Abstract
The core planar polarity proteins are essential mediators of tissue morphogenesis, controlling both the polarised production of cellular structures and polarised tissue movements. During development the core proteins promote planar polarisation by becoming asymmetrically localised to opposite cell edges within epithelial tissues, forming intercellular protein complexes that coordinate polarity between adjacent cells. Here we describe a novel protein complex that regulates the asymmetric localisation of the core proteins in the Drosophila pupal wing. DAnkrd49 (an ankyrin repeat protein) and Bride of Doubletime (Bdbt, a non-canonical FK506 binding protein family member) physically interact, and regulate each other’s levels in vivo. Loss of either protein results in a reduction in core protein asymmetry and disruption of the placement of trichomes at the distal edge of pupal wing cells. Post-translational modifications are thought to be important for the regulation of core protein behaviour and their sorting to opposite cell edges. Consistent with this, we find that loss of DAnkrd49 or Bdbt leads to reduced phosphorylation of the core protein Dishevelled and to decreased Dishevelled levels both at cell junctions and in the cytoplasm. Bdbt has previously been shown to regulate activity of the kinase Discs Overgrown (Dco, also known as Doubletime or Casein Kinase Iε), and Dco itself has been implicated in regulating planar polarity by phosphorylating Dsh as well as the core protein Strabismus. We demonstrate that DAnkrd49 and Bdbt act as dominant suppressors of Dco activity. These findings support a model whereby Bdbt and DAnkrd49 act together to modulate the activity of Dco during planar polarity establishment.
Author summary
In many animal tissues, sheets of cells are polarised in the plane of the tissue, which is evident by the production of polarised structures, such as hairs on the fly wing that point in the same direction or cilia that beat in the same direction. One group of proteins controlling this coordinated polarity are the core planar polarity proteins, which localise asymmetrically within cells such that some core proteins localise to one cell end and others to the opposite cell end. It is thought that modifications such as phosphorylation may locally regulate core protein stability, and this promotes sorting of proteins to different cell ends. We identify two proteins, DAnkrd49 and Bdbt, that form a complex and regulate core protein asymmetry. Loss of either protein causes a reduction in overall levels of the core protein Dishevelled (Dsh), and a reduction in its phosphorylation. We provide evidence that the effect on core protein asymmetry is mediated via regulation of the kinase activity of Discs overgrown (Dco, also known as Doubletime/Casein Kinase Iε) by DAnkrd49 and Bdbt. We propose that modulation of Dco activity by DAnkrd49 and Bdbt is a key step in the sorting of core proteins to opposite cell ends.
Introduction
Planar polarity describes the phenomenon whereby cells coordinate their polarity in the plane of a tissue: for example the hairs on the skin point in the same direction, cilia coordinate their beating, and cells coordinate their movements during tissue morphogenesis [1–3]. Understanding the mechanisms by which this coordinated polarisation occurs is of prime importance, as disruption of polarity can have diverse consequences, including neural tube closure defects, hydrocephalus and defects in neuronal migration [3, 4].
The fly wing is a well-characterised model system in which to study planar polarity. Each cell within the adult wing produces a single hair, or trichome, which points towards the distal end of the wing. Furthermore, viable mutations that cause characteristic swirling of the trichomes have been identified, and the genes associated with these mutations were subsequently found to be highly conserved, and to regulate planar polarity throughout the animal kingdom [5].
The core planar polarity proteins (hereafter known as the core proteins) are the best characterised group of proteins that regulate planar polarity. In the pupal wing, the core proteins adopt asymmetric subcellular localisations prior to trichome emergence, and in their absence trichomes emerge from the centre of the cell rather than at the distal cell edge [6]. The core proteins comprise the atypical cadherin Flamingo (Fmi, also known as Starry Night [Stan]), the transmembrane proteins Frizzled (Fz) and Strabismus (Stbm, also known as Van Gogh [Vang]), and three cytoplasmic proteins Dishevelled (Dsh), Prickle (Pk) and Diego (Dgo). Fmi localises to proximal and distal cell edges in the pupal wing, but is excluded from lateral cell edges, while Fz, Dsh and Dgo localise to distal cell edges and Stbm and Pk to proximal cell edges (Fig 1A). These proteins form intercellular complexes at cell junctions, that couple neighbouring cells and allow them to coordinate their polarity [1, 2].
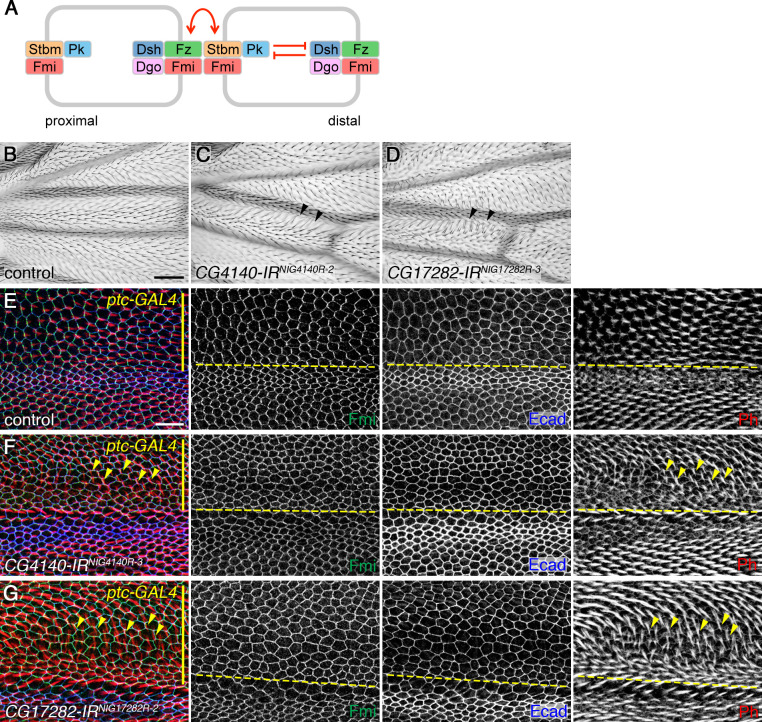
Fig 1. RNAi knockdown of DAnkrd49 and Bdbt disrupts planar polarity.
(A) Diagram to illustrate asymmetric localisation of the core planar polarity proteins. Fz, Dsh and Dgo localise to distal cell ends, while Stbm and Pk localise proximally and Fmi localises both proximally and distally. Feedback interactions are thought to lead to mutual inhibition between proximal and distal complex components (red inhibitory lines), while intercellular interactions between proximal and distal components are thought to stabilise asymmetric complexes (red arrows). (B-D) Dorsal surface of adult male wings from control wild-type flies (B), or from flies expressing RNAi against CG4140/DAnkrd49 (C, NIG line 4140R-2), or CG17282/Bdbt (D, NIG line 17282R-3), under control of the MS1096-GAL4 driver at 25°C. Distal is to the right and anterior is up in these and all other images. Black arrowheads mark a proximal region of the wing, between veins 3 and 4, in which trichomes point distally in wild-type wings, but swirl anteriorly in DAnkrd49 and Bdbt knockdown wings. Note that anterior to vein 3, trichomes tend to point towards the vein in wild-type as well as in mutants wings. Scale bar 50 μm. (E-G) Pupal wings expressing a control RNAi (E, VDRC line 39864, targeting Sik1, a gene unrelated to planar polarity), or RNAi against CG4140/DAnkrd49 (F, NIG line 4140R-3), or CG17282/Bdbt (G, NIG line 17282R-2), under control of the ptc-GAL4 driver. Larvae were raised at 25°C, collected as white prepupae and then aged for 27hr at 29°C. Wings immunolabelled for Fmi (green) and Ecad (blue), and trichomes labelled with Phalloidin (red). ptc-GAL4 is expressed at the top of the image (yellow bar), and the yellow dotted line marks the anterior-posterior boundary. Yellow arrowheads mark some trichomes which emerge from posterior cell edges, rather than from distal cell edges as normally. Scale bar 10 μm.
The mechanisms by which the core proteins become asymmetrically localised are poorly understood. The overall direction of polarity is thought to be determined by tissue-specific ‘global’ cues: these may include gradients of morphogens or Fat/Dachsous cadherin activity, or other cues such as mechanical tension [7]. In the wing these global cues may directly regulate core protein localisation or act indirectly via effects on growth and tissue morphogenesis [7]. Global cues are thought to lead to subtle biases in core protein localisation within cells that are subsequently amplified by feedback between the core proteins, in which positive (stabilising) interactions between complexes of the same orientation are coupled with negative (destabilising) interactions between complexes of opposite orientation (Fig 1A). In mathematical models, such feedback interactions have been demonstrated to be sufficient to amplify weak biases in protein localisation, leading to sorting of complexes and robust asymmetry (e.g. [8–11]).
Experimental evidence for feedback is only beginning to be elucidated. Cell culture experiments have demonstrated competitive binding between several of the core proteins, which may be important for feedback [8, 12–14]. Furthermore, we have recently shown that the core protein Pk acts through Dsh to destabilise Fz in the same cell, while stabilising Fz across cell junctions via Stbm [15].
In order for feedback to operate, cells must utilise the general cellular machinery: for example active endocytosis is necessary for Pk to destabilise Fz [15]. Furthermore, post-translational modifications of the core proteins are likely to be key mediators of feedback. For example loss of ubiquitination pathway components and some protein kinases have been shown to disrupt planar polarity. In flies, a Cullin-3/Diablo/Kelch ubiquitin ligase complex regulates Dsh levels at cell junctions, while the de-ubiquitinase Fat Facets regulates Fmi levels [16]. Stbm also negatively regulates Pk levels, and ubiquitination of Pk by Cullin-1/SkpA/Slimb promotes internalisation of Fmi-Stbm-Pk complexes [17–19]. Similarly, in vertebrates, the Stbm homologue Vangl2 may promote local degradation of Pk via ubiquitination by Smurf E3 ubiquitin ligases [20, 21]. Furthermore, Drosophila Fz phosphorylation is mediated by atypical Protein Kinase C [22], and Dsh is a target of phosphorylation by the Discs Overgrown (Dco, also known as Doubletime [Dbt] or Casein Kinase Iε [CKIε]) and Abelson kinases [23–25]. Dco/CKIε has also been implicated in phosphorylation of Stbm in both flies and vertebrates [26–29].
Here we describe the identification of two new regulators of planar polarity in the Drosophila wing. We show that Bride of Doubletime (Bdbt) and DAnkrd49 are binding partners that regulate each other’s levels. Loss of either protein disrupts asymmetric localisation of the core proteins. Furthermore, they regulate overall levels of Dsh and Dsh phosphorylation in the pupal wing, and we provide evidence that they act by modulating the activity of the kinase Dco.
Results
Identification of two novel regulators of planar polarity
To identify novel regulators of planar polarity in Drosophila, we performed an unbiased RNAi screen, using the MS1096-GAL4 driver to express RNAi lines throughout the developing wing blade and examining the adult wings for evidence of planar polarity defects in trichome (wing hair) orientation (see also [18, 30]). We identified two RNAi lines which gave a similar phenotype, in which trichomes were misoriented in the proximal region of the wing (Fig 1B–1D). These RNAi lines targeted two different loci: CG4140 and CG17282. Additional RNAi lines corresponding to these loci were obtained, and when screened using MS1096-GAL4, or ptc-GAL4 in the presence of UAS-Dcr2 (S1 Table), gave qualitatively similar trichome planar polarity defects.
To confirm that these phenotypes were due to mislocalisation of the core polarity proteins, we examined pupal wings in which RNAi constructs were expressed along the anterior-posterior compartment boundary using ptc-GAL4. As in adult wings (S1A–S1C Fig), trichome polarity was disrupted, with trichomes emerging from incorrect cell edges and swirling towards the anterior-posterior boundary (Fig 1E–1G). Trichome initiation was also delayed within the ptc-GAL4 domain, and asymmetric localisation of the core protein Fmi was disrupted, such that it showed a more uniform distribution around the apical junctions (Fig 1E–1G and S1D–S1F Fig). This loss of asymmetry was not caused by a general defect in membrane organisation or apical-basal polarity, as E-cadherin localisation was not affected. However an increase in cell size was evident.
CG4140 (also known as l(2)35Be) encodes a 215 amino acid protein with ankyrin repeats (Fig 2A). It is a homologue of the poorly characterised human gene Ankyrin Repeat Domain-Containing Protein 49 (ANKRD49, S2A Fig), and we will hereafter refer to the fly gene as DAnkrd49. CG17282 is also known as Bride of Doubletime (Bdbt), and encodes a member of the FKBP (FK506 binding protein) family. Bbdt has 3 tetratricopeptide repeats (Fig 2A) that may mediate protein-protein interactions. Like other FKBP family members, its N-terminus has structural similarity to peptidyl prolyl isomerases (PPIases), but it lacks critical residues for catalytic activity [31]. Interestingly, its N-terminus binds to the kinase Dco: loss of Bdbt in adult flies promotes hyperphosphorylation of Dco, and reduces its ability to phosphorylate target proteins (Fig 2B) [31, 32].
Fig 2.
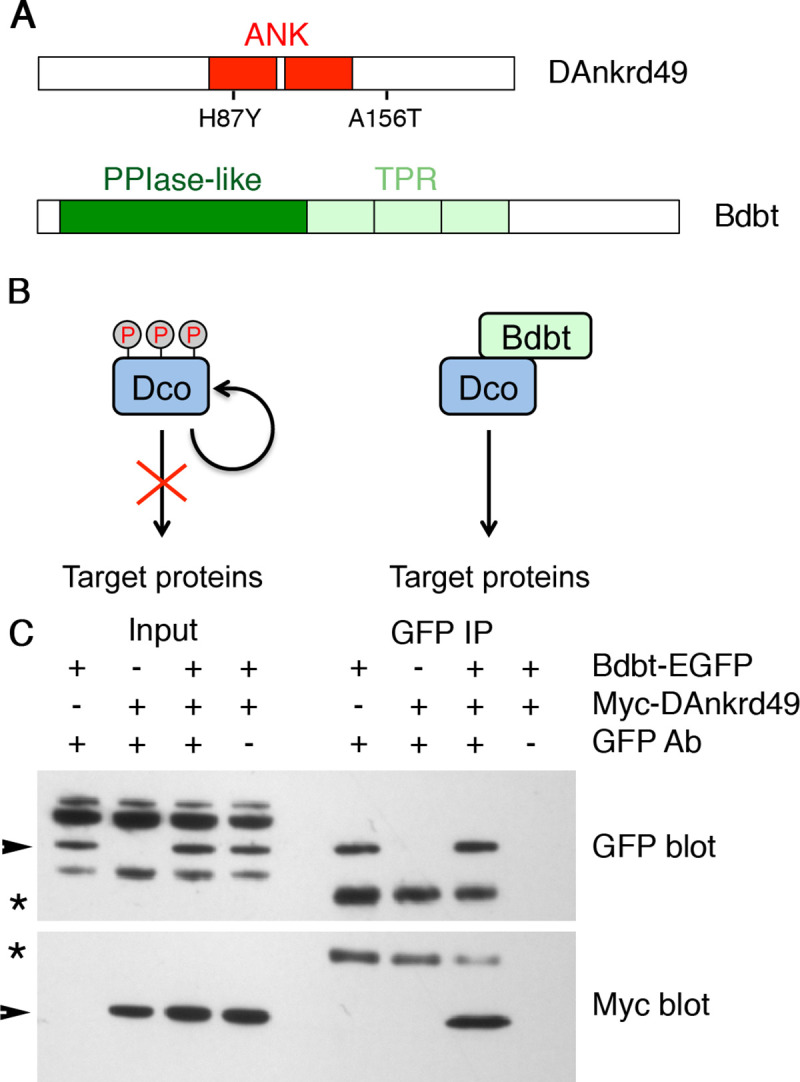
Physical interaction between DAnkrd49 and Bdbt (A) Schematics of DAnkrd49 (top) and Bdbt (bottom) protein structures. The ankyrin repeats of DAnkrd49 are shown in red, and the positions of the two point mutations seen in the DAnkrd49l(2)35Be4 allele are indicated. The peptidyl prolyl isomerase-like domain of Bdbt is shown in dark green and the tetratricopeptide repeats in pale green. (B) Regulation of Dco kinase by Bdbt, based on Fan et al 2013 [31]. In the absence of Bdbt, Dco is hyperphosphorylated and has reduced kinase activity on its target proteins [42]. Binding of Bdbt to Dco prevents hyperphosphorylation of Dco, and leads to increased Dco kinase activity. (C) Western blot showing co-immunoprecipitation of Myc-DAnkrd49 by Bdbt-EGFP, after co-transfection into S2 cells. Arrowheads indicate specific bands and asterisks indicate antibody heavy chains.
Physical interaction between Bdbt and DAnkrd49
Bdbt and DAnkrd49 were identified as binding partners in an early release of the Drosophila Protein Interaction Mapping project [33], but were not considered to be high confidence interactors in the published analysis (DPIM-2) [34]. In order to confirm this interaction, EGFP-tagged Bdbt and Myc-tagged DAnkrd49 were co-transfected into Drosophila S2 cells. Immunoprecipitation with GFP antibody led to pulldown of Myc-DAnkrd49 (Fig 2C). This suggests that Bdbt and DAnkrD49 interact and act as a complex to regulate core protein asymmetry.
DAnkrd49 and Bdbt mutants cause loss of core protein asymmetry
To confirm that the RNAi phenotypes were due to knockdown of the expected target genes, we examined loss of function mutations of DAnkrd49 and Bdbt. EMS-induced alleles of DAnkrd49 have been identified, which are homozygous lethal, but otherwise uncharacterised [35]. Sequencing of one of these alleles, DAnkrd49l(2)35Be4, revealed H87Y and A156T mutations (Fig 2A and S2A Fig). H87 is highly conserved in all species, and mutation to Y is predicted to be highly deleterious, while A156 is less well conserved, and mutation to T predicted to be tolerated (SIFT analysis) [36]. Mutant clones of DAnkrd49l(2)35Be4 were generated in the pupal wing, which revealed reduced asymmetric localisation of Fmi (Fig 3A and 3C), accompanied by delayed trichome initiation, and formation of trichomes in the cell centre rather than at the distal cell edge (Fig 3E). Levels of the junctional protein Armadillo (Arm) were not affected in these clones (S3A Fig), suggesting there is no general defect in junctional integrity. When small patches of wild-type cells were surrounded by DAnkrd49l(2)35Be4 mutant cells, polarity of the wild-type cells was often slightly disrupted. However, we did not see consistent non-autonomous effects on proximal or distal clone boundaries.
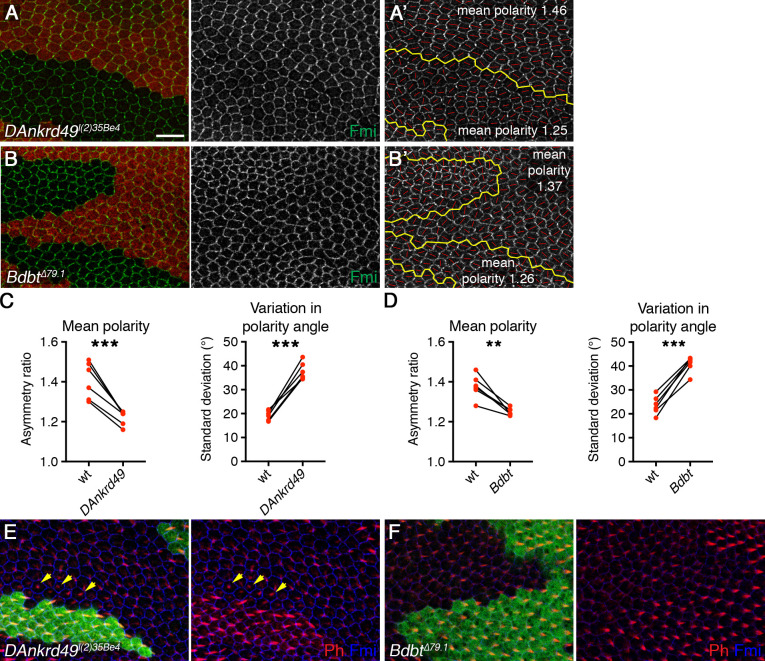
Fig 3. DAnkrd49 and Bdbt regulate asymmetric localisation of core proteins.
(A,B) 28hr APF pupal wings carrying clones of cells lacking DAnkrd49 (DAnkrd49l(2)35Be4, A), or Bdbt (BdbtΔ79.1, B), marked by loss of GFP or β-gal respectively (red). Wings immunolabelled for Fmi (green). Scale bar 10 μm. (A',B') Clones in A and B showing the clone outline (yellow line) and the polarity nematic for each cell (red lines). Length of red line (polarity magnitude) is reduced in DAnkrd49 and Bdbt clones compared to wild-type tissue. (C,D) Quantitation of mean polarity and variation in polarity angle, in wild-type and DAnkrd49 (C) or Bdbt (D) mutant tissue, values from the same wing are linked by black bars. Wild-type tissue was quantitated several cells away from clone boundaries, due to some disruption in polarity on clone boundaries. Paired t-tests were used to compare values in the same wing, **p<0.01, ***p<0.001. 6 wings of each genotype were quantified. See S2 Table for numerical data. (E,F) 32.5hr APF pupal wings carrying clones of cells lacking DAnkrd49 (DAnkrd49l(2)35Be4, E), or Bdbt (BdbtΔ79.1, F), marked by loss of GFP or β-gal respectively (green). Wings immunolabelled for Fmi (blue) and labelled with Phalloidin (red). Yellow arrows indicate trichomes which are delayed and initiate in the cell centre rather than at the cell edge.
As DAnkrd49l(2)35Be4 is unlikely to be a null mutation, and the chromosome also carries additional recessive markers, we made an independent allele by deleting the entire gene by homologous recombination (S2B Fig, see also Materials and Methods). Multiple independent alleles were generated, all of which were homozygous lethal, and lethal when transheterozygous with DAnkrd49l(2)35Be4. We then made mutant clones of DAnkrd49ΔD3.1; however few clones were recovered, and most clones that were seen consisted of only a few cells, suggesting a proliferation defect. However rare clones that were slightly larger gave a qualitatively similar polarity phenotype to DAnkrd49l(2)35Be4 mutant clones, with reduced core protein asymmetry and delayed trichome formation (S3D and S3F Fig). Due to the poor proliferation of DAnkrd49ΔD3.1 mutant clones, we used the DAnkrd49l(2)35Be4 hypomorph for subsequent experiments.
No mutant alleles were available for Bdbt, so we again used homologous recombination to knock out the entire coding region (S2B Fig, see also Materials and Methods). A single mutant allele was generated, which was homozygous lethal and failed to complement deficiencies for the region. In pupal wing clones, Bdbt mutations gave a similar phenotype to DAnkrd49 mutations, notably reduced Fmi asymmetry and delayed trichome initiation (Fig 3B, 3D and 3F). In some clones we saw a disruption in tissue structure, as seen by reduced Armadillo (Arm) staining at junctions (S3C Fig). However core protein asymmetry was reduced even in clones with normal Arm staining (S3B Fig). No obvious effect on cell proliferation was seen, unlike in DAnkrd49 clones. This suggests that DAnkrd49 may have additional functions, independent of Bdbt, or that differential perdurance of the two gene products within mutant clones may cause different effects on cell growth and viability.
EGFP-tagged DAnkrd49 and Bdbt localise uniformly in the cytoplasm and DAnkrd49 and Bdbt regulate each other’s levels
Planar polarity proteins belonging to the ‘core’ localise asymmetrically within the pupal wing, and loss of any of these proteins disrupts the localisation of the others. In contrast, other regulatory proteins such as kinases regulate the asymmetry of the core proteins but have not been observed to themselves localise asymmetrically (e.g. [16, 24]). To determine to which class DAnkrd49 and Bdbt belong, we expressed EGFP-tagged DAnkrd49 and Bdbt in the pupal wing, under control of the ubiquitous Act5C promoter. In both cases we saw uniform expression in the cytoplasm, with some localisation to the plasma membrane; but no asymmetric localisation (Fig 4A and 4B). Notably, the transgenes rescued their respective mutant phenotypes (Fig 4A–4D, compare with Fig 3A–3D), confirming both that the transgenes are functional and that the mutant phenotype is associated with mutations in the expected gene locus. Cellular levels of DAnkrd49-EGFP were increased in the absence of endogenous DAnkrd49.
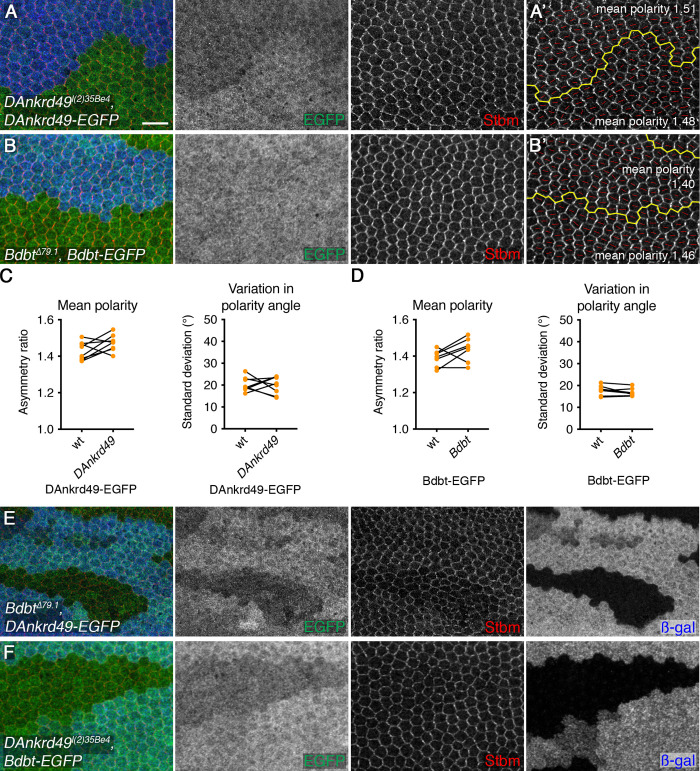
Fig 4. DAnkrd49 and Bdbt mutually regulate each other's protein levels.
(A,B) 28hr APF pupal wings carrying clones of cells lacking DAnkrd49 (DAnkrd49l(2)35Be4) in a background expressing ActP-DAnkrd49-EGFP (A), or clones of cells lacking Bdbt (BdbtΔ79.1) in a background expressing ActP-Bdbt-EGFP (B). Wings immunolabelled for GFP (green) and Stbm (red). Clones marked by loss of β-gal (blue). Note that levels of DAnkrd49-EGFP are slightly increased in the absence of endogenous DAnkrd49. Scale bar 10 μm. (A',B') Clones in A and B showing the clone outline (yellow line) and the polarity nematic for each cell (red lines). Length of red line (polarity magnitude) is not significantly different in wild-type tissue and inside the DAnkrd49 and Bdbt clones, showing that the mutant phenotype is rescued by DAnkrd49-EGFP and Bdbt-EGFP respectively. (C,D) Quantitation of mean polarity and variation in polarity angle, in clones of DAnkrd49 in a background expressing ActP-DAnkrd49-EGFP (C) or clones of Bdbt in a background expressing ActP-Bdbt-EGFP (D), values from clone and non-clone regions of the same wing are linked by black bars. Paired t-tests were used to compare values in the same wing, no significant differences were found. 8 wings were quantified in (C) and 7 wings were quantified in (D). See S2 Table for numerical data. (E,F) 28hr APF pupal wings carrying clones of cells lacking Bdbt (BdbtΔ79.1) in a background expressing ActP-DAnkrd49-EGFP (E), or clones of cells lacking DAnkrd49 (DAnkrd49l(2)35Be4) in a background expressing ActP-Bdbt-EGFP (F). Wings immunolabelled for GFP (green) and Stbm (red). Clones marked by loss of β-gal (blue). Note that levels of the EGFP-tagged proteins are reduced within the clones.
As our co-immunoprecipitation experiments suggest that DAnkrd49 and Bdbt act as a complex, we examined whether they regulated each other’s localisation or levels. Interestingly, loss of Bdbt causes a reduction in overall levels of DAnkrd49-EGFP in the pupal wing, and vice versa (Fig 4E and 4F). This is consistent with a model in which DAnkrd49 and Bdbt act in a complex in which they promote each other’s stability.
DAnkrd49 and Bdbt regulate overall levels of Dsh
To determine the basis for the loss of core protein asymmetry, we further investigated the effects of loss of DAnkrd49 and Bdbt on core protein localisation. For mutations in both genes, we found that levels of Dsh at junctions were severely reduced (Fig 5A and 5B and S3E Fig), while we did not observe changes in levels of any other core proteins we examined (S3A, S3B Fig and S4A–S4D Fig). In particular, levels of Fz, which recruits Dsh to junctions, were similar to wild-type (S4A and S4B Fig). As our Dsh antibody gives variable amounts of background staining, we confirmed this result using a P[acman]-EGFP-dsh rescue construct. This revealed a reduction in Dsh levels, not only at the junctions but also in the cytoplasm (Fig 5C and 5D). Levels of Dsh-ECFP expressed under a heterologous arm promoter were also reduced, consistent with the effects being post-transcriptional (S4E and S4F Fig). Furthermore Dsh levels were rescued in DAnkrd49 and Bdbt mutant clones when DAnkrd49-EGFP and Bdbt-EGFP were expressed, respectively (S4G and S4H Fig).
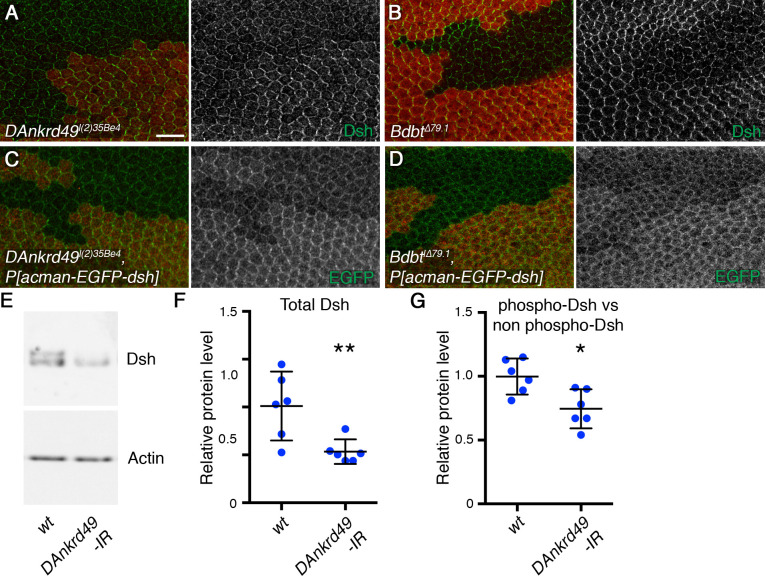
Fig 5. DAnkrd49 and Bdbt regulate Dsh levels.
(A,B) 28hr APF pupal wings carrying clones of cells lacking DAnkrd49 (DAnkrd49l(2)35Be4), marked by loss of GFP (red, A), or lacking Bdbt (BdbtΔ79.1), marked by loss of β-gal (red, B). Wings immunolabelled for Dsh (green). Scale bar 10 μm. (C-D) 28hr APF pupal wings carrying clones of cells lacking DAnkrd49 (C, DAnkrd49l(2)35Be4), or clones of cells lacking Bdbt (D, BdbtΔ79.1), in a background expressing one copy of P[acman]-EGFP-dsh. Wings immunolabelled for GFP (green), clones marked by loss of β-gal (red). (E) Representative western blot from wild-type pupal wings, or pupal wings expressing RNAi against DAnkrd49 (line 4140R-3), under control of the MS1096-GAL4 driver. Blot probed with Dsh (top) and Actin (bottom). Overall Dsh levels decrease in wings expressing DAnkrd49 RNAi. (F) Quantitation of Dsh levels from western blotting, normalised to Actin, from 6 biological replicates. Error bars are 95% confidence intervals, **p = 0.01 (unpaired t-test). See S2 Table for numerical data. (G) Comparison of levels of slow migrating Dsh (hyperphosphorylated) relative to fast migrating Dsh (less phosphorylated). The graph shows a ratio of the maximum intensity of the two bands averaged across the width of each band. Error bars are 95% confidence intervals, *p = 0.011 (unpaired t-test). See S2 Table for numerical data.
To confirm that overall cellular levels of Dsh are reduced in DAnkrd49 and Bdbt mutants, we carried out western blots on pupal wing extracts. As loss-of-function mutants were lethal, we expressed RNAi in the pupal wing using the MS1096-GAL4 driver. Expressing RNAi against Bdbt caused variable lethality (see S1 Table) and insufficient pupal wings were obtained for western analysis. However, expression of RNAi against DAnkrd49 did not affect pupal wing development, and a clear decrease in cellular levels of Dsh on western blots was observed (Fig 5E and 5F).
Bdbt and DAnkrd49 regulate levels of Dsh phosphorylation
Bdbt is a non-canonical member of the FKBP family, which lacks the critical residues for PPIase activity [31]. However, FKBP family members can also function as molecular chaperones, assisting in protein folding independent of PPIase activity [37, 38]. One possibility therefore is that Bdbt and DAnkrd49 act as molecular chaperones, directly promoting Dsh stability. To investigate this, we expressed Myc-tagged Bdbt or DAnkrd49 in S2 cells, and attempted to co-immunoprecipitate Dsh-ECFP. No co-immunoprecipitation was seen (S5 Fig): furthermore no interaction was seen in the genome-wide interaction screen that found the Bdbt-DAnkrd49 interaction [33, 34]. Thus we cannot find evidence to support a chaperone model.
An alternative model is suggested by the fact that Bdbt physically interacts with Dco [31, 32], and that Dco is known to regulate Dsh phosphorylation in both canonical Wnt signalling and in planar polarity [23, 24, 39–41]. Dco can autophosphorylate its own C-terminus, and this autophosphorylation reduces its activity [42]. Deletion of the C-terminus of Dco increases Dco activity in in vitro assays, consistent with it being an inhibitory domain [42]. Binding of Bdbt to Dco is dependent on the C-terminus of Dco, and loss of Bdbt causes hyperphosphorylation of Dco and reduced Dco activity in vivo (Fig 2B) [31]. This leads to an attractive model in which Bdbt and DAnkrd49 regulate Dsh indirectly, through regulation of Dco activity. Consistent with this, we see a reduction of relative levels of Dsh phosphorylation in pupal wings expressing RNAi against DAnkrd49 (Fig 5G), phenocopying the reduced Dsh phosphorylation that was previously shown in dco hypomorphs [24].
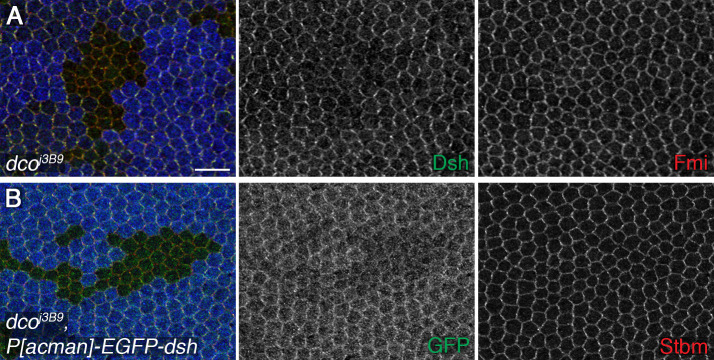
Previously we reported a loss of asymmetry in dco hypomorphic mutant clones [24], but we did not report any effect on overall Dsh levels. However, upon re-examination of dco mutant clones, we saw a reduction in levels of Dsh at junctions in mutant tissue, while no reduction in other core proteins such as Fmi was seen (Fig 6A). Furthermore, when P[acman]-EGFP-dsh was expressed in pupal wings, a reduction in both junctional and cytoplasmic levels of Dsh was seen in dco mutant tissue (Fig 6B), again phenocopying DAnkrd49 and Bdbt. Expression of dominant negative Dco has also been shown to reduce levels of P[acman]-EGFP-dsh [29].
Fig 6. Loss of Dco activity reduces Dsh levels.
(A) 28hr APF pupal wings carrying dco hypomorphic mutant clones of cells (dcoj3B9), marked by loss of β-gal (blue). Wings immunolabelled for Dsh (green) or Fmi (red). Scale bar 10 μm. (B) 28hr APF pupal wings carrying clones of cells lacking dco (dcoj3B9), in a background expressing one copy of P[acman]-EGFP-dsh. Wings immunolabelled for GFP (green), or Stbm (red), and clones marked by loss of β-gal (blue).
Bdbt and DAnkrd49 interact genetically with Dco
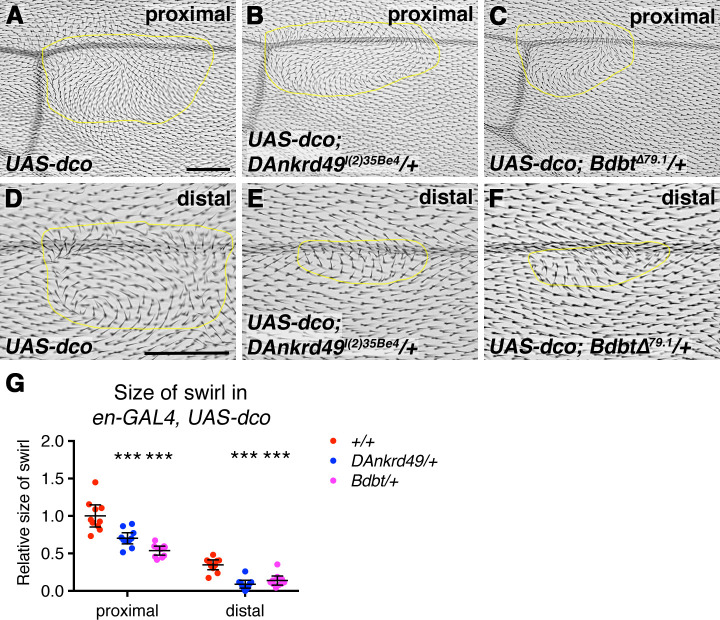
Overexpression of dco causes defects in trichome orientation in the adult wing [23, 24, 29, 41]. As DAnkrd49 and Bdbt are expected to promote Dco activity, to seek support for our model we investigated whether a reduction in their activity would suppress the dco overexpression phenotype. dco was overexpressed in the posterior compartment of the wing using the en-GAL4 driver, which reproducibly caused a large trichome swirl close to the posterior cross vein, and a smaller swirl in the distal region of the wing (Fig 7A and 7D). Interestingly, the size of the proximal swirl was reduced, and the distal swirl was almost entirely abolished in flies heterozygous for either DAnkrd49 or Bdbt (Fig 7B, 7C and 7E–7G).
Fig 7. Genetic interactions between dco, DAnkrd49 and Bdbt.
(A-F) Dorsal surface of adult wings from UAS-dco/+; en-GAL4/+ (A,D), UAS-dco/+; en-GAL4/DAnkrd49l(2)35Be4 (B,E) and UAS-dco/+; en-GAL4/+; BdbtΔ79.1/+ (C,F) female flies, raised at 25°C. (A-C) Images near the posterior cross vein. (D-F) Images around vein 4 near the distal tip of the wing. The yellow line marks the area of wing in which trichomes swirl. Scale bars 100 μm. (G) Quantitation of the area of wing in which trichomes swirl, in 10 wings of the genotypes from A-F. Samples were compared to UAS-dco/+; en-GAL4/+ using one-way ANOVA with Dunnett's multiple comparisons test, ***p≤0.001. Error bars are 95% confidence intervals. See S2 Table for numerical data.
Overall the genetic interactions between dco and DAnkrd49 and Bdbt support them acting in a common pathway. Coupled with the phenotypic similarities, we propose that DAnkrd49 and Bdbt regulate Dsh levels via modulating Dco activity.
Discussion
Asymmetric localisation of the core proteins depends on the tight regulation of protein dynamics and stability. Current models suggest that in order for core protein complexes to become aligned in the same direction, complexes must destabilise each other when they are in opposite orientations. In contrast when complexes are in the same orientation, they become more stable. Understanding how core protein stability is regulated is therefore of key importance.
Here we present evidence for a novel protein complex regulating planar polarity, consisting of the ankyrin repeat protein DAnkrd49 and the non-canonical FKBP family member Bdbt. DAnkrd49 and Bdbt physically interact in vitro and regulate each other's levels in vivo. This suggests that they may act in a complex in which each is required to stabilise the other, a model supported by the phenotypic similarity in mutant clones. We show that their activity is required for core protein asymmetry in the pupal wing, and for normal levels and phosphorylation of the cytoplasmic core protein Dsh.
Previous work on circadian rhythms has established a physical interaction between Bdbt and the kinase Dco, and that Dco activity is regulated by Bdbt [31]. Notably, Dco has previously been implicated in promoting both Dsh and Stbm phosphorylation in planar polarity signalling [23, 24, 26–29]. Thus, our data are consistent with both DAnkrd49 and Bdbt acting to regulate Dco activity in planar polarity. Firstly, loss of function clones of dco and DAnkrd49/Bdbt share a common phenotype: in their absence we see reduced overall levels of Dsh and reduced levels of Dsh phosphorylation (this work and [24]). Secondly, we see strong genetic interactions between dco and DAnkrd49/Bdbt. Although a physical interaction has been observed between Dco and Bdbt [31], we do not see a direct interaction between Dco and DAnkrd49. Therefore, a simple model is that the role of DAnkrd49 is to stabilise Bdbt, while Bdbt directly regulates Dco activity. Thus we define a regulatory cascade, whereby DAnkrd49 and Bdbt promote Dco activity, which in turn regulates phosphorylation of core proteins and asymmetric localisation.
An alternative model is that DAnkrd49 and Bdbt act to stabilise Dsh, independently of Dco. Proteins of the FKBP family are known to regulate the stability of target proteins [37, 38]. In canonical FKBP family members this stabilisation is a result of PPIase activity, which assists protein folding; whereas other family members stabilise their target proteins by direct binding [37]. The catalytic sites for PPIase are not conserved in Bdbt [31]. We were also unable to see a binding interaction between Dsh and either DAnkrd49 or Bdbt, arguing that Bdbt is unlikely to directly stabilise Dsh.
In addition to defects in planar polarity, we also see other pleiotropic defects in DAnkrd49 clones and Bdbt clones, including cell size defects, reduced proliferation and poor viability. These could plausibly be explained by regulation of Dco activity by DAnkrd49 and Bdbt. Dco acts in multiple signalling pathways. It phosphorylates the tumour suppressor Fat, which regulates cell growth and survival via the Hippo signalling pathway [43–47]. Interestingly, a hypomorphic mutation in dco causes tissue overgrowth and increased activity of the caspase inhibitor DIAP1, phenocopying loss of Hippo signalling pathway components [43, 44], while cells completely lacking Dco activity have reduced expression of the caspase inhibitor DIAP1 and reduced proliferation [45]. Dco may also act in additional signalling pathways, and these are thought to include Hedgehog signalling and canonical Wnt signalling [48]. Hence, the multitude of signalling pathways regulated by Dco may explain the complex phenotypes seen in the absence of DAnkrd49 and Bdbt. Alternatively, it is possible that DAnkrd49 and Bdbt may regulate other downstream targets in addition to Dco.
As Dco has also been implicated in phosphorylating Stbm [26–28], loss of DAnkrd49 and Bdbt is also expected to reduce Stbm phosphorylation in pupal wings. However we have not been able to verify this directly, as Stbm mobility in SDS-PAGE is only marginally increased when Dco activity is reduced [27, 29], and is not noticeably altered in extracts from animals with reduced activity of DAnkrd49 or Bdbt. Nevertheless, our recent work has shown that phosphorylation of both Stbm and Dsh by Dco is functionally important in establishing correct planar polarity [29], making the regulation of Dco activity of great interest.
Interestingly, protein interactions studies in human cell lines have identified a STRING network between Ankrd49, CKIδ/CKIε and FKBP family members [49–51]. Although the FKBP proteins identified in these studies are not the closest orthologues to Drosophila Bdbt, this nevertheless suggests conservation of a regulatory cascade of Ankrd49/FKBP promoting Dco activity, which in turn might phosphorylate core planar polarity proteins. Furthermore, the DISEASES online resource, which integrates results from text mining, manually curated disease-gene associations and genome-wide association studies, has linked Ankrd49 with brachydactyly subtypes (diseases.jensenlab.org) [52]. Brachydactyly is a key feature of Robinow syndrome, a disease closely associated with core planar polarity mutations [53]; thus understanding this regulatory cascade may be of importance for human health.
Materials and methods
Fly stocks and genetics
RNAi lines are from the Vienna Drosophila Resource Centre or the National Institute of Genetics in Japan. Transgenic fly lines were UAS-dco [54] and P[acman]-EGFP-dsh [55]. dsh-ECFP was cloned into an pArm-polyA [56], and DAnkrd49-EGFP was cloned in pActP-FRT-polyA-FRT [56], followed by P-element mediated insertion into flies. Bdbt-EGFP was cloned in pAttB-ActP-FRT-polyA-FRT [18] and then inserted into the attP2 landing site.
Mutant alleles are described in FlyBase. dcoj3B9 is a hypomorphic loss of function P-element insertion in the 5'UTR [57, 58]. l(2)35Be1 and l(2)35Be4 were isolated in an analysis of the Adh genomic region region [59] and subsequently found to carry mutations in CG4140/DAnkrd49 [35]. Both alleles were obtained from the Bloomington Drosophila Stock Centre, but l(2)35Be1 was not used as it was found to complement Df(2L)ED3, Df(2L)ED800 and Df(2L)BSC254 that uncover the l(2)35Be locus. l(2)35Be4 however failed to complement all three deficiencies as expected. The l(2)35Be4 chromosome carries additional mutations (full genotype is AdhUF l(2)35Be4 Pol32rd-s pr1 cn1). Sequencing of l(2)35Be4 revealed H87Y and A156T mutations within the DAnkrd49 coding region. Homologues were identified using HomoloGene (https://www.ncbi.nlm.nih.gov/homologene), and protein alignments were carried out using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). SIFT analysis on putative homologues was carried out to determine residues likely to be deleterious (http://sift.jcvi.org).
Null alleles of CG4140/DAnkrd49 and CG17282/Bdbt were made by deletion of the entire open reading frame by homologous recombination using the pRK2 vector (S2B Fig) [60]. 4 independent mutations of DAnkrd49 were obtained. All were homozygous lethal and lethal over DAnkrd49l(2)35Be4, Df(2L)ED3 and Df(2L)ED800. A single mutation in Bdbt was obtained: BdbtΔ79.1 was homozygous lethal, and failed to complement Df(3R)BSC508 and Df(3R)ED10845 that uncover the Bdbt locus. The phenotypes of Ankrd49 and Bdbt null mutations were rescued by transgenes expressing the appropriate gene.
Mitotic clones were induced using the FLP/FRT system and Ubx-FLP. Overexpression of UAS lines or RNAi lines used the GAL4/UAS system with MS1096-GAL4 [61], ptc-GAL4 [62] or en-GAL4 [63].
Immunostaining and imaging
Adult wings were dehydrated in isopropanol and mounted in GMM (50% methyl salicylate, 50% Canada Balsam).
Unless otherwise indicated, pupal wings were dissected at 28 hr after puparium formation (APF) at 25°C, as previously described [56]. Primary antibodies for immunostaining were mouse monoclonal anti-Fmi (Flamingo #74, DSHB) [64], rat anti-Dsh [24], affinity purified rabbit anti-Fz [65], rabbit anti-Stbm [15], affinity purified rat anti-Pk [18], affinity purified rabbit anti-GFP (ab6556, Abcam), mouse monoclonal anti-β gal (DSHB), rabbit anti-β gal (#55976, Cappel), rat monoclonal anti-Ecad (DSHB) and mouse monoclonal anti-Arm N2 7A1 (DSHB). Actin was labelled with Alexa568-conjugated Phalloidin (A12380, Molecular Probes).
Pupal wings were imaged on a Nikon A1R GaAsP confocal microscope using a 60x NA1.4 apochromatic lens. 9 Z slices separated by 150 nm were imaged, and then the 3 brightest slices around cell junctions were selected and averaged for each channel in ImageJ. Membrane masks were generated in Packing Analyzer [66]. Polarity magnitude (maximum asymmetry ratio on a cell-by-cell basis) and the variation in polarity angle were calculated as previously described [55]. Values were compared using one-way ANOVA with Dunnett's multiple comparisons test, or using paired t-tests for clones, comparing control and mutant regions of the same wings.
Biochemistry
For pupal wing westerns, 28 hr APF pupal wings were dissected directly into sample buffer. One pupal wing equivalent was used per lane. Westerns were probed with affinity purified rabbit anti-Dsh [24] and Actin AC-40 mouse monoclonal (A4700, Sigma). Detection was using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific). A BioRad ChemiDoc XRS+ was used for imaging, and band intensities from six biological replicates were quantified using ImageJ. Data were compared used unpaired t-tests.
For immunoprecipitations, cDNAs were tagged with EGFP or Myc, and cloned into the pAc5.1-V5/His vector (Invitrogen). Immunoprecipitations between Bdbt-EGFP and Myc-DAnkrd49 used GFP rabbit serum (ab290, Abcam), in 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% TritonX-100, 1x protease inhibitor cocktail (#11697498001, Roche). Immunoprecipitations between Myc-DAnkrd49 or Myc-Bdt and Dsh-ECFP used Myc antibody resin (ab1253, Abcam), in RIPA buffer (50 mM Tris-HCl pH 7.5, 100 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1x protease inhibitor cocktail). Westerns were probed with mouse monoclonal anti-GFP JL8 (#632381, Clontech) or mouse monoclonal anti-Myc 9E10 (DSHB).
Supporting information
(A-C) Dorsal surface of adult male wings from control wild-type flies (A), or from flies expressing RNAi against CG4140/DAnkrd49 (B, NIG line 4140R-2), or CG17282/Bdbt (C, NIG line 17282R-2), under control of the ptc-GAL4 driver at 25°C, in the presence of UAS-Dcr2. Scale bar 100 μm. (D-F) Pupal wings expressing a control RNAi (D, VDRC line 39864, targeting Sik1, a gene unrelated to planar polarity), or RNAi against CG4140/DAnkrd49 (E, NIG line 4140R-3) or CG17282/Bdbt (F, NIG line 17282R-2), under control of the ptc-GAL4 driver (as in Fig 1E–1G),. Wings immunolabelled for Fmi. The yellow bar shows the ptc-GAL4 expression domain. The polarity nematic for each cell is shown as red lines. Polarity magnitude (length of red line) is quantitated in the ptc-GAL4 expression domain, and is reduced in wings expressing RNAi against DAnkrd49 and Bdbt (E,F) compared to control wings (D). The polarity angle is also more regular in wild-type wings.
(TIF)
(A) Clustal alignment of DAnkrd49 with homologues. Asterisks (*) indicate conserved residues (also in red), colons (:) indicates conservation between groups of strongly similar properties, and full stop (.) indicates conservation between groups of weakly similar properties. Blue shading indicates the position of the ankyrin repeats, and blue arrows point to residues mutated in DAnkrd49l(2)35Be. (B) Diagram showing the open reading frames (blue) in the genomic regions surrounding DAnkrd49 (top) and Bdbt (bottom). Homology arms corresponding to approximately 3 kb on either side of the target gene were inserted into the pRK2 vector on either side of a white gene flanked by LoxP sites (green). Recombination between the pRK2 transgene and the genomic DNA (red dashed lines) results in the target gene being exchanged for white (Δ in red). Regions of the homology arms encoding open reading frames in pRK2 were sequenced, to ensure no additional mutations were introduced.
(TIF)
(A-C) 28hr APF pupal wings carrying clones of cells lacking DAnkrd49 (A, l(2)35Be4) or Bdbt (B and C, BdbtΔ79.1), marked by loss of β-gal (green). Wings immunolabelled for Stbm (red) and Arm (blue). (A,B) Arm staining is similar to wild-type in DAnkrd49 clones and some Bdbt clones. (C) In other Bdbt clones cells are abnormal, as seen by disrupted Arm localisation. Scale bar 10 μm. (D-F) 28hr APF pupal wings carrying clones of cells lacking DAnkrd49 (DAnkrd49ΔD3.1), marked by loss of β-gal (red in D and E or blue in F). Wings immunolabelled for Fmi (green in D and F), Dsh (green in E) and Phalloidin (red in F).
(TIF)
(A-D) 28hr APF pupal wings carrying clones of cells lacking DAnkrd49 (A,C, DAnkrd49l(2)35Be4), or Bdbt (B,D, BdbtΔ79.1) marked by loss of β-gal (red). Wings immunolabelled for Fz (green in A,B), or Pk (green in C,D). Scale bar 10 μm. (E,F) 28hr APF pupal wings carrying clones of cells lacking DAnkrd49 (E, DAnkrd49l(2)35Be4), or Bdbt (F, BdbtΔ79.1), in a background expressing one copy of Arm-dsh-ECFP. Wings immunolabelled for GFP (green), clones marked by loss of β-gal (red). (G,H) 28hr APF pupal wings carrying clones of cells lacking DAnkrd49 (DAnkrd49l(2)35Be4) in a background expressing ActP-DAnkrd49-EGFP (G), or clones of cells lacking Bdbt (BdbtΔ79.1) in a background expressing ActP-Bdbt-EGFP (H). Wings immunolabelled for GFP (green) and Dsh (red). Clones marked by loss of β-gal (blue). Dsh levels within the clone are similar to wild type in the presence of DAnkrd49-EGFP or Bdbt-EGFP (compare Dsh labelling inside and outside the clone tissue).
(TIF)
(A) Western blot showing co-immunoprecipitation experiment. S2 cells transfected with Dsh-ECFP and either Myc-DAnkrd49 or Myc-Bdbt. Immunoprecipitation with Myc antibody resin did not pull down Dsh-ECFP.
(TIF)
Lines targeting CG4140 are 4140R-2 and 4140R-3, from the NIG RNAi collection, and 26396 and 109913 which are GD and KK lines respectively, from the VDRC. 4140R-2 and 4140R-3 are two insertions of the same target sequence, and target sequences overlap with lines 26396 and 109913. Lines targeting CG17282 are 17282R-2 and 17282R-3 from the NIG RNAi collection, and GD line 40059 from the VDRC. 17282R-2 and 17282R-3 are two insertions of the same target sequence, and target sequences overlap with line 40059.
(DOCX)
Acknowledgments
We thank Mike Young, the Bloomington Drosophila Stock Center, Vienna Drosophila Resource Center and the National Institute of Genetics Japan for fly stocks, and the Developmental Studies Hybridoma Bank for antibodies. cDNAs were provided by the Drosophila Genomics Resource Centre and P[acman] constructs by BacPac Resources. BestGene and Genetivision are thanked for making transgenics, Rosalind Hale for making the Bdbt mutant and Victoria Thomas-MacArthur for excellent support for the RNAi screen. Amy Brittle, Simon Fellgett and Katie Fisher are thanked for comments on the manuscript, and the fly room staff for technical support. Imaging was performed in the Wolfson Light Microscopy Facility.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The work was funded by Wellcome Trust Senior Fellowship awards (https://wellcome.ac.uk, 084469/Z/07/Z, 100986/Z/13/Z and 210630/Z/18/Z) to D.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011; 138:1877–1892. 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014; 207:171–179. 10.1083/jcb.201408039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MT, Wallingford JB. Planar cell polarity in development and disease. Nat Rev Mol Cell Biol. 2017; 18:375–388. 10.1038/nrm.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davey CF, Moens CB. Planar cell polarity in moving cells: think globally, act locally. Development. 2017; 144:187–200. 10.1242/dev.122804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hale R, Strutt D. Conservation of planar polarity pathway function across the animal kingdom. Annu Rev Genet. 2015; 49:529–551. 10.1146/annurev-genet-112414-055224 [DOI] [PubMed] [Google Scholar]
- 6.Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol. 1993; 123:209–221. 10.1083/jcb.123.1.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aw WY, Devenport D. Planar cell polarity: global inputs establishing cellular asymmetry. Curr Opin Cell Biol. 2016; 44:110–116. 10.1016/j.ceb.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005; 307:423–426. 10.1126/science.1105471 [DOI] [PubMed] [Google Scholar]
- 9.Le Garrec JF, Lopez P, Kerszberg M. Establishment and maintenance of planar epithelial cell polarity by asymmetric cadherin bridges: a computer model. Dev Dyn. 2006; 235:235–246. 10.1002/dvdy.20617 [DOI] [PubMed] [Google Scholar]
- 10.Burak Y, Shraiman BI. Order and stochastic dynamics in Drosophila planar cell polarity. PLoS Comput Biol. 2009; 5:e1000628 10.1371/journal.pcbi.1000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schamberg S, Houston P, Monk NA, Owen MR. Modelling and analysis of planar cell polarity. Bull Math Biol. 2010; 72:645–680. 10.1007/s11538-009-9464-0 [DOI] [PubMed] [Google Scholar]
- 12.Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002; 109:371–381. 10.1016/s0092-8674(02)00715-8 [DOI] [PubMed] [Google Scholar]
- 13.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003; 130:4037–4046. 10.1242/dev.00567 [DOI] [PubMed] [Google Scholar]
- 14.Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005; 7:691–697. 10.1038/ncb1271 [DOI] [PubMed] [Google Scholar]
- 15.Warrington SJ, Strutt H, Fisher KH, Strutt D. A dual function for Prickle in regulating Frizzled stability during feedback-dependent amplification of planar polarity. Curr Biol. 2017; 27:2784–2797. 10.1016/j.cub.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strutt H, Searle E, Thomas-Macarthur V, Brookfield R, Strutt D. A Cul-3-BTB ubiquitylation pathway regulates junctional levels and asymmetry of core planar polarity proteins. Development. 2013; 140:1693–1702. 10.1242/dev.089656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003; 130:3007–3014. 10.1242/dev.00526 [DOI] [PubMed] [Google Scholar]
- 18.Strutt H, Thomas-MacArthur V, Strutt D. Strabismus promotes recruitment and degradation of farnesylated Prickle in Drosophila melanogaster planar polarity specification. PLoS Genet. 2013; 9:e1003654 10.1371/journal.pgen.1003654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho B, Pierre-Louis G, Sagner A, Eaton S, Axelrod JD. Clustering and negative feedback by endocytosis in planar cell polarity signaling is modulated by ubiquitinylation of prickle. PLoS Genet. 2015; 11:e1005259 10.1371/journal.pgen.1005259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009; 137:295–307. 10.1016/j.cell.2009.02.025 [DOI] [PubMed] [Google Scholar]
- 21.Butler MT, Wallingford JB. Control of vertebrate core planar cell polarity protein localization and dynamics by Prickle 2. Development. 2015; 142:3429–3439. 10.1242/dev.121384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djiane A, S. Y, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005; 121:621–631. 10.1016/j.cell.2005.03.014 [DOI] [PubMed] [Google Scholar]
- 23.Klein TJ, Jenny A, Djiane A, Mlodzik M. CKIepsilon/discs overgrown promotes both Wnt-Fz/beta-catenin and Fz/PCP signaling in Drosophila. Curr Biol. 2006; 16:1337–1343. 10.1016/j.cub.2006.06.030 [DOI] [PubMed] [Google Scholar]
- 24.Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Curr Biol. 2006; 16:1329–1336. 10.1016/j.cub.2006.04.041 [DOI] [PubMed] [Google Scholar]
- 25.Singh J, Yanfeng WA, Grumolato L, Aaronson SA, Mlodzik M. Abelson family kinases regulate Frizzled planar cell polarity signaling via Dsh phosphorylation. Genes Dev. 2010; 24:2157–2168. 10.1101/gad.1961010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011; 20:163–176. 10.1016/j.devcel.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly LK, Wu J, Yanfeng WA, Mlodzik M. Frizzled-induced Van Gogh phosphorylation by CK1epsilon promotes asymmetric localization of core PCP factors in Drosophila. Cell Rep. 2016; 16:344–356. 10.1016/j.celrep.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang W, Garrett L, Feng D, Elliott G, Liu X, Wang N, et al. Wnt-induced Vangl2 phosphorylation is dose-dependently required for planar cell polarity in mammalian development. Cell Res. 2017; 27:1466–1484. 10.1038/cr.2017.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strutt H, Gamage J, Strutt D. Reciprocal action of Casein Kinase I epsilon on core planar polarity proteins regulates clustering and asymmetric localisation. Elife. 2019; 8:e45107 10.7554/eLife.45107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas C, Strutt D. Rabaptin-5 and Rabex-5 are neoplastic tumour suppressor genes that interact to modulate Rab5 dynamics in Drosophila melanogaster. Dev Biol. 2014; 385:107–121. 10.1016/j.ydbio.2013.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan JY, Agyekum B, Venkatesan A, Hall DR, Keightley A, Bjes ES, et al. Noncanonical FK506-binding protein BDBT binds DBT to enhance its circadian function and forms foci at night. Neuron. 2013; 80:984–996. 10.1016/j.neuron.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatesan A, Fan JY, Nauman C, Price JL. A Doubletime Nuclear Localization Signal Mediates an Interaction with Bride of Doubletime to Promote Circadian Function. J Biol Rhythms. 2015; 30:302–317. 10.1177/0748730415588189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mintseris J, Obar RA, Celniker S, Gygi SP, VijayRaghavan K, Artavanis-Tsakonas S. DPiM: the Drosophila Protein Interaction Mapping project Personal communication to FlyBase. 2009:FBrf0209452. [Google Scholar]
- 34.Guruharsha KG, Rual JF, Zhai B, Mintseris J, Vaidya P, Vaidya N, et al. A protein complex network of Drosophila melanogaster. Cell. 2011; 147:690–703. 10.1016/j.cell.2011.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huen D, Roote J. Personal communication to FlyBase. 2010:FBrf0210708. [Google Scholar]
- 36.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001; 11:863–874. 10.1101/gr.176601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang CB, Hong Y, Dhe-Paganon S, Yoon HS. FKBP family proteins: immunophilins with versatile biological functions. Neurosignals. 2008; 16:318–325. 10.1159/000123041 [DOI] [PubMed] [Google Scholar]
- 38.Rein T. FK506 binding protein 51 integrates pathways of adaptation: FKBP51 shapes the reactivity to environmental change. Bioessays. 2016; 38:894–902. 10.1002/bies.201600050 [DOI] [PubMed] [Google Scholar]
- 39.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999; 401:345–350. 10.1038/43830 [DOI] [PubMed] [Google Scholar]
- 40.McKay RM, Peters JM, Graff JM. The casein kinase I family in Wnt signaling. Dev Biol. 2001; 235:388–396. 10.1006/dbio.2001.0308 [DOI] [PubMed] [Google Scholar]
- 41.Cong F, Schweizer L, Varmus H. Casein kinase Iε modulates the signaling specificities of Dishevelled. Mol Cell Biol. 2004; 24:2000–2011. 10.1128/mcb.24.5.2000-2011.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan JY, Preuss F, Muskus MJ, Bjes ES, Price JL. Drosophila and vertebrate casein kinase Idelta exhibits evolutionary conservation of circadian function. Genetics. 2009; 181:139–152. 10.1534/genetics.108.094805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zilian O, Burke R, Brentrup D, Gutjahr T, Bryant P, Noll M. double-time is identical to discs overgrown, which is required for cell survival, proliferation and growth arrest in Drosophila imaginal discs. Development. 1999; 126:5409–5420. [DOI] [PubMed] [Google Scholar]
- 44.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006; 38:1142–1150. 10.1038/ng1887 [DOI] [PubMed] [Google Scholar]
- 45.Guan J, Li H, Rogulja A, Axelrod JD, Cadigan KM. The Drosophila casein kinase Iepsilon/delta Discs Overgrown promotes cell survival via activation of DIAP1 expression. Dev Biol. 2007; 303:16–28. 10.1016/j.ydbio.2006.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng Y, Irvine K. Processing and phosphorylation of the Fat receptor. Proc Natl Acad Sci USA. 2009; 106:11989–11994. 10.1073/pnas.0811540106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sopko R, Silva E, Clayton L, Gardano L, Barrios-Rodiles M, Wrana J, et al. Phosphorylation of the tumor suppressor Fat Is regulated by Its ligand Dachsous and the kinase Discs Overgrown. Curr Biol. 2009; 19:1112–1117. 10.1016/j.cub.2009.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price MA. CKI, there's more than one: casein kinase I family members in Wnt/Wingless and Hedgehog signaling. Genes Dev. 2006; 20:399–410. 10.1101/gad.1394306 [DOI] [PubMed] [Google Scholar]
- 49.Kategaya LS, Hilliard A, Zhang L, Asara JM, Ptacek LJ, Fu YH. Casein kinase 1 proteomics reveal prohibitin 2 function in molecular clock. PLoS One. 2012; 7:e31987 10.1371/journal.pone.0031987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taipale M, Tucker G, Peng J, Krykbaeva I, Lin ZY, Larsen B, et al. A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell. 2014; 158:434–448. 10.1016/j.cell.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, et al. Architecture of the human interactome defines protein communities and disease networks. Nature. 2017; 545:505–509. 10.1038/nature22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pletscher-Frankild S, Palleja A, Tsafou K, Binder JX, Jensen LJ. DISEASES: text mining and data integration of disease-gene associations. Methods. 2015; 74:83–89. 10.1016/j.ymeth.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 53.Gao B, Yang Y. Planar cell polarity in vertebrate limb morphogenesis. Curr Opin Genet Dev. 2013; 23:438–444. 10.1016/j.gde.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sekine T, Yamaguchi T, Hamano K, Young MW, Shimoda M, Saez L. Casein kinase I epsilon does not rescue double-time function in Drosophila despite evolutionarily conserved roles in the circadian clock. J Biol Rhythms. 2008; 23:3–15. 10.1177/0748730407311652 [DOI] [PubMed] [Google Scholar]
- 55.Strutt H, Gamage J, Strutt D. Robust asymmetric localization of planar polarity proteins is associated with organization into signalosome-like domains of variable stoichiometry. Cell Rep. 2016; 17:2660–2671. 10.1016/j.celrep.2016.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strutt DI. Asymmetric localization of Frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001; 7:367–375. 10.1016/s1097-2765(01)00184-8 [DOI] [PubMed] [Google Scholar]
- 57.Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, Wesley CS, et al. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell. 1998; 94:97–107. 10.1016/s0092-8674(00)81225-8 [DOI] [PubMed] [Google Scholar]
- 58.Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, Young MW. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998; 94:83–95. 10.1016/s0092-8674(00)81224-6 [DOI] [PubMed] [Google Scholar]
- 59.O'Donnell J, Mandel HC, Krauss M, Sofer W. Genetic and cytogenetic analysis of the Adh region in Drosophila melanogaster. Genetics. 1977; 86:553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J, Zhou W, Watson AM, Jan YN, Hong Y. Efficient ends-out gene targeting in Drosophila. Genetics. 2008; 180:703–707. 10.1534/genetics.108.090563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capdevila J, Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 1994; 13:4459–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hinz U, Giebel B, Campos-Ortega JA. The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell. 1994; 76:77–87. 10.1016/0092-8674(94)90174-0 [DOI] [PubMed] [Google Scholar]
- 63.Johnson RL, Grenier JK, Scott MP. patched overexpression alters wing disc size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development. 1995; 121:4161–4170. [DOI] [PubMed] [Google Scholar]
- 64.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999; 98:585–595. 10.1016/s0092-8674(00)80046-x [DOI] [PubMed] [Google Scholar]
- 65.Bastock R, Strutt D. The planar polarity pathway promotes coordinated cell migration during Drosophila oogenesis. Development. 2007; 134:3055–3064. 10.1242/dev.010447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aigouy B, Farhadifar R, Staple DB, Sagner A, Röper J-C, Julicher F, et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010; 142:773–786. 10.1016/j.cell.2010.07.042 [DOI] [PubMed] [Google Scholar]