Abstract
Background
Potassium disturbances are associated with adverse prognosis in patients with chronic conditions. Its prognostic implications in stable patients attending the emergency department (ED) is poorly described.
Aims
This study aimed to assess the prevalence of dyskalemia, describe its predisposing factors and prognostic associations in a population presenting the ED without unstable medical illness.
Methods
Post-hoc analysis of a prospective, cross-sectional, multicenter study in the ED of 11 French academic hospitals over a period of 8 weeks. All adults presenting to the ED during this period were included, except instances of self-drug poisoning, inability to complete self-medication questionnaire, presence of an unstable medical illness and decline to participate in the study. All-cause hospitalization or deaths were assessed.
Results
A total of 1242 patients were included. The mean age was 57.2±22.3 years, 51% were female. The distribution according to potassium concentrations was: hypokalemia<4mmol/L(n = 620, 49.9%), normokalemia 4-5mmol/L(n = 549, 44.2%) and hyperkalemia >5mmol/L(n = 73, 0,6%). The proportion of patients with a kalemia<3.5mmol/L was 8% (n = 101). Renal insufficiency (OR [95% CI] = 3.56[1.94–6.52], p-value <0.001) and hemoglobin <12g/dl (OR [95% CI] = 2.62[1.50–4.60], p-value = 0.001) were associated with hyperkalemia. Female sex (OR [95% CI] = 1.31[1.03–1.66], p-value = 0.029), age <45years (OR [95% CI] = 1.69 [1.20–2.37], p-value = 0.002) and the use of thiazide diuretics (OR [95% CI] = 2.04 [1.28–3.32], p-value = 0.003), were associated with hypokalemia<4mmol/l. Two patients died in the ED and 629 (52.7%) were hospitalized. Hypokalemia <3.5mmol/L was independently associated with increased odds of hospitalization or death (OR [95% CI] = 1.47 [1.00–2.15], p-value = 0.048).
Conclusions
Hypokalemia is frequently found in the ED and was associated with worse outcomes in a low-risk ED population.
Introduction
Potassium disturbances are common and have been associated with increased mortality in several populations, namely in those with diabetes [1–3], chronic kidney disease (CKD) [2–7], myocardial infarction (MI) [8, 9], hypertension [9, 10] and heart failure (HF) [2, 3, 6, 9, 11–14]. In these populations, potassium levels have been associated with outcomes in a U-shaped manner, where both hypo- and hyperkalemia portend worse prognosis.
Most studies in the ED have been performed in specific populations, including CKD [15], acute MI [8, 16] and HF [12] or subsequently admitted to critical care [17], or not centered on potassium levels [18, 19] or otherwise descriptive of severe hyperkalemia [20–23]. Among these, only one study was conducted prospectively [12]. Patients included in these previous studies thus represent a high-risk subset, and not the general population presenting at the ED except for one retrospective and monocentric study [21]. On the other hand, potassium disturbances and its clinical implications have been less described among people from the general population presenting at the emergency department (ED [5, 6, 8, 17, 18, 24, 25].
The present study aims to assess the prevalence of dyskalemia in the general population presenting at the ED without unstable medical conditions. Moreover, we aim to describe the factors and the prognostic implications of potassium disturbances.
Materials and methods
The present study is a post-hoc analysis of the Adverse Drug Events and Self-medication in Emergency Departments (ADES-ED) cohort [26], which aimed at determining the frequency and severity of adverse drug reactions (ADR) related to self-medication (ADR-SM) among patients attending the ED, and also to describe their main characteristics.
The ADES-ED study was a prospective, cross-sectional, observational study conducted over a period of 8 weeks (1 March to 20 April 2010), in the ED of 11 French academic hospitals. The centers were: CHU of Clermont-Ferrand, CHU of Caen, CHU of Toulouse, CHU of Nantes, CHU of Paris-Hôtel-Dieu Hospital, CHU of Rennes, CHU of Paris-Saint Antoine Hospital, CHU of Paris-Mondor Hospital, CHU of Grenoble, CHU of Paris-Cochin Hospital, and CHU of Angers.
Recruitment method in the ADES-ED cohort
The source population was all subjects 18 years of age and older who were likely to be admitted to a participating University Hospital emergency department during the study period.
The study population was identified by a pair of students (hospital medical students and fifth year hospital-university pharmacy students) trained in patient selection and data collection, with the help of the Nurse Organizer of the Reception Centre.
A high volume of visits in participating EDs precluded an uninterrupted prospective screening for inclusion throughout the study period. Therefore, we included patients presenting during different time-periods that were randomly selected (at each center level) by a central computer, thereby limiting selection bias associated with ED circadian cycles [26].
Criteria for inclusion:
All adult patients 18 years of age and older admitted to one of the 11 investigative centers during one of the collection periods.
Exclusion criteria:
deliberate drug intoxication;
patient refusal to participate in the study;
patient’s inability to give consent.
ADES-ED study design
Patient demographics were collected from administrative data of each participating center over the same 8-week enrolment period. These data were compared with the overall study population to verify the representativeness of the studied ED population [26]. All adult patients presenting to the ED during one of the randomly assigned time slots were eligible for study participation.
All patients entering the study signed an informed consent form.
Self-medication and medical history were assessed by a standardized questionnaire. This questionnaire was developed and validated by Roulet et al. [27]. The questionnaire contained a set of 20 closed-ended questions exploring all indications and dimensions of self-medication, and data regarding medical history.
Post-hoc study
Only patients with available potassium measurements were included in the present study.
Hypokalemia was defined according to two independently explored thresholds (<4 and <3.5mmol/L) [10], while hyperkalemia was defined as a plasma potassium >5mmol/L [28]. We chose to use two thresholds because most authors define a potassium level <4mmol/L as hypokalemia [29], but potassium levels <3.5mmol/L may have a stronger prognostic association3 and were considered by others [30]. A normal potassium range was considered when potassium levels were between 4 and 5mmol/L or 3.5 to 5mmol/L depending on the threshold considered. CKD was defined as eGFR <60 ml/min/1.73 m2. The primary study endpoint was all-cause hospitalization or death.
The study protocol and patient informed consent procedures were approved by the Ethics Committee (St. Etienne CHU on February 10 2008, ref:N/A) and by the Committee on Information in Health Research (CCTIRS ref:08.369/CNIL ref:AT/YPA/SV/SN/GDP/EM/AR081393) according to French regulations in clinical research.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) if normally distributed or as median and interquartile range if the distribution was skewed. Categorical variables are expressed as frequencies and proportions (%). Association between patient characteristics and dyskalemia was assessed using multinomial logistic regression including all variables with a p value < 0.1 in Table 1 followed by a stepwise backward procedure for retaining the variables selected at a p value < 0.05. Associations with the in-ED combined outcome (hospitalization and/or death) were studied with logistic regression models, using potassium as independent variable and adjusting for age, gender, estimated glomerular filtration rate, hemoglobin and thiazide diuretics, found to be associated with potassium levels in the previous model. We adjusted on age, gender, estimated glomerular filtration rate, hemoglobin and Thiazide: patients with K>5 were indeed older, had lower eGFR and lower hemoglobin (Table 1), all of which being associated with higher risk for death.
Table 1. Characteristics of the study population overall and by potassium level categories (mmol/L).
| Overall | By potassium category | ||||
|---|---|---|---|---|---|
| Potassium concentrations (mmol/L) | <4.0 | 4.0–5.0 | >5.0 | ||
| N | 1242 | 620 | 549 | 73 | |
| Variables | p-value | ||||
| Age (years) | 57.2 ± 22.3 | 53.2±22.0 | 59.5±22.1 | 72.3±16.1 | <0.001 |
| Female gender | 634 | 342 (55.2%) | 263 (47.9%) | 29 (39.7%) | 0.006 |
| SBP (mmHg) | 139.2 ± 25.4 | 138±25 | 140±24 | 140±32 | 0.30 |
| DBP (mmHg) | 77.7 ± 15.3 | 78±15 | 77±14 | 72±16 | 0.011 |
| Heart rate (bpm) | 85.7 ± 19.4 | 85±18 | 86±19 | 86±21 | 0.72 |
| Diabetes | 132 (10.6%) | 7 (1.1%) | 10 (1.8%) | 2 (2.7%) | 0.43 |
| Hypertension | 436 (35.1%) | 189 (30.5%) | 202 (36.8%) | 45 (61.6%) | <0.001 |
| Heart failure | 120 (9.7%) | 8 (1.3%) | 10 (1.8%) | 2 (2.7%) | 0.56 |
| Atrial fibrillation | 90 (7.9%) | 7 (1.1%) | 9 (1.6%) | 0 (0.0%) | 0.45 |
| Alcoolism | 42 (3.7%) | 45 (7.9%) | 38 (7.5%) | 7 (11.1%) | 0.60 |
| eGFR (ml/min/1.73m2) | 92.4 ± 56.6 | 90±28 | 82±30 | 53±33 | <0.001 |
| eGFR <60 ml/min/1.73m2 | 223 (18.0%) | 53 (10.6%) | 116 (18.1%) | 42 (59.2%) | <0.001 |
| Glucose (mmol/L) | 6.4 ± 2.4 | 6.2±2.3 | 6.5±2.5 | 6.6±2.3 | 0.18 |
| Na+ (mmol/L) | 138.9 ± 4.2 | 139±3 | 138±4 | 137±6 | 0.002 |
| K+ (mmol/L) | 4.1 ± 0.6 | 3.7±0.2 | 4.3±0.2 | 5.5±0.6 | <0.001 |
| Hemoglobin (g/dL) | 13.3 ± 2.2 | 13±1 | 13±2 | 11±2 | <0.001 |
| NSAIDs | 168(13.5%) | 87 (14.0%) | 75 (13.7%) | 6 (8.2%) | 0.39 |
| MRAs | 41 (3.3%) | 12 (1.9%) | 21 (3.8%) | 8 (11.0%) | <0.001 |
| ACEi/ARB | 312(25.1%) | 122 (19.7%) | 152 (27.7%) | 38 (52.1%) | <0.001 |
| Beta-blockers | 217(17.5%) | 83 (13.4%) | 112 (20.4%) | 22 (30.1%) | <0.001 |
| Corticosteroids | 76 (6.1%) | 30 (4.8%) | 36 (6.6%) | 10 (13.7%) | 0.010 |
| CCBs | 143(11.5%) | 67 (10.8%) | 59 (10.7%) | 17 (23.3%) | 0.005 |
| Insulin | 73 (5.9%) | 28 (4.5%) | 33 (6.0%) | 12 (16.4%) | <0.001 |
| Loop-diuretic | 155 (12.5%) | 58 (9.4%) | 79 (14.4%) | 18 (24.7%) | <0.001 |
| Thiazide-diuretic | 115 (9.3%) | 62 (10.0%) | 42 (7.7%) | 11 (15.1%) | 0.081 |
| K binders | 11 (0.9%) | 3 (0.5%) | 5 (0.9%) | 3 (4.1%) | 0.007 |
| K supplements | 67 (5.4%) | 32 (5.2%) | 26 (4.7%) | 9 (12.3%) | 0.025 |
| Deaths at emergency | 2 (0.2%) | 0 (0.0%) | 2 (0.4%) | 0 (0.0%) | 0.28 |
| Hospitalization in ward | 629 (52.7%) | 300 (49.8%) | 288 (55.1%) | 41 (59.4%) | 0.11 |
| Hospital critical care | 16 (1.3%) | 5 (0.8%) | 9 (1.7%) | 2 (2.9%) | 0.22 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; RR, respiratory rate; ACEi/ARBs, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; NSAIDs, nonsteroidal anti-inflammatory drugs; eGFR, estimated glomerular filtration rate (CKD-EPI formula), MRAs, mineralocorticoid receptor antagonists; CCBs, Calcium channel blockers.
Continuous variables were categorized to attain log-linearity. Odds-ratios are presented with their 95% confidence intervals as OR (CI 95%).
All analyses were performed using R® software.
Results and discussion
Characteristics of the study population
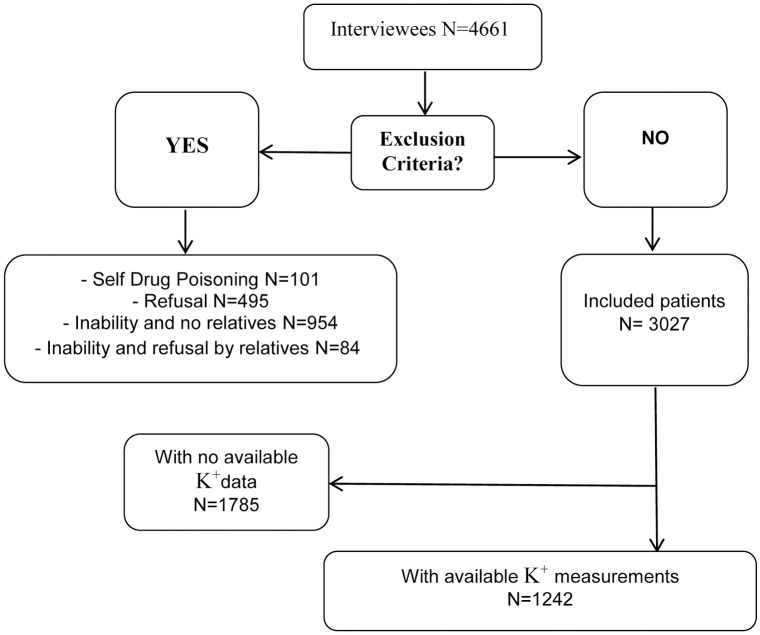
Overall, 3017 patients were included in the main study, while 1242 patients with available potassium measurements were included in the present sub-study (Fig 1). The chief complaint was abdominal pain in approximately one-quarter of the patients, with the other most frequent complaints being cardiovascular diseases, trauma and weakness.
Fig 1. Flowchart.
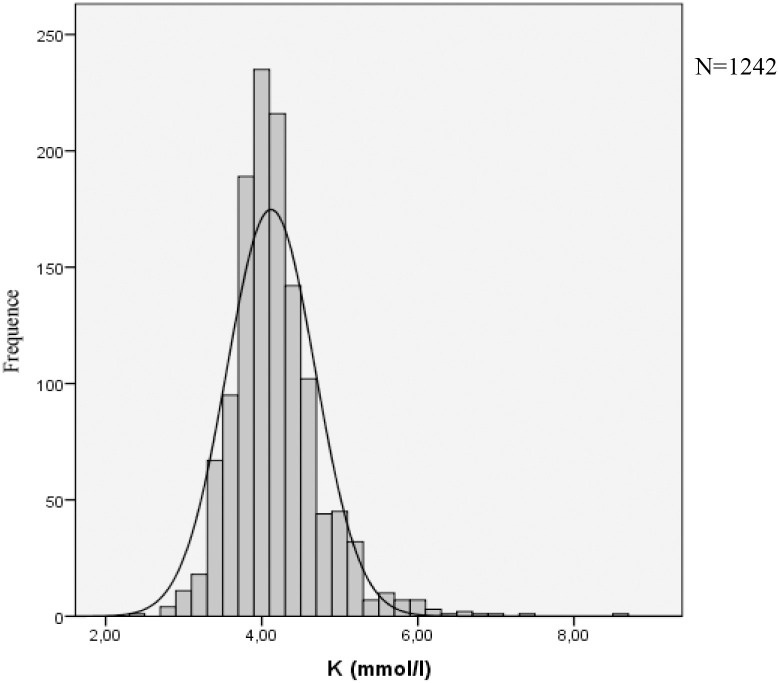
Patient characteristics are presented in Table 1. The mean age was 57.2±22.3 years. Approximately one third of the study population presented with a history of hypertension, 11% had diabetes mellitus, and 10% had a history of HF. The mean estimated glomerular filtration rate (eGFR) was 92±57 ml/min/1.73m2 (n = 223, 18% had an eGFR <60 ml/min/1.73 m2). A minority of patients had potassium levels above 5mmol/L (n = 73; 6%). Of note, very few patients had a potassium level above 5.5mmol/L (n = 20; 2%), or above 6mmol/L (n = 1; 0.1%). In contrast, hypokalemia below 4mmol/L was highly prevalent (n = 620; 50%), while fewer patients displayed hypokalemia below 3.5mmol/L (n = 101; 8%).
Potassium distribution is presented in Fig 2. Patients with hypokalemia were younger and more often female, whereas patients with hyperkalemia had a lower eGFR and hemoglobin (compared with patients with normokalemia).
Fig 2. Potassium distribution.
Patients treated with angiotensin-converting enzyme (ACEi) and angiotensin receptor blockers (ARB), mineralocorticoid receptor antagonists (MRA), calcium channel blockers (CCB), loop diuretics, potassium supplements and insulin had a higher hyperkalemia rate (Table 1).
Factors associated with hypokalemia
In multivariate analyses, female gender (OR [95%CI] = 1.31 [1.03–1.66], p-value = 0.029), younger age (age <45 years: OR [95% CI] = 1.69 [1.20–2.37], p-value = 0.002) and thiazide diuretic use (OR [95% CI] = 2.04 [1.28–3.32], p-value = 0.003) were associated with hypokalemia <4mmol/L (Table 2).
Table 2. Multinomial logistic regression analysis targeting factors associated with dyskalemia.
| Variable | Hypokalemia | p-value | Normokalemia | Hyperkalemia | p-value |
|---|---|---|---|---|---|
| OR (95% CI) | (Reference) | OR (95% CI) | |||
| Female gender | 1.31 (1.03–1.66) | 0.029 | - | 0.54 (0.31–0.92) | 0.024 |
| Age <45 year | 1.69 (1.20–2.37) | 0.002 | - | - | - |
| eGFR <60 ml/min/1.73m2 | 0.64 (0.44–0.93) | 0.020 | - | 3.56 (1.94–6.52) | <0.001 |
| Thiazide diuretics | 2.04 (1.28–3.32) | 0.003 | - | - | - |
| Hb <12 g/dL | - | - | - | 2.62 (1.50–4.60) | 0.001 |
eGFR, estimated glomerular filtration rate based on the CKD-EPI formula; Hb, hemoglobin.
All variables with a p-value < 0.1 in Table 1 were introduced into the model. A backward selection process was conducted with a 500x sampling bootstrap method.
Variables were categorized to attain log-linearity.
Hypokalemia defined as K+ <4.0mmol/L.
Normokalemia defined as K+ 4.0–5.0mmol/L.
Hyperkalemia defined as K+ >5.0mmol/L.
In a further sensitivity analysis of hypokalemia <3.5mmol/L (with 3.5-5mmol/L as a reference), only thiazide diuretics were found associated with hypokalemia (OR [95% CI] = 2.32 [1.28–4.17], p-value = 0.005) (S1 Table).
Factors associated with hyperkalemia
Renal insufficiency (OR [95% CI] = 3.56 [1.94–6.52], p-value <0.001) and hemoglobin <12g/dl (OR [95% CI] = 2.62 [1.50–4.60], p-value = 0.001) were found associated with hyperkalemia, while female gender was negatively associated (OR [95% CI] = 0.54 [0.31–0.92], p-value = 0.0024) (Table 2).
Hospitalization and death
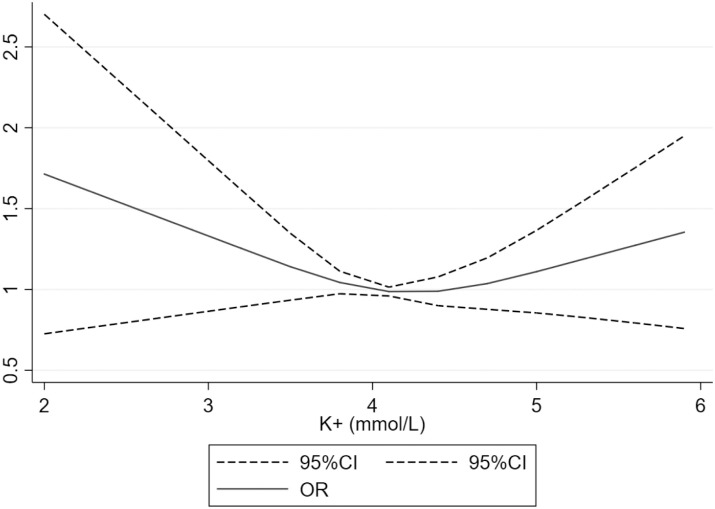
Two patients died in the ED, 629 (52.7%) were hospitalized and 16 (1.3%) were admitted to an intensive care unit (Table 3). Fig 3 presents the relationship between potassium level in blood and the combined outcome of hospitalization or death, the latter of which was found to be U-shaped, with a nadir at approximately 4.2mmol/L. In multivariate analysis, potassium was not associated with the combined outcome (Table 3).
Table 3. Associations between K+ levels and the outcome of hospitalization or death.
| K+ levels (mmol/L) | Crude OR (95% CI) | p-value | Adjusted OR (95% CI)* | p-value |
|---|---|---|---|---|
| K+ <4.0 | 0.75 (0.59–0.95) | 0.015 | 0.89 (0.69–1.15) | 0.38 |
| K+ 4.0–5.0 (ref.)** | - | - | - | - |
| K+ >5.0 | 1.25 (0.75–2.07) | 0.40 | 0.68 (0.39–1.17) | 0.16 |
*Model adjusted for age, gender, estimated glomerular filtration rate, hemoglobin and thiazide diuretics.
**The reference group is normokalemia: 4.0–5.0mmol/L.
Fig 3. Relationship between potassium level in blood and combined outcome of hospitalization or death.
*Model adjusted for age, gender, estimated glomerular filtration rate, hemoglobin and thiazide diuretics.
In a sensitivity analysis, hypokalemia< 3.5mmol/L was associated with the combined outcome of hospitalization or death (OR [95% CI] = 1.47 [1.00–2.15], p-value = 0.048) (Table 4).
Table 4. Associations between K+ levels and the outcome of hospitalization or death.
| K+ levels (mmol/L) | Crude OR (95% CI) | p-value | Adjusted OR (95% CI)* | p-value |
|---|---|---|---|---|
| K+ <3.5 | 1.37 (0.96–1.96) | 0.079 | 1.47 (1.00–2.15) | 0.048 |
| K+ 3.5–5.0 (ref.)** | - | - | - | - |
| K+ >5.0 | 1.51 (0.92–2.47) | 0.10 | 0.61 (0.35–1.08) | 0.09 |
*Model adjusted for age, gender, estimated glomerular filtration rate, hemoglobin and thiazide diuretics.
**The reference group is normokalemia: 3.5–5.0mmol/L.
Our study reports the prevalence, associated factors and prognostic implications of dyskalemia assessed in patients presenting at the ED in the absence of unstable medical illness. Hypokalemia was present in almost 50% of these patients, particularly among those less than 45 years old, in women, and those taking thiazide diuretics. Importantly, hypokalemia below 3.5mmol/L was associated with poorer outcomes. Conversely, hyperkalemia was less frequent in this population and not associated with adverse prognosis.
Our population characteristics are comparable to other low-risk cohorts [31, 32]. Moreover, similarly to Krokager et al. [33], who reported short-term mortality risk of potassium levels in hypertensive patients from the nationwide Danish registry, or in the Lindner et al. ED retrospective monocentric study [21], women were globally more susceptible to hypokalemia. The hypokalemia rates found in the present study were higher than in other reports [20, 21, 34–36].
For external validity purposes, we compared our prevalence results with another ED French cohort—the PARADISE cohort "Pathway of dyspneic patients in Emergency”, consisting of including all patients over one year aged 18 years or older admitted to the ED of the Nancy University Hospital (France) for dyspnea., as previously reported by our group [37].
In this setting, encompassing various nosological conditions, among 1318 dyspneic patients with potassium measurements, hypokalemia below 4mmol/ L was present in 36.6% of the patients, which was similar to what we found herein. Hyperkalemia above 5mmol/L was present in 6.8% of the patients.
An increase in the in-hospital short-term morbidity-mortality was observed in patients with potassium levels <3.5mmol/L, a finding also observed in another ED study [35] as well as in myocardial infarction patient cohorts [8] and in both hypertensive [33] and chronic HF [29] patients or in meta-analysis [9]. In specific population, as our results, Zhang et al. [7] demonstrated in meta-analysis that hypokalemia (<3.5mEq/L) was significantly associated with higher mortality risk among patients with CKD and dominant among women. Zhang et al also found that serum K within 3.5–4.0mEq/L among CKD patients was associated with increased all-cause risk. Potassium is an important determinant of myocardial function, and low blood potassium levels may cause arrhythmias and sudden cardiac death by accelerating depolarization, increasing automaticity and lengthening the action potential [29, 38–42].
The use of thiazide diuretics was associated with hypokalemia in our study. Their use may increase urinary potassium loss [43, 44]; hence, an appropriate electrolyte (and creatinine) monitoring is warranted in patients taking thiazides. However, certain studies have shown that the monitoring of potassium levels is suboptimal under potassium-sparing diuretics [45], particularly in primary care patients [46]. Upstream of the ED, i.e. in the primary care setting, there is indeed widespread use of thiazide diuretics, notably as long-run prescriptions in hypertensive patients (53.4% in hypertensive patients in the Danish registry [33], where the administration of diuretics in the low normal potassium level was slightly higher than the administration of ACEIs/ARBs). Thiazide diuretics were also found associated with hypokalemia in the ED in the present study, while “diuretic therapy” was found associated with both hypokalemia and hyperkalemia by Lindner et al. in their retrospective analysis of a single-center database in Switzerland [21]. Thus, an adequate biological monitoring is also warranted in hypertensive patients who are at risk of hypokalemia.
Our study has several limitations. First, the initial reasons for measuring potassium were not recorded and assessed, although the chief complaint of our population was comparable to those of the ADES-ED population (trauma in about one-third of the patients, with the other most frequent complaints being abdominal pain, weakness and cardiovascular diseases) [26], except for trauma which was second. This fact can be easily explained since patients with no potassium measurements were excluded in our study and patients with non-surgical trauma had no blood test. Secondly, data collection on drug consumption did not include dietary supplements, which can also generate iatrogenic effects, especially on potassium levels. Thirdly, the present dataset does not allow ruling out a pseudo-hyperkalemia phenomenon [47], in addition we don’t know which machines were used to test their potassium values and what are the hospitals cut-offs for potassium. However, hyperkalemia was very uncommon in the present series. Another important limitation was that mortality was only assessed in the ED, while a more long-term analysis (i.e. during hospital stay) may also be relevant. Lastly, our population only included people who have had a blood test with electrolytes. This fact selects patients who are probably sicker than those who did not have blood tests.
Notwithstanding, the strength of the present study lies in its multicenter and prospective recruitment approach while describing for the first time a population presenting at the ED without unstable medical illness.
Conclusions
Hypokalemia was frequently found in the ED. It was associated with worse outcomes in a low-risk ED population. Thus, ED patients presenting with hypokalemia should be appropriately treated and monitored both in the ED and after discharge. Furthermore, more effective prevention strategies encompassing an adequate biological monitoring of hypertensive patients treated with thiazide diuretics should be implemented upstream.
Supporting information
eGFR, estimated glomerular filtration rate based on the CKD-EPI formula; Hb, hemoglobin. All variables with a p-value of less than 0.1 in Table 1 were introduced into the model. A backward selection process was conducted with 500x sampling bootstrap method. Variables were categorized to attain log-linearity. Hypokalemia defined as K+ <3.5 mmol/L (N = 148). Normokalemia defined as K+ 3.5–5.0 mmol/L (N = 1021). Hyperkalemia defined as K+ >5.0 mmol/L (N = 73).
(DOCX)
Acknowledgments
The authors deeply thank the study steering committee (Françoise Ballereau, Jacques Bouget, Nadine Foucher, Patrice Queneau, Bertrand Renaud, Lucien Roulet, Gerald Kierzek, Aurore Armand-Perroux, Gilles Potel, Jeannot Schmidt and Françoise Carpentier) for granting us access to the clinical database. The authors thank Pierre Pothier for the editing the manuscript.
Data Availability
There are ethical restrictions for data access per French Regulation (sensitive healthcare data). Requests for access require the agreement of the study investigators and the study sponsor studying the applicant’s project. The contact person for this committee is Ms Béatrice Trombert-Paviot. (Beatrice.TROMBERT-PAVIOT@chu-st-etienne.fr; CHU of Saint Etienne, France) or Pr Patrice Queneau (pat-queneau@orange.fr, Saint Etienne University, France).
Funding Statement
The National Medicine Academy approved the study design and provided funding. The French Society for Clinical Pharmacy (SFPC) approved the protocol, supported the enrolment and training of pharmacy students and provided funding. The French Association of Pharmaceutical Manufacturers for a responsible self-medication (AFIPA) approved the protocol and the conduct of the study. Sanofi Aventis approved the protocol and the conduct of the study. Patrick Rossignol reports receiving consulting fees and travel support from Novartis, consulting fees from Novo Nordisk, AstraZeneca, Grünenthal, and Corvidia, consulting fees, lecture fees, fees for serving on a steering committee, and travel support from Relypsa/Vifor/Vifor Fresenius Medical Care, fees for serving on a steering committee and fees for serving on a critical event committee from Idorsia, lecture fees and travel support from Bayer and Servier, owning stock options in G3 Pharmaceuticals, and fees for serving as co-founder and owning stock in CardioRenal. TC reports honoraria from Novartis, Astra Zeneca. ML reports receiving lecture fees from Baxter and Fresenius, research support from Sphingotec, and consulting fees from Novartis. None of these funding sources intervened in the collection, management, analysis or interpretation of the data; nor in the preparation, review or approval of the manuscript.
References
- 1.Uribarri J, Oh MS and Carroll HJ. Hyperkalemia in diabetes mellitus. J Diabet Complications. 1990;4:3–7. 10.1016/0891-6632(90)90057-c [DOI] [PubMed] [Google Scholar]
- 2.Khanagavi J, Gupta T, Aronow WS, Shah T, Garg J, Ahn C, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10:251–7. 10.5114/aoms.2014.42577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossignol P, Legrand M, Kosiborod M, Hollenberg SM, Peacock WF, Emmett M, et al. Emergency management of severe hyperkalemia: Guideline for best practice and opportunities for the future. Pharmacol Res. 2016;113:585–591. 10.1016/j.phrs.2016.09.039 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, et al. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–43. 10.1093/eurheartj/ehm091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–62. 10.1001/archinternmed.2009.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–3. 10.1016/j.amjcard.2012.01.367 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Chen P, Chen J, Wang L, Wei Y and Xu D. Association of Low Serum Potassium Levels and Risk for All-Cause Mortality in Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Ther Apher Dial. 2019;23:22–31. 10.1111/1744-9987.12753 [DOI] [PubMed] [Google Scholar]
- 8.Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, et al. Serum potassium levels and mortality in acute myocardial infarction. Jama. 2012;307:157–64. 10.1001/jama.2011.1967 [DOI] [PubMed] [Google Scholar]
- 9.Hoppe LK, Muhlack DC, Koenig W, Carr PR, Brenner H and Schottker B. Association of Abnormal Serum Potassium Levels with Arrhythmias and Cardiovascular Mortality: a Systematic Review and Meta-Analysis of Observational Studies. Cardiovasc Drugs Ther. 2018;32:197–212. 10.1007/s10557-018-6783-0 [DOI] [PubMed] [Google Scholar]
- 10.Pitt B and Rossignol P. The association between serum potassium and mortality in patients with hypertension: ‘a wake-up call’. Eur Heart J. 2017;38:113–115. 10.1093/eurheartj/ehw209 [DOI] [PubMed] [Google Scholar]
- 11.Khan SS, Campia U, Chioncel O, Zannad F, Rossignol P, Maggioni AP, et al. Changes in serum potassium levels during hospitalization in patients with worsening heart failure and reduced ejection fraction (from the EVEREST trial). Am J Cardiol. 2015;115:790–6. 10.1016/j.amjcard.2014.12.045 [DOI] [PubMed] [Google Scholar]
- 12.Legrand M, Ludes PO, Massy Z, Rossignol P, Parenica J, Park JJ, et al. Association between hypo- and hyperkalemia and outcome in acute heart failure patients: the role of medications. Clin Res Cardiol. 2017. [DOI] [PubMed] [Google Scholar]
- 13.Núñez J, Bayés-Genís A, Zannad F, Rossignol P, Núñez E, Bodí V, et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality Circulation. 2017. [DOI] [PubMed] [Google Scholar]
- 14.Rossignol P, Dobre D, Gregory D, Massaro J, Kiernan M, Konstam MA, et al. Incident hyperkalemia may be an independent therapeutic target in low ejection fraction heart failure patients: insights from the HEAAL study. Int J Cardiol. 2014;173:380–7. 10.1016/j.ijcard.2014.02.034 [DOI] [PubMed] [Google Scholar]
- 15.Ronksley PE, Tonelli M, Manns BJ, Weaver RG, Thomas CM, MacRae JM, et al. Emergency Department Use among Patients with CKD: A Population-Based Analysis. Clin J Am Soc Nephrol. 2017;12:304–314. 10.2215/CJN.06280616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madias JE, Shah B, Chintalapally G, Chalavarya G and Madias NE. Admission Serum Potassium in Patients With Acute Myocardial Infarction: Its Correlates and Value as a Determinant of In-Hospital Outcome. Chest. 2000;118:904–913. 10.1378/chest.118.4.904 [DOI] [PubMed] [Google Scholar]
- 17.McMahon GM, Mendu ML, Gibbons FK and Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834–42. 10.1007/s00134-012-2636-7 [DOI] [PubMed] [Google Scholar]
- 18.Arampatzis S, Funk G, Leichtle AB, Fiedler GM, Schwarz C, Zimmermann H, et al. Impact of diuretic therapy-associated electrolyte disorders present on admission to the emergency department: a cross-sectional analysis. BMC Med 2013;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vroonhof K, Van Solinge W, Rovers M and Huisman A. Differences in mortality on the basis of laboratory parameters in an unselected population at the Emergency Department. Clin Chem Lab Med. 2005;43:536–41. 10.1515/CCLM.2005.093 [DOI] [PubMed] [Google Scholar]
- 20.Pfortmuller CA, Leichtle AB, Fiedler GM, Exadaktylos AK and Lindner G. Hyperkalemia in the emergency department: etiology, symptoms and outcome of a life threatening electrolyte disorder. Eur J Intern Med. 2013;24:e59–60. 10.1016/j.ejim.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 21.Lindner G, Pfortmuller CA, Leichtle AB, Fiedler GM and Exadaktylos AK. Age-related variety in electrolyte levels and prevalence of dysnatremias and dyskalemias in patients presenting to the emergency department. Gerontology. 2014;60:420–3. 10.1159/000360134 [DOI] [PubMed] [Google Scholar]
- 22.Acikgoz SB, Genc AB, Sipahi S, Yildirim M, Cinemre B, Tamer A, et al. Agreement of serum potassium measured by blood gas and biochemistry analyzer in patients with moderate to severe hyperkalemia. Am J Emerg Med. 2016;34:794–7. 10.1016/j.ajem.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 23.Chon SB, Kwak YH, Hwang SS, Oh WS and Bae JH. Severe hyperkalemia can be detected immediately by quantitative electrocardiography and clinical history in patients with symptomatic or extreme bradycardia: a retrospective cross-sectional study. J Crit Care. 2013;28:1112 e7–1112 e13. [DOI] [PubMed] [Google Scholar]
- 24.An JN, Lee JP, Jeon HJ, Kim DH, Oh YK, Kim YS, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16:R225 10.1186/cc11872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen HK, Brabrand M, Vinholt PJ, Hallas J and Lassen AT. Hypokalemia in acute medical patients: risk factors and prognosis. Am J Med. 2015;128:60–7 e1. 10.1016/j.amjmed.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 26.Asseray N, Ballereau F, Trombert-Paviot B, Bouget J, Foucher N, Renaud B, et al. Frequency and Severity of Adverse Drug Reactions Due to Self-Medication: A Cross-Sectional Multicentre Survey in Emergency Departments. Drug Safe. 2013;36:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roulet L, Asseray N, Foucher N, Potel G, Lapeyre-Mestre M and Ballereau F. A questionnaire to document self-medication history in adult patients visiting emergency departments. Pharmacoepidemiol Drug Saf. 2013;22:151–9. 10.1002/pds.3364 [DOI] [PubMed] [Google Scholar]
- 28.Rossignol P, Zannad F, Pitt B and Writing group of 10th Global Cardio Vascular Clinical Trialist forum held on December 6th-7th in Paris F. Time to retrieve the best benefits from renin angiotensin aldosterone system (RAAS) inhibition in heart failure patients with reduced ejection fraction: lessons from randomized controlled trials and registries. Int J Cardiol. 2014;177:731–3. 10.1016/j.ijcard.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 29.Rossignol P, Girerd N, Bakris G, Vardeny O, Claggett B, McMurray JJV, et al. Impact of eplerenone on cardiovascular outcomes in heart failure patients with hypokalaemia. Eur J Heart Fail. 2017; 6 792–799. [DOI] [PubMed] [Google Scholar]
- 30.Cooper LB, Benson L, Mentz RJ, Savarese G, DeVore AD, Carrero JJ, et al. Association between potassium level and outcomes in heart failure with reduced ejection fraction: a cohort study from the Swedish Heart Failure Registry. Eur J Heart Fail. 2020. [DOI] [PubMed] [Google Scholar]
- 31.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2014;23:3–16. 10.3109/08037051.2014.868629 [DOI] [PubMed] [Google Scholar]
- 32.Ferreira JP, Girerd N, Bozec E, Merckle L, Pizard A, Bouali S, et al. Cohort Profile: Rationale and design of the fourth visit of the STANISLAS cohort: a familial longitudinal population-based cohort from the Nancy region of France. Int J Epidemiol. 2018;47:395–395j. 10.1093/ije/dyx240 [DOI] [PubMed] [Google Scholar]
- 33.Krogager ML, Torp-Pedersen C, Mortensen RN, Kober L, Gislason G, Sogaard P, et al. Short-term mortality risk of serum potassium levels in hypertension: a retrospective analysis of nationwide registry data. Eur Heart J. 2017;38:104–112. 10.1093/eurheartj/ehw129 [DOI] [PubMed] [Google Scholar]
- 34.Singer AJ, Thode HC Jr. and Peacock WF. A retrospective study of emergency department potassium disturbances: severity, treatment, and outcomes. Clin Exp Emerg Med. 2017;4:73–79. 10.15441/ceem.16.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conway R, Creagh D, Byrne D, O’Riordan D and Silke B. Serum potassium levels as an outcome determinant in acute medical admissions. Clinical Medicine. 2015;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marti G, Schwarz C, Leichtle AB, Fiedler GM, Arampatzis S, Exadaktylos AK, et al. Etiology and symptoms of severe hypokalemia in emergency department patients. Eur J Emerg Med. 2014;21:46–51. 10.1097/MEJ.0b013e3283643801 [DOI] [PubMed] [Google Scholar]
- 37.Chouihed T, Rossignol P, Bassand A, Duarte K, Kobayashi M, Jaeger D, et al. Diagnostic and prognostic value of plasma volume status at emergency department admission in dyspneic patients: results from the PARADISE cohort. Clin Res Cardiol. 2019;108:563–573. 10.1007/s00392-018-1388-y [DOI] [PubMed] [Google Scholar]
- 38.Bielecka-Dabrowa A, Mikhailidis DP, Jones L, Rysz J, Aronow WS and Banach M. The meaning of hypokalemia in heart failure. Int J Cardiol. 2012;158:12–7. 10.1016/j.ijcard.2011.06.121 [DOI] [PubMed] [Google Scholar]
- 39.Tomaselli GF and Marbán E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res 1999;42:270–83. 10.1016/s0008-6363(99)00017-6 [DOI] [PubMed] [Google Scholar]
- 40.Macdonald JE and Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43:155–61. 10.1016/j.jacc.2003.06.021 [DOI] [PubMed] [Google Scholar]
- 41.Dépret F, Peacock WF, Liu KD, Rafique Z, Rossignol P and Legrand M. Management of hyperkalemia in the acutely ill patient. Annals of Intensive Care. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafique Z, Chouihed T, Mebazaa A and Frank Peacock W. Current treatment and unmet needs of hyperkalaemia in the emergency department. Eur Heart J Suppl. 2019;21:A12–A19. 10.1093/eurheartj/suy029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang JL, Yu H, Hou YW, Wang K, Bi WS, Zhang L, et al. Impact of long-term potassium supplementation on thiazide diuretic-induced abnormalities of glucose and uric acid metabolisms. J Hum Hypertens. 2018;32:301–310. 10.1038/s41371-018-0036-3 [DOI] [PubMed] [Google Scholar]
- 44.Agarwal R. Hypertension, hypokalemia, and thiazide-induced diabetes: a 3-way connection. Hypertension. 2008;52:1012–3. 10.1161/HYPERTENSIONAHA.108.121970 [DOI] [PubMed] [Google Scholar]
- 45.Cooper LB, Hammill BG, Peterson ED, Pitt B, Maciejewski ML, Curtis LH, et al. Consistency of Laboratory Monitoring During Initiation of Mineralocorticoid Receptor Antagonist Therapy in Patients with Heart Failure. JAMA. 2015;10:1973–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson E, De Deco P, Trevisan M, Bellocco R, Lindholm B, Lund LH, et al. A real-world cohort study on the quality of potassium and creatinine monitoring during initiation of mineralocorticoid receptor antagonists in patients with heart failure. Eur Heart J Qual Care Clin Outcomes. 2018;4:267–273. 10.1093/ehjqcco/qcy019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sevastos N, Theodossiades G and Archimandritis AJ. Pseudohyperkalemia in serum: a new insight into an old phenomenon. Clin Med Res. 2008;6:30–2. 10.3121/cmr.2008.739 [DOI] [PMC free article] [PubMed] [Google Scholar]