Abstract
Osteoporosis is a major concern worldwide and can be attributed to an imbalance between osteoblastic bone formation and osteoclastic bone resorption due to the natural aging process. Heritable factors account for 60–80% of optimal bone mineralization; however, the finer details of pathogenesis remain to be elucidated. Micro RNA (miRNA) and long-non-coding RNA (lncRNA) are two targets that have recently come into the spotlight due to their ability to control gene expression at the post-transcriptional level and provide epigenetic modification. miRNAs are a class of non-coding RNAs that are approximately 18–25 nucleotides long. It is thought that up to 60% of human protein-coding genes may be regulated by miRNAs. They have been found to regulate gene expression that controls osteoblast-dependent bone formation and osteoclast-related bone remodeling. lncRNAs are highly structured RNA transcripts longer than 200 nucleotides that do not translate into proteins. They have very complex secondary and tertiary structures and the same degradation processes as messenger RNAs. The fact that they have a rapid turnover is due to their sponge function in binding the miRNAs that lead to a degradation of the lncRNA itself. They can act as signaling, decoy, and framework molecules, or as primers. Current evidence suggests that lncRNAs can act as chromatin and transcriptional as well as post-transcriptional regulators. With regards to osteoporosis, lncRNA is thought to be involved in the proliferation, apoptosis, and inflammatory response of the bone. This review, which is based on a systematic appraisal of the current literature, provides current molecular and genetic opinions on the roles of miRNAs and lncRNAs in osteoporosis. Further research into the epigenetic modification and the regulatory roles of these molecules will bring us closer to potential disease-modifying treatment for osteoporosis. However, more issues regarding the detailed actions of miRNAs and lncRNAs in osteoporosis remain unknown and controversial and warrant future investigation.
Keywords: osteoporosis, micro RNA, miRNA, long-non-coding RNA, lncRNA
1. Overview
Osteoporosis is increasing in prevalence around the world due to the aging population. Although osteoporosis can be attributed to an imbalance between osteoblastic bone formation and osteoclastic bone resorption [1] as part of the natural aging process, the finer details of pathogenesis remain to be elucidated. Current treatment methods, including bisphosphonates, hormone replacement therapy, and immunotherapy, all carry the risk of side effects related to their mechanisms of action. Therefore, novel therapies are being developed. Further understanding of the underlying pathophysiology is paramount to developing these new therapies.
Micro RNA (miRNA) and long-non-coding RNA (lncRNA) are two such targets that have recently come into the spotlight due to their ability to control gene expression at the post-transcriptional level, providing epigenetic modification [2,3]. miRNAs are a class of non-coding RNAs that are approximately 18–25 nucleotides long [4]. It is thought that up to 60% of human protein-coding genes may be regulated by miRNAs [2,5]. They bind to the 3-untranslated regions (3-UTR) of target genes, leading to messenger RNA (mRNA) degradation and transcription inhibition [2,6]. The process of miRNA regulation is complex, as each miRNA binds to a number of targets, and several miRNAs target the same mRNA [2]. They have been found to regulate most biological processes, including cell development, differentiation, proliferation, metabolism, and cell cycle regulation [2]. They have also been found to regulate gene expression that controls osteoblast-dependent bone formation and osteoclast-related bone remodeling [3,7].
lncRNAs are highly structured RNA transcripts longer than 200 nucleotides that do not translate into proteins [8]. In fact, lncRNAs have very complex secondary and tertiary structures and the same degradation processes as mRNAs. The fact that they have a rapid turnover is due to their sponge function in binding the miRNAs that lead to a degradation of the lncRNA itself [6,9]. They can act as signaling, decoy, and framework molecules, or as primers [9]. Current evidence suggests that lncRNAs can act as chromatin and transcriptional as well as post-transcriptional regulators [8]. With regards to osteoporosis, lncRNA is thought to be involved in the proliferation, apoptosis, and inflammatory response of the bone [10].
The interaction between miRNAs and lncRNAs is also of current interest. Studies have attempted to link lncRNAs, miRNAs, and mRNAs together in a complex network, such as Hao et al.’s systematic analysis using the mandibles from ovariectomized mice [11]. Fei and colleagues performed a small study in five Chinese women to identify the key lncRNAs in postmenopausal osteoporosis (PMOP) through RNA sequencing [12]. After identifying various differentially expressed mRNAs (DEmRNAs) and differentially expressed lncRNAs (DElncRNAs), they constructed a DElncRNA-DEmRNA co-expression network [12]. In a larger study, Zhou et al. identified lncRNAs in 73 Caucasian women with PMOP and established an mRNA/lncRNA co-expression network [13]. The shared goal of the above studies was to provide a foundation for future investigations of lncRNAs in PMOP and help to develop biomarkers and drugs. In this review, we aim to summarize the current evidence on the actions of various miRNA and lncRNA. IsomiRNAs are grouped together.
2. The Role of Micro RNA (miRNA) and Long-Non-Coding RNA (lncRNA) in Osteoporosis
2.1. Micro RNA (miRNA)
2.1.1. miR-9-5p
miR-9-5p inhibits osteogenesis and promotes adipogenesis via directly binding to Wnt3a [14]. It also promotes osteoclastogenesis [15]. In a study of 30 osteoporosis patients, miR-9-5p was found to be more highly expressed in the serum of osteoporosis patients compared to healthy controls [14].
2.1.2. miR-21
miR-21 has been found to promote osteoclastogenesis. Through a positive feedback loop that involves programmed cell death, miR-21 is upregulated by osteoclastogenesis factor c-Fos and then promotes RANKL (receptor activator of nuclear factor-κB ligand)-induced osteoclastogenesis [1]. Jiang and colleagues found that miR-21 targeted SMAD7 and inhibited osteogenesis [16]. A review by Cheng et al. has concluded that studies consistently showed a positive role of miR-21 in osteoclastogenesis [17]. miR-21 was found to be increased in the serum and bone tissue of osteoporotic patients [18]. However, miR-21-5p expression has been found to be significantly lower among osteoporotic/osteopenic women with vertebral fractures [19]. The action of miR-21 is doubtful as it appears to be both up- and downregulated in postmenopausal osteoporosis trials [18,19,20,21,22]. In some in vitro studies, it is reported that it promotes osteogenesis, while in others, it inhibits osteoclastogenesis [23,24]. Therefore, further studies are needed to determine whether it can be used as a clinical biomarker for osteoporosis.
2.1.3. miR-29
Decreased miR-29 is a potential marker of osteoporosis. Lower serum miR-29 levels were associated with vertebral fractures in postmenopausal women [25]. In osteoblast-specific miR-29a transgenic mice, overexpression of miR-29a increased bone formation and decreased osteoclastic resorption by inhibiting RANKL and CXCL12 (C-X-C Motif Chemokine Ligand 12) expression in osteoblasts [26]. miR-29 is described to act in favoring of osteogenesis and limiting osteoclastogenesis [27,28,29], and it is downregulated in patients with osteoporosis [26,30]. miR-29b-3p, on the other hand, is known to downregulate multiple genes involved in osteoblast formation and is induced in osteoclast formation [31]. Other studies suggest miR-29 has a role in osteoclast differentiation, but reports on its mechanism of action are conflicting [1].
2.1.4. miR-30b-5p
In osteoporotic women, serum levels of miR-30b-5p were low. In vitro studies of osteogenesis have validated that miR-30b-5p targets RUNX2 (runt-related transcription factor 2) and decreases during the late stages of osteoblast differentiation [31].
2.1.5. miR-31
miR-31-3p has been found to inhibit osteoclastic bone resorption through the repression of osteoclast formation [1]. However, other studies have found that miR-31a-5p expression in BMSCs (bone mesenchymal stem cells) increases with age, and increases osteoclastogenesis, thereby contributing to age-related bone loss [5]. miR-31-5p was found to be downregulated in patients with WNT1 osteoporosis, a primary osteoporosis due to heterozygous p.C218G WNT1 mutation [32]. miR-31-5p is known to inhibit WNT signaling, leading to low bone formation and, therefore, the increased risk of bone fractures [32].
2.1.6. miR-100
Levels of miR-100 are increased in the serum and bone tissue of osteoporotic patients [18]. Cheng et al.’s systematic review found that miR-100 inhibited bone formation [17]. Osteoporotic patients had upregulation of miR-100-5p in both osteoblasts and osteoclasts, so it was speculated that an overall increase in expression led to bone loss. miR-100 expression was decreased during osteoblastic differentiation in vitro, and overexpression of miR-100 in MSC inhibited osteogenic differentiation. The role of miR-100 in osteoclastogenesis has not yet been established [17].
2.1.7. miR-103-3p
The levels of serum miR-103-3p are lower in osteoporotic women when compared with healthy controls [31]. In vitro studies of osteogenesis have suggested that miR-103-3p inhibits osteoblast differentiation and proliferation [31].
2.1.8. miR-122-5p
An analysis of 139 serum samples using RT-qPCR (real-time quantitative polymerase chain reaction) showed that lower levels of miR122-5p were found in patients with lower BMD (bone mineral density), and, therefore, may be associated with the development of osteoporosis [33]. This makes it a likely diagnostic biomarker for osteoporosis [33].
2.1.9. miR-124
miR-124 could potentially alleviate the progression of osteoporosis. Studies have shown miR-124 to suppress differentiation and migration of osteoclast precursors, thereby inhibiting osteoclast formation [1]. However, it also inhibits osteogenesis [34]. Similarly, serum miR-124-3p was found to be significantly upregulated in postmenopausal women with low BMD [19]. The authors of this study suggested that a possible explanation would be a compensatory mechanism of bone tissue in response to menopause-induced bone destruction [19].
2.1.10. miR-133 Family
miR-133 increases osteoclastogenesis due to mRNA targeting of the proteins that inhibit osteoclastogenesis [35]. miR-133a is upregulated in osteoporosis. It targets the RUNX2 gene 3′-UTR when overexpressed in an osteoblast cell line, and suppresses alkaline phosphatase (ALP) (a marker of osteoblast formation) production, and, therefore, osteoblast differentiation [1]. Using bioinformatics analysis, three osteoclast-related potential target genes have been identified for miR-133a (CXCL11, CXCR3, and SLC39A1), but the sample size was limited [31]. A new study led by Kocijan found miRNA-133 to be downregulated in the serum of animals receiving zoledronic acid, which suggests a positive effect of bisphosphonates on RUNX2 and thus, bone formation [36]. Cheng et al. also summarized that miR-133a promoted bone resorption and could potentially inhibit bone formation [17]
2.1.11. miR-135a-5p
miR-135a-5p levels were elevated in bone tissue of postmenopausal women with osteoporosis compared with postmenopausal women without osteoporosis [37]. miR-135a-5p was found to be potentially downregulated during osteogenic differentiation [37]. The study also suggested that miR-135a-5p inhibited osteogenic differentiation by targeting RUNX2 directly [37].
2.1.12. miR-146a
Another potential target for treating osteoporosis is miR-146a, found in bone tissue, which can be induced by TNF-a/RANKL treatment, and has been found to inhibit osteoclastogenesis in mouse models [1].
2.1.13. miR-148a
miR-148a overexpression induces osteoclast formation [1]. In an ovariectomized rat model, overexpression of microRNA-148a in the serum was associated with apoptosis and inhibition of cell growth [38]. It significantly reduced the expression of estrogen receptor alpha (ERα), phosphoinositide-3-kinase regulatory subunit 1 (PI3K), and phosphorylated-protein kinase B (AKT) in osteoblasts in vitro [38]. One can infer that suppressing miR-148a could potentially help treat osteoporosis.
2.1.14. miR-155
miR-155 plays an important role in bone destruction, as demonstrated by its involvement in rheumatoid arthritis [1]. It is now known that miR-155 regulates osteoclastogenesis through several essential transcriptional factors, such as inhibiting MITF (microphthalmia-associated transcription factor) [1].
2.1.15. miR-182-5p
In Pan et al.’s animal study, ovariectomized rats treated with alendronate were used to assess the effects of miR-182-5p [39]. They found that in the alendronate treatment group, miR-182-5p was downregulated in the serum, ADCY6 (Adenylate Cyclase 6) was upregulated, and the Rap1/MAPK (mitogen-activated protein kinase) signaling pathway was activated [39]. Then, RT-qPCR and Western blot analysis showed that miR-182-5p inhibited ADCY6 expression and Rap1/MAPK signaling pathway activation, while downregulation of miR-182-5p inhibited cell cycle progression as well as osteoblastic cell apoptosis [39]. Therefore, decreasing miR-182-5p could be a potential goal of osteoporosis treatment.
2.1.16. miR-194-5p
In whole blood lysates of postmenopausal Chinese women, miR-194-5p was found to be upregulated by over five-fold, and could potentially be used to discriminate against osteopenia and osteoporosis [5].
2.1.17. miR-200a-3p
Blood was collected from 30 postmenopausal women, and the miR-200a-3p level was found to be higher in the serum of these patients compared with controls [40]. High levels of miR-200a-3p suppressed osteogenic differentiation of BMSCs [40].
2.1.18. miR-203a
miR-203a is found to be upregulated in the bone tissue from postmenopausal women with a history of low-traumatic fractures and slows osteoblast differentiation [36].
2.1.19. miR-214-5p
An in vitro study showed that miR-214-5p overexpression promoted adipogenic differentiation, thereby promoting osteoporosis progression [41]. miR-214-5p was found to promote adipogenic differentiation of human BMSCs through the regulation of the TGF (transforming growth factor)-β/Smad2/COL4A1 (collagen type IV α1 chain) signaling pathway and would downregulate the expression of ALP, RUNX2, osteocalcin, collagen α-1 (I) chain (COL1A1) mRNA, TGF-β, phosphorylated (p)-Smad2, and COL4A1 protein [41].
2.1.20. miR-221
Bony fragments extracted from 12 women with PMOP fractures undergoing hip replacements showed that miR-221 was underexpressed compared to the 12 healthy controls (osteoarthritis without osteoporosis) [42]. miR-221 was found to inhibit osteogenic inhibition by negatively regulating RUNX2 expression [42].
2.1.21. miR-223
Upregulation of miR-223 is thought to inhibit osteoclast differentiation [1]. miR-223-5p was found to be upregulated in the serum of osteoporotic patients with hip fractures compared to nonosteoporotic women [18]. However, miR-223-5p was not associated with incident osteoporotic fractures in a large cohort of 217 women [43], causing some doubt on its clinical use.
2.1.22. miR-338 Cluster
In Guo et al.’s study, miR-338-3p was found to be decreased during osteoblast differentiation, and that miR-338-3p knockout upregulated RUNX2 at the mRNA level [44]. In a small sample (n = 15) of PMOP patients, significantly increased miR-338 levels in the serum were found [45]. Ovariectomized mice also had increased levels of miR-338, and this was detected earlier than the decrease in bone density measured by micro-CT [45]. An estrogen-dependent Runx2/Sox4 (SRY-Box Transcription Factor 4)/miR-338 positive feedback loop could afford to regulate osteoblast differentiation. The use of a miR-388 inhibitor in the mice significantly prevented osteoporosis after an ovariectomy [45].
2.1.23. miR-365
In glucocorticoid-induced osteoporosis (GIOP) mice, levels of miR-365 were found to be suppressed in bone tissue [46]. MMP-9 (matrix metalloproteinase-9) is produced by osteoclasts and assists in the degradation of the extracellular matrix [46]. Bioinformatics analysis suggests that activation of miR-365 suppresses MMP-9 [46]. Therefore, activation of miR-365 could be a novel target of osteoporosis treatment.
2.1.24. miR-410
In 26 postmenopausal women with osteoporosis, and also in ovariectomized mice, there is elevated miR-410 and reduced BMP-2 (bone morphogenetic protein 2) in serum samples [47]. Bioinformatics analysis has shown that miR-410 binds to BMP-2 and regulates its expression. It is known that BMP-2 can control miRNA expression, including the switch between bone and muscle differentiation [3]. However, this study had a small sample size with a lack of genetic diversity, and further studies are needed to help elucidate the role of miR-410 in osteoporosis.
2.1.25. miR-422a
miR-422a is significantly upregulated in the circulating monocytes of postmenopausal women with low BMD in a study by Cao et al. [48]. Using bioinformatics analysis, five potential osteoclast-related target genes were identified for miR-422a (CBL, CD226, IGF1, PAG1, TOB2), but the sample size was limited [31]. miR-422a may stimulate osteoclastogenesis, but further research is required to elucidate its mechanism of action [48].
2.1.26. miR-449b-5p
In vivo findings suggest that miR-449 overexpression could inhibit the osteogenic differentiation of BMSCs by binding directly to the 3-UTR terminus of SATB2 (Special AT-rich sequence-binding protein 2) and suppressing SATB2 [49]. Human studies are required to determine the serum levels of miR-449b-5p in PMOP.
2.1.27. miR-503
miR-503 expression was found to be markedly reduced in PMOP [1]. miR-503 overexpression in human peripheral blood monocytes led to inhibition of RANKL-induced osteoclast differentiation [1].
2.1.28. miR-543
In ovariectomized rats, overexpression of miRNA-543 was found to significantly suppress cell growth and promote apoptosis in osteoblasts [50]. In addition, miRNA-543 upregulation inhibited YAF-2 (YY1-associated factor 2) expression in osteoblasts [50]. The effects of miRNA-543 were further enhanced by YAF-2 knockdown [50]. These findings suggest that inhibiting miRNA-543 could potentially protect osteoblasts against ovariectomy-induced osteoporosis through the AKT/p38 MAPK signaling pathway and targeting YAF2 [50]. This could provide a potential therapeutic target for PMOP.
2.1.29. miR-579-3p
Micro RNA-579-3P expression in the serum of osteoporotic patients was significantly higher than those of normal controls, and this inhibited osteogenic differentiation of human BMSCs [51].
2.1.30. miR-874
In osteoporotic rats, there was inactivation of miR-874 and SUFU (suppressor of fused gene) overexpression in bone tissue. Both upregulation of miR-874 and downregulation of SUFU were found to promote osteoblast proliferation [52]. Human studies are required to establish the role of miR-874 in PMOP.
2.1.31. miR-1297
miR-1297, highly expressed in osteoporosis, has been found to inhibit osteogenic differentiation of human BMSCs [53]. It affects the Wnt signaling pathway; its direct target is WNT5A [53]. An in vitro study has shown that levels of miR-1297 decreased after osteogenic induction [53], providing a novel way to monitor PMOP treatment.
2.1.32. miR-2861
Decreased miR-2861 may contribute to the pathogenesis of osteoporosis. miR-2861 was identified in primary mouse osteoblasts, and it was found to promote osteoblast differentiation by suppressing histone deacetylase 5 (HDAC5) expression at the post-transcriptional level [54]. HDAC5 is an enhancer of RUNX2 degradation [54]. Overexpression of miR-2861 also enhances BMP2-induced osteoblastogenesis, and inhibition attenuates it [54]. In vivo silencing of miR-2861 in mice was found to reduce RUNX2 protein expression, which led to inhibited bone formation and decreased bone mass [54]. Surprisingly, miR-2861 was found to be conserved in humans, and a homozygous mutation that blocked miR-2861 expression was shown to cause primary osteoporosis in two related adolescents [54]. Therefore, this study shows that miR-2861 plays an important physiological role in osteoblast differentiation. Serum levels of miR-2861 were found to be higher in postmenopausal women with low BMD, which could reflect a compensatory mechanism of the human body towards menopause-induced bone destruction [19].
Table 1 and Table 2 provide a brief overview of the molecules listed.
Table 1.
List of micro RNAs (miRNAs), their actions, and expression in postmenopausal osteoporosis (PMOP). Studies were performed in humans unless otherwise stated in parentheses.
| miRNA | Action | Expression in PMOP | Sources | References |
|---|---|---|---|---|
| miR-9-5p | inhibit osteogenesis, promote adipogenesis promote osteoclastogenesis |
high | serum | Zhang et al. [14] Wang et al. [15] |
| miR-21 | promote osteoclastogenesis promote osteogenesis and inhibit osteoclastogenesis |
unclear | Jiang et al. [16] Cheng at al. [17] Seeliger et al. [18] Yavropoulou et al. [19] Yang et al. [23] Hu et al. [24] |
|
| miR-29 | unclear | low | serum | Tang et al. [1] Lian et al. [25] Kocijan et al. [26] Li et al. [27] Kapinas et al. [28] Rossi et al. [29] Bottani et al. [31] |
| miR-30b-5p | negatively regulate osteoblast differentiation | low | serum | Bottani et al. [31] |
| miR-31 | unclear | low | bone | Tang et a. [1] Foessl et al. [5] Mäkitie et al. [32] |
| miR-100 | inhibit osteogenic differentiation | high | bone and serum | Cheng at al. [17] Seeliger et al. [18] |
| miR-103-3p | inhibit osteoblast differentiation and proliferation | low | serum | Bottani et al. [31] |
| miR-122-5p | inhibit osteoblast differentiation | low | serum | Mandourah et al. [33] |
| miR-124 | inhibit osteoclast formation inhibit osteogenesis |
high | serum | Tang et al. [1] Yavropoulou et al. [19] Qadiret et al. [34] |
| miR-133 | inhibit osteoblast differentiation increase osteoclastogenesis |
high | bone and serum (mouse) | Tang et al. [1] Cheng at al. [17] Wang et al. [35] Kocijan et al. [36] |
| miR-135a-5p | inhibit osteogenic differentiation | high | bone | Shi et al. [37] |
| miR-146a | inhibit osteoclastogenesis | high | bone (mouse) | Tang et al. [1] |
| miR-148a | induce osteoclast formation | high | Serum (mouse) | Tang et al. [1] Xiao et al. [38] |
| miR-155 | regulate osteoclastogenesis | high | unclear (mouse) | Tang et al. [1] |
| miR-182-5p | inhibited ADCY6 expression and Rap1/MAPK signaling pathway activation | high | bone and serum (mouse) | Pan et al. [39] |
| miR-194-5p | unclear | high | whole blood lysate | Foessl et al. [5] |
| miR-200a-3p | inhibit osteogenic differentiation | high | serum | Lv et al. [40] |
| miR-203a | slow osteoblast differentiation | high | bone | Kocijan et al. [36] |
| miR-214-5p | promote adipogenic differentiation | high | (in vitro) | Qiu et al. [41] |
| miR-221 | inhibit osteogenic inhibition | low | bone | Zhang et al. [42] |
| miR-223 | inhibit osteoclast differentiation | unclear | serum | Tang et al. [1] Seeliger et al. [18] Pickering et al. [43] |
| miR-338 | regulate osteoblast differentiation | high | serum | Guo et al. [44] Lin et al. [35] |
| miR-365 | suppresses MMP-9 | low | bone (mouse) | Li et al. [46] |
| miR-410 | regulate BMP-2 expression | high | serum | van Wijnen et al. [3] Zhang et al. [47] |
| miR-422a | may stimulate osteoclastogenesis | high | human circulating monocytes | Bottani et al. [31] Cao et al. [48] |
| miR-449b-5p | inhibit osteogenic differentiation | unclear | (in vivo) | Li et al. [49] |
| miR-503 | inhibit osteoclast differentiation | low | human circulating monocytes | Tang et al. [1] |
| miR-543 | promote osteoblast apoptosis | high | bone (mouse) | Li et al. [50] |
| miR-579-3p | inhibit osteogenic differentiation | high | serum | Luo et al. [51] |
| miR-874 | promote osteoblast proliferation | low | bone (mouse) | Lin et al. [52] |
| miR-1297 | inhibit osteogenic differentiation | high | bone | Wang et al. [53] |
| miR-2861 | promote osteoblast differentiation | high | serum | Yavropoulou et al. [19] Li et al. [54] |
Table 2.
List of long-non-coding RNAs (lncRNAs), their actions, and expression in PMOP. Studies were performed in humans unless otherwise stated in parentheses.
| lncRNA | Action | Expression in PMOP | Sources | References |
|---|---|---|---|---|
| ANCR | inhibit osteoblasts, increase osteoclastogenesis | high | blood mononuclear cells | Wu et al. [9] Cai et al. [55] Tong et al. [56] |
| BMNCR | inhibit osteoporosis | low | bone (mouse) | Chen et al. [57] |
| CASC11 | lead to TNF-α upregulation in osteoclasts | high | plasma | Yu et al. [58] |
| CRNDE | regulate cell apoptosis | high | bone | Li et al. [10] |
| GAS5 | regulate osteogenic differentiation | low | bone | Feng et al. [59] |
| MALAT1 | unclear | low | bone (mouse) | Yang et al. [60] Zheng et al. [61] |
| MEG3 | unclear | high | bone | Wu et al. [9] Wang et al. [62] Sun et al. [63] |
| MSC-AS1 | induce osteogenic differentiation | unclear | bone (mouse) | Zhang et al. [64] |
| NEF | interact with IL-6 | low | plasma | Ma et al. [65] |
| SNHG1 | unclear | low | plasma | Huang et al. [66] |
| TUG1 | may promote osteoclast differentiation | high | plasma | Han et al. [67] |
| XIXT | promote osteogenic differentiation of BMSCs | low | Serum | Zhang et al. [68] |
2.2. Long-Non-Coding RNA (lncRNA)
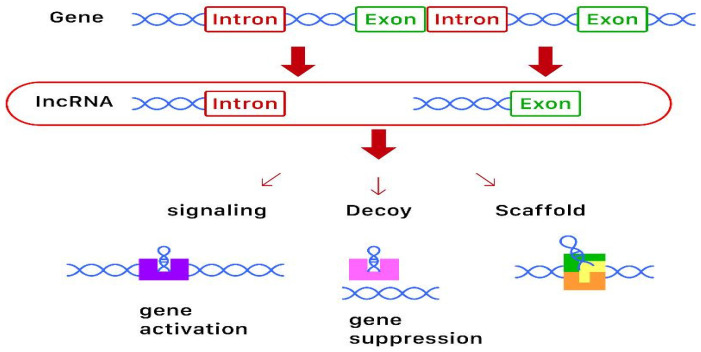
lncRNAs are highly structured RNA transcripts longer than 200 nucleotides that do not translate into proteins [8]. They have very complex secondary and tertiary structures and the same degradation processes as mRNAs. Although similar to mRNAs, lncRNAs degrade more easily due to their sponge function in binding the miRNAs that lead to a degradation of the lncRNA itself [6,9]. They can act as signaling, decoy, and framework molecules, or as primers. Current evidence suggests that lncRNAs can act as chromatin and transcriptional as well as post-transcriptional regulators. With regards to osteoporosis, lncRNA is thought to be involved in the proliferation, apoptosis, and inflammatory response of the bone. A diagram of the biosynthesis, action, and function of lncRNAs is shown in Figure 1.
Figure 1.
Diagram of the biosynthesis and function of long-non-coding RNAs (lncRNAs). lncRNAs are derived from genes, some containing introns and some containing exons. These lncRNAs then act as signaling (for gene activation), decoy (for gene suppression), or scaffold molecules to exert epigenetic modification.
2.2.1. lncRNA-ANCR
There is evidence that lncRNA-ANCR (anti-differentiation non-coding RNA), also known as DANCR (differentiation antagonizing non-protein coding RNA), promotes osteoporosis [9]. QRT-PCR detection showed that lncRNA-ANCR was increased in the osteoblast group in the PMOP mice model [55]. When ANCR was silenced through the transfection of postmenopausal mice, their osteoblast cells showed decreased apoptosis and increased proliferation [55]. It is also upregulated in the blood mononuclear cells in postmenopausal women with low BMD and is found to promote IL6 and TNF-α expression [56]. As IL6 and TNF-α are inflammatory markers involved in osteoclastogenesis [56], it can be inferred that DANCR could serve as a potential biomarker for osteoporosis.
2.2.2. lncRNA BMNCR
lncRNA BMNCR (bone marrow associated non-coding RNA) has been suggested to alleviate the progression of osteoporosis. Chen et al. found that lncRNA BMNCR expression was decreased in the bone marrow and spleen of osteoporotic mice [57]. Specifically, its expression was decreased during RANKL-induced osteoclast differentiation [57]. This would suggest that lncRNA BMNCR helps inhibit osteoporotic change.
2.2.3. lncRNA CASC11
lncRNA CASC11 (cancer susceptibility 11) has been shown to be upregulated in PMOP [58]. A small study consisting of blood samples from 67 patients with PMOP showed that CASC11 and TNF-α were both increased in their plasma [58]. The researchers deduced that overexpression of CASC11 led to TNF-α upregulation in osteoclasts [58]. Furthermore, plasma levels of CASC11 and TNF-α were found to be decreased after treatment with elcatonin [58]. Higher CASC11 levels were also associated with a prolonged treatment period [58]. However, the molecular mechanism by which CASC11 regulates TNF-α remains unknown. For now, the measurement of plasma levels of CASC11 may indicate which patients may need a longer treatment course for PMOP.
2.2.4. lncRNA CRNDE
The lncRNA colorectal neoplasia differentially expressed (CRNDE), first identified in colorectal tumors, is found to be increased in the osteoclasts of postmenopausal women compared to healthy women in a study by Li et al. [10]. Overexpression of CRNDE in osteoclasts of healthy women improved cell proliferation rate, while CRNDE knockdown in osteoclasts of osteoporotic women inhibited cell proliferation. In addition, cell percentage declined in the S-phase during CRDNE knockdown compared to overexpression, causing apoptosis [10]. The study inferred that CRNDE played a role in regulating cell apoptosis, and knockdown could halt the proliferation of osteoclasts.
2.2.5. lncRNA GAS5
Low levels of lncRNA GAS5 (growth arrest-specific 5) have been found in bone tissue of patients with PMOP [59]. Feng et al. showed that GAS5 could regulate the expression of RUNX2 through mRNA-498, which negatively regulates osteogenic differentiation [59]. Thus, overexpression of GAS5 could halt the progression of osteoporosis.
2.2.6. lncRNA MALAT1
The lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), also known as nuclear-enriched transcript 2 (NEAT2), is involved in osteoporosis, as well as serving as a prognostic biomarker for lung cancer metastases [60]. However, studies have shown conflicting results in the mouse model. Yang et al. concluded that exosomal MALAT1 derived from BMSCs could afford to enhance osteoblastic activity and improve symptoms of osteoporosis [60]. Zheng et al., on the other hand, found that MALAT1 inhibited osteogenic differentiation of BMSCs through the enhancement of the MAPK signaling pathway, and promoted the progression of osteoporosis [61]. Further studies are required to clarify these findings.
2.2.7. lncRNA MEG3
The lncRNA MEG3 (maternally expressed 3) was found to promote osteoporosis in non-cancerous subjects [62]. In both ovariectomized mice and women with PMOP, expression of miR-133a-3p and MEG3 were found to be significantly higher in bone tissue compared to controls [62]. There was a positive correlation between miR-133a-3p and MEG3 expression in BMSCs; MEG3 overexpression significantly increases miR-133a-3p expression by direct binding, downregulating osteogenic differentiation [62,63]. On the other hand, MEG3 has been found to play a critical role in osteoblastic differentiation in the treatment of multiple myeloma [9], suggesting disease-dependent effects and calling for further studies to clarify these findings.
2.2.8. lncRNA MSC-AS1
lncRNA MSC-AS1 (MSC antisense RNA 1) may alleviate osteoporosis. Expression of MSC-AS1 was found to increase with osteogenic differentiation of mice BMSCs, as well as osteogenesis-related genes, such as RUNX2, osteopontin, and osteocalcin [64]. Knockdown of MSC-AS1, on the other hand, downregulated BMP2, p-smad1/5/8, and RUNX2 [64]. The above findings suggest that MSC-AS1 plays a role in inducing osteogenic differentiation, thus alleviating osteoporosis.
2.2.9. lncRNA NEF
Plasma levels of lncRNA NEF (neighboring enhancer of FOXA2) are downregulated in PMOP women [65]. In addition, this study found that low plasma levels of lncRNA NEF were significantly correlated to a longer treatment course of elcatonin until BMD returned to normal range (which was within three months for all patients). lncRNA NEF may interact with IL-6 to produce these effects [65] and can be used as a biomarker of the disease.
2.2.10. lncRNA SNHG1
lncRNA SNHG1 (small nucleolar RNA host gene 1) has been found to be downregulated in osteoporosis [66]. Compared with healthy postmenopausal women, postmenopausal women with osteoporosis had lower plasma levels of SNHG1 in a 6-year follow-up study [66]. The study also found that anti-osteoportic treatment, such as bisphosphonates and hormone replacement therapy, could upregulate plasma SNHG1 [66]. Although SNHG1 is well-established in cancer biology as a regulator of cancer cell behavior [66], the molecular mechanism of SNHG1 in osteoporosis is still unknown. It is known that SNHG1 is involved with multiple miRNAs, such as miR-145, miR-195, miR-338, and miR-497, which are involved in the differentiation of osteoblasts and osteoclasts [66]. Therefore, SNHG1 may potentially be used as a biomarker for both the diagnosis and treatment for PMOP.
2.2.11. lncRNA TUG1
lncRNA TUG1 (taurine-upregulated gene 1) is thought to be upregulated in osteoporosis. This theory is based on the fact that it is inhibited in ankylosing spondylitis, which is commonly thought of as an inverse pathological process to osteoporosis [67]. In the plasma of patients of both genders at various stages of osteoporosis, there was upregulation of lncRNA TUG1 [67]. Although the mechanism of action of lncRNA TUG1 has not yet been elucidated, it is thought to sponge miR-204-5p to promote osteoblast differentiation [67]. It is, therefore, a potential diagnostic marker for osteoporosis.
2.2.12. lncRNA XIXT
lncRNA XIXT (X-inactive specific transcript), which could potentially alleviate osteoporosis, was found to be downregulated in the serum of osteoporotic patients [68]. It was found to promote osteogenic differentiation of BMSCs and to halt osteoporosis progression through targeting miRNA-30a-5p [68]. In addition, knockdown of miRNA-30a-5p enhances the expression of RUNX2, and vice versa, suggesting RUNX2 is the downstream target of miRNA-30a-5p [68]. Therefore, XIXT could be a potential novel therapeutic target for PMOP.
3. Discussion
The field of miRNAs and lncRNAs with regards to osteoporosis is still relatively new, and this is reflected in the quantity of research currently available. Often, there are conflicting results in the literature, such as miR-223 affording to both promote and inhibit osteoclastogenesis [3]. Wijnen et al. suggest that miRNAs provide both positive and negative cross-talk between different regulatory pathways [3], thereby leading to this phenomenon. Another possible explanation is that miRNA is present in different clinical specimens. For example, Mandourah et al. found that, while both miR-122-5p and miR-4516 were suitable biomarkers for osteoporosis, miR-122-5p was detectable in the serum, while miR-4516 was found in the plasma [33]. A large cohort study of 682 women found that there was a lack of association between bone parameters and circulating levels of miRNAs, stating results were canceled out after age adjustment [69]. The authors suggest this could be due to the fact that age was also strongly correlated with the serum levels of the 32 miRNAs they selected [69]. On the other hand, another pilot study suggests that the combination of many miRNAs can help predict fragility fracture risk [70]. Perhaps it makes sense that one cannot look at each miRNA in isolation, as the cellular processes of OP work in a synergistic fashion. Finally, many studies on miRNA deregulation lack control groups [7]. These factors are worth bearing in mind when conducting future clinical trials.
The current review of literature was retrieved from studies of different sources (human and animals) and tissues (bone cells, serum, and tissue fluid), which may explain the many conflicting results. Thus, conflicting conclusions have been reported for most miRNAs and lncRNAs depending on cellular models used, animal studies or cohorts of humans, and even analytical methods. We have specified these sources and differences where possible. One of the advantages of animal studies is the convenience in conducting these studies. Some animals (such as mice) have a shorter growth cycle than that of a human being and are subject to investigations in a limited research period. Furthermore, gene knockout can be implemented in an animal model to investigate the depletion effect of genes, DNAs, as well as RNAs on the target organs, which cannot be performed in a human being. However, the major disadvantage of animal studies lies in the differences of genes and subsequent RNAs between animals and human beings, and thus generalization of the conclusions of animal studies to human beings is limited. In contrast, the results of human studies are observational but direct evidence. The major disadvantage of human studies is that intervention (such as gene knockout) cannot be implemented due to the consideration of moral hazards. In addition, the expression of genes, miRNAs, or lncRNAs in variant tissues (bone cells, serum, and tissue fluid) may be different, thus resulting in conflicting conclusions.
Due to the lack of clear-cut associations between PMOP and the expression of miRNAs and lncRNAs at present, there is still a long way to go before they can be used as potential noninvasive biomarkers. In addition, there are also many barriers still to overcome to transfer miRNA and lncRNA knowledge to the synthesis of clinical therapeutic drugs. Synthetic oligonucleotides mimicking miRNAs have a limited half-life due to degradation by nucleases in the bloodstream, and they also have a poor capacity to penetrate host cell membranes to reach their target cells [71]. lncRNA degrades even more easily than miRNA due to their low structural stability [9]. At present, antagomiRs, viruses, scaffold-based miRNA delivery, and extracellular vesicles have all been used as vectors in miRNA studies [71]. AntagomiRs are direct mRNA inhibitors, but, unfortunately, are required at a high dose to work [71]. Adeno-associated viruses are small vectors non-pathogenic to humans but are expensive [71]. Scaffold-based miRNA delivery provides not only structural support but also a convenient environment for bone tissue growth [71]. Finally, extracellular vesicles are natural bioabsorbable gene carriers that can recognize target cells, with the great advantages of oral administration and ease of long-term storage [71]. There are also currently no reports of lncRNAs in current osteoporosis treatment up to date.
4. Conclusions
In conclusion, miRNAs and lncRNAs are two potential targets that are the logical next step in osteoporosis research. Further research into the epigenetic modification and the regulatory roles of these molecules will bring us closer to potential disease-modifying treatment for PMOP. This review provides current opinions on the roles of miRNAs and lncRNAs in osteoporosis. However, more issues regarding the detailed actions of miRNAs and lncRNAs in osteoporosis remain unknown and controversial and warrant future investigation.
Abbreviations
| 3-UTR | 3-untranslated regions |
| ADCY6 | adenylate cyclase 6 |
| AKT | phosphorylated-protein kinase B |
| ALP | alkaline phosphatase |
| BMD | bone mineral density |
| BMSC | bone mesenchymal stem cell |
| BMP-2 | bone morphogenetic protein 2 |
| COL1A1 | collagen α-1 (I) chain |
| COL4A1 | collagen type IV α1 chain |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| DElncRNAs | differentially expressed lncRNAs |
| DEmRNAs | differentially expressed mRNAs |
| ERα | estrogen receptor alpha |
| GIOP | glucocorticoid-induced osteoporosis |
| HDAC5 | histone deacetylase 5 |
| lncRNA | long non-coding RNA |
| MAPK | mitogen-activated protein kinase |
| mRNA | messenger RNA |
| miRNA | micro RNA |
| MITF | microphthalmia-associated transcription factor |
| MMP-9 | matrix metalloproteinase-9 |
| PI3K | phosphoinositide-3-kinase regulatory subunit 1 |
| PMOP | postmenopausal osteoporosis |
| RANKL | receptor activator of nuclear factor-κB ligand |
| RNA | ribonucleic acid |
| RT-qPCR | real-time quantitative polymerase chain reaction |
| RUNX2 | runt-related transcription factor 2 |
| SATB2 | special AT-rich sequence-binding protein 2 |
| SOX4 | SRY-box transcription factor 4 |
| SUFU | suppressor of fused gene |
| TGF | transforming growth factor |
| YAF-2 | YY1-associated factor 2 |
Author Contributions
N.-Y.K. and K.-H.C. conceived and designed the study. N.-Y.K., L.-R.C., and K.-H.C. performed the data collection. N.-Y.K., L.-R.C., and K.-H.C. analyzed the data. N.-Y.K., L.-R.C., and K.-H.C. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work and A.P.C. were funded by a grant of the Taipei Tzu-Chi Hospital, Taiwan (TCRD-TPE-109-10) for K.H. Chen. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tang P., Xiong Q., Ge W., Zhang L. The Role of MicroRNAs in Osteoclasts and Osteoporosis. RNA Biol. 2014;11:1355–1363. doi: 10.1080/15476286.2014.996462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Q., Zheng S., Zheng J. The Emerging Role of MicroRNAs in Bone Remodeling and Its Therapeutic Implications for Osteoporosis. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Wijnen A., van de Peppel J., van Leeuwen J., Lian J., Stein G., Westendorf J., Oursler M., Im H.J., Taipaleenmäki H., Hesse E., et al. MicroRNA Functions in Osteogenesis and Dysfunctions in Osteoporosis. Cur. Osteoporos. Rep. 2013;2:72–82. doi: 10.1007/s11914-013-0143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge D., Wang W., Chen H., Yang L., Cao X. Functions of MicroRNAs in Osteoporosis. Eur. Rev. Med. and Pharmacol. Sci. 2017;21:4784–4789. [PubMed] [Google Scholar]
- 5.Foessl I., Kotzbeck P., Obermayer-Pietsch B. MiRNAs as Novel Biomarkers for Bone Related Diseases. [(accessed on 2 July 2020)];J. Lab. Precis. Med. 2019 4 doi: 10.21037/jlpm.2018.12.06. Available online: http://jlpm.amegroups.com/article/view/4655. [DOI] [Google Scholar]
- 6.Yoon J.H., Kim J., Gorospe M. Long noncoding RNA turnover. Biochimie. 2015;117:15–21. doi: 10.1016/j.biochi.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellavia D., De Luca A., Carina V., Costa V., Raimondi L., Salamanna F., Alessandro R., Fini M., Giavaresi G. Deregulated MiRNAs in Bone Health: Epigenetic Roles in Osteoporosis. Bone. 2019;122:52–75. doi: 10.1016/j.bone.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Silva A., Moura S., Teixeira J., Barbosa M., Santos S., Almeida M. Long Noncoding RNAs: A Missing Link in Osteoporosis. Bone Res. 2019:7. doi: 10.1038/s41413-019-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Q., Li X., Miao Z., Ye J., Wang B., Zhang F., Xu R., Jiang D., Zhao M., Yuan F. Long Non-Coding RNAs: A New Regulatory Code for Osteoporosis. Front. Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W., Zhu H., Xu H., Zhang B., Huang S. CRNDE Impacts the Proliferation of Osteoclast by Estrogen Deficiency in Postmenopausal Osteoporosis. Eur. Rev. Med. Pharmacol. Sci. 2018;22:5815–5821. doi: 10.26355/eurrev_201809_15907. [DOI] [PubMed] [Google Scholar]
- 11.Hao L., Fu J., Tian Y., Wu J. Systematic Analysis of LncRNAs, MiRNAs and MRNAs for the Identification of Biomarkers for Osteoporosis in the Mandible of Ovariectomized Mice. Int. J. Mol. Med. 2017;40:689–702. doi: 10.3892/ijmm.2017.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fei Q., Bai S., Lin J., Meng H., Yang Y., Guo A. Identification of Aberrantly Expressed Long Non-Coding RNAs in Postmenopausal Osteoporosis. Int. J. Mol. Med. 2018;41:3537–3550. doi: 10.3892/ijmm.2018.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y., Xu C., Zhu W., He H., Zhang L., Tang B., Zeng Y., Tian Q., Deng H. Long Noncoding RNA Analyses for Osteoporosis Risk in Caucasian Women. Calcif. Tissue Int. 2019;105:183–192. doi: 10.1007/s00223-019-00555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H., Wang X., Zhao H., Zhou C. MicroRNA-9-5p Promotes Osteoporosis Development through Inhibiting Osteogenesis and Promoting Adipogenesis via Targeting Wnt3a. Eur. Rev. Med. Pharmacol. Sci. 2019;23:456–463. doi: 10.26355/eurrev_201901_16855. [DOI] [PubMed] [Google Scholar]
- 15.Wang S., Tang C., Zhang Q., Chen W. Reduced miR-9 and miR-181a expression down-regulates Bim concentration and promote osteoclasts survival. Int. J. Clin. Exp. Pathol. 2014;7:2209–2218. [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang L., Tian L., Zhang C. Bone Marrow Stem Cells-Derived Exosomes Extracted from Osteoporosis Patients Inhibit Osteogenesis via MicroRNA-21/SMAD7. Eur. Rev. Med. Pharmacol. Sci. 2018;22:6221–6229. doi: 10.26355/eurrev_201810_16028. [DOI] [PubMed] [Google Scholar]
- 17.Cheng V., Au P., Tan K., Cheung C. MicroRNA and Human Bone Health. JBMR Plus. 2019;3:2–13. doi: 10.1002/jbm4.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeliger C., Karpinski K., Haug A., Vester H., Schmitt A., Bauer J., van Griensven M. Five Freely Circulating MiRNAs and Bone Tissue MiRNAs Are Associated with Osteoporotic Fractures. J. Bone Miner. Res. 2014;29:1718–1728. doi: 10.1002/jbmr.2175. [DOI] [PubMed] [Google Scholar]
- 19.Yavropoulou M., Anastasilakis A., Makras P., Tsalikakis D., Grammatiki M., Yovos J. Expression of MicroRNAs That Regulate Bone Turnover in the Serum of Postmenopausal Women with Low Bone Mass and Vertebral Fractures. Eur. J. Endocrinol. 2017;176:169–176. doi: 10.1530/EJE-16-0583. [DOI] [PubMed] [Google Scholar]
- 20.Kelch S., Balmayor E.R., Seeliger C., Vester H., Kirschke J.S., van Griensven M. miRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients. Sci. Rep. 2017;7:15861. doi: 10.1038/s41598-017-16113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H., Wang Z., Fu Q., Zhang J. Plasma miRNA levels correlate with sensitivity to bone mineral density in postmenopausal osteoporosis patients. Biomarkers. 2014;19:553–556. doi: 10.3109/1354750X.2014.935957. [DOI] [PubMed] [Google Scholar]
- 22.Weilner S., Skalicky S., Salzer B., Keider V., Wagner M., Hildner F., Gabriel C., Dovjak P., Pietschmann P., Grillari-Voglauer R., et al. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone. 2015;79:43–51. doi: 10.1016/j.bone.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Yang N., Wang G., Hu C., Shi Y., Liao L., Shi S., Cai Y., Cheng S., Wang X., Liu Y., et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J. Bone Miner. Res. 2013;28:559–573. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]
- 24.Hu C.H., Sui B.D., Du F.Y., Shuai Y., Zheng C.X., Zhao P., Yu X.R., Jin Y. miR-21 deficiency inhibits osteoclast function and prevents bone loss in mice. Sci. Rep. 2017;7:43191. doi: 10.1038/srep43191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lian W., Ko J., Chen Y., Ke H., Hsieh C., Kuo C., Wang S., Huang B., Tseng J., Wang F. MicroRNA-29a Represses Osteoclast Formation and Protects against Osteoporosis by Regulating PCAF-Mediated RANKL and CXCL12. Cell Death Dis. 2019;10:705. doi: 10.1038/s41419-019-1942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kocijan R., Muschitz C., Geiger E., Skalicky S., Baierl A., Dormann R., Plachel F., Feichtinger X., Heimel P., Fahrleitner-Pammer A., et al. Circulating MicroRNA Signatures in Patients With Idiopathic and Postmenopausal Osteoporosis and Fragility Fractures. J. Clin. Endocrinol. Metab. 2016;101:4125–4134. doi: 10.1210/jc.2016-2365. [DOI] [PubMed] [Google Scholar]
- 27.Li Z., Hassan M.Q., Jafferji M., Aqeilan R.I., Garzon R., Croce C.M., Van Wijnen A.J., Stein J.L., Stein G.S., Lian J.B. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J. Biol. Chem. 2009;284:15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapinas K., Kessler C., Ricks T., Gronowicz G., Delany A.M. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J. Biol. Chem. 2010;285:25221–25231. doi: 10.1074/jbc.M110.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi M., Pitari M.R., Amodio N., Di Martino M.T., Conforti F., Leone E., Botta C., Paolino F.M., Del Giudice T., Iuliano E., et al. miR-29b negatively regulates human osteoclastic cell differentiation and function: Implications for the treatment of multiple myeloma-related bone disease. J. Cell. Physiol. 2013;228:1506–1515. doi: 10.1002/jcp.24306. [DOI] [PubMed] [Google Scholar]
- 30.Feichtinger X., Muschitz C., Heimel P., Baierl A., Fahrleitner-Pammer A., Redl H., Resch H., Geiger E., Skalicky S., Dormann R., et al. Bone-related Circulating MicroRNAs miR-29b-3p, miR-550a-3p, and miR-324-3p and their Association to Bone Microstructure and Histomorphometry. Sci. Rep. 2018;8:4867. doi: 10.1038/s41598-018-22844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bottani M., Banfi G., Lombardi G. Perspectives on MiRAs as Epigenetic Markers in Osteoporosis and Bone Fracture Risk: A Step Forward in Personalized Diagnosis. Front. Genet. 2019;10 doi: 10.3389/fgene.2019.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mäkitie R., Hackl M., Niinimäki R., Kakko S., Grillari J., Mäkitie O. Altered MicroRNA Profile in Osteoporosis Caused by Impaired WNT Signaling. J. Clin. Endocrinol. Metab. 2018;103:1985–1996. doi: 10.1210/jc.2017-02585. [DOI] [PubMed] [Google Scholar]
- 33.Mandourah A., Ranganath L., Barraclough R., Vinjamuri S., Van’T Hof R., Hamill S., Czanner G., Dera A., Wang D., Barraclough D. Circulating MicroRNAs as Potential Diagnostic Biomarkers for Osteoporosis. Sci. Rep. 2018;8:8421. doi: 10.1038/s41598-018-26525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qadir A.S., Um S., Lee H., Baek K., Seo B.M., Lee G., Kim G.S., Woo K.M., Ryoo H.M., Baek J.H. miR-124 Negatively Regulates Osteogenic Differentiation and In vivo Bone Formation of Mesenchymal Stem Cells. J. Cell. Biochem. 2015;116:730–742. doi: 10.1002/jcb.25026. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Li L., Moore B.T., Peng X.H., Fang X., Lappe J.M., Recker R.R., Xiao P. MiR-133a in human circulating monocytes: A potential biomarker associated with postmenopausal osteoporosis. PLoS ONE. 2012;7:e34641. doi: 10.1371/journal.pone.0034641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kocijan R., Weigl M., Skalicky S., Geiger E., Ferguson J., Leinfellner G., Heimel P., Pietschmann P., Grillari J., Redl H., et al. MicroRNA Levels in Bone and Blood Change during Bisphosphonate and Teriparatide Therapy in an Animal Model of Postmenopausal Osteoporosis. Bone. 2020;131:115104. doi: 10.1016/j.bone.2019.115104. [DOI] [PubMed] [Google Scholar]
- 37.Shi X., Zhang Z. MicroRNA-135a-5p Is Involved in Osteoporosis Progression through Regulation of Osteogenic Differentiation by Targeting RUNX2. Exp. Ther. Med. 2019;18:2393–2400. doi: 10.3892/etm.2019.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao Y., Li B., Liu J. MicroRNA-148a Inhibition Protects against Ovariectomy-induced Osteoporosis through PI3K/AKT Signaling by Estrogen Receptor α. Mol. Med. Rep. 2018;17:7789–7796. doi: 10.3892/mmr.2018.8845. [DOI] [PubMed] [Google Scholar]
- 39.Pan B., Tong Z., Li S., Wu L., Liao J., Yang Y., Li H., Dai Y., Li J., Pan L. Decreased MicroRNA-182-5p Helps Alendronate Promote Osteoblast Proliferation and Differentiation in Osteoporosis via the Rap1/MAPK Pathway. Biosci. Rep. 2018:38. doi: 10.1042/BSR20180696. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Lv R., Pan X., Song L., Sun Q., Guo C., Zou S., Zhou Q. MicroRNA-200a-3p Accelerates the Progression of Osteoporosis by Targeting Glutaminase to Inhibit Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Biomed. Pharmacother. 2019;116:108960. doi: 10.1016/j.biopha.2019.108960. [DOI] [PubMed] [Google Scholar]
- 41.Qiu J., Huang G., Na N., Chen L. MicroRNA-214-5p/TGF-β/Smad2 Signaling Alters Adipogenic Differentiation of Bone Marrow Stem Cells in Postmenopausal Osteoporosis. Mol. Med. Rep. 2018;17:6301–6310. doi: 10.3892/mmr.2018.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Gao Y., Cai L., Li F., Lou Y., Xu N., Kang Y., Yang H. MicroRNA-221 Is Involved in the Regulation of Osteoporosis through Regulates RUNX2 Protein Expression and Osteoblast Differentiation. Am. J. Transl. Res. 2017;9:126–135. [PMC free article] [PubMed] [Google Scholar]
- 43.Pickering M., Millet M., Rousseau J., Croset M., Szulc P., Borel O., Rendu E., Chapurlat R. Selected Serum MicroRNA, Abdominal Aortic Calcification and Risk of Osteoporotic Fracture. PLoS ONE. 2019;14:e0216947. doi: 10.1371/journal.pone.0216947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo D., Han Y., Cong L., Liang D., Tu G. Resveratrol Prevents Osteoporosis in Ovariectomized Rats by Regulating MicroRNA-338-3p. Mol. Med. Rep. 2015;12:2098–2106. doi: 10.3892/mmr.2015.3581. [DOI] [PubMed] [Google Scholar]
- 45.Lin C., Yu S., Jin R., Xiao Y., Pan M., Pei F., Zhu X., Huang H., Zhang Z., Chen S., et al. Circulating MiR-338 Cluster Activities on Osteoblast Differentiation: Potential Diagnostic and Therapeutic Targets for Postmenopausal Osteoporosis. Theranostics. 2019;9:3780–3797. doi: 10.7150/thno.34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li G., Bu J., Zhu Y., Xiao X., Liang X., Zhang R. Curcumin Improves Bone Microarchitecture in Glucocorticoid-Induced Secondary Osteoporosis Mice through the Activation of MicroRNA-365 via Regulating MMP-9. Int. J. Clin. Exp. Pathol. 2015;8:15684–15695. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H., Ding W., Ji F., Wu D. MicroRNA-410 Participates in the Pathological Process of Postmenopausal Osteoporosis by Downregulating Bone Morphogenetic Protein-2. Exp. Ther. Med. 2019;18:3659–3666. doi: 10.3892/etm.2019.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao Z., Moore B., Wang Y., Peng X., Lappe J., Recker R., Xiao P. MiR-422a as a Potential Cellular MicroRNA Biomarker for Postmenopausal Osteoporosis. PLoS ONE. 2014;9:e97098. doi: 10.1371/journal.pone.0097098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J., Wei X., Sun Q., Zhao X., Zheng C., Bai C., Du J., Zhang Z., Zhu L., Jia Y. MicroRNA-449b-5p Promotes the Progression of Osteoporosis by Inhibiting Osteogenic Differentiation of BMSCs via Targeting Satb2. Eur. Rev. Med. Pharmacol. Sci. 2019;23:6394–6403. doi: 10.26355/eurrev_201908_18519. [DOI] [PubMed] [Google Scholar]
- 50.Li X., Ning L., Zhao X., Wan S. MicroRNA-543 Promotes Ovariectomy-Induced Osteoporosis through Inhibition of AKT/P38 MAPK Signaling Pathway by Targeting YAF2. J. Cell. Biochem. 2018 doi: 10.1002/jcb.28143. [DOI] [PubMed] [Google Scholar]
- 51.Luo B., Yang J., Wang Y., Qu G., Hao P., Zeng Z., Yuan J., Yang R., Yuan Y. MicroRNA-579-3p Promotes the Progression of Osteoporosis by Inhibiting Osteogenic Differentiation of Mesenchymal Stem Cells through Regulating Sirt1. Eur. Rev. Med. Pharmacol. Sci. 2019;23:6791–6799. doi: 10.26355/eurrev_201908_18717. [DOI] [PubMed] [Google Scholar]
- 52.Lin J., Liu Z., Yu B., Zhang X. MicroRNA-874 Targeting SUFU Involves in Osteoblast Proliferation and Differentiation in Osteoporosis Rats through the Hedgehog Signaling Pathway. Biochem. Biophys. Res. Comm. 2018;506:194–203. doi: 10.1016/j.bbrc.2018.09.187. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q., Wang C., Meng Y. MicroRNA-1297 Promotes the Progression of Osteoporosis through Regulation of Osteogenesis of Bone Marrow Mesenchymal Stem Cells by Targeting WNT5A. Eur. Rev. Med. Pharmacol. Sci. 2019;23:4541–4550. doi: 10.26355/eurrev_201906_18029. [DOI] [PubMed] [Google Scholar]
- 54.Li H., Xie H., Liu W., Hu R., Huang B., Tan Y.F., Liao E.Y., Xu K., Sheng Z.F., Zhou H.D., et al. A Novel MicroRNA Targeting HDAC5 Regulates Osteoblast Differentiation in Mice and Contributes to Primary Osteoporosis in Humans. J. Clin. Invest. 2009;119:3666–3677. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai N., Li C., Wang F. Silencing of LncRNA-ANCR Promotes the Osteogenesis of Osteoblast Cells in Postmenopausal Osteoporosis via Targeting EZH2 and RUNX2. Yonsei Med. J. 2019;60:751–759. doi: 10.3349/ymj.2019.60.8.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tong X., Gu P., Xu S., Lin X. Long Non-Coding RNA-DANCR in Human Circulating Monocytes: A Potential Biomarker Associated with Postmenopausal Osteoporosis. Biosci. Biotechnol. Biochem. 2015;79:732–737. doi: 10.1080/09168451.2014.998617. [DOI] [PubMed] [Google Scholar]
- 57.Chen R., Zhang X., Zhu X., Wang C. LncRNA Bmncr Alleviates the Progression of Osteoporosis by Inhibiting RANML-Induced Osteoclast Differentiation. Eur. Rev. Med. Pharmacol. Sci. 2019;23:9199–9206. doi: 10.26355/eurrev_201911_19411. [DOI] [PubMed] [Google Scholar]
- 58.Yu H., Zhou W., Yan W., Xu Z., Xie Y., Zhang P. LncRNA CASC11 Is Upregulated in Postmenopausal Osteoporosis and Is Correlated with TNF-α. Clin. Interv. Aging. 2019;14:1663–1669. doi: 10.2147/CIA.S205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng J., Wang J., Li C. LncRNA GAS5 Overexpression Alleviates the Development of Osteoporosis through Promoting Osteogenic Differentiation of MSCs via Targeting MicroRNA-498 to Regulate RUNX2. Eur. Rev. Med. Pharmacol. Sci. 2019;23:7757–7765. doi: 10.26355/eurrev_201909_18985. [DOI] [PubMed] [Google Scholar]
- 60.Yang X., Yang J., Lei P., Wen T. LncRNA MALAT1 Shuttled by Bone Marrow-Derived Mesenchymal Stem Cells-Secreted Exosomes Alleviates Osteoporosis through Mediating MicroRNA-34c/SATB2 Axis. Aging. 2019;11:8777–8791. doi: 10.18632/aging.102264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng S., Wang Y., Yang Y., Chen B., Wang C., Li R., Huang D. LncRNA MALAT1 Inhibits Osteogenic Differentiation of Mesenchymal Stem Cells in Osteoporosis Rats through MAPK Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:4609–46017. doi: 10.26355/eurrev_201906_18038. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q., Li Y., Zhang Y., Ma L., Lin L., Meng J., Jiang L., Wang L., Zhou P., Zhang Y. LncRNA MEG3 Inhibited Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells from Postmenopausal Osteoporosis by Targeting MiR-133a-3p. Biomed. Pharmacother. 2017;89:1178–1186. doi: 10.1016/j.biopha.2017.02.090. [DOI] [PubMed] [Google Scholar]
- 63.Sun H., Peng G., Wu H., Liu M., Mao G., Ning X., Yang H., Deng J. Long non-coding RNA MEG3 is involved in osteogenic differentiation and bone diseases (Review) Biomed. Rep. 2020;13:15–21. doi: 10.3892/br.2020.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang N., Hu X., He S., Ding W., Wang F., Zhao Y., Huang Z. LncRNA MSC-AS1 Promotes Osteogenic Differentiation and Alleviates Osteoporosis through Sponging MicroRNA-140-5p to Upregulate BMP2. Biochem/ Biophys. Res. Comm. 2019;519:790–796. doi: 10.1016/j.bbrc.2019.09.058. [DOI] [PubMed] [Google Scholar]
- 65.Ma X., Guo Z., Gao W., Wang J., Liu Y., Gao F., Sun S., Zhou X., Yang Z., Zheng W. LncRNA-NEF Is Downregulated in Postmenopausal Osteoporosis and Is Related to Course of Treatment and Recurrence. J. Int. Med. Res. 2019;47:3299–3306. doi: 10.1177/0300060519847854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang S., Zhu X., Xiao D., Zhuang J., Liang G., Liang C., Zheng X., Ke Y., Chang Y. LncRNA SNHG1 Was Down-Regulated after Menopause and Participates in Postmenopausal Osteoporosis. Biosci. Rep. 2019;39 doi: 10.1042/BSR20190445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han Y., Liu C., Lei M., Sun S., Zheng W., Niu Y., Xia X. LncRNA TUG1 Was Upregulated in Osteoporosis and Regulates the Proliferation and Apoptosis of Osteoclasts. J. Orthop. Surg. Res. 2019;14 doi: 10.1186/s13018-019-1430-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Zhang H., Du X., Dong Q. LncRNA XIXT Promotes Osteogenic Differentiation of Bone Mesenchymal Stem Cells and Alleviates Osteoporosis Progression by Targeting MiRNA-30a-5p. Eur. Rev. Med. Pharmacol. Sci. 2019;23:8721–8729. doi: 10.26355/eurrev_201910_19266. [DOI] [PubMed] [Google Scholar]
- 69.Feurer E., Kan C., Croset M., Sornay-Rendu E., Chapurlat R. Lack of Association Between Select Circulating miRNAs and Bone Mass, Turnover, and Fractures: Data from the OFELY Cohort. J. Bone Miner. Res. 2019;34:1074–1085. doi: 10.1002/jbmr.3685. [DOI] [PubMed] [Google Scholar]
- 70.Ladang A., Beaudart C., Locquet M., Reginster J.-Y., Bruyère O., Cavalier E. Evaluation of a Panel of MicroRNAs That Predicts Fragility Fracture Risk: A Pilot Study. Calcif. Tissue Int. 2020;106:239–247. doi: 10.1007/s00223-019-00628-8. [DOI] [PubMed] [Google Scholar]
- 71.Sun X., Guo Q., Wei W., Robertson S., Yuan Y., Luo Y. Current Progress on MicroRNA-Based Gene Delivery in the Treatment of Osteoporosis and Osteoporotic Fracture. Int. J. Endocrinol. 2019;3:1–17. doi: 10.1155/2019/6782653. [DOI] [PMC free article] [PubMed] [Google Scholar]