Madam — South Korea reported the highest number of patients with coronavirus disease 2019 (COVID-19) outside China between 20 February and 9 March 2020, primarily in Daegu city [1]. At that time, there were no guidelines for the treatment of cancer patients during the pandemic. Between 18 February and 30 April 2020, 2295 breast cancer patients visited our regional cancer centres. Of these, 569 (24.8%) patients received systemic treatment, including cytotoxic chemotherapy (n = 230, 40.4%), targeted therapies (n = 23, 4.0%) and hormonal therapy (n = 316, 55.5%) (Table 1 ). In total, 229 (9.9%) patients were tested for COVID-19. All were negative except for two patients undergoing adjuvant trastuzumab treatment. They both showed a mild COVID-19 disease course and completed the planned schedule. Other than those two patients, we found no significant effect on increasing infection risk. Considering the prognosis of breast cancer patients, our cancer centre focused on personal hygiene and patient education to reduce the risk of COVID-19 infection rather than changing the treatment strategy. Notwithstanding, we did not find an increased risk of infection in combination with active anticancer treatment, including cytotoxic chemotherapy, even during the pandemic in Daegu, Korea. With our institutional experience, an effective way to protect cancer patients from COVID-19 has been suggested [2,3].
-
•
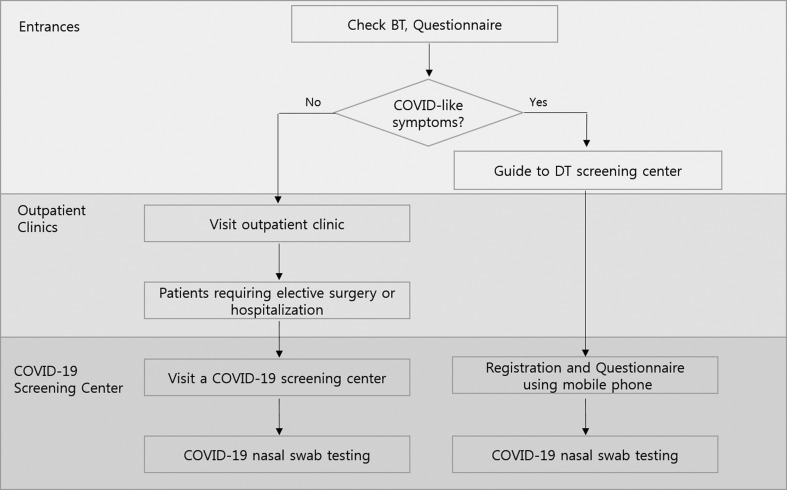
Routine screening for COVID-19 when the patients are hospitalised or before a major surgical procedure (Figure 1).
-
•
A screening facility for COVID-19 in a separate building to the main hospital, including a drive-through screening centre
-
•
Close co-operation with governmental and regional Centers for Disease Control and Prevention.
Table 1.
Breast cancer patient characteristics (total, n = 569)
| Characteristics | n (%) |
|---|---|
| Age (years) | |
| ≤40 | 48 (8.4) |
| 40–50 | 158 (27.8) |
| 50–60 | 211 (37.1) |
| 60–70 | 100 (17.6) |
| >70 | 52 (9.1) |
| Gender | |
| Female | 566 (99.5) |
| Male | 3 (0.5) |
| ECOG performance status | |
| 0 or 1 | 399 (70.1) |
| 2 | 120 (21.1) |
| Unknown | 50 (8.8) |
| Stage (AJCC eighth edition), anatomic | |
| I | 168 (29.5) |
| IIA/B | 209 (36.7) |
| IIIA/B/C | 87 (15.3) |
| IV | 105 (18.5) |
| Type of anticancer therapy | |
| Cytotoxic chemotherapy | 230 (40.4) |
| Docetaxel/paclitaxel | 109 (19.1) |
| Adriamycin | 45 (7.9) |
| Gemcitabine | 29 (5.1) |
| Vinorelbine | 8 (1.4) |
| CMF | 19 (3.3) |
| Eribulin | 8 (1.4) |
| Clinical trial | 12 (2.0) |
| Non-cytotoxic therapy | 339 (59.6) |
| Endocrine therapy | 316 (55.5) |
| Palbociclib with letrozole | 7 (1.2) |
| Targeted therapy including trastuzumab | 23 (4.0) |
Fig 1.
Patient care policy during the coronavirus disease 2019 (COVID-19) pandemic in our cancer centre. The processes Kyungpook National University Chilgok Hospital have in place to keep our cancer patients safe from COVID-19 while receiving anticancer treatment. BT, body temperature; DT, drive-through.
Based on our experience in treating breast cancer patients in a pandemic region, we recommend that breast cancer patients can undergo their planned treatment with minimal impact of COVID-19 where there are active hygiene and institutional measures against COVID-19.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Korean Society of Infectious Diseases Korean Society of Pediatric Infectious Diseases, Korean Society of Epidemiology, Korean Society for Antimicrobial Therapy, Korean Society for Healthcare-associated Infection Control and Prevention, Korea Centers for Disease Control and Prevention. Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon K.T., Ko J.H., Shin H., Sung M., Kim J.Y. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J Korean Med Sci. 2020;35:e123. doi: 10.3346/jkms.2020.35.e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S.Y., Choi S.H., Park J.E., Hwang S., Kwon K.T. Crucial role of temporary airborne infection isolation rooms in an intensive care unit: containing the COVID-19 outbreak in South Korea. Crit Care. 2020;24:238. doi: 10.1186/s13054-020-02944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]