Abstract
Coronavirus disease 2019 (COVID-19) is a newly emerging human infectious disease that has quickly become a worldwide threat to health, mainly causing severe acute respiratory syndrome. In addition to the widely described respiratory syndrome, COVID-19 may cause life-treating complications directly or indirectly related to this infection. Among these, thrombotic complications have emerged as an important issue in patients with COVID-19 infection, particularly in patients in intensive care units. Thrombotic complications due to COVID-19 are likely to occur due to a pro-coagulant pattern encountered in some of these patients or to a progressive endothelial thrombo-inflammatory syndrome causing microvascular disease. In the present authors' experience, from five different hospitals in Italy and the UK, imaging has proved its utility in identifying these COVID-19-related thrombotic complications, with translational clinical relevance. The aim of this review is to illustrate thromboembolic complications directly or indirectly related to COVID-19 disease. Specifically, this review will show complications related to thromboembolism due to a pro-coagulant pattern from those likely related to an endothelial thrombo-inflammatory syndrome.
Background
Coronavirus disease 2019 (COVID-19) is a newly emerging human infectious disease that has quickly spread worldwide, mainly causing a severe acute respiratory syndrome.1 , 2 In addition to lung disease, COVID-19 may cause a wide spectrum of non-respiratory COVID-19 disease due to involvement of organs by the virus or to complications directly or indirectly related to this infection. Among these, thrombotic complications due to abnormal coagulation status have emerged as an important issue in patients with COVID-19 infection,3 , 4 and may occur in up to 31% of intensive care unit (ICU) COVID-19 patients.5 , 6 Bikdeli et al. 7 suggested some possible causative mechanisms for COVID-19-associated thrombotic disease, including direct effects of COVID-19 through severe illness and hypoxia or severe inflammatory response, or an indirect effect of infection related to investigational therapies used for treating COVID-19, which may have adverse drug–drug interactions with antiplatelet agents and anticoagulants.
Interestingly, some COVID-19 patients have haemostatic abnormalities, including mild thrombocytopenia, increased D-dimer levels, increased clot strength, and hyperfibrinogenaemia, which all indicate a pro-coagulant pattern. Increased levels of D-dimer and fibrin degradation products and prothrombin time prolongation are associated with a higher risk of death in these patients.8 , 9 In addition, anticoagulant therapy mainly with low molecular weight heparin appears to be associated with better prognosis in severe COVID-19 patients meeting sepsis-induced coagulopathy criteria or with markedly elevated D-dimer.3
The pro-coagulant pattern identified in many patients with COVID-19 explains the relatively high rate of thrombotic complications, including pulmonary embolism, deep-vein thrombosis of leg, catheter-related upper extremity thrombosis, renal infarct, and ischaemic stroke, which have also been identified in patients without known prior hypercoagulable conditions and no prior history of atrial fibrillation.5 , 10
Thrombotic complications due to COVID-19 have also been suggested to occur due to a progressive endothelial thrombo-inflammatory syndrome causing microvascular disease.11 Some theories have suggested that this endothelial thrombo-inflammatory syndrome may be related to the presence of ACE2, which is the target receptor of COVID-19, in the endothelial cells of many small vessels12 , 13 or to complement-mediated microvascular injury.14 Varga et al. 15 also recently demonstrated at histopathology endothelial cell involvement across the vascular beds of different organs in a series of patients with COVID-19, including glomerular capillary loops of the kidney, vessels feeding the small bowel in three patients causing mesenteric ischaemia, and lymphocytic endotheliitis in the lung, heart, kidney, and liver causing multi-organ failure in one patient. In one of the three reported patients with small bowel mesenteric ischaemia, computed tomography (CT) demonstrated pneumatosis intestinalis of the jejunum and air within the portal vein as signs of mesenteric ischaemia.15 COVID-19 endotheliitis could explain the systemic impaired microcirculatory function in different vascular beds and their clinical sequelae in patients with COVID-19.
Although evidence regarding the mechanism of thromboembolic complications of COVID-19 is accumulating rapidly, there are few imaging presentation reports of these complications in COVID-19. In the authors' experience from four different COVID hospitals in Italy, including three centres in the Lombardy region and one in Sicily, and a hospital in the UK, imaging has proved its utility in identifying these COVID-19-related thrombotic complications, with translational clinical relevance.
The aim of this review is to illustrate the direct or indirect thromboembolic effects of COVID-19 disease, including those caused by pro-coagulation thrombosis and endothelial thrombo-inflammatory syndromes.
Pulmonary embolism
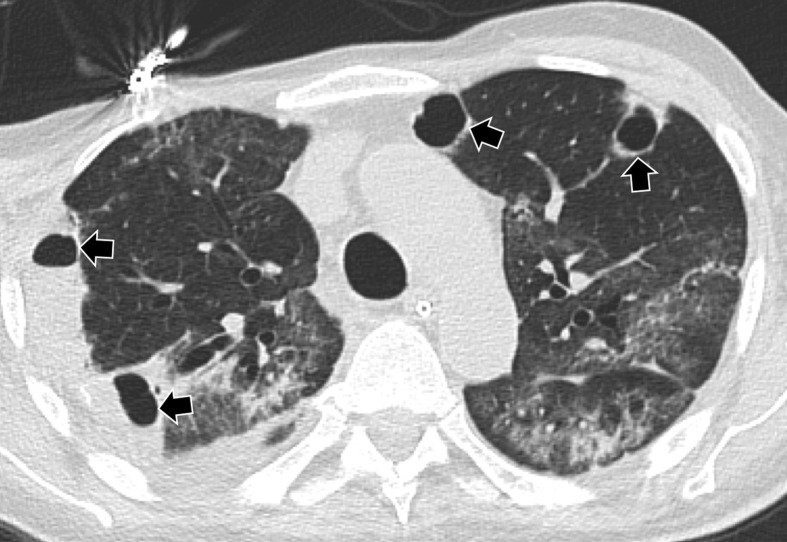
Pulmonary embolism has been demonstrated radiologically in up to 30% of patients with COVID-19 on pulmonary CT angiography, and in 14% patients with proven COVID-19 pneumonia admitted to the ICU.5 , 16 , 17 Pulmonary emboli detected in COVID-19 patients are located in main pulmonary artery in 22% of the cases, lobar artery in 34%, segmental artery in 28%, and subsegmental artery in 16% (Figure 1, Figure 2 ).16 COVID-19 patients with pulmonary embolism have higher D-dimer levels than those without pulmonary emboli and are more likely to be admitted to the ICU.16 , 17 In case of elevated D-dimer levels on hospital admission or sudden clinical worsening, CT pulmonary angiography should be considered, as pulmonary embolism is a life-threatening, but potentially curable, condition.18 Interestingly, in patients with COVID-19 infection, D-dimer >2,660 ng/mL had a sensitivity of 100% and a specificity of 67% for predicting the diagnosis of pulmonary embolism.16 Therefore, in patients that are likely to be admitted to the ICU, CT pulmonary angiography should be performed in addition to unenhanced chest CT on hospital admission, particularly if D-dimer levels are elevated. Indeed, once these patients with severe acute respiratory distress syndrome (ARDS) are admitted to the ICU, CT pulmonary angiography for pulmonary embolism may be not feasible or particularly difficult to perform due to critical illness and prone position.7 Pulmonary embolism may cause acute right-sided heart failure leading to increased central venous pressure and potentially passive hepatic congestion with hepatomegaly and nutmeg liver aspect on contrast-enhanced CT (Fig 3 ).19, 20, 21, 22 Lung infarction as a complication of pulmonary embolisms is rare due to the double arterial circulation of the lung, but it has been described in COVID-19 patients (Fig 4 )23; if present, it appears on CT as ground-glass opacities in the early phases in unobstructed lung zones representing pulmonary haemorrhage and peripheral wedge-shaped pulmonary consolidation and may rarely show a reverse halo sign or excavation.24, 25, 26 Therefore, lung infarction from pulmonary embolism needs to be differentiated from ground-glass opacities or consolidations related to COVID-19, also considering that these consolidations may show a reverse halo in about 4% of patients, or from cavitation due to pulmonary septic embolism (Fig 5 ) and from ground-glass opacities related to small vessel thrombosis considering that alveolar capillary microthrombi and pulmonary vascular microangiopathy have been described on autopsy series of COVID-19 patients.27 The wedge-shaped appearance of the consolidation and the identification of associated pulmonary embolism may be helpful for the differential diagnosis.
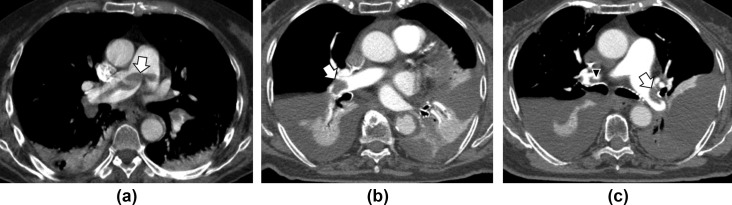
Figure 1.
Embolisms of the saddle and main pulmonary arteries in two patients with COVID-19. (a) CT pulmonary angiogram of a 72-year-old woman with D-dimer level >20,000 ng/ml on admission to the emergency department reveals a thrombus (arrow) in the main pulmonary artery at the saddle extending across the origin of both the right and left pulmonary arteries. (b,c) A 72-year-old woman with D-dimer level >2,000 ng/ml: (b) CT pulmonary angiogram on admission shows a pulmonary thrombus (arrow) in the right main pulmonary artery, subsegmental thrombi in the left, bilateral pleural effusion with atelectasis. (c) CT pulmonary angiogram at 1-week follow-up for clinical worsening shows pulmonary thrombus (arrow) in the left main pulmonary artery, bilateral pleural effusion with atelectasis, and a small clot is also visible in the right pulmonary artery (arrowhead).
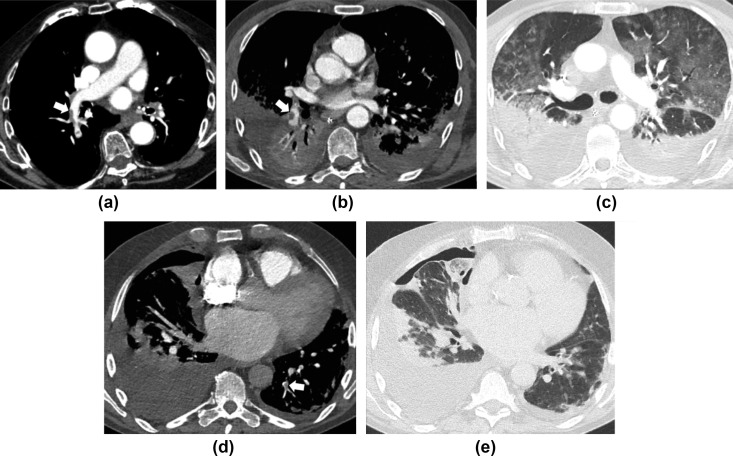
Figure 2.
Embolisms in the lobar, segmental, and subsegmental pulmonary arteries in three COVID-19 patients. (a) CT pulmonary angiogram of a 74-year-old man with D-Dimer level >800 ng/ml on admission reveals thrombus (arrow) in the right lobar pulmonary artery for the inferior lobe. (b,c) A 63-year-old man on day 23 after admission and D-dimer level of 15,662 ng/ml. CT pulmonary angiogram reveals pulmonary thrombus (arrow) in the right segmental pulmonary artery for the basal pyramid, bilateral pleural effusion with atelectasis, consolidations in the posterior segments of the inferior lobes (gravitational segments), and ground-glass opacities in the anterior segments (anti-gravitational segments), consistent with acute respiratory distress syndrome. (d,e) A 79-year-old man on day 1 after admission and D-dimer level of 54,158 ng/ml with pulmonary thrombus (arrow) in the subsegmental pulmonary artery for the postero-basal segment of the left inferior lobe, bilateral pleural effusion with consolidations and pneumothorax on the right side.
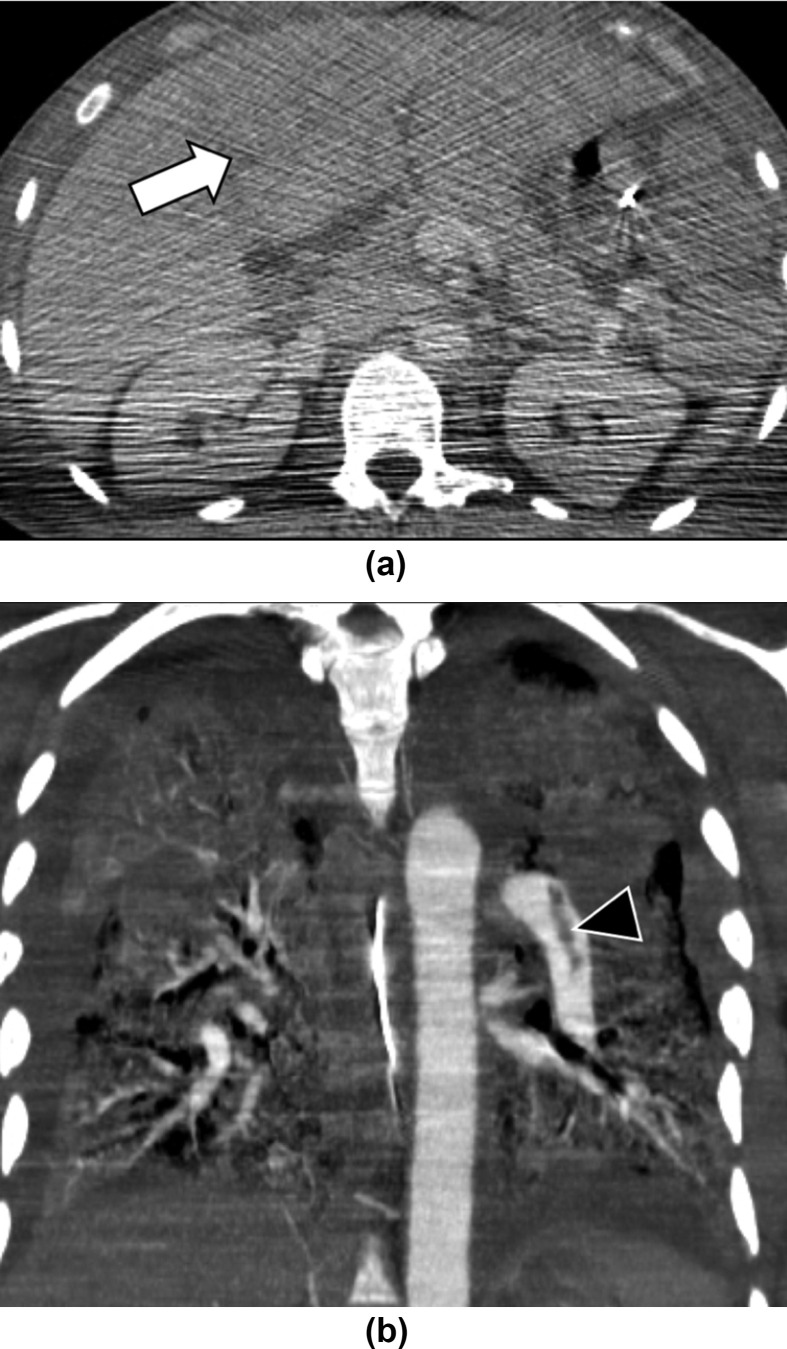
Figure 3.
A 48-year-old man with multiorgan failure and COVID-19. (a) Abdominal CT demonstrates hepatomegaly and abnormal perfusion of left liver (arrow), consistent with nutmeg liver. Of note, the quality of the images is low due to positioning of the arms along the body. (b) Pulmonary CT angiography performed in the same patient 2-days earlier showed left pulmonary embolism (arrowhead), bilateral ground glass opacities, and consolidations.
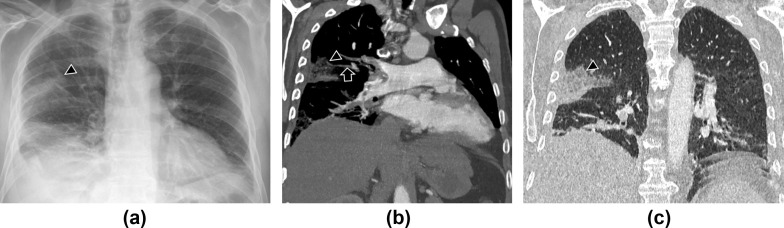
Figure 4.
A 82-year-old man with COVID-19, admitted for fever and chest pain. (a) Posteroanterior chest radiograph, (b) coronal chest CT with soft windows, and (c) coronal chest CT with lung windows demonstrate a triangular-shaped consolidation (arrowheads) in the middle lobe and a filling defect (arrow in b) of the corresponding segmental branch of the pulmonary artery, indicating a thrombus. These findings are consistent with lung infarction as a complication of pulmonary embolism. Pleural effusion is also identified.
Figure 5.
A 61-year-old man with septic embolism and COVID-19 infection. Axial CT with lung windows demonstrates multiple bilateral subpleural cavitated nodules (arrows), some on the right within consolidated areas, bilateral ground glass opacities and right pleural effusion. No pulmonary artery thromboembolism was demonstrated on CT pulmonary angiogram in this patient (not shown).
Ischaemic stroke
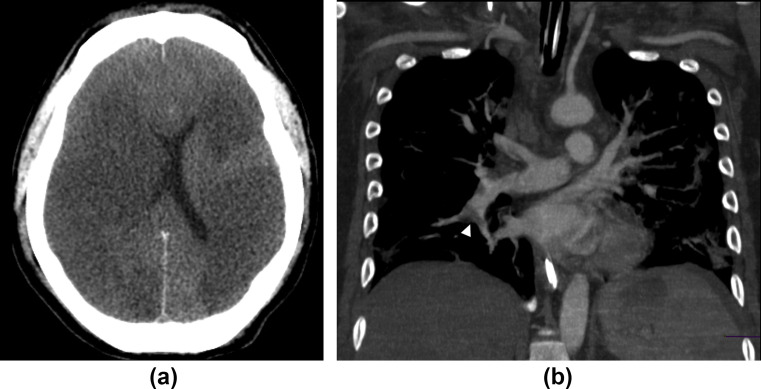
Neurological symptoms manifest in up to 36% of COVID-19 patients, and are more common in patients with severe infection.28 , 29 In a series of 214 COVID-19 patients,28 acute cerebrovascular disease, which includes ischaemic stroke and cerebral haemorrhage, was demonstrated in 2.8% (6/214) of the patients on head CT. In most cases (83%, 5/6), it was an ischaemic stroke (Fig 6 ) and occurred in critically ill patients with increased D-dimer levels.28 These data are confirmed by the study of Klok et al. 5 that reported ischaemic stroke in approximately 2% (3/184) of ICU patients with proven COVID-19 pneumonia. Recently, Kandemirli et al. 30 showed that 44% of ICU COVID-19 patients with neurological symptoms who underwent brain magnetic resonance imaging (MRI) had abnormal findings, such as increased cortical diffusion-weighted signal with corresponding low ADC values, subtle leptomeningeal enhancement, and punctate cortical blooming artefact. Although the mechanisms and phenotype of ischaemic stroke in COVID-19 patients are not yet understood, it is usually caused by large-vessel occlusion due to a prothrombotic state.
Figure 6.
A 45-year-old man with ischaemic stroke, pulmonary embolism, and COVID-19 infection. (a) CT of the head shows bilateral loss of grey–white matter differentiation, cortical hypodensity with associated parenchymal swelling with resultant mass effect, and compression on the lateral ventricles, findings consistent with ischaemic stroke. (b) CT pulmonary angiogram in the same patient reveals right pulmonary embolism (arrowhead).
Acral ischaemia and endothelial thrombo-inflammatory syndrome
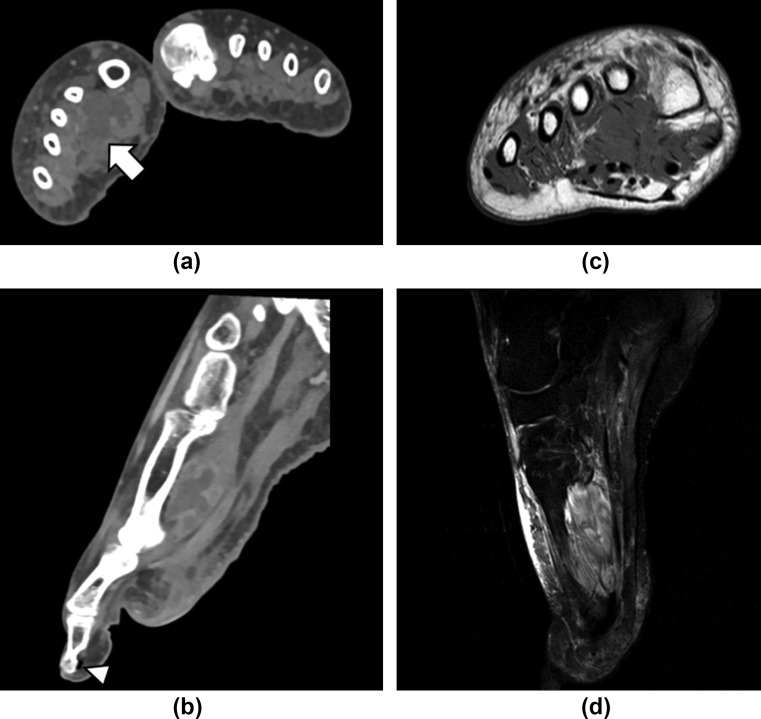
Microvascular injury related to progressive endothelial thrombo-inflammatory syndrome may explain skin manifestations of COVID-19 disease, which include perniosis-like skin lesions, purpuric skin rash, or acro-ischaemia presentations (i.e., finger/toe cyanosis, red-purple papules and dry gangrene).14 , 31, 32, 33 In these patients, CT and MRI can reliably diagnose the location and severity of arterial stenosis or occlusion and may demonstrate collections, abscesses, and oedematous muscular fibres (Fig 7 ).
Figure 7.
A 51-year-old woman with COVID-19. The patient was admitted after a few weeks of cough and dyspnoea and then subsequently developed ARDS. In addition to lung involvement, the patient developed unilateral foot necrosis with clinical features of peripheral ischaemia. (a) Contrast-enhanced CT in the axial and (b) reconstructed sagittal images showed a medium-density structure resembling a collection with hyperattenuating rims in the adductor halluces muscle (arrow), slightly decreased small artery opacification, with patent major arteries, gas locules (white arrowhead) around the first distal phalange without osseous destruction. (c) MRI performed 1 day after CT showed on the axial T1-weighted and (d) sagittal T2-weighted spectral presaturation with inversion recovery (SPIR) sequence thickened and oedematous but intact muscular fibres of the adductor halluces muscle.
Other thromboembolic events
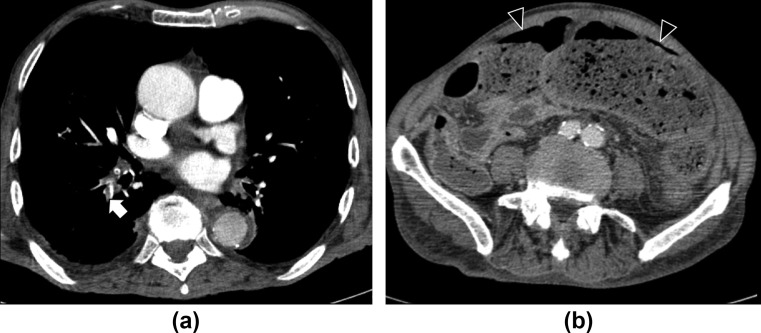
COVID-19 has been also associated with cardiac, renal, and gastrointestinal thromboembolic complications.15 Bhayana et al. 34 described abdominal bowel wall abnormalities, such as large or small bowel thickening, pneumatosis, or portal vein gas or perforation (Fig 8 ), in approximately 42% of COVID-19 patients who underwent abdominal CT, and bowel ischaemia or necrosis was demonstrated in some of the patients who underwent surgery. In addition, acute infarct in abdominal solid organs was demonstrated in 4.8% of patients. Cardiac manifestations due to COVID-19 are usually delayed from the onset of symptoms, and include acute ischaemic stroke, myocardial injury, and cardiogenic shock.35, 36, 37, 38 Other thromboembolic complications in COVID-19 include catheter-related deep vein thrombosis and disseminated intravascular coagulation38 , 39
Figure 8.
A 75-year-old man with stercoral perforation and COVID-19 infection 1 month earlier. (a) Contrast-enhanced chest CT performed during hospitalization for COVID-19 lung infection showed pulmonary embolism (arrow). The patient recovered and was then discharged. (b) Contrast-enhanced abdominal CT performed after 1 month for fever and abdominal pain showed gastrointestinal perforation (arrowheads) with stercoral peritonitis, but the mesenteric vessels were patent.
Conclusions
To conclude, the rate of thromboembolic complications in COVID-19 patients is relatively high. The likely mechanism includes a pro-coagulant pattern and an endothelial thrombo-inflammatory syndrome, which may result in multisystemic thrombotic disease. Therefore, in addition to the widely described role of imaging for assessment of COVID-19 pneumonia, CT may be useful for identification of thromboembolic complications, such as pulmonary embolism, ischaemic stroke, mesenteric ischaemia, and acro-ischaemia. Specifically, in patients with elevated D-dimer levels and those likely to be admitted to the ICU, pulmonary CT angiography should be considered for suspicion of pulmonary embolism in addition to chest CT.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.World Health Organization WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int Available at:
- 2.Rubin G.D., Ryerson C.J., Haramati L.B., et al. The Role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner society. Chest. 2020 doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Bai H., Chen X., et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang T., Chen R., Liu C., et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30109-5. S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. S0049-3848(20)30120-30121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui S., Chen S., Li X., et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. S0735-1097(20)35008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G., Plebani M., Michael Henry B. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020 doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranucci M., Ballotta A., Di Dedda U., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lushina N., Kuo J.S., Shaikh H.A. Pulmonary, cerebral, and renal thromboembolic disease associated with COVID-19 infection. Radiology. 2020:201623. doi: 10.1148/radiol.2020201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciceri F., Beretta L., Scandroglio A.M., et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamming I., Timens W., Bulthuis M.L., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020 doi: 10.1016/j.trsl.2020.04.007. S1931-5244(20)30070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30937-5. S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard-Lorant I., Delabranche X., Severac F., et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020:201561. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grillet F., Behr J., Calame P., et al. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020:201544. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotzinger D.C., Beigelman-Aubry C., von Garnier C., et al. Pulmonary embolism in patients with COVID-19: time to change the paradigm of computed tomography. Thromb Res. 2020;190:58–59. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watts J.A., Marchick M.R., Kline J.A. Right ventricular heart failure from pulmonary embolism: key distinctions from chronic pulmonary hypertension. J Card Fail. 2010;16:250–259. doi: 10.1016/j.cardfail.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Matthews J.C., McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008;4:49–59. doi: 10.2174/157340308783565384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correale M., Tarantino N., Petrucci R., et al. Liver disease and heart failure: back and forth. Eur J Intern Med. 2018;48:25–34. doi: 10.1016/j.ejim.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Giallourakis C.C. Liver complications in patients with congestive heart failure. Gastroenterol Hepatol (N Y) 2013;9:244–246. [PMC free article] [PubMed] [Google Scholar]
- 23.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 14 May 2020 doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha S.I., Shin K.M., Lee J., et al. Clinical relevance of pulmonary infarction in patients with pulmonary embolism. Thromb Res. 2012;130(3):e1–e5. doi: 10.1016/j.thromres.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 25.He H., Stein M.W., Zalta B., et al. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imag. 2006;21(1):1–7. doi: 10.1097/01.rti.0000187433.06762.fb. [DOI] [PubMed] [Google Scholar]
- 26.Godoy M.C., Viswanathan C., Marchiori E., et al. The reversed halo sign: update and differential diagnosis. Br J Radiol. 2012;85(1017):1226–1235. doi: 10.1259/bjr/54532316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 28.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kandemirli S.G., Dogan L., Sarikaya Z.T., et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020:201697. doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recalcati S., Barbagallo T., Frasin L.A., et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Cao W., Xiao M., et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischaemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 33.Alramthan A., Aldaraji W. A case of COVID-19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 doi: 10.1111/ced.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhayana R., Som A., Li M.D., et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020:201908. doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]