Abstract
Objectives
In 2016, WHO estimated 376 million new cases of the four main curable STIs: gonorrhoea, chlamydia, trichomoniasis and syphilis. Further, an estimated 290 million women are infected with human papillomavirus. STIs may lead to severe reproductive health sequelae. Low-income and middle-income countries carry the highest global burden of STIs. A large proportion of urogenital and the vast majority of extragenital non-viral STI cases are asymptomatic. Screening key populations and early and accurate diagnosis are important to provide correct treatment and to control the spread of STIs. This article paints a picture of the state of technology of STI point-of-care testing (POCT) and its implications for health system integration.
Methods
The material for the STI POCT landscape was gathered from publicly available information, published and unpublished reports and prospectuses, and interviews with developers and manufacturers.
Results
The development of STI POCT is moving rapidly, and there are much more tests in the pipeline than in 2014, when the first STI POCT landscape analysis was published on the website of WHO. Several of the available tests need to be evaluated independently both in the laboratory and, of particular importance, in different points of care.
Conclusion
This article reiterates the importance of accurate, rapid and affordable POCT to reach universal health coverage. While highlighting the rapid technical advances in this area, we argue that insufficient attention is being paid to health systems capacity and conditions to ensure the swift and rapid integration of current and future STI POCT. Unless the complexity of health systems, including context, institutions, adoption systems and problem perception, are recognised and mapped, simplistic approaches to policy design and programme implementation will result in poor realisation of intended outcomes and impact.
Keywords: point of care, testing, resource-limited settings, public health
Introduction
In 2016, WHO estimated 376 million new cases per year of the four main curable STIs: gonorrhoea (Neisseria gonorrhoeae (NG)), chlamydia (Chlamydia trachomatis (CT)), trichomoniasis (Trichomonas vaginalis (TV)) and syphilis (Treponema pallidum subspecies pallidum (TP)).1 In addition to CT, NG, TV and TP, Mycoplasma genitalium (MG) infections are prevalent in many settings.2 An estimated 290 million women are infected with human papillomavirus (HPV),3 and an estimated 3.9 million people live with HIV.4 Low- and middle-income countries (LMICs) carry the highest global burden of non-viral and viral STIs.5 6 STIs may lead to severe reproductive sequelae and neonatal death, are associated with the development of cancers resulting in high mortality, and can facilitate the transmission and acquisition of HIV.7
A large proportion of urogenital and the vast majority of extragenital non-viral STI cases are asymptomatic. These cases are most often identified through sexual contact notification, and particularly opportunistic testing and screening. Screening or significantly enhanced testing of key populations and early and accurate diagnosis of infection are important to provide correct treatment and to control the spread of STIs and their sequelae.7 Recent publications have called for the advancement of point-of-care testing (POCT) for STIs, as they promise significantly improved performance compared with syndromic management. POCT potentially avoids overuse and misuse of antimicrobials and could thus decrease the selection of antimicrobial resistance (AMR) in STI pathogens and bystander commensal or pathogenic bacterial species, lower testing costs for patients and healthcare systems, reduce waiting times, speed up and increase accurate treatment, and improve patient follow-up.8–11 Accurate, rapid and affordable POCT could increase access to testing and identification of STIs in a single-patient visit in both LMICs and high-income countries (HICs) and could be used at all levels of the healthcare system while also contributing to the improvement of STI surveillance through POCT connectivity.12
Diagnostics are often undervalued, but they are just as important to the attainment of the United Nations Sustainable Development Goals (SDGs) as medicines and vaccines. In particular, improved access to diagnostics will be essential to reach SDG 3.7: ‘ensure universal access to sexual and reproductive health care services, including for family planning, information and education, and the integration of reproductive health into national strategies and programs’ and SDG 3.8: ‘achieve universal health coverage (UHC), including financial risk protection, access to quality essential health care services and access to safe, effective, quality and affordable essential medicines and vaccines for all’.13 In close alignment with the SDGs are WHO's triple billion goals, which include improved access to diagnostics for primary healthcare as a means to enable UHC for one billion more individuals.14 To achieve this goal, moving testing closer to the patient will be essential. WHO's Global health sector strategy for the Control and Prevention of STIs recognises STI POCT as an innovation that enables improvement in all steps of the STI services cascade (online supplementary appendix 1).7
sextrans-2019-054358supp001.pdf (1,004.2KB, pdf)
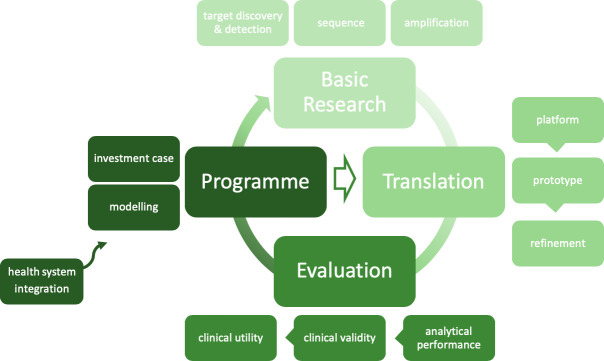
To move the agenda forward, WHO has (1) facilitated several STI POCT landscape analyses, the first in 2014, and stakeholder meetings15 16; (2) developed target product profiles (TPPs); (3) established a global network of centres for and implemented studies to evaluate commercially available STI POC tests (POCTs); (4) developed a standard methodology for evaluation of STI POCTs and (5) developed a roadmap to accelerate the STI POCT agenda (figure 1).
Figure 1.
Roadmap to advance point-of-care testing for the control and prevention of STIs (adapted from Toskin et al 10).
Inspired by the global roadmap for STI vaccine development, the WHO International Advisory Group on STI POCT visually structured the roadmap along four main development phases necessary for advancing products from the concept stage to routine use: basic research, translation, evaluation and programmatic implementation. Rather than linear, this is a cyclical roadmap, each element being informed by previous experiences. Notwithstanding their potential to strengthen healthcare systems,17 the introduction of STI POCTs, allowing for more decentralisation, is not without risks. Currently, evidence from implementation studies and guidance and recommendations to sustain an effective introduction of such tools in primary care settings, particularly in LMICs, are lacking.
In the following sections, the present article summarises the STI POCT landscape, followed by a discussion of key health systems issues to be considered, as well as opportunities to strengthen health systems and improve health outcomes.
STI POCT landscape in 2018/2019
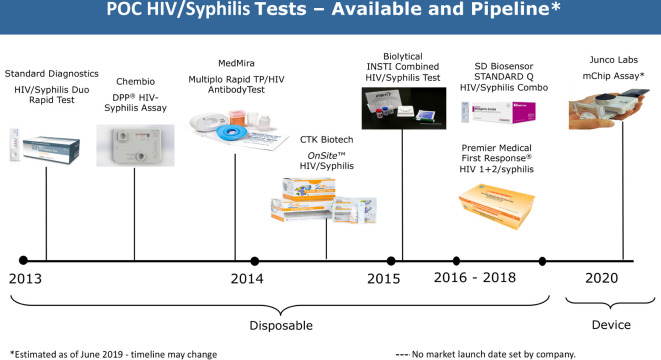
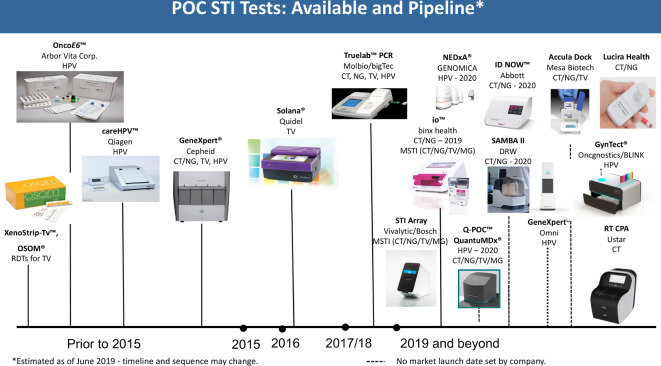
Over the past years, there have been an increasing number of POCTs for STIs developed, most of which are still in the pipeline, with expected launch in 2019 and beyond (figures 2 and 3).
Figure 2.
Landscape for HIV/syphilis POCTs. TP, Treponema pallidum subspecies pallidum.
Figure 3.
Landscape for NG, CT, TV and HPV point-of-care testing. CT, Chlamydia trachomatis; HPV, human papillomavirus; NG, Neisseria gonorrhoeae; TV, Trichomonas vaginalis; MSTI, multi-STI; CPA, cross priming amplification;.
HIV/syphilis
Simple, rapid, sensitive and specific POCTs for syphilis and HIV are already commercially available.18 However, some performance anomalies suggest the need for additional evaluation studies. Optimisation of the available HIV/syphilis dual tests is still required to be in line with the WHO TPPs for use in primary healthcare settings in LMICs, as there are issues with multiple and complicated steps, timing, the inflexibility of the reading window, shelf life, environmental tolerances and, finally, cost.9 There is only one novel assay in the pipeline, the mChip from Junco Labs (New York, New York, USA) and Columbia University in collaboration with OPKO Health (Miami, Florida, USA), which has been evaluated in Rwanda with good results,19 but no commercial launch date has been set.
NG/CT/TV/MG
Simple, rapid and equipment-free POCTs for the detection of CT, NG and TV are available but have a suboptimal sensitivity for screening or widely implemented testing.20–23 Nevertheless, emerging technologies promise major advances in the coming years.
Molecular tests for CT, NG and TV are now available from Molbio Diagnostics (Goa, India) for its Truelab Real Time micro PCR System and Cepheid (Sunnyvale, California, USA) for its GeneXpert system, both near-patient. A combined CT/NG test from Binx Health (formerly Atlas Genetics) (Trowbridge, UK) for its molecular io platform was also recently launched; a CT/NG/TV/MG test is in the pipeline. A test for CT/NG is currently in the pipeline for the ID NOW platform (Abbott Laboratories, Lake Bluff, Illinois, USA), an instrument-based, molecular diagnostic test using isothermal nucleic acid amplification technology for the qualitative detection of CT/NG targets. Lucira Health (formerly Diassess) (Emeryville, California, USA) has an assay for CT/NG in the pipeline that is an instrument-free, deployable, disposable and simple-to-use molecular test with a usability profile equivalent to current rapid immunoassay diagnostic tests. Further, QuantuMDx (Newcastle upon Tyne, UK) is planning to develop a CT/NG/TV/MG multiplex assay run direct from swab samples on a small, handheld diagnostic device, Q-POC, that can deliver patient results in under 15 min. The system runs end-point PCR chemistries and quantitative PCR chemistries, and includes a microarray after the amplification step. Randox (Crumlin, UK) has developed an STI array assay for the Bosch Vivalytic (Waiblingen, Germany) molecular platform, which uses microfluidic techniques to detect 10 bacterial, viral and protozoan ‘STIs’, providing a comprehensive infection profile from a single urethral/vaginal swab and/or swab from ulcer samples, for example, Haemophilus ducreyi, TP, and herpes simplex virus type 1 (HSV-1) and 2 (HSV-2). The test panel for STIs includes CT, NG, TV and MG, as well as additional bacterial species Mycoplasma hominis and Ureaplasma urealyticum, which are frequently only colonising commensal bacteria that are not recommended to be routinely tested and treated as STIs.24
Blusense ViroTrack (Copenhagen, Denmark), which currently offers an assay for dengue virus, is planning to add certain STIs to its platform. It uses microfluidics and next-generation latex immune-turbidometry in the form of a patented optomagnetic nanoparticle-based readout technology which can be used to detect antigens, antibodies, small molecules, RNA, DNA and micro-RNA.
Antimicrobial resistance
AMR to all therapeutic antimicrobials in NG and MG has emerged, which compromises the treatment of these infections globally.2 25 26 In response, WHO has revitalised the WHO Global Gonococcal Antimicrobial Surveillance Programme.25 However, in many countries, cultured gonococcal isolates are becoming increasingly rare because of use of syndromic management or non-culture-based diagnostic methods.27 Accordingly, sensitive and specific tests that detect NG and MG AMR determinants to predict AMR for use particularly, but not exclusively, in primary and secondary healthcare settings would be exceedingly valuable. Rapid, accurate POCTs simultaneously detecting infection and predicting AMR could significantly impact treatment precision and infection management. This strategy would enable diagnosis and individualised treatment at the first healthcare visit, reducing selection pressure on currently recommended antimicrobials, reducing transmission of resistant NG and MG strains, and providing means for AMR surveillance.11 28 29 A modelling study in the UK showed that if a 30 min AMR POCT for NG and penicillin resistance would be available, 79% of the 33 431 annual ceftriaxone treatments could possibly be replaced by benzylpenicillin.11 If a similar test with ciprofloxacin resistance were available, 66% of current treatment in the UK could be replaced by ciprofloxacin, both with considerable cost savings.
A number of prototypic POC devices for molecular detection, primarily targeting ciprofloxacin resistance or susceptibility in NG, are in the pipeline, but none is approved for routine use. However, developing appropriate AMR POCTs for most other therapeutic antimicrobials for NG, such as ceftriaxone and azithromycin, remain challenging.29 Furthermore, keys for successful deployment of these AMR POCTs will include understanding the cost consequences, cost-effectiveness and acceptability for key stakeholders.
A molecular POCT detecting ciprofloxacin susceptibility in NG is under development by Binx Health in cooperation with the St George’s Hospital, London, and QuantuMDx is developing an AMR NG POCT to complement its CT/NG/TV/MG multiplex assay. WHO supports the development and validation of NG AMR POCTs, and TPPs for these types of POCTs are currently under development.
Human papillomavirus
Molecular tests suitable for near-patient testing for HPV are already available from Cepheid, Qiagen (Hilden, Germany) and Molbio Diagnostics. New HPV assays are expected in the near future from GENOMICA (Madrid, Spain) for its NEDxA platform and from Cepheid for its portable Omni platform. Furthermore, a partnership has been set up between Oncgnostics (Jena, Germany) and BLINK (Jena, Germany). Oncgnostics will develop its GynTect HPV assay for use on the BLINK platform. The system is anticipated to be both multiplexed and multianalyte and designed for use at POC.
Several of the available tests need to be evaluated independently both in the laboratory, and of particular importance, in different POC. It is clear that the development of STI POCTs is moving rapidly, but there is a gap when considering the readiness of health systems to support the integration at several levels. These gaps range from identifying opportunities at global level for strategic procurement through global funding initiatives, normative technical implementation guidance and integration of such tests into national health systems.
STI POCT health systems integration
Although aimed at the individual, health innovations such as POCT are implemented within health systems and often have implications that are more complex than first appreciated.30 This recognition builds on the WHO definition of the health system as ‘consisting of all organisations, people and action whose primary intent is to promote, restore or maintain health’.[w1] This notion of the health system requires moving beyond consideration of linear approaches comprising static health system building blocks (ie, service delivery, health workforce, health information, medical technologies, health financing, leadership and governance) to recognising the complex web of inter-relationships and pathways of influence that transform these blocks into people-centred systems.30 This requires taking an aerial view of the whole system when considering the implementation of innovations.[w2]
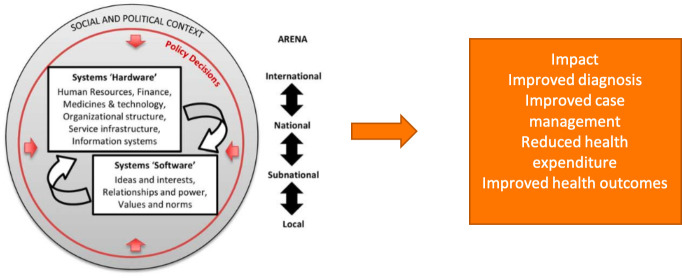
As illustrated in figure 4, health systems comprise both 'hardware' and 'software'; moreover, rather than being a vehicle for technological innovations, they are grounded in political and social contexts, on international and national as well as subnational and local levels, with underlying power structures, interests and interdependencies.[w3]
Figure 4.
Social construction perspective on health policy and systems (adapted from Sheikh et al).[w3].
Top-down, simplistic approaches to the introduction of innovations such as POCT, which only focus on the implications for the hardware components of the system (eg, training of health workers to apply a new technology or improvements in supply-chain management) and ignore the software and system-wide elements, including cultural norms and historical trajectories, run the risk of unexpected and, in some instances, adverse outcomes. This implies and demands a health systems research agenda encompassing:
Normative/evaluation or exploratory/explanatory questions driven by social science research methods in addition to clinical and epidemiological research employing both qualitative and quantitative methodological tools.
Inquiry at multiple levels of analysis, including mapping the context, institutions and adoption systems. This requires ‘macro-level focuses on the architecture of systems, meso-level on the functioning of organizations and systemic interventions, and micro level on the role of individuals involved in activities of health provision, utilization, and governance, and how systems respectively shape and are shaped by their decisions and behaviour’.[w3]
This health system research agenda should not be undertaken in isolation. Much knowledge and lessons have been learnt from the introduction of POCT into national health systems for other STIs, such as HIV. Ongoing global initiatives for the dual elimination of mother-to-child transmission (MTCT) of HIV and syphilis and the triple elimination of MTCT of HIV, syphilis and hepatitis B are examples of how countries can leverage the efforts and resources that have been invested in HIV programmes to improve prenatal screening for syphilis, an STI that is often forgotten.[w4] STIs and POCT feature prominently in the Global HIV/STI and Viral Hepatitis Strategy and the 2018 Astana Declaration on primary healthcare. SDG 3.8.1., ‘Coverage of essential health services’ and the Global Health Sector Strategy on STIs provide further opportunities for building STI POCT into global and national initiatives. For HPV, the director-general of WHO has made a global call for action towards the elimination of cervical cancer. Together with HPV vaccination, screening programmes will be essential to achieve this goal. POCT for HPV should be clearly positioned as the tool par excellence for screening, both in HICs and LMICs, as well as for routine monitoring of HPV prevalence.[w5] POCT is also indispensable in the battle against AMR. WHO's Global Action Plan calls on the research community to invest in the development of effective and low-cost POCTs for detection of AMR.[w6]
The publication of the WHO Essential Diagnostics List (EDL) in 2018 and 2019 highlighted the importance of diagnostics that can be used at lower levels of the healthcare system and will likely influence regional and national policy.[w7, w8] While the current EDL includes mainly laboratory based diagnostics for STIs (HIV, syphilis, NG and CT) and only POCTs for HIV and syphilis, there is a need for advocating for more STI POCTs in forthcoming editions, accompanied by a set of easy-to-use guidelines for developing STI POCT policy and programme implementation.
Equally important is securing funding to support policy and programme implementation within LMICs. Sustainable funding dedicated to STI POCTs is direly needed. However, as Kuupiel et al (2017) observed,[w9] even if sufficient investment is made in securing the availability of POCT in general, there are health system barriers that challenge their adoption in LMICs. These include a combination of hardware and software challenges at the national, subnational and local levels, including financial, human resources, policy regulatory, infrastructure, quality control and quality assurance, work–flow balance, training of personnel, supply chain, relationships between healthcare workers and patients and cultural acceptability of POCTs. It is important to re-emphasise that a well-functioning POCT programme should be informed by findings of the research agenda as described previously, but countries should also consider investing in data connectivity so that test results from thousands of POCT sites around a country are not just being recorded on registers and only reported or analysed periodically. Digitising laboratory and POC test results can standardise the interpretation of results and allows data to be linked to proficiency testing to ensure testing quality, reducing interpretation and transcription errors. Weak health systems in LMICs contribute to almost a quarter of healthcare equipment being out of service.[w10] Remote monitoring of POC instrument functionality and use through connectivity allows programmes to optimise instrument placement, algorithm adoption and supply management. Alerts can be built into the system to raise alarm at unusual trends such as outbreaks. Connected diagnostics have become a useful tool for epidemiology, patient care and tracking, research, and AMR and outbreak surveillance.[w11]17
When introducing health innovations such as STI POCT, context-appropriate communication is important. As Atun et al (2012) argue ‘…the reasons for slow adoption and diffusion of health innovations are less to do with the perceived benefits of the innovation, but the way the problem, which the innovation is designed to address, is perceived by the individuals and the adoption system within health institutions, the health system and the broad context’.[w2] If stakeholder perception of the problem and the innovation to address the problem are disparate with the evidence of the benefits of the innovation, then poor adoption and, in some instances, resistance may ensue. For example, in Russia, the incorrect perception that HIV/AIDS was a problem associated mainly with injecting drug users and sex workers resulted in poor uptake of antiretroviral therapy.[w12] The introduction of POCT for syphilis has been shown to strengthen health systems by increasing access to prenatal care for a greater percentage of the population, increasing system efficiency by reducing the number of patient visits and improving patient outcomes.[w13, w14]
It is also important to recognise the role of key stakeholders in getting buy-in and ensuring the successful diffusion of innovations. For example, in relation to scaling up innovations in Ethiopia, Spicer et al note: “…if an innovation is a new technology, vaccine or medication and/or requires changes in national legislation they first approach federal government, whereas expanding programs accepted by federal government required targeting relevant Regional Health Bureaus”.[w15]
Hence stakeholder engagement and buy-in and effective communication should reflect that investment in the proper introduction and scale-up of STI POCT is an investment in health system strengthening in LMICs.
Conclusion
For STI POCT to support WHO's goal of ending STI epidemics as public health concerns by 2030, it must be recognised that health systems barriers challenge the successful adoption and implementation of STI POCT.[w16] A health systems research agenda integrating effective and existing STI POCT technologies into health systems can and should be taken forward immediately through a combination of demonstration and implementation research projects. This is essential for building a body of knowledge that recognises the complexity of developing a responsive and effective health system for addressing the transmission and burden of STIs through POCT.
References from w1 to w16 are available in online supplementary appendix 2.
sextrans-2019-054358supp002.pdf (128.1KB, pdf)
Key messages.
Accurate, rapid and affordable point-of-care testing (POCT) for sexually transmitted infections are important tools to reach the goal of the WHO STI Global health sector strategy, ending STI epidemics as major public health concerns.
The development of STI POCTs is moving rapidly; although they will not all make it to the finish line, there have never been more POCTs in the pipeline to detect gonorrhoea, chlamydia, trichomoniasis and the human papillomavirus.
The roll-out of STI POCT is not sufficiently prepared: there are no opportunities at a global level for strategic procurement; normative technical implementation guidance for integration of STI POCT into national health systems is urgently needed.
sextrans-2019-054358supp003.pdf (57.7KB, pdf)
Acknowledgments
The authors acknowledge the work of the WHO International Advisory Group on STI point-of-care testing.
Footnotes
Handling editor: Federico Garcia
Contributors: IT conceived the presented idea. MM developed the landscape of STI point-of-care testing. MU developed the sections on antimicrobial resistance. VG and RWP the section on health systems. CZ and KB compiled information and developed the draft of the manuscript. JK reviewed the manuscript. All authors discussed, contributed to and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: “Some of the authors are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the views, decisions or policies of the World Health Organization.”
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing not applicable as no datasets generated and/or analysed for this study. The full updated landscape is available on: https://www.who.int/reproductivehealth/topics/rtis/Diagnostic-Landscape-for-STIs-2019.pdf
References
- 1. World Health Organization Report on global sexually transmitted infection surveillance, 2018. Geneva: WHO, 2018. [Google Scholar]
- 2. Unemo M, Jensen JS. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol 2017;14:139–52. 10.1038/nrurol.2016.268 [DOI] [PubMed] [Google Scholar]
- 3. de Sanjosé S, Diaz M, Castellsagué X, et al. . Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007;7:453–9. 10.1016/S1473-3099(07)70158-5 [DOI] [PubMed] [Google Scholar]
- 4. Joint United Nations Programme on HIV/AIDS Global report 2018. Geneva: UNAIDS, 2018. [Google Scholar]
- 5. World Health Organization Global strategy for the prevention and control of sexually transmitted infections: 2006-2015. Geneva: WHO, 2007. [Google Scholar]
- 6. World Health Organization Fact sheet: human papillomavirus (HPV) and cervical cancer. Geneva: WHO, 2018. http://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer [Google Scholar]
- 7. World Health Organization Global health sector strategy on sexually transmitted infections 2016-2021. Geneva: WHO, 2016. [Google Scholar]
- 8. Zemouri C, Wi TE, Kiarie J, et al. . The performance of the vaginal discharge syndromic management in treating vaginal and cervical infection: a systematic review and meta-analysis. PLoS One 2016;11:e0163365 10.1371/journal.pone.0163365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toskin I, Murtagh M, Peeling RW, et al. . Advancing prevention of sexually transmitted infections through point-of-care testing: target product profiles and landscape analysis. Sex Transm Infect 2017;93:S69–80. 10.1136/sextrans-2016-053071 [DOI] [PubMed] [Google Scholar]
- 10. Toskin I, Blondeel K, Peeling RW, et al. . Advancing point of care diagnostics for the control and prevention of STIs: the way forward. Sex Transm Infect 2017;93:S81–8. 10.1136/sextrans-2016-053073 [DOI] [PubMed] [Google Scholar]
- 11. Turner KM, Christensen H, Adams EJ, et al. . Analysis of the potential for point-of-care test to enable individualised treatment of infections caused by antimicrobial-resistant and susceptible strains of Neisseria gonorrhoeae: a modelling study. BMJ Open 2017;7:e015447 10.1136/bmjopen-2016-015447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wedderburn CJ, Murtagh M, Toskin I, et al. . Using electronic readers to monitor progress toward elimination of mother-to-child transmission of HIV and syphilis: an opinion piece. Int J Gynaecol Obstet 2015;130:S81–3. 10.1016/j.ijgo.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 13. General assembly resolution 70/1, transforming our world: the 2030 agenda for sustainable development, A/RES/70/1, 2015. Available: undocs.org/A/RES/70/1
- 14. World Health Organization Executive board 144th session, provisional agenda item 5.1, EB144/5, 2018. Available: apps.who.int/gb/ebwha/pdf_files/EB144/B144_5-en.pdf
- 15. World Health Organization STI POCT meeting report: target product profiles and research questions. Geneva: WHO, 2014. www.who.int/reproductivehealth/POTC-TPPs-2016.pdf [Google Scholar]
- 16. Murtagh MM. The point-of-care diagnostic landscape for sexually transmitted infections (STIs). The Murtagh group, LLC, 2018. Available: www.who.int/reproductivehealth/topics/rtis/Diagnostic_Landscape_2018.pdf
- 17. Peeling RW. Diagnostics in a digital age: an opportunity to strengthen health systems and improve health outcomes. Int Health 2015;7:384–9. 10.1093/inthealth/ihv062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gliddon HD, Peeling RW, Kamb ML, et al. . A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sex Transm Infect 2017;93:S3–15. 10.1136/sextrans-2016-053069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chin CD, Cheung YK, Laksanasopin T, et al. . Mobile device for disease diagnosis and data tracking in resource-limited settings. Clin Chem 2013;59:629–40. 10.1373/clinchem.2012.199596 [DOI] [PubMed] [Google Scholar]
- 20. Guy RJ, Causer LM, Klausner JD, et al. . Performance and operational characteristics of point-of-care tests for the diagnosis of urogenital gonococcal infections. Sex Transm Infect 2017;93:16–21. 10.1136/sextrans-2017-053192 [DOI] [PubMed] [Google Scholar]
- 21. Kelly H, Coltart CEM, Pant Pai N, et al. . Systematic reviews of point-of-care tests for the diagnosis of urogenital Chlamydia trachomatis infections. Sex Transm Infect 2017;93:S22–30. 10.1136/sextrans-2016-053067 [DOI] [PubMed] [Google Scholar]
- 22. Gaydos CA, Klausner JD, Pai NP, et al. . Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect 2017;93:S31–5. 10.1136/sextrans-2016-053063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peeling RW, Holmes KK, Mabey D, et al. . Rapid tests for sexually transmitted infections (STIs): the way forward. Sex Transm Infect 2006;82:v1–6. 10.1136/sti.2006.024265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horner P, Donders G, Cusini M, et al. . Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women? - a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol 2018;32:1845–51. 10.1111/jdv.15146 [DOI] [PubMed] [Google Scholar]
- 25. Wi T, Lahra MM, Ndowa F, et al. . Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 2017;14:e1002344 10.1371/journal.pmed.1002344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014;27:587–613. 10.1128/CMR.00010-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Low N, Unemo M. Molecular tests for the detection of antimicrobial resistant Neisseria gonorrhoeae: when, where, and how to use? Curr Opin Infect Dis 2016;29:45–51. 10.1097/QCO.0000000000000230 [DOI] [PubMed] [Google Scholar]
- 28. Sadiq ST, Mazzaferri F, Unemo M. Rapid accurate point-of-care tests combining diagnostics and antimicrobial resistance prediction for Neisseria gonorrhoeae and Mycoplasma genitalium. Sex Transm Infect 2017;93:S65–8. 10.1136/sextrans-2016-053072 [DOI] [PubMed] [Google Scholar]
- 29. Donà V, Low N, Golparian D, et al. . Recent advances in the development and use of molecular tests to predict antimicrobial resistance in Neisseria gonorrhoeae. Expert Rev Mol Diagn 2017;17:845–59. 10.1080/14737159.2017.1360137 [DOI] [PubMed] [Google Scholar]
- 30. Adam T, de Savigny D. Systems thinking for strengthening health systems in LMICs: need for a paradigm shift. Health Policy Plan 2012;27:S1–3. 10.1093/heapol/czs084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sextrans-2019-054358supp001.pdf (1,004.2KB, pdf)
sextrans-2019-054358supp002.pdf (128.1KB, pdf)
sextrans-2019-054358supp003.pdf (57.7KB, pdf)