of PC was observed in PCOS patients, while the contrary trend (up-regulation) was shown for Lyso PC [46]. In the case of fatty acyls, the general direction demonstrates down-regulation. However, Liu et al. (2018) reported an increased level of 1-Hydroxy-2,12,15-heneicosatrien-4-one in PCOS patients in comparison with the control subjects. The diversity was also observed in the case of AAs metabolism. For example, increased level of phenylalanine, valine, and isoleucine was observed, while a decreased amount of alanine, glutamine, and tyrosine was reported. The results are presented in Table 3.
Table 3.
The most significant changes in FF metabolites in women with PCOS in comparison with the control subjects.
| Metabolites | PCOS vs. Control | Metabolic Pathways | Studies | Techniques |
|---|---|---|---|---|
| Paxilline | Naphthopyrans | Liu et al., 2018 | LC-MS | |
| PC (o-22:0/20:4(8Z,11Z,14Z,17Z)) | Glycerophospholipid | Liu et al., 2018 | LC-MS | |
| PC (o22:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | Glycerophospholipid | Liu et al., 2018 | LC-MS | |
| LysoPC (16:1(9Z)) | Glycerophospholipid | Liu et al., 2018 | LC-MS | |
| LysoPC (16:0) | Glycerophospholipid | Liu et al., 2018 | LC-MS | |
| Sun et al., 2019 | LC-MS | |||
| LysoPC (14:0) | Glycerophospholipid | Sun et al., 2019 | LC-MS | |
| LysoPC (18:0) | Glycerophospholipid | Sun et al., 2019 | LC-MS | |
| LysoPC (20:4(8Z,11Z,14Z,17Z)) | Glycerophospholipid | Liu et al., 2018 | LC-MS | |
| PGP (16:0/20:4(5Z,8Z,11Z,14Z) | Glycerophospholipid | Liu et al., 2018 | LC-MS | |
| Glycerophosphocholine | Glycerophospholipid | Chen et al., 2020 | LC-MS | |
| Ceramide (d18:0/16:0) | Sphingolipids | Liu et al., 2018 | LC-MS | |
| Ceramide (d18:0/24:0) | Sphingolipids | Liu et al., 2018 | LC-MS | |
| Galabiosylceramide (d18:1/24:1(15Z)) | Sphingolipids | Liu et al., 2018 | LC-MS | |
| Tetrahexosylceramide (d18:1/24:0) | Sphingolipids | Liu et al., 2018 | LC-MS | |
| 7β-Hydroxycholesterol | Lipid metabolism | Chen et al., 2020 | LC-MS | |
| Malyl-CoA | Fatty Acyls | Liu et al., 2018 | LC-MS | |
| 1-Hydroxy-2,12,15-heneicosatrien-4-one | Fatty Acyls | Liu et al., 2018 | LC-MS | |
| 16-hydroxypalmitic acid | Fatty Acyls | Liu et al., 2018 | LC-MS | |
| Tridecanol | Fatty Acyls | Liu et al., 2018 | LC-MS | |
| Carnitine | Fatty acids metabolism | Chen et al., 2020 | LC-MS | |
| 4-Hydroxy-3-(16-methylheptadecyl)-2H-pyran-2-one | Pyrans | Liu et al., 2018 | LC-MS | |
| Anandamide | Organonitrogen compounds | Liu et al., 2018 | LC-MS | |
| Indan-1-ol | Indanes | Liu et al., 2018 | LC-MS | |
| 2-p-Tolyl-1-propene, p-Mentha-1,3,5,8-tetraene | Phenylpropenes | Liu et al., 2018 | LC-MS | |
| β -Ionol | Sesquiterpenoids | Liu et al., 2018 | LC-MS | |
| Androstenol | Androstane steroids | Liu et al., 2018 | LC-MS | |
| (3R, 6′Z)-3,4-Dihydro-8-hydroxy-3-(6-pentadecenyl)-1H-2-benzopyran-1-one | Benzopyrans | Liu et al., 2018 | LC-MS | |
| 6-Tridecylsalicylic acid | Benzoic acids and derivatives | Liu et al., 2018 | LC-MS | |
| 2,3-dihydroxypropyl dodecanoate | Glycerol metabolism | Chen et al., 2020 | LC-MS | |
| Methylmalonic acid | Carboxylic acids | Liu et al., 2018 | LC-MS | |
| Lysyl-Valine | Carboxylic acids and derivatives | Liu et al., 2018 | LC-MS | |
| Prolyl-Methionine | Carboxylic acids and derivative | Liu et al., 2018 | LC-MS | |
| VPGPR Enterostatin | Carboxylic acids and derivative | Liu et al., 2018 | LC-MS | |
| 1H-Indol-3-ylacetyl-myo-inositol | Indoles and derivatives | Liu et al., 2018 | LC-MS | |
| 1-Pentadecene | Unsaturated hydrocarbons | Liu et al., 2018 | LC-MS | |
| Lithocholic acid glycine conjugate | Non classified | Liu et al., 2018 | LC-MS | |
| Lactate | Gluconeogenesis/Glycolysis | Zhang et al., 2017 | NMR | |
| Liu el al., 2018 | GC-MS | |||
| Glyceraldehyde | Gluconeogenesis/Glycolysis | Chen et al., 2020 | LC-MS | |
| Pyruvate | Glucose glycolysis | Zhang et al., 2017 | NMR | |
| Zhao et al., 2015 | GC-MS | |||
| Valine | Amino acid metabolism | Zhao et al., 2015 | MS/MS | |
| Isoleucine | Amino acid metabolism | Zhao et al., 2015 | MS/MS | |
| Leucine | Amino acid metabolism | Zhao et al., 2015 | MS/MS | |
| Sun et al., 2019 | LC-MS | |||
| Alanine | Amino acid metabolism | Zhang et al., 2017 | NMR | |
| Glutamine | Amino acid metabolism | Zhang et al., 2017 | NMR | |
| Tyrosine | Amino acid metabolism | Zhang et al., 2017 | NMR | |
| Phenylalanine | Amino acid metabolism | Sun et al., 2019 | LC-MS | |
| d-Glutamic acid | Amino acid metabolism | Chen et al., 2020 | LC-MS | |
| Ferulic acid | Amino acid metabolism | Chen et al., 2020 | LC-MS | |
| Salicylic acid | Amino acid metabolism | Chen et al., 2020 | LC-MS | |
| Lysine | Amino acid metabolism | Chen et al., 2020 | LC-MS | |
| 3-Methylhistidine | Amino acid metabolism | Chen et al., 2020 | LC-MS | |
| α-Keto-β-methylvalerate | Alpha-keto acids | Zhao et al., 2015 | GC-MS | |
| α-Ketoisovalerate | Alpha-keto acids | Zhao et al., 2015 | GC-MS | |
| α-Ketoisocaproate | Alpha-keto acids | Zhao et al., 2015 | GC-MS | |
| Hexanoyl (C6) | Acylcarnitines | Zhao et al., 2015 | MS/MS | |
| Malonyl (C3DC) | Acylcarnitines | Zhao et al., 2015 | MS/MS | |
| Hydroxyisovaleryl (C5OH) | Acylcarnitines | Zhao et al., 2015 | MS/MS | |
| Octenoyl (C8:1) | Acylcarnitines | Zhao et al., 2015 | MS/MS | |
| Adipyl (C6DC) | Acylcarnitines | Zhao et al., 2015 | MS/MS | |
| β-Hydroxybutyrate | Ketones | Zhao et al., 2015 | GC-MS | |
| Succinate | TCA cycle metabolites | Zhao et al., 2015 | GC-MS | |
| Malate | TCA cycle metabolites | Zhao et al., 2015 | GC-MS | |
| Oxaloacetate | TCA cycle metabolites | Zhao et al., 2015 | GC-MS | |
| Cis-aconitate | TCA cycle metabolites | Zhao et al., 2015 | GC-MS | |
| Acetate | TCA cycle metabolites | Zhang et al., 2017 | NMR | |
| Acetoacetate | TCA cycle metabolites | Zhang et al., 2017 | NMR | |
| 3-Hyroxybutyrate | TCA cycle metabolites | Zhang et al., 2017 | NMR | |
| N-Methylnicotinamide | Metabolites of NAD catabolism | Zhao et al., 2015 | LC-MS/MS | |
| N1-Methyl-2-pyridone-5-carboxamide (2PY) | Metabolites of NAD catabolism | Zhao et al., 2015 | LC-MS/MS | |
| N1-Methyl-4-pyridone-3-carboxamide (4PY) | Metabolites of NAD catabolism | Zhao et al., 2015 | LC-MS/MS | |
| Deoxycorticosterone | Steroid metabolism | Sun et al., 2019 | LC-MS | |
| Pregnenolone | Steroid metabolism | Chen et al., 2020 | LC-MS | |
| 17-Hydroxyprogesterone | Steroid metabolism | Chen et al., 2020 | LC-MS | |
| 3-Hydroxynonanoyl carnitine | Fatty acid metabolism | Sun et al., 2019 | LC-MS | |
| Eicosapentaenoic acid | Fatty acid metabolism | Sun et al., 2019 | LC-MS | |
| Phytosphingosine | Sphingolipid metabolism | Sun et al., 2019 | LC-MS | |
| N-acetylneuraminic acid | Sialic acid metabolism | Chen et al., 2020 | LC-MS | |
| Pyridoxal 5′-phosphate | Vitamin B6 metabolism | Chen et al., 2020 | LC-MS | |
| Purine | Purines metabolism | Chen et al., 2020 | LC-MS | |
| 1,3-Dimethyluracil | Purines metabolism | Chen et al., 2020 | LC-MS | |
| Oxalic acid | Glyoxylic acid metabolism | Chen et al., 2020 | LC-MS | |
| Phenylglyoxylic acid | Glyoxylic acid metabolism | Chen et al., 2020 | LC-MS |
PC = phosphatidylcholine, (LysoPC) = lysophosphatidylcholine, PGP = glycerol-3-phosphate, CoA = coenzyme A; ↑ up-regulation; ↓ down-regulation.
3. Discussion
The summary of all the metabolites detected in three different biological matrices gives us a complementary overview of the metabolomic profile of women with PCOS and allows us to look for a correlation between altered levels of metabolites from different biochemical pathways.
Lipids are the largest group of molecules whose metabolism is deranged in women with PCOS. They are involved in various metabolic pathways, such as steroid hormone biosynthesis, sphingolipid, and fatty acids metabolisms like oxidation or amide metabolism. Phospholipids, which take part in multiple biological pathways, are down-regulated in PCOS. There are few studies confirming the decreased levels of sphinganine, LPE, especially LPE (22:5) and LPC, mainly LPC (18:2) in women with PCOS [27,28,34]. LPCs are involved in glucose metabolism, and low levels of LPC (18:2) correlate with IR and increased risk of T2DM. Thus, this would be in accordance with the observation that women with PCOS are more prone to these metabolic alterations [34]. Hauola et al. (2015) noticed that the differences in the lipid profiles between PCOS and healthy women correlate with the phase of the menstrual cycle. The most significant changes were detected between samples collected from healthy women during the luteal phase of the menstrual cycle and women with PCOS [35]. Among sphingolipids, phytosphingosine (PHS), which stimulates the transcriptional activity of the peroxisome proliferator-activated receptor γ (PPARγ), is downregulated in women with PCOS. According to the literature, PPARs are ligand-dependent transcription factors that regulate the expression of numerous genes associated with the metabolism of carbohydrates, lipids, and proteins [30]. Dysfunction of these receptors may also contribute to the increased risk of MetS and T2DM. The latest lipidomic studies indicate that elevated levels of DG and cholesterol ester, and lower levels of LysoPC correlate with IR, irrespective of a person being overweight or obese [49].
Szczuko et al. (2017) analysed plasma fatty acids in women with PCOS. Their results showed that the levels of all free fatty acids were lower in women with PCOS; however, the concentration of nervonic acid was several times (almost 330 times) higher than in the control subjects. This level of nervonic acid was observed in two analysed groups of women, namely women with PCOS with a biochemical indication of hyperandrogenism and women with normal androgen levels. Poly-unsaturated fatty acids (PUFAs), the precursors of eicosanoids, were significantly increased in women with PCOS compared with the control subjects, which may be due to the presence of low-grade systemic inflammation. In recent years, some studies reported that this process could be stimulated by the pro-inflammatory interleukin-1 (IL-1), which is overexpressed in women with PCOS [50].
One of the detected metabolites, which may be considered as a potential biomarker of PCOS, is DHEA. Excess levels of DHEA are mostly detected in the metabolome of women with PCOS [29,30,33,34]. Serum concentrations of DHEA-S are also evaluated in clinical practice for the evaluation of biochemical hyperandrogenism in women. Besides, 19-oxotestosterone levels are also elevated in women with POCS. This could be due to the higher activity of aromatase, which catalyses the formation of C18 estrogens from C19 androgens [30]. Zhao et al. (2014) showed that, apart from elevated DHEA levels in women with PCOS, there is a significant increase of DHTS and ANDS, which points to the exaggerated androgen synthesis [29]. Dhayat et al. (2018) reported that in women with PCOS, there is an alternative pathway of 11-oxygenated androgen production. They also reported that four steroids such as androstanediol, estriol, 20-β-dihydrocortisone, and cortisol are found as potential markers of PCOS [41].
Lipidomic analysis was also the main goal of the study conducted by Vonica et al. (2019). The authors reported that alterations in acylglycerols, PGs and LTs, phosphocholines, and carnitine metabolites occur in women with PCOS. As a result, cholestane-5α (18:1/0:0), triacylglycerol (18:2/18:2/0-18:0), cholestane-3β, 5α, 6β-triol (18:0/0:0) were found as the crucial metabolites to identify women with PCOS from the controls. Serum levels of these metabolites were decreased. Moreover, elevated acylcarnitine: 2-hydroxylauroylcarnitine with decreased phosphocholines metabolites (18:1/18:4, 18:3/18:2) were also observed. The authors assumed that this alteration might be linked to the lipid peroxidation. Levels of some of the TG (18:2/18:2/0-18:0) and DG species (18:1/20:0/0:0, 18:0/0:0/20:1, 22:4n6/0:0/18:4n3, 18:3/0:0/22:5, 20:0/0:0/20:0, 18:1/24:0/0:0, 18:0/0:0/24:1) were also elevated in women with PCOS in comparison with the control subjects [37]. This can be explained by a higher BMI and increased intra-abdominal fat deposition, which is also a hallmark of PCOS. Dyslipidemia, which occurs in PCOS is characterized by the increased concentrations of total cholesterol (TCh), low density lipoproteins (LDL), very-low density lipoproteins (VLDL), and TGs coupled with decreased high-density lipoproteins (HDL) and HDL-cholesterol. During intestinal absorption, TGs are degraded to fatty acids. After that, they are again resynthesized and transported by the chylomicrons through the bloodstream to the adipose and muscle tissue, where their degradation into free fatty acids and monoacylglycerol takes place. Elevated levels of TGs in PCOS women may be caused by a high fat intake or reduced fat energy consumption at night. It was confirmed that in women with PCOS, the reduced ability to switch to lipid oxidation during fasting also occurs at night [37].
7β-Hydroxycholesterol is another lipid metabolite that could also be a potential biomarker of PCOS. It highly correlates with a PCOS diagnosis. This oxysterol is found as a metabolic intermediate or the end-product of cholesterol metabolism. Due to its bioactivity, it could induce oxidative stress and disturb the metabolism of fatty acids. Chen et al. (2020) showed that elevated levels of 7β-hydroxycholesterol measured in the FF by the induction of the oxidative stress may disturb the growth of oocytes in PCOS [48].
There also are some specific alterations of the bile acid metabolism. These abnormalities and dysfunction of fat absorption in women with PCOS is due to the decreased level of glycocholic acid, which was reported by Zhao et al. (2014) [29] and confirmed by Jia et al. (2019) [34].
The metabolism of prostaglandins and carnitine also seems to be deranged in women with PCOS. Vonica et al. (2019) reported an increased level of the prostaglandin (PG) E2 pathway and oxo-leukotrienes (LT) known to play a pivotal role in inflammation [37]. Dong et al. (2015) showed an increase of prostaglandin F2a (FPG-2a) and a decrease of l-carnitine in women with PCOS [30]. Elevated levels of FPG-2a may also be related to the presence of a low-grade systemic inflammation in PCOS. In turn, l-carnitine plays an essential role in fatty acid metabolism as well as their transport across the mitochondrial membrane to the mitochondrial matrix where β-oxidation of fatty acids (FAs) occurs. Decreased levels of l-carnitine may point to the impairment of these processes in PCOS. It could result in an accumulation of the FAs in the cytosol. It is noticed that l-carnitine supplementation may improve oocyte quality and therefore, may have a positive effect on fertility [30]. Carnitine is also involved in stabilizing acetylCoA and coenzyme A levels and plays an essential role during fetal maturation [34]. Elevated levels of acetate found by Whigham et al. (2014) and RoyChodhury et al. (2016) also indicate reduced FAs oxidation in women with PCOS [28].
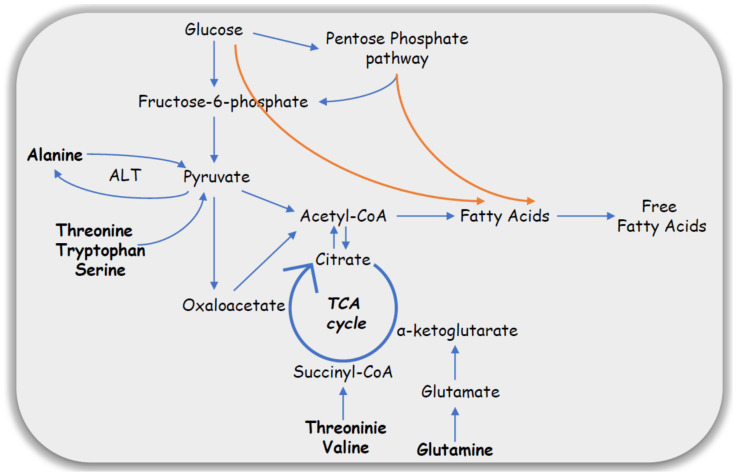
Impaired carbohydrate metabolism is a hallmark of PCOS. Elevated serum lactate and reduced level of glucose determined in plasma samples suggest alterations in glucose metabolism accompanied with elevated glycolytic activity associated with the TCA cycle impairment. Whigham et al. (2014) suggested that in women with PCOS, some AAs are utilized as a source of alternative energy metabolism [24]. Alanine is an important amino acid in the process of gluconeogenesis. Serum alanine-amino transaminase (ALT) activity is elevated in women with PCOS, which usually is the result of NAFLD and lead to increased alanine transformation [27]. According to the literature, ALT is involved in urea cycle and AAs metabolism, where alanine may be converted to pyruvate by donating an amine group and enter the TCA cycle as well as be formed from pyruvate by accepting an amine group, respectively. Serum levels of glucogenic AAs acids such as valine, leucine, and threonine, which may also enter the TCA cycle or be involved in the process of gluconeogenesis were also significantly elevated in women with PCOS. The higher number of growing antral follicles in PCOS utilizes more energy. For this purpose, carbohydrate metabolism may not be sufficient and may thus lead to the utilization of other energy substrates like glutamine, glutamate, or 3-hydroxybutyric acid. Whigham et al. (2014) pointed out that the TCA cycle and glucose metabolism are the major pathways deranged in PCOS [28]. As reported, glucose metabolism can be carried out via alternative pathways such as glycolysis or pentose phosphate pathways and lead to an increased FAs synthesis, which may explain the observed increased FAs accumulation in the adipocytes (Figure 2).
Figure 2.
Alteration of glucose metabolism in PCOS.
In women with PCOS elevated levels of phenylalanine and glycated phenylalanine were also detected. The accumulation of the glycated AAs was also reported in T2DM [25]. The lower level of other AAs such as proline and histidine may be due to the increased utilization of these AAs as antioxidants during the oxidative stress present in PCOS.
Vitamin B6 metabolism may also be deranged in women with PCOS. One of its metabolic pathways is the synthesis and degradation of the AAs. The results of Chen et al. (2020) showed a significant increase of pyridoxal 5′-phosphate (PLP) and d-glutamic acid in the FF of women with PCOS. The authors claimed that both compounds are linked with the vitamin B6 metabolism. On the other hand, it is known that PLP is a coenzyme in the metabolism of homocysteine. Moreover, increased levels of homocysteine were observed in women with PCOS. Taking this into the account, disruption of the homocysteine metabolism in PCOS may impair the oocyte microenvironment. The second metabolite detected by the authors was glutamic acid. Its levels are strictly associated with efficiency of the glutamate decarboxylase, while the activity of this enzyme is regulated by the presence of vitamin B6. Glutamic acid is essential for the growth of oocytes, because it can be utilized as an alternative source of energy. These authors also suggested that the increased level of this metabolite is due to its accumulation in the FF [48].
Tang et al. (2019) pointed out that amino acid metabolic abnormalities are also characteristic of PCOS [51]. Among the branched-chain AAs, three were pointed out: valine, leucine, and isoleucine. All of them were up-regulated in women with PCOS. It is assumed that increased levels of valine, leucine, and isoleucine may affect the progression of the IR and obesity. From a biological point of view, branched-chain AAs can serve as substrates for the synthesis of glucose. It occurs in the case of IR, where abnormal glucose metabolism is carried out and alanine is obtained by the transamination of pyruvic acid. Second subgroup of AAs, which are associated with IR are lysine, phenylalanine, and 2-aminoadipic acid. Studies conducted by Tang et al. (2019) pointed to significantly higher concentrations of these metabolites in women with PCOS, which can also be associated with the IR [51]. Chen et al. (2020) reported that phenylalanine may be important for the growth and development of oocytes and could therefore be associated with ovulatory dysfunction [48].
IR plays a key role in the pathophysiology of PCOS and is associated with ovulatory dysfunction and hyperandrogenism. Some mechanisms of IR in women with PCOS are connected to an excessive activity of 17 α-hydroxylase, which regulates the conversion of 17-hydroxyprogesterone into androstenedione, excessive stimulation of IGF-I receptors, and diminished synthesis of insulin-like growth factor binding protein 1 (IGF-BP1) [52].
The Rotterdam diagnostic criteria yield four separate PCOS phenotypes (A, B, C, and D). Phenotype A includes all the three features (HA, AnO, and PCOM), whereas phenotype B, C, and D include only two (HA and AnO or HA and PCOM or AnO and PCOM, respectively). Among the mentioned PCOS studies, only one analyzed the metabolites among four different PCOS phenotypes (A, B, C, and D). Zhao et al. (2012) reported elevated levels of leucine and decreased levels of serine and threonine in women with the C (HA + PCOM) phenotype in comparison to the other phenotypes [25].
Limitations of Metabolomic Studies
The range of described metabolites identified during metabolomic studies is enormous and gives an overview of the metabolic profile of women with PCOS. Several different compounds and many biochemical pathways seem to be involved in the pathogenesis of PCOS, which indicates the complexity of this common endocrinopathy. These alterations may be caused by an increased or reduced efficiency of different biochemical reactions, up- or down-regulation of genes, increased or decreased activity of enzymes, as well the formation of alternative metabolic pathways. Metabolomics enables us to study these biochemical pathways, which might be involved in the pathogenesis of PCOS. However, it is important to remember that there are some limitations of metabolomic studies. An enormous challenge in research, which is performed with the use of human matrices is inter-individual variability, especially in women, in whom the range of the detected metabolites could be correlated to different hormone levels during the menstrual cycle. Some metabolomic studies were performed relatively in a small group of women, with only tentatively identified metabolites. There could also be some difficulties with the efficiency of the analysers, which sometimes yield false positive results. Furthermore, differences in sample preparation also have a significant impact on the final results.
Therefore, in metabolomics research, every step of the study is significant—from appropriate patient requirements through analytical accuracy to the identification and statistical analysis of the obtained results. That is why there is the need to confirm if the detected compounds could become reliable biomarkers, which would selectively distinguish PCOS from other endocrinopathies.
4. Conclusions
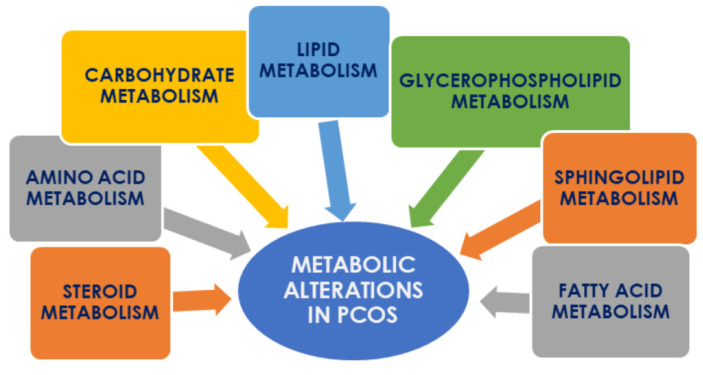
Metabolomics has proven to be a potential tool in studying the pathophysiology of PCOS. The application of metabolomics allows us to discover metabolic pathways that have been shown to be deranged. These abnormalities are associated mainly with the metabolism of lipids, fatty acids, sphingolipids and glycerophospholipids, steroids as well as carbohydrates and amino acids (Figure 3). Additionally, some alternative biochemical processes have been shown to be up-regulated in women with PCOS; however, their clinical significance should be confirmed and evaluated. Determination of disturbed pathways allows identification of the specific compounds characteristic of PCOS, which might be considered as biomarkers and became potential targets for future metabolomic research. Finding appropriate biochemical markers could be a milestone in early diagnosis of this endocrinopathy and a starting point for targeted future pharmacological interventions.
Figure 3.
The main biochemical pathways disturbed in PCOS.
Abbreviations
| HA | Hyperandrogenism |
| AnO | Anovulatory oligomenorrhea |
| TV-US | Transvaginal ultrasound |
| PCOS | Polycystic ovary syndrome |
| PCOM | Polycystic ovary morphology |
| T2DM | Type 2 diabetes mellitus |
| AH | Arterial hypertension |
| MetS | Metabolic syndrome |
| NAFLD | Non-alcoholic fatty liver disease |
| CVD | Cardiovascular disease |
| IR | Insulin resistance |
| HMDB | Human Metabolome Database |
| MS | Mass spectrometry |
| GC-MS | Gas chromatography–mass spectrometry |
| LC-MS | Liquid chromatography–mass spectrometry |
| NMR | Nuclear magnetic resonance |
| FF | Follicular fluid |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PUFAs | Poly-unsaturated fatty acids |
| IGF-BP1 | Insulin-like growth factor binding protein 1 |
Author Contributions
Conceptualization, A.R.; resources, A.R.; data curation, A.R.; writing—original draft preparation, A.R. and M.B.-F.; writing—review and editing, M.B.-F., D.R.; visualization, A.R.; supervision, M.B.-F., D.R. and M.J.M.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This review article was financed by the Polish National Science Centre (grant number 2018/31/N/NZ7/03781).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Apridonidze T., Essah P.A., Iuorno M.J., Nestler J.E. Prevalence and Characteristics of the Metabolic Syndrome in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2005;90:1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 2.Crespo R., Bachega T., Mendonça B., Gomes L. An update of genetic basis of PCOS pathogenesis. Arch. Endocrinol. Metab. 2018;62:352–361. doi: 10.20945/2359-3997000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfiel R.L. The Diagnosis of Polycystic Ovary Syndrome in Adolescents. Pediatr. Vol. 2015;136:1154–1165. doi: 10.1542/peds.2015-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amiri F.N., Tehrani F.R., Simbar M., Montazeri A., Ali R., Thamtan M. The Experience of Women Affected by Polycystic Ovary Syndrome: A Qualitative Study From Iran. Int. J. Endocrinol. Metab. 2014;12:e13612. doi: 10.5812/ijem.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha A.L.L., Faria L.C., Guimarães T.C.M., Moreira G.V., Cândido A.L., Couto C.A., Reis F.M. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: Systematic review and meta-analysis. J. Endocrinol. Investig. 2017;40:1279–1288. doi: 10.1007/s40618-017-0708-9. [DOI] [PubMed] [Google Scholar]
- 6.Carmina E., Bucchieri S., Esposito A., Del Puente A., Mansueto P., Orio F., Fede G.D., Rini G. Abdominal Fat Quantity and Distribution in Women with Polycystic Ovary Syndrome and Extent of Its Relation to Insulin Resistance. J. Clin. Endocrinol. Metab. 2007;92:2500–2505. doi: 10.1210/jc.2006-2725. [DOI] [PubMed] [Google Scholar]
- 7.Shukla P., Mukherjee S. Mitochondrial dysfunction: An emerging link in the pathophysiology of polycystic ovary syndrome. Mitochondrion. 2020;52:24–39. doi: 10.1016/j.mito.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q., Xie Y.-J., Qu L., Zhang M., Mo Z. Dyslipidemia involvement in the development of polycystic ovary syndrome. Taiwan J. Obstet. Gynecol. 2019;58:447–453. doi: 10.1016/j.tjog.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Papadakis G., Kandarako E., Papalou O., Vryonidou A., Diamanti-Kandarakis E. Is cardiovascular risk in women with PCOS a real risk? Current insights. Minerva Endocrinol. 2017;42:340–355. doi: 10.23736/S0391-1977.17.02609-8. [DOI] [PubMed] [Google Scholar]
- 10.Azziz R., Carmina E., Chen Z., Dunaif A., Laven J.S.E., Legro R.S., Lizneva D., Natterson-Horowtiz B., Teede H.J., Yildiz B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Prim. 2016;2:16057. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 11.Murri M., Insenser M., Escobar-Morreale H.F. Metabolomics in polycystic ovary syndrome. Clin. Chim. Acta. 2014;429:181–188. doi: 10.1016/j.cca.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Yan D.-K., Liu R.-H., Jin H.-Z., Liu X.-R., Ye J., Shan L., Zhang W.-D. “Omics” in pharmaceutical research: Overview, applications, challenges, and future perspectives. Chin. J. Nat. Med. 2015;13:3–21. doi: 10.1016/S1875-5364(15)60002-4. [DOI] [PubMed] [Google Scholar]
- 13.Diamanti-Kandarakis E., Piperi C., Spina J., Argyrakopoulou G., Papanastasiou L., Bergiele A., Panidis D. Polycystic Ovary Syndrome: The influence of environmental and genetic factors. Hormones. 2006;5:17–34. doi: 10.14310/horm.2002.11165. [DOI] [PubMed] [Google Scholar]
- 14.Lindon J.C., Holmes E., Bollard M.E., Stanly E.G., Nicholson J.K. Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers. 2004;9:1–31. doi: 10.1080/13547500410001668379. [DOI] [PubMed] [Google Scholar]
- 15.Luque-Ramírez M., Millán J.L.S., Escobar-Morreale H.F. Genomic variants in polycystic ovary syndrome. Clin. Chim. Acta. 2006;366:14–26. doi: 10.1016/j.cca.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Atiomo W., Khalid S., Parameshweran S., Houda M., Layfield R. Proteomic biomarkers for the diagnosis and risk stratification of polycystic ovary syndrome: A systematic review. Int. J. Obstet. Gynaecol. 2009;116:137–143. doi: 10.1111/j.1471-0528.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 17.Barderas M.G., Laborde C.M., Posada M., de la Cuesta F., Zubiri I., Vivanco F., Alvarez-Llamas G. Metabolomic profiling for identification of novel potential biomarkers in cardiovascular diseases. J. Biomed. Biotechnol. 2011;2011:790132. doi: 10.1155/2011/790132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei Z., Huhman D.V., Sumner L.W. Mass spectrometry strategies in metabolomics. J. Biol. Chem. 2011;286:25435–25442. doi: 10.1074/jbc.R111.238691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villas-Bôas S.G., Mas S., Åkesson M., Smedsgaard J., Nielsen J. Mass spectrometry in metabolome analysis. Mass. Spectrom. Rev. 2005;24:613–646. doi: 10.1002/mas.20032. [DOI] [PubMed] [Google Scholar]
- 20.Edison A.S., Markley J.L., Bru R., Eghbalnia H.R., Powers R., Raftery D., Wishart D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017;43:34–40. doi: 10.1016/j.copbio.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma X., Fan L., Meng Y., Hou Z., Mao Y.-D., Wang W., Ding W., Liu J.-Y. Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol. Hum. Reprod. 2007;13:527–535. doi: 10.1093/molehr/gam036. [DOI] [PubMed] [Google Scholar]
- 22.Matharoo-Ball B., Hughes C., Lancashire L., Tooth D., Ball G., Creaser C., Elgasim M., Rees R., Layfield R., Atiomo W. Characterization of biomarkers in polycystic ovary syndrome (PCOS) using multiple distinct proteomic platforms. J. Proteome Res. 2007;6:3321–3328. doi: 10.1021/pr070124b. [DOI] [PubMed] [Google Scholar]
- 23.Schmid C.W. Metabolomics: What’s happening downstream of DNA. Environ. Health Perspect. 2004;112:410–415. doi: 10.1289/ehp.112-a410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L., Hu W., Liu Q., Hao Q., Sun B., Zhang Q., Mao S., Qiao J., Yan X. Metabonomics Reveals Plasma Metabolic Changes and Inflammatory Marker in Polycystic Ovary Syndrome Patients. J. Proteome Res. 2012;11:2937–2946. doi: 10.1021/pr3000317. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y., Fu L., Li R., Wang L.-N., Yang Y., Liu N.-N., Zhang C.-M., Ying W., Ping L., Tu B.-B., et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: Plasma metabolomics analysis. BMC Med. 2012;10:2012. doi: 10.1186/1741-7015-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atiomo W., Daykin C.A. Metabolomic biomarkers in women with polycystic ovary syndrome: A pilot study. Mol. Hum. Reprod. 2012;18:546–553. doi: 10.1093/molehr/gas029. [DOI] [PubMed] [Google Scholar]
- 27.Escobar-Morreale H.F., Samino S., Insenser M., Vinaixa M., Luque-Ramírez M., Lasunción M.A., Correig X. Metabolic heterogeneity in polycystic ovary syndrome is determined by obesity: Plasma metabolomic approach using GC-MS. Clin. Chem. 2012;58:999–1009. doi: 10.1373/clinchem.2011.176396. [DOI] [PubMed] [Google Scholar]
- 28.Whigham L.D., Butz D.E., Dashti H., Tonelli M., Johnson L.K., Cook M.E., Porter W.P., Eghbalnia H.R., Markley J.L., Steven R., et al. Metabolic Evidence of Diminished Lipid Oxidation in Women With Polycystic Ovary Syndrome. Curr. Metabolomics. 2014;2:269–278. doi: 10.2174/2213235X01666131203230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X., Xu F., Qi B., Hao S., Li Y., Zou L., Lu C., Xu G., Hou L. Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography-mass spectrometry. J. Proteome Res. 2014;13:1101–1111. doi: 10.1021/pr401130w. [DOI] [PubMed] [Google Scholar]
- 30.Dong F., Deng D., Chen H., Cheng W., Li Q., Luo R., Ding S. Serum metabolomics study of polycystic ovary syndrome based on UPLC-QTOF-MS coupled with a pattern recognition approach. Anal. Bioanal. Chem. 2015;407:4683–4695. doi: 10.1007/s00216-015-8670-x. [DOI] [PubMed] [Google Scholar]
- 31.RoyChoudhury S., Mishra B.P., Khan T., Chattopadhayay R., Lodh I., Datta Ray C., Bose G., Sarkar H.S., Srivastava S., Joshi M.V., et al. Serum metabolomics of Indian women with polycystic ovary syndrome using 1 H NMR coupled with a pattern recognition approach. Mol. Biosyst. 2016;12:3407–3416. doi: 10.1039/C6MB00420B. [DOI] [PubMed] [Google Scholar]
- 32.Szczuko M., Zapałowska-Chwyć M., Drozd A., Maciejewska D., Starczewski A., Stachowska E. Metabolic pathways of oleic and palmitic acid are intensified in PCOS patients with normal androgen levels. Prostaglandins Leukot. Essent. Fat. Acids. 2017;126:105–111. doi: 10.1016/j.plefa.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Buszewska-Forajta M., Rachoń D., Stefaniak A., Wawrzyniak R. Identification of the metabolic fingerprints in women with polycystic ovary syndrome using the multiplatform metabolomics technique. J. Steroid Biochem. Mol. Biol. 2019;186:176–184. doi: 10.1016/j.jsbmb.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Jia C., Xu H., Xu Y., Xu Y., Shi Q. Serum metabolomics analysis of patients with polycystic ovary syndrome by mass spectrometry. Mol. Reprod. Dev. 2019;86:292–297. doi: 10.1002/mrd.23104. [DOI] [PubMed] [Google Scholar]
- 35.Haoula Z., Ravipati S., Stekel D.J., Ortori C.A., Hodgman C., Daykin C., Raine-Fenning N., Barrett D.A., Atiomo W. Lipidomic analysis of plasma samples from women with polycystic ovary syndrome. Metabolomics. 2015;11:657–666. doi: 10.1007/s11306-014-0726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan X., Jiang J., Huang Z., Gong J., Wang Y., Xue W., Deng Y., Wang Y., Zheng T., Sun A., et al. UPLC/Q-TOF-MS based plasma metabolomics and clinical characteristics of polycystic ovarian syndrome. Mol. Med. Rep. 2019;19:280–292. doi: 10.3892/mmr.2018.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vonica C.L., Ilie I.R., Socaciu C., Moraru C., Georgescu B., Farcaş A., Roman G., Mureşan A.A., Georgescu C.E. Lipidomics biomarkers in women with polycystic ovary syndrome (PCOS) using ultra-high performance liquid chromatography–quadrupole time of flight electrospray in a positive ionization mode mass spectrometry. Scand. J. Clin. Lab. Investig. 2019;79:437–442. doi: 10.1080/00365513.2019.1658215. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Peralbo M.A., Luque de Castro M.D. Preparation of urine samples prior to targeted or untargeted metabolomics mass-spectrometry analysis. Trends Anal. Chem. 2012;41:75–85. doi: 10.1016/j.trac.2012.08.011. [DOI] [Google Scholar]
- 39.Wang W., Wang S., Tan S., Wen M., Qian Y., Zeng X., Guo Y., Yu C. Detection of urine metabolites in polycystic ovary syndrome by UPLC triple-TOF-MS. Clin. Chim. Acta. 2015;448:39–47. doi: 10.1016/j.cca.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Zou Y., Zhu F.-F., Fang C.-Y., Xiong X.-Y., Li H.-Y. Identification of Potential Biomarkers for Urine Metabolomics of Polycystic Ovary Syndrome Based on Gas Chromatography-Mass Spectrometry. Chin. Med. J. (Engl.) 2018;131:945–949. doi: 10.4103/0366-6999.229899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhayat N.A., Marti N., Kollmann Z., Troendle A., Bally L., Escher G., Grössl M., Ackermann D., Ponte B., Pruijm M., et al. Urinary steroid profiling in women hints at a diagnostic signature of the polycystic ovary syndrome: A pilot study considering neglected steroid metabolites. PLoS ONE. 2018;13:e0203903. doi: 10.1371/journal.pone.0203903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arya B.K., Haq A.U., Chaudhury K. Oocyte quality reflected by follicular fluid analysis in poly cystic ovary syndrome (PCOS): A hypothesis based on intermediates of energy metabolism. Med. Hypotheses. 2012;78:475–478. doi: 10.1016/j.mehy.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 43.McRae C., Sharma V., Fisher J. Metabolite Profiling in the Pursuit of Biomarkers for IVF Outcome: The Case for Metabolomics Studies. Int. J. Reprod. Med. 2013;2013:1–16. doi: 10.1155/2013/603167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao H., Zhao Y., Li T., Li M., Li J., Li R., Liu P., Yu Y., Qiao J. Metabolism alteration in follicular niche: The nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic. Biol. Med. 2015;86:295–307. doi: 10.1016/j.freeradbiomed.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Liu L., Yin T., Yang J., Xiong C.-L. Follicular metabolic changes and effects on oocyte quality in polycystic ovary syndrome patients. Oncotarget. 2017;8:80472–80480. doi: 10.18632/oncotarget.19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L., Yin T.-L., Chen Y., Li Y., Yin L., Ding J., Yang J., Feng H.L. Follicular dynamics of glycerophospholipid and sphingolipid metabolisms in polycystic ovary syndrome patients. J. Steroid Biochem. Mol. Biol. 2018;185:142–149. doi: 10.1016/j.jsbmb.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Sun Z., Chang H., Wang A., Song J., Zhang X., Guo J., Leung P.C.K., Lian F. Identification of potential metabolic biomarkers of polycystic ovary syndrome in follicular fluid by SWATH mass spectrometry. Reprod. Biol. Endocrinol. 2019;17:45. doi: 10.1186/s12958-019-0490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X., Lu T., Wang X., Sun X., Zhang J., Zhou K., Ji X., Sun R., Wang X., Chen M., et al. Metabolic alterations associated with polycystic ovary syndrome: A UPLC Q-Exactive based metabolomic study. Clin. Chim. Acta. 2020;502:280–286. doi: 10.1016/j.cca.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Tonks K.T., Coster A.C., Christopher M.J., Chaudhuri R., Xu A., Gagnon-Bartsch J., Chisholm D.J., James D.E., Meikle P.J., Greenfield J.R., et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity. 2016;24:908–916. doi: 10.1002/oby.21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popovic M., Sartorius G., Christ-Crain M. Chronic low-grade inflammation in polycystic ovary syndrome: Is there a (patho)-physiological role for interleukin-1? Semin. Immunopathol. 2019;41:447–459. doi: 10.1007/s00281-019-00737-4. [DOI] [PubMed] [Google Scholar]
- 51.Tang L., Yuan L., Yang G., Wang F., Fu M., Chen M., Liu D. Changes in whole metabolites after exenatide treatment in overweight/obese polycystic ovary syndrome patients. Clin. Endocrinol. (Oxf.) 2019;91:508–516. doi: 10.1111/cen.14056. [DOI] [PubMed] [Google Scholar]
- 52.Lewandowski K.C., Skowrońska-Jóźwiak E., Łukasiak K., Gałuszko K., Dukowicz A., Cedro M., Lewiński A. How much insulin resistance in polycystic ovary syndrome? Comparison of HOMA-IR and insulin resistance (Belfiore) index models. Arch. Med. Sci. 2019;15:613–618. doi: 10.5114/aoms.2019.82672. [DOI] [PMC free article] [PubMed] [Google Scholar]