Abstract
Purpose
Acute respiratory failure (ARF) is a common cause of admission to intensive care units (ICUs). Mucoactive agents are medications that promote mucus clearance and are frequently administered in patients with ARF, despite a lack of evidence to underpin clinical decision making. The aim of this systematic review was to determine if the use of mucoactive agents in patients with ARF improves clinical outcomes.
Methods
We searched electronic and grey literature (January 2020). Two reviewers independently screened, selected, extracted data and quality assessed studies. We included trials of adults receiving ventilatory support for ARF and involving at least one mucoactive agent compared with placebo or standard care. Outcomes included duration of mechanical ventilation. Meta-analysis was undertaken using random-effects modelling and certainty of the evidence was assessed using Grades of Recommendation, Assessment, Development and Evaluation.
Results
Thirteen randomised controlled trials were included (1712 patients), investigating four different mucoactive agents. Mucoactive agents showed no effect on duration of mechanical ventilation (seven trials, mean difference (MD) −1.34, 95% CI −2.97 to 0.29, I2=82%, very low certainty) or mortality, hospital stay and ventilator-free days. There was an effect on reducing ICU length of stay in the mucoactive agent groups (10 trials, MD −3.22, 95% CI −5.49 to −0.96, I2=89%, very low certainty).
Conclusion
Our findings do not support the use of mucoactive agents in critically ill patients with ARF. The existing evidence is of low quality. High-quality randomised controlled trials are needed to determine the role of specific mucoactive agents in critically ill patients with ARF.
PROSPERO registration number
CRD42018095408.
Keywords: critical care, ARDS, assisted ventilation, non invasive ventilation
Key messages.
What is the key question?
Do mucoactive agents improve clinically important outcomes in critically ill, ventilated patients with acute respiratory failure?
What is the bottom line?
This systematic review found limited trials and evidence that mucoactives improved clinically important outcomes in patients ventilated with acute respiratory failure.
Why read on?
We describe four mucoactive agents across 13 trials and explain why we urge mucoactive use with caution in this population.
Introduction
Acute respiratory failure (ARF) is a common cause of admission into intensive care units (ICUs) and is associated with high acute and late mortality rates.1–3 The mainstay of respiratory supportive therapy is invasive and non-invasive mechanical ventilation.4 A considerable proportion of patients within ICU are mechanically ventilated, with audit data from the USA suggesting up to 40% of patients may be receiving mechanical ventilation at any given time. In the UK, an estimated 116 000 adult ICU admissions alone are for respiratory support per annum.5
Patients mechanically ventilated in the ICU for ARF can experience mucus accumulation due to several reasons including infection, muscle weakness, mucociliary escalator dysfunction and suppression of cough reflex due to analgesia and sedation used to facilitate ventilation.6 Targeted respiratory physiotherapy treatments, including mucoactive agents, are used to manage secretion retention.7 8 Mucoactive agents are a class of medications that aid the removal of mucus from the lung and are generally subclassified according to their mechanism of action.9 Mucolytics, such as N-acetylcysteine (NAC), work by breaking mucin crosslinking, mucoregulators such as carbocisteine decrease mucus production, expectorants such as hypertonic saline (HTS) work by increasing water content, and mucokinetics such as ambroxol work by increasing pulmonary mucus transport by acting on the cilia. This classification is not strict as it is known that some mucoactive agents, such as HTS, have multiple mechanisms of action.10 They can be delivered to patients either via inhalation direct to the lungs or systemically; oral or intravenous.
There are no systematic reviews on the use of mucoactive agents in mechanically ventilated or high-flow nasal oxygen (HFNO) populations specific to ARF. Furthermore, the National Institute for Health and Care Excellence,11 the British Thoracic Society12 and the Intensive Care Society12 13 do not provide published recommendations regarding mucoactive agent use in this population. In contrast to the lack of evidence of efficacy, surveys show mucoactive agent usage is common14–16 with a recent survey of ICU- level practice finding mucoactive agents were used in 83% of ICU in the UK, although with notable variation in prescribing practices.17 This survey indicated high use of NAC, HTS and carbocisteine, with the most highly ranked indication for use being thick secretions, and the most widely expected benefit that of reduced duration of mechanical ventilation. Practice relating to the use of mucoactive agents is currently guided by anecdotal evidence, local experience and clinician preference.
Objective
To systematically review the current evidence for the use of mucoactive agents in critically ill patients with ARF, with the objective of determining improvement on clinically important outcomes.
Methods
Deviations between this review and protocol are listed in the online supplementary material.
thoraxjnl-2019-214355supp001.pdf (350.3KB, pdf)
Types of studies
We included any design of clinical trial that compared at least one mucoactive agent with standard care or placebo in ventilated patients (including HFNO) with ARF. We excluded observational, case series, pilot/feasibility and longitudinal studies.
Types of participants
We included trials that studied adults (at least 15 years old) who were mechanically ventilated, either invasively, non-invasively or had received HFNO for treatment of ARF. Invasive mechanical ventilation was defined as a participant requiring an artificial tracheal airway. Non-invasive ventilation was defined as application of respiratory support via a sealed face-mask, nasal mask, mouthpiece, or full face visor or helmet, without the need for tracheal intubation. HFNO was defined as any cannula sitting within the nostrils and delivering heated and humidified oxygen with a gas flow rate of ≥20 L/min.18 ARF was defined to include the following conditions: acute hypoxic respiratory failure (type 1), acute hypercapnic respiratory failure (type 2), acute respiratory distress syndrome (ARDS) and acute lung injury (ALI). Patients could present with one or more of these conditions. Patients with pre-existing chronic respiratory conditions with sputum abnormalities such as bronchiectasis or cystic fibrosis were excluded, however, patients with other chronic respiratory conditions including chronic obstructive pulmonary disease (COPD) were included.
Types of interventions
We included trials involving at least one mucoactive agent. A mucoactive agent was defined as any agent used to assist the removal of mucus/sputum from the lungs.9 19 We included any method of delivery as mucoactive mechanisms of action impact on the same clinical outcomes.20 We did not regard isotonic saline as an active mucoactive agent as it was used as the placebo/control group in several trials.
Outcomes
The primary outcome for invasive/non-invasive ventilated patients was duration of ventilation and for HFNO patients was intubation rate. Secondary outcomes were time to extubation, reintubation, number of ventilator free days at day 28, duration of ICU stay, duration of hospital stay, mortality (all cause within 28, 60 and 90 days), health-related quality of life, adverse events/development of other pathologies and healthcare related costs. All outcomes included were from a recently published core outcome set for trials of interventions intending to modify the duration of mechanical ventilation.21
Search strategy
We searched three electronic databases (MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials), three clinical trial registries (EU Clinical Trial Register, ClinicalTrials.gov, WHO trial registry) and grey literature (OpenGrey). With the aid of a medical librarian, a comprehensive search strategy was developed using appropriate Medical Subject Headings (MeSH) and keywords (online supplementary material). The references of relevant articles were also checked for potentially eligible trials. All searches were from database inception to 9 January 2020. The search was for English language only articles.
Data collection and analysis
Search results were imported into a systematic review manager (Covidence: www.covidence.org) for screening and data extraction.
Selection of studies
Two review authors (RA and CY) independently screened titles and abstracts from the searches for inclusion. The full text of publications was retrieved for potentially eligible studies. Full-text publications were additionally also independently screened for inclusion by a third review author (DM) to confirm inclusion. Any disagreements or uncertainties were resolved through discussion or with consultation of the other review authors.
Data extraction
Data from included trials were extracted independently by two review authors (RA and CY) including: the type and setting of the study, number of participants, eligibility criteria (online supplementary material), nature of the intervention(s) in each group (dose and route of administration of mucoactive agent), time points of outcome measurement and results of outcomes. Any disagreements were resolved through discussion or with consultation of the other review authors. Study authors were contacted to request data that were not reported or insufficient in the main publication.
Assessment of risk of bias
Two review authors (RA and CY) independently used the Cochrane Risk of Bias tool for randomised trials within Covidence.22 Risk of bias was assessed across eight domains: sequence generation, allocation concealment, blinding of outcome assessors, blinding of participants, blinding of personnel, selective outcome reporting, incomplete outcome data and other sources of bias. Each potential source of bias was marked as high, low or unclear.
Measures of effect
The unit of analysis was the participant. Binary variables were calculated as odds ratios (ORs) with 95% confidence intervals (CIs). Continuous variables were calculated as mean differences (MDs) with 95% CIs. When analysing for mortality, we used the longest fully reported follow-up.23 Meta-analyses were performed using Review Manager V.5.3 and statistical heterogeneity evaluated using the I2 statistic, with a value of >50% implying substantial heterogeneity.24 Trials with standard care or a placebo as a comparator were grouped as ‘non-mucoactive agent’ and synthesised together. This was then compared with the pooled mucoactive group. We estimated mean values from trials that reported any relevant median values using methodologies from Wan et al.25 Analysis was undertaken using random methods models.
For the NEBULAE trial (Effect of On-Demand vs Routine Nebulization of Acetylcysteine With Salbutamol on Ventilator-Free Days in Intensive Care Unit Patients Receiving Invasive Ventilation),26 the control group (routine nebulisation of a mucoactive agent) was regarded as the mucoactive agent group in the analyses and the ‘on-demand’ group was regarded as the control/standard care. The analyses compared the routine nebulisation group, that is, the intervention group to a subset of patients from the on-demand group that received no nebulisations, that is, the control group (n=268). This excluded patients that received NAC and salbutamol treatment in the control group. In the original trial, the control group was the routine nebulisation group, and the intervention group was the on-demand group.
Subgroup analysis
Where possible we performed meta-analysis based on specific mucoactive agent when there was adequate data. This included a subgroup analysis of mucoactive agent type for duration of ventilation, mortality and duration of ICU stay. There were insufficient trials to test subgroup differences in other outcomes.
Sensitivity analysis
Sensitivity analyses were not undertaken due to the small number of trials and a lack of aggregate data.27
Quality of evidence
Five authors (RA, JB, BB, BC and BO) determined an overall certainty of the evidence for the outcomes using the principles of the GRADE system (Grades of Recommendation, Assessment, Development and Evaluation) to rate the strength of findings into one of four levels: high, moderate, low or very low.28 We generated a ‘Summary of Findings’ table using GRADEpro software (www.gradepro.org) to present outcome-specific information regarding overall certainty of evidence from studies. Certainty of evidence was assessed according to study design, risk of bias, inconsistency, indirectness, imprecision, publication bias and any other factors, for example, confounding and downgraded by one (for serious) or two (for very serious) levels as necessary.
Results
Literature search
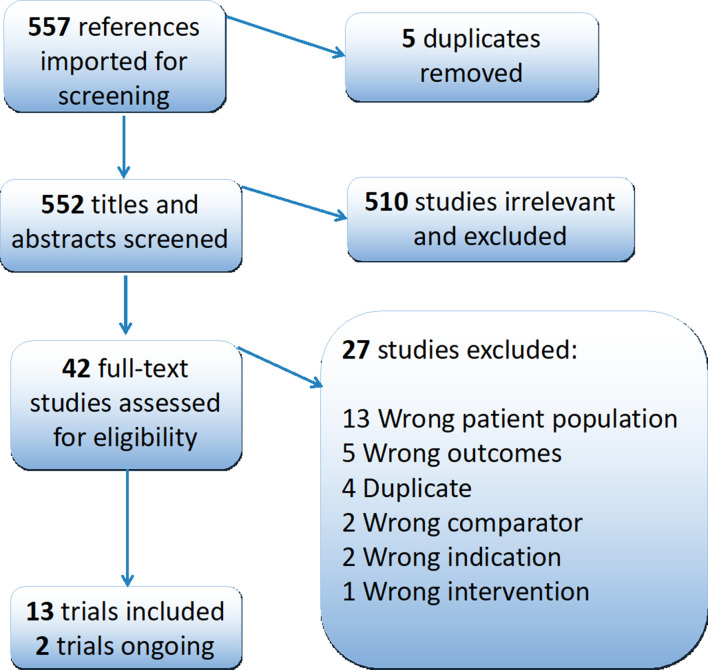
The search identified 557 results (figure 1). After importing into covidence, five duplicates were identified and removed. After title and abstract screening, 510 records were excluded leaving 42 records for full text screening. After full-text screening, 13 completed trials were eligible for inclusion in the review26 29–40 with two trials identified as ongoing.41 42 A list of the 27 excluded studies, with reasons for exclusion, are within the online supplementary material. Also included within the online supplementary material are the numbers of search results found from each data resource.
Figure 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics and risk of bias
Characteristics of included trials are summarised in table 1. All studies were prospective randomised parallel group controlled trials, totalling 1712 patients. Mucoactive agents investigated included NAC (n=8 trials), heparin (n=3 trials), ambroxol (n=1 trial) and HTS (n=1). Nine trials included patients with ALI or ARDS, and four trials with a mixed ARF population. The number of participants in the trials ranged from 27 to 922. The longest follow-up was reported at 90 days. No trials involved patients receiving HFNO.
Table 1.
Characteristics of included studies
| Author and year | No of participants | Age (years, mean±SD) | Population (ventilation as described in paper) |
Mucoactive agent intervention and dose | Control | Duration to last follow-up (days) | Outcomes measured relevant for this r eview |
| Bandeshe38 2016* | 214 | Placebo: 59 Usual care: 62 Intervention: 57 (reported as medians) |
Mixed (invasive mechanical ventilation) |
Heparin (Nebulised; 2 mL 5000 units every 6 hours) |
Placebo (0.9%) NaCl Usual care |
Not reported | Mortality, ICU stay, hospital stay, duration of mechanical ventilation and adverse events. |
| Bernard33 1997 | 46 | Control: 47±4 Intervention: 43±6 |
ARDS (mechanically ventilated) |
NAC (Intravenous; 70 mg/kg, every 8 hours, for 10 days) |
Placebo (5% dextrose in water) |
30 | Mortality, ventilator free days at day 30 and adverse events. |
| Dixon40 2010 | 50 | Control: 55.5±17.0 Intervention: 56.0±16.5 |
Mixed (invasive mechanical ventilation) |
Heparin (Nebulised; 25 000 U/5 mL every 4 or 6 hours) |
Placebo (0.9% NaCl) |
60 | Mortality, ventilator free days at day 28, ICU stay, hospital stay and adverse events. |
| Domenighetti31 1997 | 42 | Control: 52.4±17 Intervention: 52.1±17.8 |
ARDS (invasive and non-invasive ventilation) |
N-acetylcysteine (Intravenous; 190 mg/kg/day, for 2 days) |
Placebo (not defined in paper) |
ICU discharge (not reported) |
Mortality, ICU stay, duration of ventilation and adverse events. |
| Jepsen35 1992 | 66 | Control: 51.5 Intervention: 50.5 |
ARDS (tracheal intubation) |
N-acetylcysteine (Intravenous; 150 mg/kg then 20 mg/kg/hour for 6 days) |
Placebo (not defined in paper) |
60 | Mortality. |
| Masoompour30 2015 | 40 | Control: 50.6±21 Intervention: 59.7±22 |
Mixed (invasive ventilation) |
N-acetylcysteine (Nebulised; 2 mL of 20%, 3 times per day) |
Control (isotonic saline) |
1 | Mortality. |
| Moradi37 2009 | 27 | Control: 49.2±4.5 Intervention: 48.4±5.5 |
ARDS/ALI (mechanically ventilated) |
N-acetylcysteine Intravenous; 150 mg/kg diluted in 5% dextrose day 1 then 50 mg/kg/day for 3 consecutive days) |
Placebo (5% dextrose) |
Not reported | Mortality, ICU stay and duration of mechanical ventilation. |
| Ortolani36 2000 | 36 | Control: 55±13 Intervention: 57±14 |
ARDS (artificial ventilation) |
N-acetylcysteine (Intravenous; 50 mg/kg in 5% dextrose, every 8 hours for 9 days) |
Placebo (5% dextrose in water) |
30 | Mortality and ICU stay. |
| Saleh39 2017 | 80 | Control: 34.8±14.8 Intervention: 34.3±14.6 |
ARDS (mechanical ventilation) |
Heparin (Nebulised; 5000 IU heparin mixed with 3 mL of normal Saline every 4 hours) |
Standard Care (Conventional management of ARDS: Mechanical ventilation with open lung protective strategy, conservative fluid management, solumedrol at a dose of 1 mg/kg/day, antimicrobials and bronchodilators if needed) |
End of ICU stay | Mortality, ICU stay and duration of mechanical ventilation. |
| Suter32 1994 | 61 | Control: 48.1±21.9 Intervention: 46.6±19.7 |
ALI (invasive ventilation) |
N-acetylcysteine (Intravenous; 40 mg/kg/day for 3 days) |
Placebo (not defined in paper) |
ICU discharge (not reported) |
Mortality, ICU stay and adverse effects. |
| Van Meenen26
(NEBULAE) 2018† |
922 | Control: 66 Intervention: 65 (median values reported) |
Mixed (invasive) |
On demand N-acetylcysteine (Nebulised; 300 mg with 2.5 mg salbutamol) |
Routine N-acetylcysteine (300 mg with 2.5 mg salbutamol, 4 times daily for upto 28 days) |
90 | Mortality, duration of ventilation, ventilator free days at day 28, ICU stay, hospital length of stay and adverse events. |
| Yong-Jun34 2014 | 68 | Control: 57±8 Intervention: 58±12 |
ARDS (invasive and non-invasive ventilation) |
Ambroxol (Intravenous; 1005 mg/day, for 7 days) |
Placebo (0.9% isotonic saline) |
Not reported | Duration of ventilation, ICU stay and hospital stay. |
| Dahruog 201729 | 60 | Control: 41.87±16.44 intervention: 41.30±14.13 |
ARDS (invasive ventilation) |
Hypertonic saline (nebulised; 3%, 4 mL once daily, for 7 days) |
Control (not defined in paper) |
30 | Mortality, duration of mechanical ventilation, ICU stay and adverse events. |
*Standard care and placebo were combined as one group using methodology from the Cochrane Handbook Table 7.7, as this was a three arm trial comparing placebo, mucoactive and standard care.
†The control group (routine nebulisation) was regarded as mucoactive group in the analyses and the on-demand group was regarded as the control/standard care. The analyses compared the routine nebulisation group to a subset of patients from the on-demand group that received no nebulisations (n=268). This excluded patients that received NAC and salbutamol treatment in the control group.
ALI, acute lung injury; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; NAC, N-acetylcysteine.
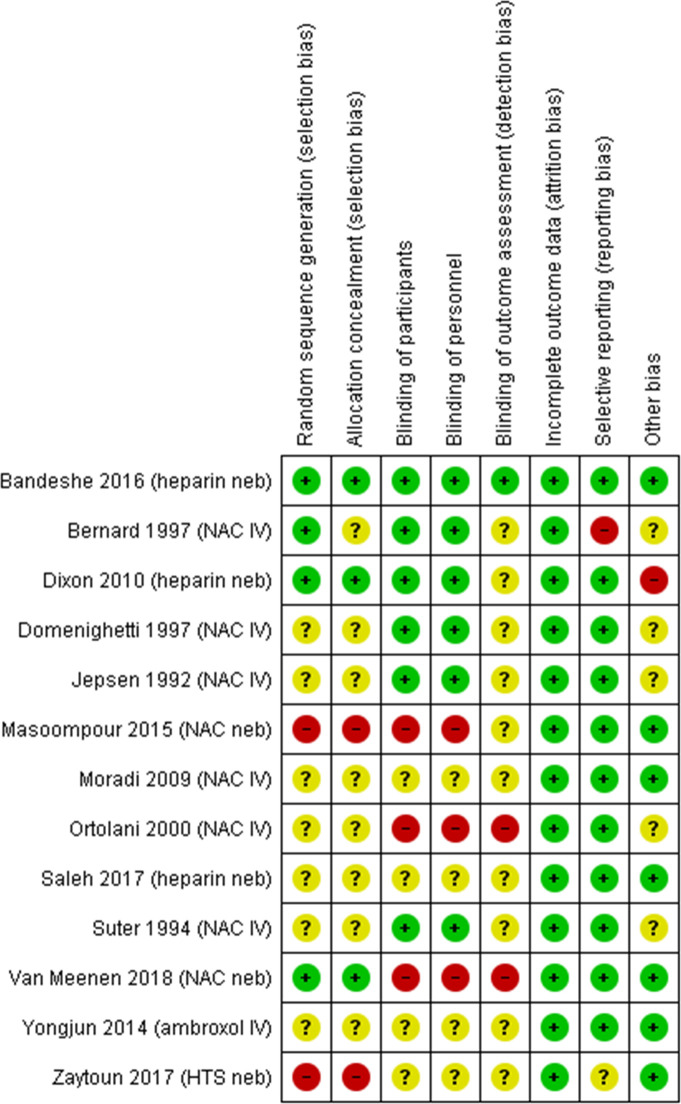
Risk of bias assessment is described in figure 2. Six trials26 29 30 33 36 40 were judged as high risk of bias in at least one domain, six trials31 32 34 35 37 39 were deemed as an unclear risk of bias across at least three domains and one trial36 overall had a low risk of bias. Ten trials29–37 39 had high or unclear risk of bias due to allocation concealment and nine29–32 34–37 39 due to sequence generation. Twelve26 29–37 39 40 trials had high or an unclear risk of bias due to blinding. All trials were at a low risk of attrition bias. One trial33 was at high risk of reporting bias.
Figure 2.
Risk of bias for included trials. (van Meenen 2018 had stated in their protocol that an economic analysis would be performed. This however was not within the trial publication. Following correspondence with authors this was a shared decision by them and the editor of JAMA. This cost related data is currently being used to prepare a separate publication to be submitted aside of the main clinical results. therefore, we assessed rob for selective outcome reporting as low.) HTS, hypertonic saline; NAC, N-acetylcysteine.
Duration of mechanical ventilation
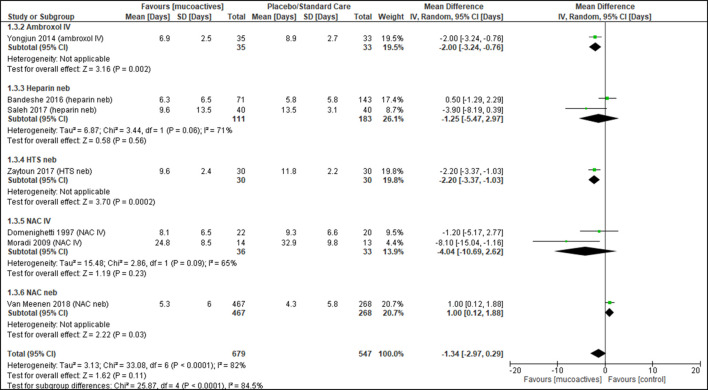
The duration of mechanical ventilation was reported in seven trials26 29 31 34 37–39 (n=1226 patients). Pooled analysis showed no effect with the use of mucoactive agents with high heterogeneity (MD −1.34, 95% CI −2.97 o 0.29, p=0.11, I2=82%) (figure 3). There was no noticeable publication bias with funnel plot estimates (online supplementary material).
Figure 3.
Duration of ventilation. Forest plot of comparison: mucoactive agent versus placebo/standard care. HTS, hypertonic saline; IV, intravenous; NAC, N-acetylcysteine; Neb, nebuliser.
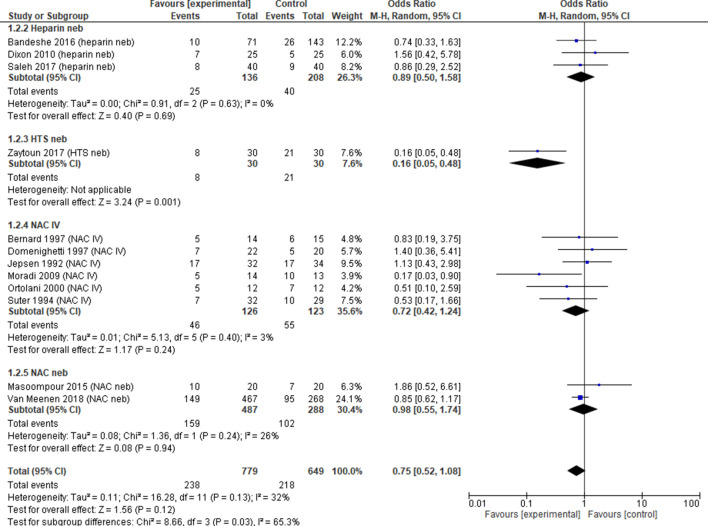
Mortality
Mortality was reported in 12 trials26 29–33 35–40 (n=1428 patients). Mucoactive agents had no effect on mortality (OR 0.75, 95% CI 0.52 to 1.08, p=0.12, I2=32%). Subgroup analysis showed no difference in reduction of mortality with use of NAC intravenous (OR 0.72, 95% CI 0.42 to 1.24, p=0.24, I2=3%), NAC neb (OR 0.98, 95% CI 0.55 to 1.74, p=0.94, I2=26%) or heparin (OR 0.89, 95% CI 0.50 to 1.58, p=0.69, I2=0%) all with low heterogeneity (figure 4). There was no noticeable publication bias with funnel plot estimates (online supplementary material).
Figure 4.
Mortality. Forest plot of comparison: mucoactive agent versus placebo/standard care. HTS, hypertonic saline; IV, intravenous; M-H, Mantel-Haenszel; NAC, N-acetylcysteine; Neb, nebuliser.
Duration of ICU stay
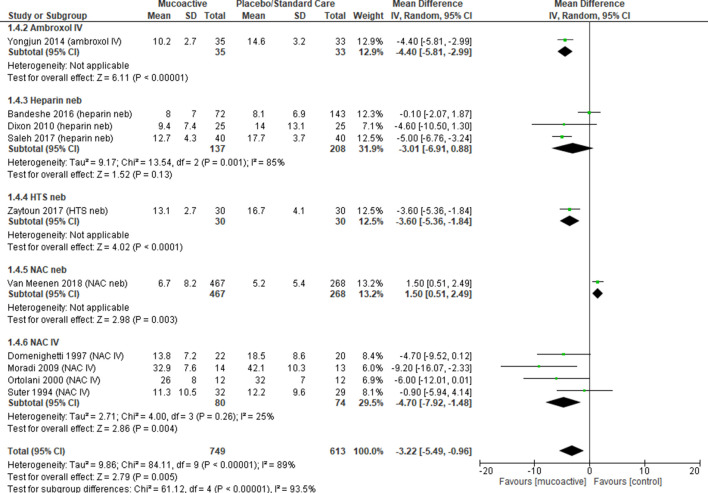
Duration of ICU stay was reported in 10 trials26 29 31 32 34 36–40 (n=1362 patients). There was a difference in duration of ICU stay favouring the use of mucoactive agents with high heterogeneity (overall MD −3.22, 95% CI −5.49 to 0.96, p=0.005, I2=89%). Subgroup analysis showed a difference in reduction of ICU stay with NAC intravenous with low heterogeneity (overall MD −4.70, 95% CI −7.92 to −1.48, p=0.004, I2=25%) and no difference in ICU stay with the use of nebulised heparin (overall MD −3.01, 95% CI −6.91 to 0.88, p=0.13, I2=85%) (figure 5). There was slight publication bias with funnel plot estimates (online supplementary material).
Figure 5.
Duration of ICU stay. Forest plot of comparison: mucoactive agent versus placebo/standard care. HTS, hypertonic saline; ICU, intensive care unit; IV, intravenous; NAC, N-acetylcysteine; Neb, nebuliser.
Duration of hospital stay
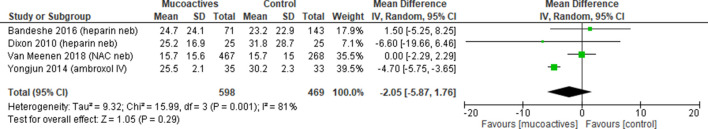
The duration of hospital stay was reported in four trials26 34 38 40 (1067=patients). Pooled analysis showed no difference with the use of mucoactive agents with high heterogeneity (overall MD −2.05, 95% CI −5.87 to 1.76, p=0.29, I2=81%) (figure 6).
Figure 6.
Duration of hospital stay. Forest plot of comparison: mucoactive agent versus placebo/standard care. IV, intravenous; NAC, N-acetylcysteine; Neb, nebuliser.
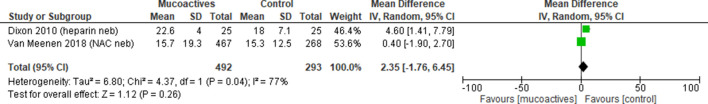
Ventilator free days
Three trials reported ventilator-free days, two at day 2826 40 and one trial reported at day 30.33 Pooled analysis showed no difference in the number of ventilator free days at day 28 with high heterogeneity (overall MD 2.35, 95% CI −1.76 to 6.45, p=0.26, I2=77%) (figure 7). For one study,33 the median number of ventilator free days was 11 for the mucoactive agent group and three for the placebo group. This study could not be incorporated into the meta-analysis as the mean (SD) could not be estimated.
Figure 7.
Ventilator free days at day 28. Forest plot of comparison: mucoactive agent versus placebo/standard care. IV, intravenous; NAC, N-acetylcysteine; Neb, nebuliser.
Intubation rate, time to extubation, reintubation, health-related quality of life and healthcare-related costs
No trials reported outcomes for intubation rate, time to extubation, reintubation, health-related quality of life or healthcare-related costs.
Adverse events and development of other pathologies
Three trials,26 33 38 investigating nebulised NAC, intravenous NAC and nebulised Heparin, respectively, reported on adverse events in both arms. Four further trials reported the number of adverse events only in the mucoactive agent arm of the study. Two trials analysed differences between groups. The NEBULAE trial26 reported that the proportion of patients developing one or more nebulisation-related adverse events was significantly higher in the mucoactive group that received nebulised NAC (p<0.001), however, the development of other pathologies/pulmonary complications were not significantly different between the two groups. One trial,29 investigating nebulised HTS, reported a significantly higher development of ventilator associated pneumonia in the control group, but did not report the original number of events in each arm (p=0.014). One trial40 investigating nebulised heparin reported no differences in number of days that patients had blood stained sputum. Further details on adverse events are summarised in the online supplementary material.
Summary of findings
Certainty of evidence is summarised in table 2. Downgrading of certainty levels was primarily related to high risk of bias and imprecision (high heterogeneity).
Table 2.
GADE and summary of findings
| Summary of findings: mucoactive agents compared with non-mucoactive agents for acute respiratory failure | |||||
| Patient or population: ventilated with acute respiratory failure Setting: intensive care unit (ICU) Intervention: mucoactive agents Comparison: non-mucoactive agents | |||||
| Outcomes | № of participants (studies) Follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
| Risk with non-mucoactives | Risk difference with mucoactives | ||||
| Duration of ventilation | 1226 (7 RCTs) |
⨁◯◯◯ VERY LOW* |
– | The mean duration of Ventilation was 0 | MD 1.34 lower (2.97 lower to 0.29 higher) |
| Mortality | 1428 (12 RCTs) |
⨁⨁⨁◯ MODERATE† |
OR 0.75
(0.52 to 1.08) |
336 per 1000 | 61 fewer per 1000 (128 fewer to 17 more) |
| Duration of ICU stay | 1362 (10 RCTs) |
⨁◯◯◯ VERY LOW‡ |
– | The mean duration of ICU stay was 0 | MD 3.22 lower (5.49 lower to 0.96 lower) |
| Duration of hospital stay | 1067 (4 RCTs) |
⨁◯◯◯ VERY LOW§ |
– | The mean duration of Hospital stay was 0 | MD 2.05 lower (5.87 lower to 1.76 higher) |
| Ventilator free days at day 28 | 785 (2 RCTs) |
⨁◯◯◯ VERY LOW¶ |
– | The mean ventilator-free days at day 28 was 0 | MD 2.35 higher (1.76 lower to 6.45 higher) |
GRADE working group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
*Risk of bias was deemed serious as the majority of trials (6/7) had an unclear or high risk of bias in at least one domain. Inconsistency was deemed as very serious due to high heterogeneity (I2=82%).
†Risk of bias was deemed serious as the majority of trials (11/12) had an unclear or high risk of bias in at least one domain.
‡Risk of bias was deemed serious as the majority of trials (9/10) had an unclear or high risk of bias in at least one domain. Inconsistency was deemed as very serious due to high heterogeneity (I2=89%).
§Risk of bias was deemed serious as the majority of trials (3/4) had an unclear or high risk of bias in at least one domain. Inconsistency was deemed as very serious due to high heterogeneity (I2=81%).
¶Risk of bias was deemed serious as both trials (2/2) had an unclear or high risk of bias in at least one domain. Inconsistency was deemed as very serious due to high heterogeneity (I2=77%).
GRADE, Grades of Recommendation, Assessment, Development and Evaluation; MD, mean difference.
Discussion
This systematic review found that use of mucoactive agents did not improve outcomes including duration of ventilation, mortality, duration of hospital stay or ventilator-free days at day 28, although they did decrease ICU stay. The GRADE evaluation showed that the certainty level of evidence for four outcomes in the review was very low, with only mortality having a moderate level of certainty of evidence. Certainty of the evidence was primarily impacted by a risk of bias mostly related to random sequence generation and allocation concealment. We note there are differences in our risk of bias assessment in some trials that were also included in a previous review, these are explained in the online supplementary material. Other reasons affecting the evidence included small sample size, mixed patient populations and high heterogeneity. Our findings indicate that further evidence is required to determine the effectiveness of mucoactive agents for critically ill patients with ARF. Future trials should be designed to address the methodological limitations identified in studies included in this review. Our systematic review echoes a previous expert narrative review in 2017 which concluded use of mucoactive agents within the critically ill is in need of evidence-based recommendations.19
Two mucoactive agents that are used within critical care units, recombinant human DNase (also known as RhDNase) and carbocisteine were not investigated in trials in this review, therefore, highlighting the lack of evidence to support their use in ventilated patients with ARF. This mirrors findings from a review of aerosolised mucoactive agents in hospitalised patients43 and guidelines by The American Association for Respiratory Care,44 although these publications were not targeted at critically ill patients in the ICU. There is evidence of effect with these mucoactive agents in other respiratory conditions. Carbocisteine shows benefit for patients with COPD45 and RhDNase for cystic fibrosis.46 However, it should not be assumed that a mucoactive agent demonstrated to be effective in one clinical population will necessarily be effective in another population and indeed there may be potential for harm. For example, although there is evidence to support RhDNase in cystic fibrosis, a well-designed and powered clinical trial showed it was not effective and potentially harmful in a bronchiectasis population.47 We note that there is an active trial exploring the use of nebulised RhDNase in ventilated ARF patients. This trial aims to recruit 500 patients to explore the use of to reduce the incidence of ARDS.48 Expected to complete in 2021, it will likely provide evidence to guide the use of RhDNase.
Eight trials in this review explored the effectiveness of intravenous NAC and a subgroup analysis highlighted that there was no benefit in mortality or duration of ventilation, however, there was a reduction in ICU stay. Our analysis of NEBULAE,26 with nebulised NAC, showed no improvement with outcomes with some evidence suggesting it may be associated with more adverse events. Our review supports the conclusions of a previous systematic review highlighting the need for more data to support use of NAC in the critically ill.49 Another review focusing on NAC use in ARDS also found a reduction of ICU stay based on three trials.50 However, they did not use the full core outcome set21 for ventilated trials or search for HFNO trials. There is a small ongoing trial expected to be complete later this year, exploring nebulised NAC in 52 ARDS patients.51
The second most common mucoactive explored in this review was nebulised heparin, showing no improvement in clinical outcomes with the exception of one trial40 showing an improvement in ventilator free days. One trial that explored ambroxol showed improvements in duration of ventilation and reduced ICU and hospital stay. However, we acknowledge that ambroxol is not available in the UK. One trial29 exploring the efficacy of HTS showed significant reductions in mortality, duration of mechanical ventilation and ICU stay but this trial was small and methodologically flawed.
Our recent survey of ICU unit level practice17 and a point prevalence survey52 exploring the use of mucoactive agents in ARF, showed HTS and carbocisteine are the most commonly used. Alongside this review, it provides justification for a clinical trial to explore the effectiveness of these mucoactive agents in ARF. These interventions could have adverse effects, and the resource use in terms of staff time to administer therapy, particularly those that are nebulised, may be significant. Conversely if any of these mucoactives have a positive significant impact on clinical outcomes there is also potential for savings such as reduced costs associated with shortened critical care and hospital stay. Also, these therapies are individually inexpensive, and a lack of robust evidence to support their use in practice may not deter some clinicians from continuing to prescribe them regardless of effectiveness. Nonetheless, as they are used in large numbers of critically ill patients, the overall scaled cost might be substantial.
The strength of our review is that it followed prespecified methods using a published protocol (PROSPERO) with duplicate and independent screening and data extraction. We also obtained a subgroup of data from one large trial to strengthen the meta-analysis providing a more accurate estimate of effect and used a full core outcome set21 with a five member panel grading the quality of evidence for each outcome. The major limitations of the results are the relatively small number and large heterogeneity of included trials, the low quality of evidence, and lack of relevant reported outcomes and associated data. Not all trials reported fully on adverse events which leaves important questions surrounding the safety and tolerability of mucoactive agents.
Conclusion
In conclusion, the evidence in this review suggests that use of mucoactive agents does not improve most clinical outcomes in ventilated patients with ARF. The findings do not support the current widespread clinical practice of various mucoactive agents in mechanically ventilated patients with ARF. However, there was considerable heterogeneity in included trials and the certainty of this evidence is low. Therefore, until further evidence is available, mucoactive agent use should be considered with caution in this population. This systematic review justifies investment in large, high-quality randomised controlled trials to explore the use of commonly used mucoactive agents, where evidence does not already exist (eg, carbocisteine) or is not adequate (eg, HTS), to inform practice. Such trials should include appropriate outcomes from the core outcome set for ventilated trials21 and report fully on adverse events.
Acknowledgments
We would like to thank Rebecca Lancaster from Queen’s University Belfast for help with the translation and screening of German language papers. We also thank the NEBULAE investigators for providing subgroup data for their trial that was funded by a grant from The Netherlands Organisation for Health Research and Development (ZonMW). We would also like to acknowledge the Professor John Glover Award for support. Lastly we acknowledge previous publication of preliminary data via conference posters in abstract books (Anand R et al. Should mucoactives be used in acute respiratory failure in the critically ill? Results from a systematic review. 2019; Anand R et al. Should mucoactives be used in acute respiratory failure in the critically ill: preliminary results from a systematic review? 2018).
Footnotes
Contributors: All authors fulfilled ICMJE guidelines for authorship criteria. RA, DM, JB, BB, CY, BO and BC contributed to the conception, data collection, data analysis, drafting the article and critical revisions. MB, MS and JW contributed to conception and provided critical revisions to the article. DvM, FP and MJS contributed to provisional data collection and critical revisions. PD provided critical revisions to the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JB, DM, BO and RA are investigators in an NIHR HTA funded trial of mucoactives in bronchiectasis.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information. Any additional details are available on reasonable request.
References
- 1. Prescott HC, Sjoding MW, Langa KM, et al. Late mortality after acute hypoxic respiratory failure. Thorax 2018;73:618–25. 10.1136/thoraxjnl-2017-210109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2., Ranieri VM, Rubenfeld GD, et al. , ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 3. Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788–800. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 4. Narendra DK, Hess DR, Sessler CN, et al. Update in management of severe hypoxemic respiratory failure. Chest 2017;152:867–79. 10.1016/j.chest.2017.06.039 [DOI] [PubMed] [Google Scholar]
- 5. Harrison D. Number of mechanically ventilated patients during 2012 The Intensive Care National Audit & Research Centre (ICNARC), 2014. [Google Scholar]
- 6. Konrad F, Schreiber T, Brecht-Kraus D, et al. Mucociliary transport in ICU patients. Chest 1994;105:237–41. 10.1378/chest.105.1.237 [DOI] [PubMed] [Google Scholar]
- 7. Magazine R, Rao S, Chogtu B. Prescribing patterns of drugs in acute respiratory distress syndrome (ARDS): an observational study. J Clin Diagn Res 2015;9:FC01–4. 10.7860/JCDR/2015/10411.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahmood NA, Chaudry FA, Azam H, et al. Frequency of hypoxic events in patients on a mechanical ventilator. Int J Crit Illn Inj Sci 2013;3:124–9. 10.4103/2229-5151.114272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balsamo R, Lanata L, Egan CG. Mucoactive drugs. Eur Respir Rev 2010;19:127–33. 10.1183/09059180.00003510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elkins MR, Bye PTP. Mechanisms and applications of hypertonic saline. J R Soc Med 2011;104 Suppl 1:2–5. 10.1258/JRSM.2011.S11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. NICE Guidance 2018 Available: https://www.nice.org.uk/guidance [Accessed 20 Mar 2018].
- 12. Davidson AC, Banham S, Elliott M, et al. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax 2016;71 Suppl 2:ii–35. 10.1136/thoraxjnl-2015-208209 [DOI] [PubMed] [Google Scholar]
- 13. The Faculty of Intensive Care Medicine Guidelines for the provision of intensive care services. 2nd Edn The Faculty of Intensive Care Medicine, 2019. [Google Scholar]
- 14. Ehrmann S, Roche-Campo F, Sferrazza Papa GF, et al. Aerosol therapy during mechanical ventilation: an international survey. Intensive Care Med 2013;39:1048–56. 10.1007/s00134-013-2872-5 [DOI] [PubMed] [Google Scholar]
- 15. Grivans C, Lindgren S, Aneman A, et al. A Scandinavian survey of drug administration through inhalation, suctioning and recruitment maneuvers in mechanically ventilated patients. Acta Anaesthesiol Scand 2009;53:710–6. 10.1111/j.1399-6576.2009.01957.x [DOI] [PubMed] [Google Scholar]
- 16. Ehrmann S, Roche-Campo F, Bodet-Contentin L, et al. Aerosol therapy in intensive and intermediate care units: prospective observation of 2808 critically ill patients. Intensive Care Med 2016;42:192–201. 10.1007/s00134-015-4114-5 [DOI] [PubMed] [Google Scholar]
- 17. Borthwick M, McAuley D, Warburton J, et al. Mucoactive agent use in adult UK Critical Care Units: a survey of health care professionals’ perception, pharmacists’ description of practice, and point prevalence of mucoactive use in invasively mechanically ventilated patients. PeerJ 2020;8:e8828 10.7717/peerj.8828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Millette BH, Athanassoglou V, Patel A. High flow nasal oxygen therapy in adult anaesthesia. Trends in Anaesthesia and Critical Care 2018;18:29–33. 10.1016/j.tacc.2017.12.001 [DOI] [Google Scholar]
- 19. Icard BL, Rubio E. The role of mucoactive agents in the mechanically ventilated patient: a review of the literature. Expert Rev Respir Med 2017;11:807–14. 10.1080/17476348.2017.1359090 [DOI] [PubMed] [Google Scholar]
- 20. Sadowska AM. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther Adv Respir Dis 2012;6:127–35. 10.1177/1753465812437563 [DOI] [PubMed] [Google Scholar]
- 21. Blackwood B, Ringrow S, Clarke M, et al. A core outcome set for critical care ventilation trials. Crit Care Med 2019;47:1324–31. 10.1097/CCM.0000000000003904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roth D, Heidinger B, Havel C, et al. Different mortality time points in critical care trials: current practice and influence on effect estimates in meta-analyses. Crit Care Med 2016;44:e737–41. 10.1097/CCM.0000000000001631 [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Meenen DMP, van der Hoeven SM, Binnekade JM, et al. Effect of on-demand vs routine nebulization of acetylcysteine with salbutamol on Ventilator-Free days in intensive care unit patients receiving invasive ventilation: a randomized clinical trial. JAMA 2018;319:993–1001. 10.1001/jama.2018.0949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deeks JJ, Higgins JP, Altman DG, Cochrane Statistical Methods Group . Analysing data and undertaking meta‐analyses In: Cochrane Handbook for systematic reviews of interventions. 23, 2019: 241–84. [Google Scholar]
- 28. Balshem H, Helfand M, Schünemann HJ, et al. Grade guidelines: 3. rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 29. Dahruog AH, Zaytoun TM, Abdella HM. Role of hypertonic saline nebulization therapy in patients with early acute respiratory distress syndrome. Intensive Care Med Exp 2017;5:74–84. [Google Scholar]
- 30. Masoompour SM, Anushiravani A, Tafaroj Norouz A. Evaluation of the effect of nebulized N-acetylcysteine on respiratory secretions in mechanically ventilated patients: randomized clinical trial. Iran J Med Sci 2015;40:309–15. [PMC free article] [PubMed] [Google Scholar]
- 31. Domenighetti G, Suter PM, Schaller MD, et al. Treatment with N-acetylcysteine during acute respiratory distress syndrome: a randomized, double-blind, placebo-controlled clinical study. J Crit Care 1997;12:177–82. 10.1016/S0883-9441(97)90029-0 [DOI] [PubMed] [Google Scholar]
- 32. Suter PM, Domenighetti G, Schaller MD, et al. N-Acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest 1994;105:190–4. 10.1378/chest.105.1.190 [DOI] [PubMed] [Google Scholar]
- 33. Bernard GR, Wheeler AP, Arons MM, et al. A trial of antioxidants N-acetylcysteine and Procysteine in ARDS. Chest 1997;112:164–72. 10.1378/chest.112.1.164 [DOI] [PubMed] [Google Scholar]
- 34. Yong-Jun L, Juan C, Bin O, et al. Effect of high dose ambroxol on the extra-vascular lung water and oxygenation of patients with extra-pulmonary acute respiratory distress syndrome. Intensive Care Med 2014;40:S233. [Google Scholar]
- 35. Jepsen S, Herlevsen P, Knudsen P, et al. Antioxidant treatment with N-acetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo-controlled study. Crit Care Med 1992;20:918–23. 10.1097/00003246-199207000-00004 [DOI] [PubMed] [Google Scholar]
- 36. Ortolani O, Conti A, De Gaudio AR, et al. Protective effects of N-acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome. Shock 2000;13:14–18. 10.1097/00024382-200013010-00003 [DOI] [PubMed] [Google Scholar]
- 37. Moradi M, Mojtahedzadeh M, Mandegari A, et al. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine. Respir Med 2009;103:434–41. 10.1016/j.rmed.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 38. Bandeshe H, Boots R, Dulhunty J, et al. Is inhaled prophylactic heparin useful for prevention and management of pneumonia in ventilated ICU patients?: the IPHIVAP Investigators of the Australian and New Zealand intensive care Society clinical Trials Group. J Crit Care 2016;34:95–102. 10.1016/j.jcrc.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 39. Saleh M, Omar E. Does nebulized heparin have value in acute respiratory distress syndrome patients in the setting of polytrauma? Egypt J Bronchol 2017;11:332–5. 10.4103/ejb.ejb_24_17 [DOI] [Google Scholar]
- 40. Dixon B, Schultz MJ, Smith R, et al. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial. Crit Care 2010;14:R180. 10.1186/cc9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ClinicalTrials.gov N-Acetyl-Cysteine in early acute respiratory distress syndrome. Available: https://clinicaltrials.gov/ct2/show/NCT03346681 [Accessed 12 Jun 2018].
- 42. ClinicalTrials.gov Inhaled dornase alpha to reduce respiratory failure after severe trauma. Available: https://clinicaltrials.gov/ct2/show/NCT03368092
- 43. Sathe NA, Krishnaswami S, Andrews J, et al. Pharmacologic agents that promote airway clearance in hospitalized subjects: a systematic review. Respir Care 2015;60:1061–70. 10.4187/respcare.04086 [DOI] [PubMed] [Google Scholar]
- 44. Strickland SL, Rubin BK, Haas CF, et al. AARC clinical practice guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care 2015;60:1071–7. 10.4187/respcare.04165 [DOI] [PubMed] [Google Scholar]
- 45. Zheng J-P, Kang J, Huang S-G, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (peace study): a randomised placebo-controlled study. Lancet 2008;371:2013–8. 10.1016/S0140-6736(08)60869-7 [DOI] [PubMed] [Google Scholar]
- 46. Yang C, Chilvers M, Montgomery M, et al. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev 2016;4:CD001127. 10.1002/14651858.CD001127.pub3 [DOI] [PubMed] [Google Scholar]
- 47. O'Donnell AE, Barker AF, Ilowite JS, et al. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. Chest 1998;113:1329–34. 10.1378/chest.113.5.1329 [DOI] [PubMed] [Google Scholar]
- 48. ClinicalTrials.gov Inhaled dornase alpha to reduce respiratory failure after severe trauma, 2017. Available: https://clinicaltrials.gov/ct2/show/NCT03368092 [Accessed 8 Mar 2019].
- 49. Tarrant BJ, Maitre CL, Romero L, et al. Mucoactive agents for adults with acute lung conditions: a systematic review. Heart Lung 2019;48:141–7. 10.1016/j.hrtlng.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 50. Lu X, Ma Y, He J, et al. N-Acetylcysteine for adults with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Hong Kong Journal of Emergency Medicine 2019;26:288–98. 10.1177/1024907918794559 [DOI] [Google Scholar]
- 51. ClinicalTrials.gov N-Acetyl-Cysteine in early acute respiratory distress syndrome, 2017. Available: https://clinicaltrials.gov/ct2/show/NCT03346681 [Accessed 8 Mar 2019].
- 52. Borthwick M, Shyamsundar M, Warburton J. Mucoactive agents in acute respiratory failure: point prevalence survey of UK adult critical care. Mucoactive agents in acute respiratory failure: point prevalence survey of UK adult critical care, Birmingham. Forthcoming poster presentation. Intensive Care Society, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2019-214355supp001.pdf (350.3KB, pdf)