Abstract
Cerebral hyperperfusion syndrome (CHS) is a clinical syndrome following a revascularization procedure. In the past decade, neurointerventional surgery has become a standard procedure to treat stenotic or occluded cerebral vessels in both acute and chronic settings, as well as endovascular thrombectomy in acute ischemic stroke. This review aims to summarize relevant recent studies regarding the epidemiology, diagnosis, and management of CHS as well as to highlight areas of uncertainty. Extracranial and intracranial cerebrovascular diseases in acute and chronic conditions are considered. The definition and diagnostic criteria of CHS are diverse. Although impaired cerebrovascular autoregulation plays a major role in the pathophysiology of CHS, the underlying mechanism is still not fully understood. Its clinical characteristics vary in different patients. The current findings on clinical and radiological presentation, pathophysiology, incidence, and risk factors are based predominantly on carotid angioplasty and stenting studies. Hemodynamic assessment using imaging modalities is the main form of diagnosis although the criteria are distinct, but it is helpful for patient selection before an elective revascularization procedure is conducted. After endovascular thrombectomy, a diagnosis of CHS is even more complex, and physicians should consider concomitant reperfusion injury. Management and preventative measures, including intensive blood pressure control before, during, and after revascularization procedures and staged angioplasty, are discussed in detail.
Keywords: complication, hemorrhage, stent, stroke
Introduction
Cerebral hyperperfusion syndrome (CHS) is a rare but severe complication, and was first described as a clinical syndrome following carotid endarterectomy (CEA). In neurointerventional surgery, CHS may complicate extracranial carotid angioplasty and stenting (CAS), and is characterized by headaches, neurological deficits, and seizures not caused by cerebral ischemia. It is commonly associated with an increase in blood pressure.
The incidence and risk factors of CHS have recently been reported in several large cohorts of patients undergoing CAS, intracranial angioplasty and stenting (INCS), and endovascular thrombectomy (EVT). Greater familiarity with recent reports of the clinical symptoms, imaging features, and management strategies for CHS may improve the vigilance necessary for effective diagnosis and prevention.
This review focuses on recent important studies on the presentation, diagnosis, pathophysiology, incidence, risk factors, prevention, and management of CHS in patients undergoing CAS, INCS, and EVT.
Search strategy and selection criteria
CHS studies for this review were searched using PubMed. The search term 'cerebral hyperperfusion' was used as the keyword, and the search was limited to clinical reports, clinical studies, clinical trials, and reviews. The search was also limited to studies with abstracts available. The search was performed up to 30 August 2019. A total of 1490 items were identified. Using the PRISMA flowchart for guidance (online supplementary material), titles and abstracts were reviewed by the authors before requesting the full text. Duplicates, reports of moyamoya disease or bypass surgery, in vitro studies, and non-English studies were excluded. Following careful review of 135 full text articles, 56 studies focusing on updated information were included after careful discussion by the authors.
neurintsurg-2019-015621supp001.pdf (18.1KB, pdf)
Overview of cerebral hyperperfusion syndrome
Clinical presentation and radiological findings
Most patients with CHS have mild symptoms and signs, while some may progress to severe and life-threatening symptoms. The most common clinical presentations include severe headache (ipsilateral to the lesion side or diffuse), and eye and facial pain. Less common and more severe symptoms include focal neurological deficits, seizures, and loss of consciousness. CHS occurred conspicuously earlier after CAS than after CEA, but the cause is unknown. The incidence of CHS peaks at 12 hours after CAS and at 6 days after CEA. Hemorrhage after CAS was also diagnosed significantly earlier following CAS (1.7±2.1 days) than after CEA (10.7±9.9 days) (p=0.0098).1 Despite the earlier presentation of CHS after CAS, it can delay its occurrence by up to 1 month after the procedure.
On radiological studies, patchy or diffuse white matter edema, predominantly involving the posterior parieto-occipital lobe, focal infarction, and petechial hemorrhage (figures 1 and 2) were potential findings, for which MRI was more sensitive. However, negative radiological finding cannot exclude CHS.
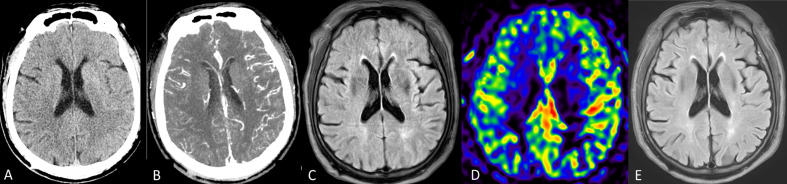
Figure 1.
(A–E) Cerebral hyperperfusion syndrome after carotid stenting. (A) CT shows regional swelling as sulcal effacement on the left temporo-parieto-occipital lobe. (B) CT angiography reveals hyperemic change on the same territory. (C) MR examination (T2 fluid attenuation inversion recovery, FLAIR) shows regional swelling without evidence of ischemic stroke (not shown). (D) Arterial spin labeling revealed relative hyperperfusion in the same region. Under strict blood pressure control, the patient improved a few days later. (E) Follow-up T2 FLAIR image 6 months later shows remission of the regional swelling.
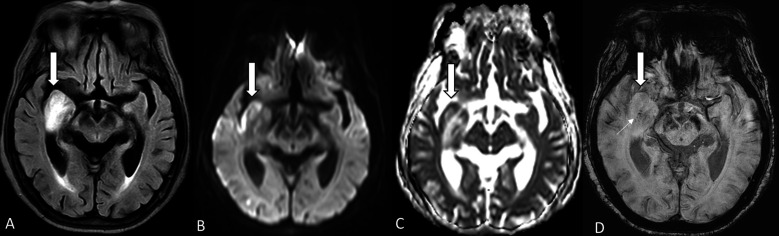
Figure 2.
(A–D) Hyperperfusion after successful recanalization of the acute occluded right M1. MR examination performed 24 hours later. (A) T2 fluid attenuation inversion recovery (FLAIR) shows focal bright signal intensity at the swelling of the right medial temporal lobe (arrow). (B, C) Diffusion weighted images and apparent diffusion coefficient mapping reveal acute cortical infarct (arrows) and white matter edema. (D) MR susceptibility image shows focal swelling (large arrow) and petechial hemorrhage (small arrow).
Imaging modalities also play a key role in the objective assessment and diagnosis of cerebral hemodynamics in CHS. Among them, transcranial color duplex (TCD) is the most widely available modality in practice. It measures cerebral blood velocity in the middle cerebral artery, which correlates adequately with cerebral blood flow (CBF). However, TCD is operator dependent and optimal image acquisition is not always feasible. Cerebral perfusion imaging, including single photon emission computed tomography (SPECT), CT perfusion, and perfusion weighted imaging, enables direct estimation of CBF and related perfusion parameters based on radioisotope or bolus injection of radioisotope or contrast agents. Among them, CT perfusion is recommended because of good availability, relatively short acquisition time, and feasibility in critical patients. The comparison of perfusion parameter to the contralateral hemisphere can be performed by commercial workstations. (figure 3). Also, these new MRI techniques, such as arterial spin labeling (ASL) and quantitative MRI, can estimate CBF without the use of a contrast agent. Periprocedural imaging evaluation and prediction of CHS was studied using color coded DSA, quantitative digital subtraction tomography, and c arm derived cerebral blood volume.2–4
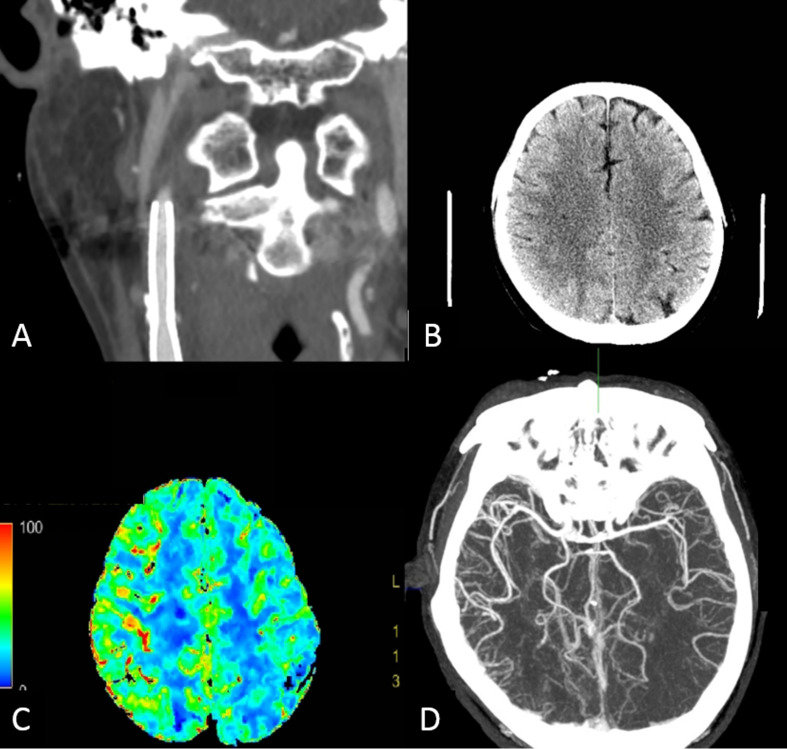
Figure 3.
(A–D) Application of imaging in cerebral hyperperfusion syndrome. A subject underwent right carotid artery stenting and presented with seizures 9 days after the procedure. Blood pressure at presentation was 158/100 mm Hg. (A) Reconstructed coronal CT angiography demonstrates stent deployment from the cervical internal carotid artery to the common carotid artery without in-stent thrombosis. (B) Non-contrast CT shows sulcal effacement and subcortical hypointensity at the right parietal lobe. (C) Perfusion CT illustrates increased cerebral blood flow in the right cerebral cortex compared with the contralateral side. (D) Reformatted axial CT angiography also shows numerous engorged cortical branches of the right middle cerebral artery.
Using quantitative methods such as SPECT, xenon enhanced CT, cerebral hyperperfusion is usually considered when the revascularized territory increases CBF by 100% or more from baseline values after CEA or CAS.1 This operational definition is impractical because of the lack of pretreatment CBF measurements. By comparing with the contralateral hemisphere, an increase in CBF is generally used in defining CHS, but this cannot be applied to patients with contralateral stenotic disease. On TCD examination, a 1.5-fold or 2.0-fold postoperative increase in middle cerebral artery mean flow velocity compared with preoperative levels may predict the occurrence of CHS.1 5 Regarding CT perfusion, many criteria have been proposed, such as elevated postoperative cerebral blood volume,6 mean transit time threshold <2 s or >4 s,7 increased regional cerebral blood flow and regional cerebral blood volume, and decreased mean transit time along with decreased time to peak in the clinically related artery territory.8 The definition of CHS is highly variable and dependent on diagnostic tools. Consensus on the definition and diagnostic criteria for CHS using different hemodynamic assessment tools is warranted.
Pathophysiology
Many pathophysiological mechanisms contributing to the development of CHS have been proposed. The most accepted mechanism is impairment of cerebral autoregulation. Normal cerebral autoregulation constricts the brain vessels in response to a sudden increase in blood flow, to maintain normal cerebral perfusion within an acceptable range (60–160 mm Hg). Autoregulation includes both a myogenic and a neurogenic component. The myogenic component is usually the first reaction to a change in blood flow. If that fails, the remaining autoregulation depends on the innervation of the sympathetic autonomic system. Impaired cerebral autoregulatory mechanisms can lead to CHS. In a chronic ischemic brain, the arterioles and capillaries are vulnerable to rupture and bleeding when perfusion pressure abruptly increases. This process explains the occurrence of CHS in patients who have normal systemic arterial pressure, especially those with small vessel disease (as a result of chronic hypertension or diabetes mellitus).9 In patients with sufficient collateral circulation, less damage occurs in the arterioles and capillaries under chronic ischemia, and thus the collateral circulation may protect against CHS.10
A second theory on the formation of CHS is damage from free radicals. Free radicals cause vasodilatation and can increase the permeability of cerebral vessels during ischemic reperfusion. Reactive oxygen species can damage the cerebrovascular endothelium, resulting in postoperative hyperperfusion which can be prevented by administration of a free radical scavenger.11 12
Third, the role of baroreceptor reflex breakdown in CHS has been proposed. The breakdown of baroreceptors damages the ability to respond to acute changes in systemic arterial blood pressure. These changes can result from various stimuli to the carotid body, such as angioplasty, stent placement, and endarterectomy.12 A less mentioned theory involves the relationship of CHS with the trigeminovascular reflex. Following exposure to vasoconstrictors, the trigeminovascular system releases vasoactive neuropeptides, resulting in increased CBF, to return vascular tone to baseline. This trigeminovascular reflex has been implicated in the development of CHS.13
Incidence and risk factors
Important updated meta-analyses, high quality large observational studies, and pertinent clinical trials in CAS were selected for the discussion. In the past decade, based on a review of nine studies (4446 patients) by Moulakakis et al, 14 the incidence of CHS was 1.16% (range 0.44–11.7%) and that of intracerebral hemorrhage (ICH) was 0.74% (range 0.36–4.5%). A recent systematic review and meta-analysis15 reported that the incidence of CHS after CAS was 4.6% (3.1–6.8%) in 8731 patients, a rate much higher than in previous reports. The incidence of CHS was variable between these studies based on the variation in research design and disease definition.
Compared with CEA, one meta-analysis16 of 236 537 procedures (218 144 CEA; 18 393 CAS) concluded that CEA had a higher risk of CHS than CAS, although the authors proposed that the difference was generated mainly from older studies on CEA. The ICH risk between the two treatments did not differ. This conclusion was contradictory to that of a large cohort study that compared 2341 cases of CAS with 14 347 cases of CEA, in which patients who had undergone CAS were significantly more likely to have ICH than those who had undergone CEA (0.85% vs 0.42%).17 However, patients referred for endovascular treatment comprised a high risk cohort of suboptimal candidates for CEA in many CAS series. This difference in patient populations may partially explain the difference in CHS incidence and severity. The stricter medication protocol in CAS, including anticoagulant use and dual antiplatelet treatment, may lead to a higher rate of hemorrhagic events. In an earlier review by Abou-Chebl et al, 18 antiplatelet and anticoagulant treatment did not have an increased risk of ICH following CAS when intraprocedural anticoagulation use was not excessive and was discontinued postoperatively. A recent Spanish national prospective multicenter study19 reported that CHS occurred in 22 (2.9%) patients and the rate of hemorrhage was 0.7% in 757 patients after CAS, revealing a lower incidence of hemorrhage compared with earlier results.
Large adjudicated randomized studies have reported composite safety outcomes that may provide some insight into risk factors for CHS. Despite older age being a major concern, the Stenting and Angioplasty with Protection for Patients at High Risk for Endarterectomy and Carotid Revascularization with Endarterectomy and Stenting Systems trials did not report an increase in major complications in patients aged >80 years.20 21 In other large ECAS studies, the three most commonly mentioned conditions associated with the development of CHS were critical intracranial atherosclerotic stenosis ≥90%, severe contralateral intracranial atherosclerotic disease, and longstanding pre-existing hypertension. Abou-Chebl et al 18 stated that the risk of CHS was 16% in patients with all three conditions. As cerebral vascular reserve is considered as a marker of cerebral autoregulation, reduced cerebral vascular reserve has been reported to be a risk factors for CHS.22 Other reported risk factors in cohort studies included female sex, chronic kidney disease, left-sided carotid disease, progressive neurological deficit, recurrent hemorrhage, pre-existing brain lesions, and microvascular disease.19 23 24
Any evidence suggesting poor collateral supply to ischemic brain shows an increased risk for reduced cerebral vascular reserve. Therefore, patients should be considered as high risk if they suffer from more frequent transit ischemic attack or prior stroke due to hemodynamic insufficiency. High grade stenosis, incomplete circle of Willis, and impaired hemodynamics on perfusion study are imaging evidence to identify those prone to develop CHS.25
Preventive measures and management
In a meta-analysis by Bouri et al,26 the cumulative incidence of CHS after CEA increased appreciably above a postoperative systolic blood pressure of 150 mm Hg. The efficacy of intensive blood pressure control to decrease the risk for hyperperfusion in CAS was also evident.27 Most CAS studies recommended maintaining postprocedural blood pressure at <140/90 mm Hg. In patients at high risk of CHS and ICH, blood pressure was maintained at <120/80 mm Hg. High risk criteria included factors previously described in patients following CAS, including hypertension at baseline, treated carotid stenosis of >90%, and poor collateral blood flow. The latter was defined by contralateral carotid occlusion or stenosis >80%, indicating an isolated ipsilateral carotid circulation. The lower limit of blood pressure was 90/60 mm Hg, except in those with concomitant cardiovascular disorders. Blood pressure should be monitored continuously via an arterial line or checked every 15 min or less, during and at least 24 hours after the procedure. Intravenous short acting antihypertensive agents should always be available. Antihypertensive agents, including labetolol and clonidine, are proposed because of their reducing effect on CBF.6 However, intravenous nicardipine is more convenient for titrating, despite its potential effect on cerebrovascular vasodilatation. After 1 day, antihypertensive agents could be changed to oral forms and education around blood pressure checking should be offered. The duration of antihypertensive treatment is unclear. We suggest cautious monitoring of blood pressure for at least 1 month for the restoration of cerebral autoregulation. Other preventive measures for CHS include delayed procedure after cerebral infarction and local anesthesia. Edaravone, a free radical scavenger, is considered to be effective in reducing CHS.28 However, randomized controlled trials that establish efficacy and safety of these measures are not available.
Corresponding to the presumed CHS mechanism of impaired cerebral autoregulation, staged angioplasty is an appealing strategy in CAS to allow gradual adaptation of increased CBF. Staged angioplasty is a two step endovascular treatment, including angioplasty and delayed CAS, used in high risk patients with ECAS with impaired cerebrovascular reserve. The first angioplasty was performed using an undersized balloon (diameter of approximately 3.0 mm). Thereafter, definitive CAS was performed after improvement in hyperperfusion, according to a cerebral hemodynamic study, 2–4 weeks later. This process could have been considered as avoiding autoregulation and reducing the possibility of CHS in acute stenting. In one report, data from 525 patients (532 lesions, mean age 72.5±7.5 years, 74 women) were analyzed.29 Scheduled procedures included staged angioplasty for 113 lesions and regular CAS for 419 lesions. The rate of CHS was lower in the staged angioplasty group than in the regular CAS group (4.4% vs 10.5%, p=0.047). Multivariate analysis showed that staged angioplasty was negatively related to CHS (OR 0.315; 95% CI 0.120 to 0.828). Staged angioplasty may be an effective and safe carotid revascularization procedure to avoid CHS in selective patients. However, there are many concerns about this method, such as the lack of a standard protocol for anesthesia, the protection method (proximal protection or not), the interval between submaximal angioplasty and stenting, the next step if recoiling is encountered in the second session, and standards for evaluating CHS. The advantages and disadvantages of this strategy should be discussed with patients.
The goal of CHS management is to avoid complications and provide the best supportive care. Cerebral edema, seizures, and hemorrhage are the main causes of neurological deterioration. Sedation, hyperventilation, treatment of fever, mannitol, or hypertonic saline are common methods to control cerebral edema. Prophylactic anticonvulsant agents are commonly given. When hemorrhage occurs, neurosurgical consultation is warranted and antiplatelet use should be carefully discussed.
Cerebral hyperperfusion syndrome in specific conditions
Intracranial atherosclerosis
Following the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis trial,30 stent use to treat intracranial atherosclerotic disease was reduced, given its stringent indications. This reduction means that less data are available in the literature about CHS in patients after INCS. Furthermore, most studies of complications after INCS report few cases and provide scant descriptions of the clinical symptoms and the conditions for developing hemorrhage. In the nine studies of INCS with over 50 patients,30–38 the average incidence of non-technical related intracranial hemorrhage was about 2.6% (0–7%), and the onset was immediate or within a few hours after the procedure.
Although predefined CHS is not reported, the current reported data on ICH after INCS range from 0% to 3.4%. Risk factors for CHS and its hemorrhagic consequence (figure 4) after INCS were similar to those involved in extracranial procedures. Because no surgical manipulation of the cervical baroreceptor occurs in INCS, the pathophysiological explanation for the development of CHS is more likely impairment of cerebral autoregulation rather than baroreceptor reflex breakdown or induction of free radicals by the surgical procedure. Because of limited data, the management and prevention of CHS after INCS have not specifically been recommended. Periprocedural strict blood pressure control is reasonable. Further research on CHS under these circumstances is warranted.
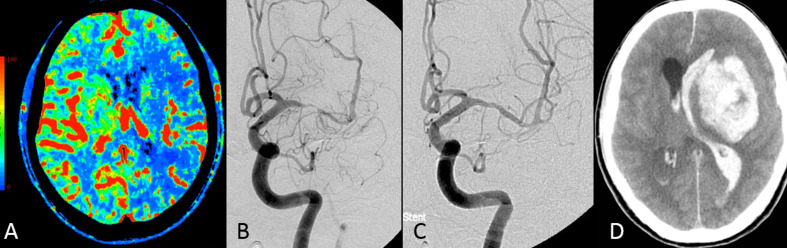
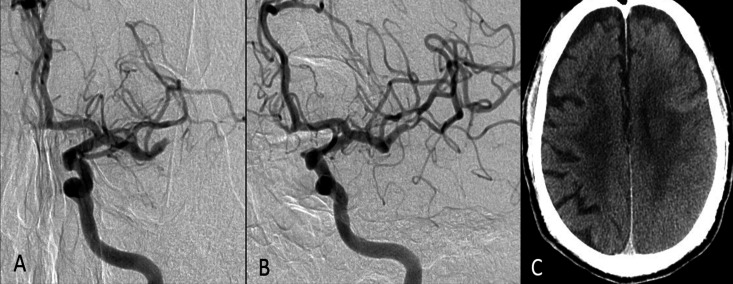
Figure 4.
(A–D) A patient with left middle artery stenosis presented with multiple strokes at the left cerebral hemispheres. (A) Perfusion CT (cerebral blood flow mapping) reveals a significant decrease in blood flow to the territory of the left middle cerebral artery, including the putamen. (B, C) Catheter angiography performed before (B) and after (C) angioplasty and stenting. The patient complained of severe left-sided headache although blood pressure was controlled at about 120/80 mm Hg after the procedure. Six hours later, a sudden onset of loss of consciousness and intracerebral hemorrhage and hematoventricle were found on CT (D). Cerebral hyperperfusion syndrome complicated by intracranial hemorrhage was diagnosed.
Acute ischemic stroke
After mechanical thrombectomy, vessel wall disruption and increasing permeability might occur, which may contribute to the development of CHS.39 Damaged cerebral vessel walls are vulnerable to a sudden increase in blood flow after EVT. Before CHS diagnosis after EVT, especially hemorrhagic CHS, technique induced injuries as a cause must first be excluded, such as guidewire perforation, vessel dissection, and vessel rupture. After successful EVT, imaging studies, especially MRI, should be used to identify the consequence of CHS, including brain edema in the culprit vascular territory (figure 5), hemorrhage beyond the vessel walls, and hemorrhage distal to the occluded vascular region.
Figure 5.
(A–C) Patient receives endovascular thrombectomy due to occlusion at the distal M1 segment of the left middle cerebral artery (MCA) (A). The final result is a modified Treatment in Cerebral Infarction score of 3 (B). The 24 hour follow-up CT (C) reveals cortical swelling in the left MCA region associated with some cortical petechial hemorrhage. Cerebral hyperperfusion syndrome was considered. The patient’s 90 day modified Rankin Scale score was 4.
Because clinical presentation is potentially delayed and obscured in AIS, hemodynamic assessment is crucial. A study that used the ASL technique found that 13 of 27 (48%) patients with AIS who had undergone successful EVT developed hyperperfusion, in which they defined CHS as visually perceived increased CBF compared with the contralateral side.40 By using these imaging modalities for hemodynamic assessment, patients with postprocedural CHS had less improvement in National Institutes of Health Stroke Scale scores at 24 hours, a longer duration of disturbance of consciousness, lower Alberta Stroke Program Early CT Score on diffusion weighted imaging, more hemorrhagic transformation, and poorer outcome at 90 days (modified Rankin Scale score >2).40–42 The application of TCD and CT perfusion in evaluating CHS after EVT has been done. The other available method includes measuring regional CBF with the ASL technique41 42 or SPECT,43 and calculation of normalization of oxygen metabolism by quantitative MRI.44
After recanalization of AIS for large vessel occlusion, the acute brain changes, including edema and hemorrhage, are sometimes referred to as reperfusion injury (RI), rather than CHS. During RI, deterioration of the salvageable penumbra by an inflammatory cascade involves leukocyte infiltration, release of neutrophil derived oxidants and proteolytic enzymes, release of cytokines, and disruption of the blood–brain barrier. Activated platelets and complementary elements can adhere to the endothelium, releasing inflammatory mediators and contributing to the 'no flow' phenomenon, further causing tissue injury through different pathways.7 Moreover, RI and CHS are different in many aspects. In AIS, the target vessel is reopened after an abrupt occlusion and loss of perfusion in the brain tissue, although this perfusion loss may be associated with underlying intracranial atherosclerotic disease. In acute occluded intracranial atherosclerotic disease, the vessel is reopened, but its territorial brain tissue suffers chronic hypoperfusion. However, elucidation of CHS from RI in EVT is difficult; a single method cannot be used to determine the possibility of additional risk of hemorrhage in EVT in underlying intracranial atherosclerotic disease. The current reported symptomatic ICH after EVT for intracranial atherosclerotic disease associated AIS range is 2.5–11.1%, which is not remarkably different from that of embolic occlusion.45 The role of CHS and RI in post-EVT brain injury is therefore unclear. Because anti-inflammatory therapy and neuroprotective agents are recommended for RI treatment, whether they have a role in CHS treatment is of interest. Future research using advanced modalities to differentiate and classify these entities may provide tailored patient treatment.
Despite the fact that intraprocedural hypotension during endovascular stroke therapy is associated with larger infarct volumes and worse functional outcomes in patients with large cerebral vessel occlusion, the optimal postprocedural blood pressure control should take CHS and RI into consideration46 Recent studies suggest lower postprocedural blood pressure is beneficial to functional outcome, and the first 6 hours is the key period.47 48 We recommend preparation of intravenous antihypotensive agents during the procedure, and immediately lower systolic blood pressure to <140 mm Hg after optimal recanalization is achieved. Keeping blood pressure in a narrow range (ideally systolic blood pressure variability <20 mm Hg) is also important as increased blood pressure variability is associated with a worse outcome.49
Miscellaneous
Many case reports or small series report CHS after recanalization of the vertebral artery and subclavian artery, and external carotid artery.50 51 Most patients had chronic high grade stenosis or occlusive disease, as opposed to the explanation of baroreceptor reflex breakdown. A few case reports have described CHS development in patients with large cerebral aneurysm treated by stent assisted coiling or flow diverter.52 53 Therefore, larger series are required to clarify the relationship between the procedures and CHS.
Conclusion
CHS demonstrated variable clinical manifestations, from mild symptoms, such as headache, to severe presentation, and radiological studies may play a role in diagnosis. An immediate postprocedure CBF study, such as ASL or perfusion scans, may help diagnose CHS. The underlying mechanism of CHS remains unclear, but impairment of cerebral autoregulation is the most likely explanation. Blood pressure control during and after recanalization appears to be effective. Staged angioplasty may be an alternative therapy for a high risk patient undergoing CAS. CHS may also occur after INCS, and blood pressure control is still a reasonable preventive measure. After EVT, despite the fact that differentiation between RI and CHS is difficult, lowering and decreasing variability of blood pressure level is probably beneficial.
Footnotes
Contributors: All authors made substantial contributions to the conception and design of the study; acquisition of data or analysis and interpretation of the data; drafting the article or revising it critically for important intellectual content; and final approval of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1. Ogasawara K, Sakai N, Kuroiwa T, et al. . Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 2007;107:1130–6. 10.3171/JNS-07/12/1130 [DOI] [PubMed] [Google Scholar]
- 2. Yamauchi K, Enomoto Y, Otani K, et al. . Prediction of hyperperfusion phenomenon after carotid artery stenting and carotid angioplasty using quantitative DSA with cerebral circulation time imaging. J Neurointerv Surg 2018;10:576–9. 10.1136/neurintsurg-2017-013259 [DOI] [PubMed] [Google Scholar]
- 3. Narita S, Aikawa H, Nagata S-I, et al. . Intraprocedural prediction of hemorrhagic cerebral hyperperfusion syndrome after carotid artery stenting. J Stroke Cerebrovasc Dis 2013;22:615–9. 10.1016/j.jstrokecerebrovasdis.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 4. Fujimoto M, Itokawa H, Moriya M, et al. . Evaluation of cerebral hyperperfusion after carotid artery stenting using C‑Arm CT measurements of cerebral blood volume. Clin Neuroradiol 2018;28:253–60. 10.1007/s00062-016-0552-x [DOI] [PubMed] [Google Scholar]
- 5. Fujimoto S, Toyoda K, Inoue T, et al. . Diagnostic impact of transcranial color-coded real-time sonography with echo contrast agents for hyperperfusion syndrome after carotid endarterectomy. Stroke 2004;35:1852–6. 10.1161/01.STR.0000133131.93900.ff [DOI] [PubMed] [Google Scholar]
- 6. van Mook WNKA, Rennenberg RJMW, Schurink GW, et al. . Cerebral hyperperfusion syndrome. Lancet Neurol 2005;4:877–88. 10.1016/S1474-4422(05)70251-9 [DOI] [PubMed] [Google Scholar]
- 7. Pan J, Konstas A-A, Bateman B, et al. . Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology 2007;49:93–102. 10.1007/s00234-006-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park H, Baek J-H, Kim BM. Endovascular treatment of acute stroke due to intracranial atherosclerotic stenosis-related large vessel occlusion. Front Neurol 2019;10:308 10.3389/fneur.2019.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo Z-N, Xing Y, Wang S, et al. . Characteristics of dynamic cerebral autoregulation in cerebral small vessel disease: diffuse and sustained. Sci Rep 2015;5:15269. 10.1038/srep15269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang F, Fukasaku K, Liu H, et al. . A computational model study of the influence of the anatomy of the circle of Willis on cerebral hyperperfusion following carotid artery surgery. Biomed Eng Online 2011;10:84. 10.1186/1475-925X-10-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimamura N, Naraoka M, Matsuda N, et al. . Intra-arterial oxidative stress correlates negatively with cognitive function and positively with postoperative ischemic lesions in carotid artery stenosis stenting. J Neurointerv Surg 2018;10:440–5. 10.1136/neurintsurg-2017-013465 [DOI] [PubMed] [Google Scholar]
- 12. Nouraei SAR, Al-Rawi PG, Sigaudo-Roussel D, et al. . Carotid endarterectomy impairs blood pressure homeostasis by reducing the physiologic baroreflex reserve. J Vasc Surg 2005;41:631–7. 10.1016/j.jvs.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 13. Macfarlane R, Moskowitz MA, Sakas DE, et al. . The role of neuroeffector mechanisms in cerebral hyperperfusion syndromes. J Neurosurg 1991;75:845–55. 10.3171/jns.1991.75.6.0845 [DOI] [PubMed] [Google Scholar]
- 14. Moulakakis KG, Mylonas SN, Sfyroeras GS, et al. . Hyperperfusion syndrome after carotid revascularization. J Vasc Surg 2009;49:1060–8. 10.1016/j.jvs.2008.11.026 [DOI] [PubMed] [Google Scholar]
- 15. Huibers AE, Westerink J, de Vries EE, et al. . Editor's choice - cerebral hyperperfusion syndrome after carotid artery stenting: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2018;56:322–33. 10.1016/j.ejvs.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 16. Galyfos G, Sianou A, Filis K. Cerebral hyperperfusion syndrome and intracranial hemorrhage after carotid endarterectomy or carotid stenting: a meta-analysis. J Neurol Sci 2017;381:74–82. 10.1016/j.jns.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 17. Hussain MA, Alali AS, Mamdani M, et al. . Risk of intracranial hemorrhage after carotid artery stenting versus endarterectomy: a population-based study. J Neurosurg 2018;129:1522–9. 10.3171/2017.8.JNS171142 [DOI] [PubMed] [Google Scholar]
- 18. Abou-Chebl A, Yadav JS, Reginelli JP, et al. . Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol 2004;43:1596–601. 10.1016/j.jacc.2003.12.039 [DOI] [PubMed] [Google Scholar]
- 19. González García A, Moniche F, Escudero-Martínez I, et al. . Clinical predictors of hyperperfusion syndrome following carotid stenting: results from a national prospective multicenter study. JACC Cardiovasc Interv 2019;12:873–82. 10.1016/j.jcin.2019.01.247 [DOI] [PubMed] [Google Scholar]
- 20. Yadav JS, Wholey MH, Kuntz RE, et al. . Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–501. 10.1056/NEJMoa040127 [DOI] [PubMed] [Google Scholar]
- 21. CaRESS Steering Committee Carotid revascularization using endarterectomy or stenting systems (CaRESS) phase I clinical trial: 1-year results. J Vasc Surg 2005;42:213–9. 10.1016/j.jvs.2005.04.023 [DOI] [PubMed] [Google Scholar]
- 22. Kaku Y, Yoshimura S-ichi, Kokuzawa J. Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol 2004;25:1403–8. [PMC free article] [PubMed] [Google Scholar]
- 23. Dua A, Romanelli M, Upchurch GR, et al. . Predictors of poor outcome after carotid intervention. J Vasc Surg 2016;64:663–70. 10.1016/j.jvs.2016.03.428 [DOI] [PubMed] [Google Scholar]
- 24. Grunwald IQ, Politi M, Reith W, et al. . Hyperperfusion syndrome after carotid stent angioplasty. Neuroradiology 2009;51:169–74. 10.1007/s00234-008-0483-6 [DOI] [PubMed] [Google Scholar]
- 25. Zhang L, Dai D, Li Z, et al. . Risk factors for hyperperfusion-induced intracranial hemorrhage after carotid artery stenting in patients with symptomatic severe carotid stenosis evaluation. J Neurointerv Surg 2019;11:474–8. 10.1136/neurintsurg-2018-013998 [DOI] [PubMed] [Google Scholar]
- 26. Bouri S, Thapar A, Shalhoub J, et al. . Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg 2011;41:229–37. 10.1016/j.ejvs.2010.10.016 [DOI] [PubMed] [Google Scholar]
- 27. Abou-Chebl A, Reginelli J, Bajzer CT, et al. . Intensive treatment of hypertension decreases the risk of hyperperfusion and intracerebral hemorrhage following carotid artery stenting. Catheter Cardiovasc Interv 2007;69:690–6. 10.1002/ccd.20693 [DOI] [PubMed] [Google Scholar]
- 28. Ogasawara K, Inoue T, Kobayashi M, et al. . Pretreatment with the free radical scavenger edaravone prevents cerebral hyperperfusion after carotid endarterectomy. Neurosurgery 2004;55:1060–7. 10.1227/01.NEU.0000140838.27450.63 [DOI] [PubMed] [Google Scholar]
- 29. Hayakawa M, Sugiu K, Yoshimura S, et al. . Effectiveness of staged angioplasty for avoidance of cerebral hyperperfusion syndrome after carotid revascularization. J Neurosurg 2019:2002–3. 10.1016/j.jvs.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 30. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. . Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003. 10.1056/NEJMoa1105335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Terada T, Tsuura M, Matsumoto H, et al. . Hemorrhagic complications after endovascular therapy for atherosclerotic intracranial arterial stenoses. Neurosurgery 2006;59:310–8. 10.1227/01.NEU.0000225326.81661.68 [DOI] [PubMed] [Google Scholar]
- 32. Fiorella D, Levy EI, Turk AS, et al. . US multicenter experience with the Wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke 2007;38:881–7. 10.1161/01.STR.0000257963.65728.e8 [DOI] [PubMed] [Google Scholar]
- 33. Guo X-bin, Ma N, Hu X-bo, et al. . Wingspan stent for symptomatic M1 stenosis of middle cerebral artery. Eur J Radiol 2011;80:e356–60. 10.1016/j.ejrad.2010.11.029 [DOI] [PubMed] [Google Scholar]
- 34. Costalat V, Maldonado IL, Vendrell J-F, et al. . Endovascular treatment of symptomatic intracranial stenosis with the Wingspan stent system and gateway PTA balloon: a multicenter series of 60 patients with acute and midterm results. J Neurosurg 2011;115:686–93. 10.3171/2011.5.JNS101583 [DOI] [PubMed] [Google Scholar]
- 35. Shin YS, Kim BM, Suh SH, et al. . Wingspan stenting for intracranial atherosclerotic stenosis: clinical outcomes and risk factors for in-stent restenosis. Neurosurgery 2013;72:596–604. 10.1227/NEU.0b013e3182846e09 [DOI] [PubMed] [Google Scholar]
- 36. SC Y, Leung TW, Lee KT, et al. . Angioplasty and stenting of intracranial atherosclerosis with the Wingspan system: 1-year clinical and radiological outcome in a single Asian center. J Neurointervent Surg 2014;6:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu S, Wu P, Shi H, et al. . Hyperperfusion syndrome after stenting for intracranial artery stenosis. Cell Biochem Biophys 2015;71:1537–42. 10.1007/s12013-014-0377-7 [DOI] [PubMed] [Google Scholar]
- 38. Alexander MJ, Zauner A, Chaloupka JC, et al. . Weave trial: final results in 152 on-label patients. Stroke 2019;50:889–94. 10.1161/STROKEAHA.118.023996 [DOI] [PubMed] [Google Scholar]
- 39. Perren F, Kargiotis O, Pignat J-M, et al. . Hemodynamic changes may indicate vessel wall injury after stent retrieval thrombectomy for acute stroke. J Neuroimaging 2018;28:412–5. 10.1111/jon.12513 [DOI] [PubMed] [Google Scholar]
- 40. Shimonaga K, Matsushige T, Hosogai M, et al. . Hyperperfusion after endovascular reperfusion therapy for acute ischemic stroke. J Stroke Cerebrovasc Dis 2019;28:1212–8. 10.1016/j.jstrokecerebrovasdis.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 41. Okazaki S, Yamagami H, Yoshimoto T, et al. . Cerebral hyperperfusion on arterial spin labeling MRI after reperfusion therapy is related to hemorrhagic transformation. J Cereb Blood Flow Metab 2017;37:3087–90. 10.1177/0271678X17718099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu S, Liebeskind DS, Dua S, et al. . Postischemic hyperperfusion on arterial spin labeled perfusion MRI is linked to hemorrhagic transformation in stroke. J Cereb Blood Flow Metab 2015;35:630–7. 10.1038/jcbfm.2014.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abumiya T, Katoh M, Moriwaki T, et al. . Utility of early post-treatment single-photon emission computed tomography imaging to predict outcome in stroke patients treated with intravenous tissue plasminogen activator. J Stroke Cerebrovasc Dis 2014;23:896–901. 10.1016/j.jstrokecerebrovasdis.2013.07.028 [DOI] [PubMed] [Google Scholar]
- 44. Seiler A, Lauer A, Deichmann R, et al. . Complete restitution of the ischemic penumbra after successful thrombectomy: a pilot study using quantitative MRI. Clin Neuroradiol 2019;29:415–23. 10.1007/s00062-018-0675-3 [DOI] [PubMed] [Google Scholar]
- 45. Park H, Baek J-H, Kim BM. Endovascular treatment of acute stroke due to intracranial atherosclerotic stenosis-related large vessel occlusion. Front Neurol 2019;10:308. 10.3389/fneur.2019.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Petersen NH, Ortega-Gutierrez S, Wang A, et al. . Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke 2019;50:1797–804. 10.1161/STROKEAHA.118.024286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cernik D, Sanak D, Divisova P, et al. . Impact of blood pressure levels within first 24 hours after mechanical thrombectomy on clinical outcome in acute ischemic stroke patients. J Neurointerv Surg 2019;11:735–9. 10.1136/neurintsurg-2018-014548 [DOI] [PubMed] [Google Scholar]
- 48. Chu H-J, Lin C-H, Chen C-H, et al. . Effect of blood pressure parameters on functional independence in patients with acute ischemic stroke in the first 6 hours after endovascular thrombectomy. J Neurointerv Surg 2019:pii: neurintsurg-2019-015412. 10.1136/neurintsurg-2019-015412 [DOI] [PubMed] [Google Scholar]
- 49. Bennett AE, Wilder MJ, McNally JS, et al. . Increased blood pressure variability after endovascular thrombectomy for acute stroke is associated with worse clinical outcome. J Neurointerv Surg 2018;10:823–7. 10.1136/neurintsurg-2017-013473 [DOI] [PubMed] [Google Scholar]
- 50. Fu C, Xu Z, Hu Z, et al. . Cortical blindness as a rare presentation of hemorrhagic cerebral hyperperfusion syndrome following vertebral angioplasty. J Neurointerv Surg 2018;10:e21. 10.1136/neurintsurg-2017-013412.rep [DOI] [PubMed] [Google Scholar]
- 51. Ito K, Yonaha H, Kai Y, et al. . Hyperperfusion syndrome after stent placement for subclavian artery stenosis: case report. Neurol Med Chir 2012;52:902–5. 10.2176/nmc.52.902 [DOI] [PubMed] [Google Scholar]
- 52. Ecker RD, Murray RD, Seder DB. Hyperperfusion syndrome after stent/coiling of a ruptured carotid bifurcation aneurysm. Neurocrit Care 2013;18:54–8. 10.1007/s12028-012-9733-x [DOI] [PubMed] [Google Scholar]
- 53. Chiu AHY, Wenderoth J. Cerebral hyperperfusion after flow diversion of large intracranial aneurysms. J Neurointerv Surg 2013;5:e48. 10.1136/neurintsurg-2012-010479.rep [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2019-015621supp001.pdf (18.1KB, pdf)