INTRODUCTION:
Chronic hepatitis B (CHB) remains a major worldwide public health concern. Besifovir dipivoxil maleate (BSV) is a new promising treatment for CHB. However, long-term efficacy and safety have not yet been evaluated. Therefore, the goal of the study is to determine the antiviral efficacy and safety of BSV treatment over a 144-week duration (BSV-BSV) in comparison with those of a sequential treatment with tenofovir disoproxil fumarate (TDF) followed by a 96-week duration BSV administration (TDF-BSV).
METHODS:
After 48 weeks of a double-blind comparison between BSV and TDF treatments, patients continued the open-label BSV study. We evaluated antiviral efficacy and drug safety up to 144 weeks for BSV-BSV and TDF-BSV groups. The primary endpoint was a virological response (hepatitis B virus DNA < 69 IU/mL).
RESULTS:
Among the 197 patients enrolled, 170 and 158 patients entered the second-year and third-year open-label phase extensional study, respectively, whereas 153 patients completed the 144-week follow-up. The virological response rate over the 144-week period was 87.7% and 92.1% in BSV-BSV and TDF-BSV groups, respectively (P = 0.36). The rates of ALT normalization and HBeAg seroconversion were similar between the groups. No drug-resistant mutations to BSV were noted. Bone mineral density and renal function were well preserved in the BSV-BSV group and were significantly improved after switching therapy in TDF-BSV patients.
DISCUSSION:
This extensional study of a phase 3 trial (NCT01937806) suggests that BSV treatment is efficacious and safe for long-term use in treatment-naïve and TDF-experienced patients with CHB.
INTRODUCTION
Chronic hepatitis B (CHB) remains a major worldwide public health problem despite the presence of effective vaccines and potent antivirals (1,2). Although the prevalence of chronic hepatitis B virus (HBV) infection was significantly reduced after the introduction of a universal HBV vaccination program in many Asian countries, new cases of HBV infection are still reported, even in low-prevalence areas (3–5). In South Korea, CHB still has a significant impact on public health, considering that the hepatitis B surface antigen (HBsAg) is detected in 60%–70% of patients with liver cirrhosis (5) and 65%–75% of patients with hepatocellular carcinoma (HCC) (6,7).
Currently, second-generation nucleos(t)ide analogs (NAs), such as entecavir, tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide fumarate (TAF), which have high genetic barriers to resistance and potent viral suppression activity, are used as primary treatments of CHB (8).
Besifovir dipivoxil maleate (BSV), an acyclic nucleotide phosphonate (a guanosine monophosphate), has been tested in several phase Ia/IIb studies and has shown potent suppression of HBV replication (9–12). BSV was approved for the treatment of chronic HBV infection in May 2017 in South Korea after we performed a randomized, multicenter, double-blind phase 3 study comparing the efficacy and safety of once-daily treatment with BSV vs TDF for 48 weeks in naïve patients with CHB (13). We reported that BSV elicited minor side effects on the kidneys and bones, with antiviral efficacy comparable with that of TDF over 48 weeks of treatment (13). This study was planned as an open-label follow-up study in which patients receive BSV for 7 years after initial treatment with BSV or TDF.
The aim of this study was to compare the antiviral efficacy and safety of a BSV solo treatment (BSV-BSV) over a 144-week period with a 48-week TDF treatment, followed by a 96-week BSV sequential treatment (TDF-BSV).
METHODS
Study design
This experimental study took over a 144-week period of treating patients with hepatitis B in South Korea. The first 48 weeks corresponded to a double-blind, randomized, multicenter, noninferiority, controlled trial comparing BSV (Ildong Pharmaceutical, Seoul, Korea) with TDF (Gilead Sciences, Foster City, CA) treatments. Patients from that study who were suited to continue a single-arm, open-label trial received BSV for an additional 96 weeks. Written informed consent for both the 48-week initial phase and the open-label phase was obtained from all study participants. This study was conducted with the approval of the Human Research Ethics Committee in the 22 participating hospitals in the Republic of Korea, following the Declaration of Helsinki and all other applicable guidelines, laws, and regulations. Detailed descriptions of the study design, sample size estimates, eligibility criteria, and overall study population have been previously reported (13).
Bone mineral density, noninvasive fibrosis test, and resistance surveillance were regularly performed (see Supplementary Material, Supplementary Digital Content 1, http://links.lww.com/AJG/B491). Particularly, FIB-4 and aspartate aminotransferase-to-platelet ratio indexes (APRI) were annually estimated to assess liver fibrosis (see Table, Supplementary Digital Content 2 for formula used, http://links.lww.com/AJG/B485).
Subjects
Patients with CHB younger than 20 years of age and HBsAg-positive for at least 6 months before screening were eligible. Subjects with HBV DNA levels >17,241 IU/mL (1 × 105 copies/mL) and > 1,724 IU/mL (1 × 104 copies/mL), who tested positive and negative for hepatitis B e-antigen (HBeAg), respectively, were randomized 1:1 into BSV or TDF treatment groups for 48 weeks. To maintain blinding of investigators and subjects, subjects in the BSV group were given BSV 150 mg with L-carnitine 660 mg as a supplement and TDF placebo, whereas those in the TDF group were given TDF 300 mg with L-carnitine as a BSV placebo. After 48 weeks, the patients continued on BSV or were switched to BSV 150 mg with L-carnitine 660 mg during the open-label phase for up to 144 weeks.
Endpoints
The efficacy endpoint was the proportion of subjects with a virological response, defined as HBV DNA <69 IU/mL at week 144. Other efficacy and safety endpoints, including the proportions of subjects with undetectable HBV DNA levels (<20 IU/mL), changes of serum HBV DNA levels, HBsAg or HBeAg seroconversion, alanine aminotransferase (ALT) normalization, drug resistance, adverse events (AEs), bone mineral density (BMD), renal function-related parameters, fibrosis parameters, incidences of HCC, and other abnormalities, were assessed.
Laboratory tests and assessments
All laboratory data during the trial were collected at 12-week intervals via the Central Lab (GC abs, Yongin, Korea). HBV DNA quantification was performed using a COBAS AmpliPrep/TaqMan test (Roche Diagnostics, Indianapolis, IN) with a lower detection limit of 20 IU/mL. Other laboratory methods were followed as previously reported (13).
Bone mineral density, non-invasive fibrosis test, and resistance surveillance were regularly performed (see Supplementary Material, Supplementary Digital Content 1, http://links.lww.com/AJG/B491). Particularly, FIB-4 and aspartate aminotransferase-to-platelet ratio indexes (APRI) were annually estimated to assess liver fibrosis (see Table, Supplementary Digital Content 2 for formula used, http://links.lww.com/AJG/B485).
Statistical analysis
For primary efficacy analysis, proportions of patients with HBV DNA <69 IU/mL were determined using the full analysis set (FAS), which included all patients who received at least one investigational product and completed at least one efficacy evaluation. Per-protocol analysis was performed after excluding consent withdrawals and major protocol violators from the FAS population.
Differences in the baseline characteristics and secondary endpoints between the groups were tested using an independent 2-sample t test or Wilcoxon rank-sum test for continuous variables and a χ2 test or Fisher exact test for categorical variables. We followed the CONSORT guidelines for randomized controlled trials.
RESULTS
Study population
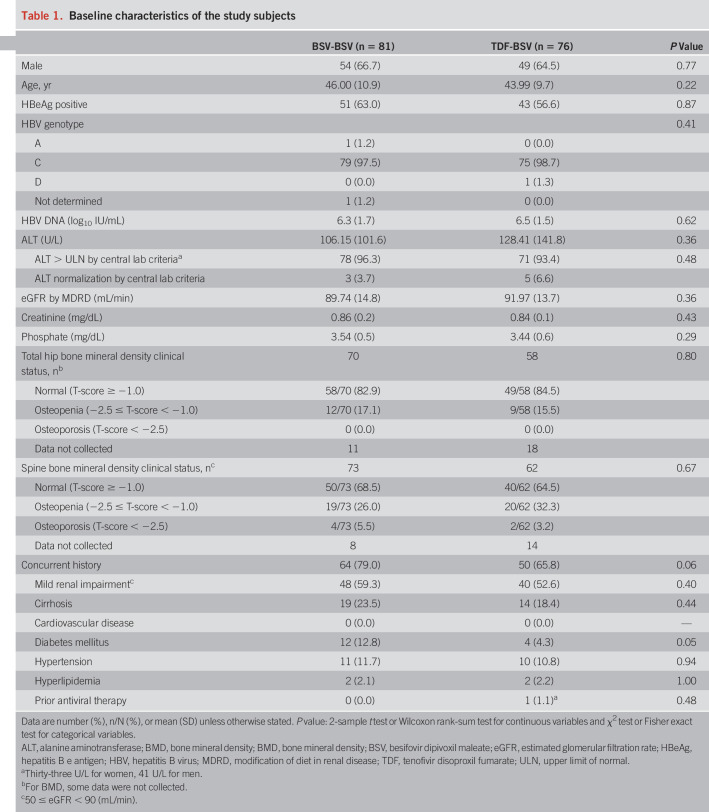
Of 197 patients who were initially randomly assigned, 170 (86%) entered the second year, 158 (80%) entered the third year extensional study, and finally 153 completed all 144 weeks of follow-up (Figure 1). Of 157 patients (81 in BSV-BSV and 76 in TDF-BSV) who qualified for the FAS at 144 weeks, 145 were included for the PPS analysis. The baseline characteristics for both treatment groups were well-balanced (Table 1). Considering both the groups, 65.6% patients enrolled were men, with a mean age of 45 years. Subjects positive for HBeAg represented 63.0% in the BSV-BSV group and 56.6% in the TDF-BSV group, with the most common HBV genotype being C. For BSV-BSV and TDF-BSV groups, the means of HBV DNA at the baseline were 6.3 and 6.5 log10 IU/mL, respectively. More than half of the patients showed a normal T-score in the hip and/or spine at the baseline (64.5%–84.5%). Although the BSV-BSV group was more likely to have concurrent histories, including cirrhosis and diabetes mellitus, at the baseline, no statistically significant differences were noted between the groups.
Figure 1.
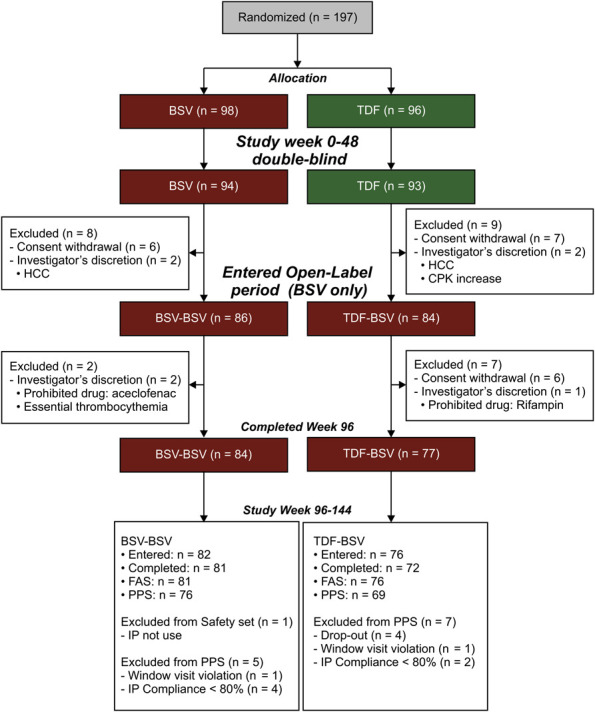
Patient disposition. BSV, besifovir dipivoxil maleate; CPK, creatinine phosphokinase; FAS, full analysis set; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; IP, investigational product; PPS, per-protocol set; TDF, tenofovir disoproxil fumarate.
Table 1.
Baseline characteristics of the study subjects
Efficacy
Virological response.
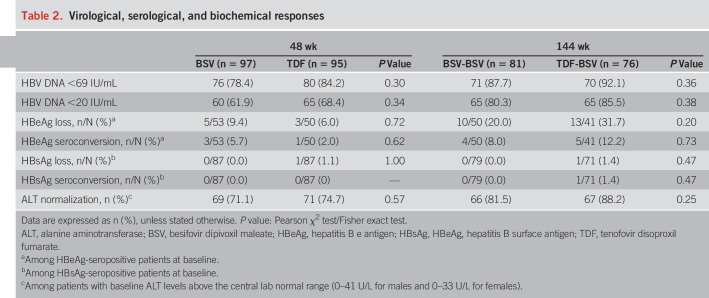
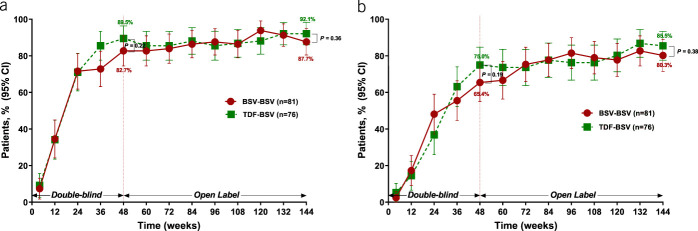
The rate of virological response (HBV DNA < 69 IU/mL) in BSV-BSV was 87.7% (71/81), whereas 92.1% (70/76) of patients who switched the treatment from TDF to BSV responded up to 144 weeks, indicating that the BSV-BSV group met the efficacy endpoint of noninferiority with predetermined −15% margin to BSV-TDF according to the FAS (lower bound 95% CI = −13.8%, P = 0.36) (Table 2 and Figure 2a). The complete virological response with the lower limit of HBV DNA quantification (<20 IU/mL) was 80.3% (65/81) among the BSV-BSV patients and 85.5% (65/76) in the TDF-BSV group at week 144 (P = 0.38) (Table 2 and Figure 2b). Similar results were observed in the PPS (see Figure, Supplementary Digital Content 3A, 3B, http://links.lww.com/AJG/B485) (HBV DNA < 69 IU/mL, 92.1% vs 95.7%, P = 0.50; HBV DNA < 20 IU/mL, 84.2% vs 88.4%, P = 0.46; in BSV-BSV vs TDF-BSV, respectively).
Table 2.
Virological, serological, and biochemical responses
Figure 2.
Viral suppression by study visit. (a) Proportions of patients with HBV DNA <69 IU/mL as determined by the FAS. (b) Proportions of patients with HBV DNA <20 IU/mL as determined by FAS. Bars represent 95% confidence intervals. BSV, besifovir dipivoxil maleate; FAS, full analysis set; HBV, hepatitis B virus; TDF, tenofovir disoproxil fumarate.
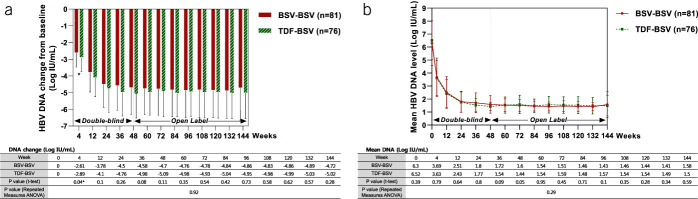
Antiviral responses from the baseline during every follow-up visit until week 144 are shown in Figure 3. During the first 48 weeks, the degree of HBV DNA reduction was not significantly different between the 2 groups (Figure 3a), except for at the end of the first month. Mean HBV DNA decreased to <2 log10 IU/mL levels at week 24 and remained stable for 144 weeks (Figure 3b). No significant differences between the 2 groups were observed during the 144 weeks (Figure 3b).
Figure 3.
Antiviral responses by study visit (weeks). (a) Degree of HBV DNA reduction (log IU/mL). (b) Mean HBV DNA level (log IU/mL). ANOVA, analysis of variance; BSV, besifovir dipivoxil maleate; HBV, hepatitis B virus; TDF, tenofovir disoproxil fumarate.
Biochemical and serological responses.
Among HBeAg-positive patients, the cumulative incidences of HBeAg loss at 144 weeks were 20.0% (10/50) in the BSV-BSV group and 31.7% (13/41) in the TDF-BSV group (P = 0.20). The incidences of HBeAg seroconversion were 8.0% (4/50) and 12.2% (5/41) in the BSV-BSV and TDF-BSV groups, respectively (P = 0.73). There was no significant difference in HBeAg serological responses between both groups (Table 2); only one patient (1.4%, 1/71) in the TDF-BSV group showed HBsAg loss during the 144 weeks (Table 2). ALT levels normalized in 81.5% (66/81) and 88.2% (67/76) subjects in the BSV-BSV and TDF-BSV groups after 144 weeks, with no significant difference between the groups (P = 0.25) (Table 2).
Subgroup analysis.
Biochemical, serological, and virological responses were separately evaluated in HBeAg-positive and HBeAg-negative patients according to the baseline HBeAg status for FAS and PPS (see Tables, Supplementary Digital Content 4 and 5, http://links.lww.com/AJG/B485; see Figure, Supplementary Digital Content 6, http://links.lww.com/AJG/B485).
In the FAS, all HBeAg-negative patients reached HBV DNA <69 IU/mL at week 144. Only one patient in each group did not achieve a complete virological response (HBV DNA < 20 IU/mL) by week 144. There was no difference in virological response rates among HBeAg-negative patients.
Among HBeAg-positive patients, the proportions who achieved HBV DNA <69 IU/mL were 80.4% (41/51) in the BSV-BSV and 86.1% (37/43) in the TDF-BSV group (P = 0.47). The rates of HBV DNA <20 IU/mL were 70.6% (36/51) and 76.7% (33/43), respectively (P = 0.50). There was no difference in virological response rates between both the groups when considering HBeAg-positive patients.
Virological breakthrough and resistance surveillance.
Both groups showed no antiviral resistance for 144 weeks. The proportions of patients who experienced virological breakthrough between 96 and 144 weeks were 4.9% (4/81) in the BSV-BSV group and 7.9% (6/76) in the TDF-BSV group, with no significant difference between the groups. However, serum HBV DNA became undetectable in all these patients without modifying the therapy during follow-up. DNA sequencing was performed for patients who experienced a virological breakthrough, confirming that new mutations that caused by amino acid substitutions were not detected in the HBV conserved sites.
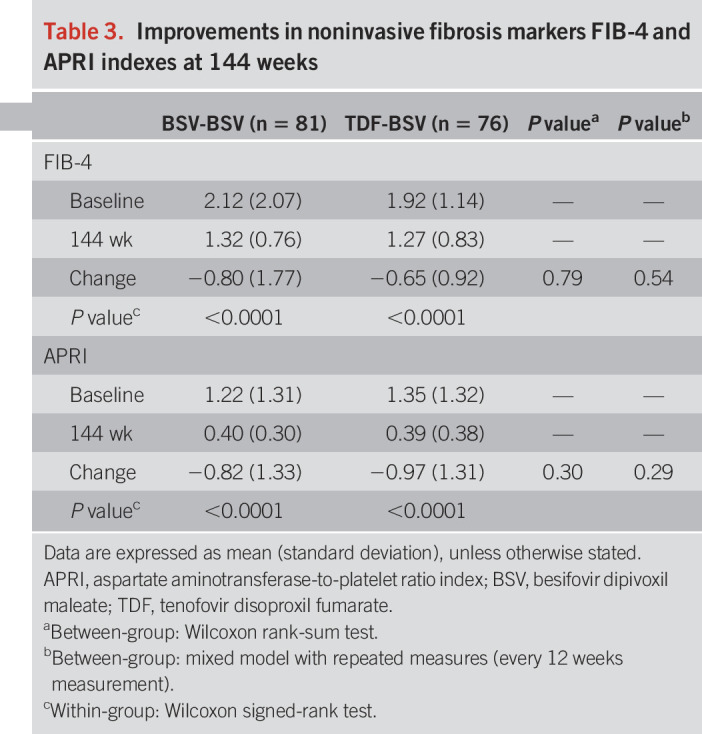
Noninvasive fibrosis markers: FIB-4 and APRI.
The FIB-4 and APRI indexes significantly improved at 144 weeks vs baseline in both groups (Table 3; see Table, Supplementary Digital Content 7, http://links.lww.com/AJG/B485). FIB-4 improved from 2.12 to 1.32 and APRI from 2.12 to 0.4 in the BSV-BSV group. Likewise, FIB-4 improved from 1.92 to 1.27 and APRI from 1.35 to 0.39 in the TDF-BSV group, respectively (all P < 0.001). According to these results, the degree of hepatic fibrosis significantly decreased in both the BSV-BSV and TDF-BSV groups over the duration of drug administration. There was no significant difference in FIB-4 and APRI values between both the groups.
Table 3.
Improvements in noninvasive fibrosis markers FIB-4 and APRI indexes at 144 weeks
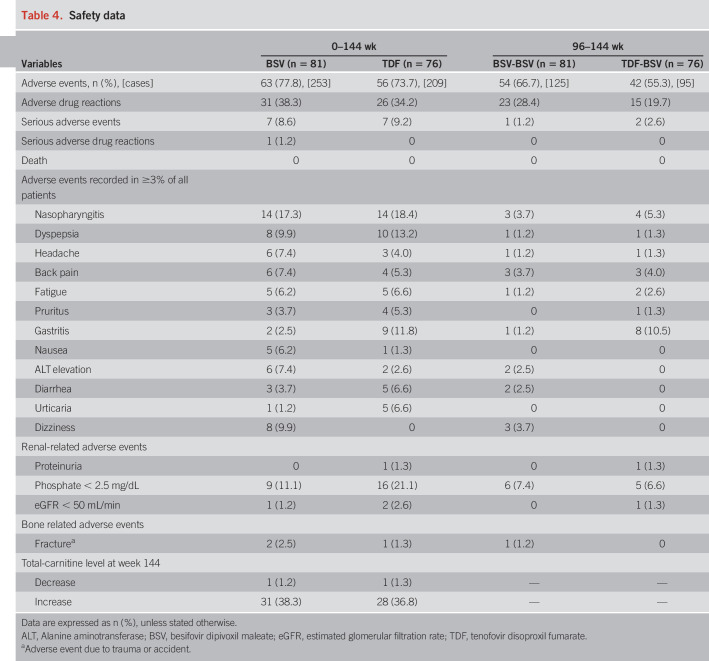
Safety
Adverse events.
After reporting AEs during the 96-week study (13), 3 serious AEs occurred: HCC in one patient from the BSV-BSV group and HCC and type 2 diabetes mellitus in 2 patients of the TDF-BSV group (Table 4). However, the investigator confirmed that all 3 patients could continue the study. No serious adverse drug reactions or death was newly reported during 96–144 weeks in the study. L-carnitine levels were measured during every follow-up visit. These levels were stably maintained, with only one patient in each group exhibiting a decreased level.
Table 4.
Safety data
The median changes in eGFR from the baseline were −0.5, −0.7, and −0.5 mL/min at 48, 96, and 144 weeks, respectively, for the BSV-BSV group, indicating no effect of BSV on renal function. On the contrary, for the TDF-BSV group, the median changes in eGFR levels from baseline were −7.8, −0.4, and −0.8 mL/min at 48, 96, and 144 weeks, respectively; therefore, the recovered renal function after switching to BSV at 49 weeks was maintained up to 144 weeks (Figure 4). There was no incidence of acute kidney injury in the 2 groups.
Figure 4.
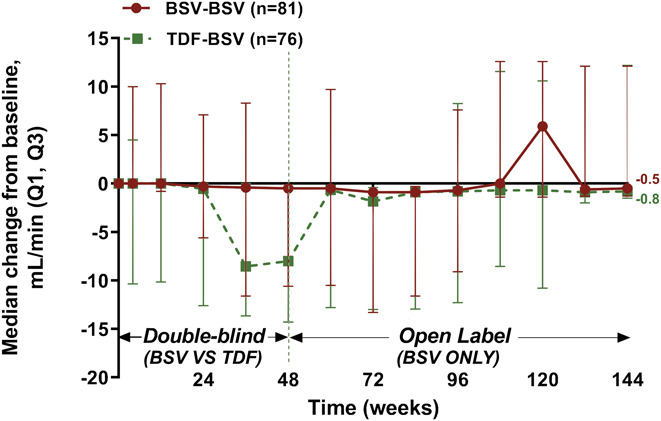
Median changes from baseline in eGFR (MDRD) by study week. Data are presented as median (Q1, Q3) values (mL/min). BSV, besifovir dipivoxil maleate; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease; TDF, tenofovir disoproxil fumarate.
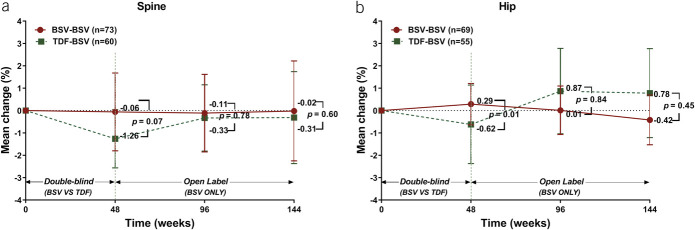
The mean percent change in spine BMD from the baseline to week 144 was −0.02% (95% CI: −2.25 to 2.22) for the BSV-BSV (P = 0.59) and −0.31% (95% CI: −2.37 to 1.74) for the TDF-BSV (P = 0.93) group. This difference was not significant (Figure 5a). The mean percent change in hip BMD from the baseline to week 144 was −0.42% (95% CI: −1.53 to 0.7) for the BSV-BSV (P = 0.10) and 0.78% (95% CI: −1.21 to 2.77) for the TDF-BSV group. This difference was not significant (Figure 5b).
Figure 5.
Changes in BMD. (a) Mean percentage changes in the spine at weeks 48, 96, and 144 of treatment. Bars represent 95% confidence interval. (b) Mean percentage changes in the hip at weeks 48, 96, and 144 of treatment. Bars represent 95% confidence intervals. BMD, bone mineral density; BSV, besifovir dipivoxil maleate; TDF, tenofovir disoproxil fumarate.
DISCUSSION
The incidence of HBV infection is decreasing worldwide because of improved socioeconomic status, universalization of vaccine programs, and effective antiviral treatments (1,4). However, population movements are now affecting the occurrence and prevalence of HBV in several low-endemic countries (14–17). Unlike most infectious diseases, deaths from HBV-related liver cirrhosis and/or HCC increased between 1990 and 2013 (18).
BSV, the newest approved agent in the NA class in Korea, is currently recommended as the first-line oral agent for CHB treatment by the Korean Association for the Study of the Liver (19). Our study followed the CHB treatment with BSV for 144 weeks, with findings consistent with those of our previous 96-week study (13). In total, 89.8% and 82.8% of patients had HBV DNA <69 and <20 IU/mL, respectively, indicating that the antiviral effect of BSV was maintained up to 144 weeks. Reduction in hip and spine BMD was observed in patients treated with TDF for 48 weeks, but it returned to the baseline level after switching to BSV, and it was maintained up to 144 weeks. BSV treatment also showed a favorable safety profile regarding renal parameters. No viral resistance was detected during the course of the 144-week treatment. We also confirmed the improvement of fibrosis applying the FIB-4 and APRI tests.
A recent 3–year study showed that TAF was associated with a lower risk of BMD decline and nephrotoxicity (20). The mean BMDs decreased from the baseline to week 144 of therapy (hip: −2.49 vs −0.41, P < 0.001; spine: −2.01 vs −0.51, P < 0.001; in TDF and TAF groups, respectively). We also observed a significant decrease in BMD in the TDF group relative to that in the BSV group at 48 weeks. However, in the group of patients receiving BSV for 144 weeks, the BMD mean changes from the baseline were −0.42 (hip) and 0.02 (spine), similar to those results obtained with TAF. Treatment with BSV and TAF also showed similar trends in eGFR changes over 3 years (eGFRMDRD for BSV, −0.5 vs eGFRCG for TAF, −1.2). Although it is difficult to perform a direct comparison between BSV and TAF treatments, they seem to be similar for preserving renal function and bone density.
Improvements in fibrosis after antiviral treatment have been previously reported by histology (21,22). Although liver biopsy is the accepted standard method for the assessment of hepatic fibrosis, repeated evaluations are difficult to perform because of cost, the risk of life-threatening complications, and a lack of available expertise (23–25). Therefore, we analyzed the extent of liver fibrosis using FIB-4 and APRI as biomarkers, as indicated by previous studies (26–28). Importantly, in 2 noninvasive fibrosis tests, our results showed a clear fibrosis improvement on BSV administration.
Carnitine depletion was the most common side effect of BSV (11,12). Therefore, BSV was supplemented with 660 mg of L-carnitine. Carnitine concentrations were closely monitored during the study period and at week 144. Except for one patient in each group who evidenced an increase in carnitine levels, patients were generally stably maintained with the supplementation and none experienced clinical features of carnitine deficiency, such as hypoglycemia, hypoketosis, and encephalopathy.
This study has several limitations. Although the general safety concerns related to nephrotoxicity can be ignored in the BSV group considering the rate of decline of eGFR, additional markers related to renal function, such as urinary albumin, fractional excretion of uric acid, and urinary beta-2 microglobulin, were not evaluated. Moreover, no assessments of bone turnover markers for the estimation of bone formation or resorption were performed, although it is recommended for patients receiving these NAs. Although noninvasive fibrosis markers were evaluated to demonstrate the improvement of liver fibrosis, histological analysis did not confirm such improvement; this calls for further evaluation. However, to our knowledge, this is the first study reporting antiviral effects of BSV providing safe renal and bone profile for an extended treatment period.
In summary, BSV treatment maintained antiviral efficacy over 144-week period without any viral resistance. BSV administration was safe, well-tolerated, and effective for treatment-naïve patients with CHB as well as those who switched from TDF to BSV treatment.
CONFLICTS OF INTEREST
Guarantor of the article: Kwang-Hyub Han, MD.
Specific author contributions: H.J.Y. wrote the manuscript and performed the formal analysis and critical revision. K.H.H.: was involved in the study conception, design, and supervision. All the other authors contributed to data acquisition and revision of the manuscript.
Financial support: This work was supported by Ildong Pharmaceutical. This sponsor had a partial role in study design, collection, analysis, and interpretation of data.
Potential competing interests: K.H.H. received grants from the clinical trial
Study Highlights.
WHAT IS KNOWN
✓ BSV is a new treatment of chronic hepatitis B virus infection.
✓ BSV has an antiviral efficacy comparable with that of TDF over 48 weeks.
✓ BSV has a better safety profile than TDF in terms of bone and renal outcomes.
WHAT IS NEW HERE
✓ BSV demonstrated durable and potent antiviral activity over 144 weeks.
✓ Approximately 88% of subjects had HBV DNA <69 IU/mL without antiviral resistance mutations.
✓ Long-term BSV treatment for chronic hepatitis B is safe and improves fibrosis.
sponsor Ildong Pharmaceutical. All the other authors declare no conflicts of interest.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing and publication support.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/B485, http://links.lww.com/AJG/B491
REFERENCES
- 1.European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015;386:1546–55. [DOI] [PubMed] [Google Scholar]
- 3.Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of hepatitis B virus infection and impact of vaccination on disease. Clin Liver Dis 2016;20:607–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho EJ, Kim SE, Suk KT, et al. Current status and strategies for hepatitis B control in Korea. Clin Mol Hepatol 2017;23:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae HB, Kim JH, Kim JK, et al. Current status of liver diseases in Korea: Hepatitis B. Korean J Hepatol 2009;15:S13–24. [DOI] [PubMed] [Google Scholar]
- 6.Cheon JH, Park JW, Park KW, et al. The clinical report of 1,078 cases of hepatocellular carcinomas: National Cancer Center experience. Korean J Hepatol 2004;10:288–97. [PubMed] [Google Scholar]
- 7.Kim SR, Kudo M, Hino O, et al. Epidemiology of hepatocellular carcinoma in Japan and Korea. A review. Oncology 2008;75:13–6. [DOI] [PubMed] [Google Scholar]
- 8.Buti M, Riveiro-Barciela M, Esteban R. Long-term safety and efficacy of nucleo(t)side analogue therapy in hepatitis B. Liver Int 2018;38(Suppl 1):84–9. [DOI] [PubMed] [Google Scholar]
- 9.Yuen MF, Kim J, Kim CR, et al. A randomized placebo controlled, dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis B. Antivir Ther 2006;11:977–83. [PubMed] [Google Scholar]
- 10.Yuen MF, Han KH, Um SH, et al. Antiviral activity and safety of LB80380 in hepatitis B e antigen-positive chronic hepatitis B patients with lamivudine-resistant disease. Hepatology 2010;51:767–76. [DOI] [PubMed] [Google Scholar]
- 11.Lai CL, Ahn SH, Lee KS, et al. Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut 2014;63:996–1004. [DOI] [PubMed] [Google Scholar]
- 12.Yuen MF, Ahn SH, Lee KS, et al. Two-year treatment outcome of chronic hepatitis B infection treated with besifovir vs. entecavir: Results from a multicentre study. J Hepatol 2015;62:526–32. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SH, Kim W, Jung YK, et al. Efficacy and safety of besifovir dipivoxil maleate compared with tenofovir disoproxil fumarate in treatment of chronic hepatitis B virus infection. Clin Gastroenterol Hepatol 2019;17:1850–9. [DOI] [PubMed] [Google Scholar]
- 14.Coppola N, Alessio L, Gualdieri L, et al. Hepatitis B virus, hepatitis C virus and human immunodeficiency virus infection in undocumented migrants and refugees in southern Italy, January 2012 to June 2013. Euro Surveill 2015;20:30009. [DOI] [PubMed] [Google Scholar]
- 15.Hampel A, Solbach P, Cornberg M, et al. Current seroprevalence, vaccination and predictive value of liver enzymes for hepatitis B among refugees in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2016;59:578–83. [DOI] [PubMed] [Google Scholar]
- 16.Levy V, Yuan J, Ruiz J, et al. Hepatitis B sero-prevalence and risk behaviors among immigrant men in a population-based household survey in low-income neighborhoods of northern California. J Immigr Minor Health 2010;12:828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppola N, Alessio L, Gualdieri L, et al. Hepatitis B virus infection in undocumented immigrants and refugees in southern Italy: Demographic, virological, and clinical features. Infect Dis Poverty 2017;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the global burden of disease study 2013. Lancet 2016;388:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol 2019;25:93–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan HLY, Lim YS, Seto WK, et al. 3-year efficacy and safety of tenofovir alafenamide compared with tenofovir disoproxil fumarate in HBeAg-negative and -positive patients with chronic hepatitis B. Hepatology 2018;68(Suppl 1):227–8A. [Google Scholar]
- 21.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011–20. [DOI] [PubMed] [Google Scholar]
- 22.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013;381:468–75. [DOI] [PubMed] [Google Scholar]
- 23.Spinzi G, Terruzzi V, Minoli G. Liver biopsy. N Engl J Med 2001;344:2030. [PubMed] [Google Scholar]
- 24.Maharaj B, Maharaj RJ, Leary WP, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet 1986;1:523–5. [DOI] [PubMed] [Google Scholar]
- 25.Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol 2010;8:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WR, Loomba R, Berg T, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer 2015;121:3631–8. [DOI] [PubMed] [Google Scholar]
- 27.Lin CL, Liu CH, Wang CC, et al. Serum biomarkers predictive of significant fibrosis and cirrhosis in chronic hepatitis B. J Clin Gastroenterol 2015;49:705–13. [DOI] [PubMed] [Google Scholar]
- 28.Sonneveld MJ, Brouwer WP, Chan HL, et al. Optimisation of the use of APRI and FIB-4 to rule out cirrhosis in patients with chronic hepatitis B: Results from the SONIC-B study. Lancet Gastroenterol Hepatol 2019;4:538–44. [DOI] [PubMed] [Google Scholar]
- 29.Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.