Abstract
In 500 children aged ≤10 years after 13-valent pneumococcal conjugate vaccine (PCV)13 immunisation in different schedules, serotypes 19A-specific and 19F-specific immunoglobulin G (IgG) were predicted to persist above 0.35 µg/mL for ≥10 years in all groups, likely due to PCV13-induced memory with natural boosting from residual diseases and colonisation. Generally, serotype-specific IgG could persist above 0.35 µg/mL longer (≥5 years) in the catch-up group than in the 2+1 and 3+1 immunisation groups. 14.5% of the carriage isolates belonged to PCV13 serotypes; statistical analysis revealed that a high serum IgG level (>10.96 µg/mL) will be required to eliminate the point-prevalence nasopharyngeal carriage of serotype 19A.
Keywords: clinical epidemiology, bacterial infection, paediatric lung disaese, pneumonia, respiratory infection, infection control
Introduction
There is a scarcity of data on the long-term persistence of immunity after 13-valent pneumococcal conjugate vaccine (PCV13) immunisation and its impact on nasopharyngeal carriage. The national PCV13 catch-up programme implemented during 2013–2014 and the inclusion of PCV13 in the national immunisation programme in 2015 had successfully reduced the incidence of invasive pneumococcal disease (IPD) from 17.8/100 000 in 2012 to 5.5/100 000 in 2017 among children aged 0–5 years; however, information about the population-wide immunogenicity post-PCV13 vaccination remains unknown.1 We conducted a cross-sectional study in 2016–2017 to assess the persistence of immunity after the administration of PCV13 in three different schedules introduced over the years in children in Taiwan and the impact of PCV13 immunisation on nasopharyngeal carriage.
Methods
This cross-sectional study in 2016–2017 enrolled 500 children aged ≤10 years who were previously administered with PCV13 according to different schedules in 2009–2017. These children were divided into four groups on the basis of the immunisation schedule, including catch-up (1–2 doses of PCV13 (n=257), 2+1 (n=60), 3+1 (n=164), and those without vaccination (n=19) (see online supplementary figure S1 and table S1).
thoraxjnl-2019-213878supp001.pdf (170.5KB, pdf)
thoraxjnl-2019-213878supp002.pdf (47KB, pdf)
Immunoglobulin G (IgG) antibody titres specific for eight serotypes (3, 6A, 6B, 7F, 14, 19A, 19F and 23F) were determined by ELISA.2 3 In a subset of 50 sera, opsonophagocytic activity (OPA) was determined for serotypes 1, 3, 5 and 19A by multiplex OPA at a WHO Reference Laboratory in Institute of Child Health, University College London, UK.3
Serotyping was performed by latex agglutination and confirmed by Quellung reaction (Statens Serum Institut, Copenhagen, Denmark). A multiplex polymerase chain reaction serotyping was also performed as described previously.4
Linear regression analyses were performed to assess the persistence of anticapsular polysaccharide IgG after PCV13 vaccination; correlation between serum IgG (μg/mL) and time (years) after vaccination was evaluated using Pearson’s correlation coefficient (r).3 A logistic regression model was used to analyse the association between nasopharyngeal carriage and IgG concentration for each serotype.3 Additional information is included in online supplementary materials and methods.
thoraxjnl-2019-213878supp003.pdf (112.4KB, pdf)
Results
No significant differences were found in the population-wide geometric mean concentrations (GMCs) among the catch-up, 2+1 and 3+1 immunisation groups for the majority of serotypes examined in this study (see online supplementary table S2 and figure S2). GMCs for serotype 3 were low at approximately 0.30 µg/mL in different groups. GMC for serotype 19A in the 3+1 group was higher but not significant (p>0.744) when compared with other groups.
thoraxjnl-2019-213878supp004.pdf (102KB, pdf)
thoraxjnl-2019-213878supp005.pdf (483KB, pdf)
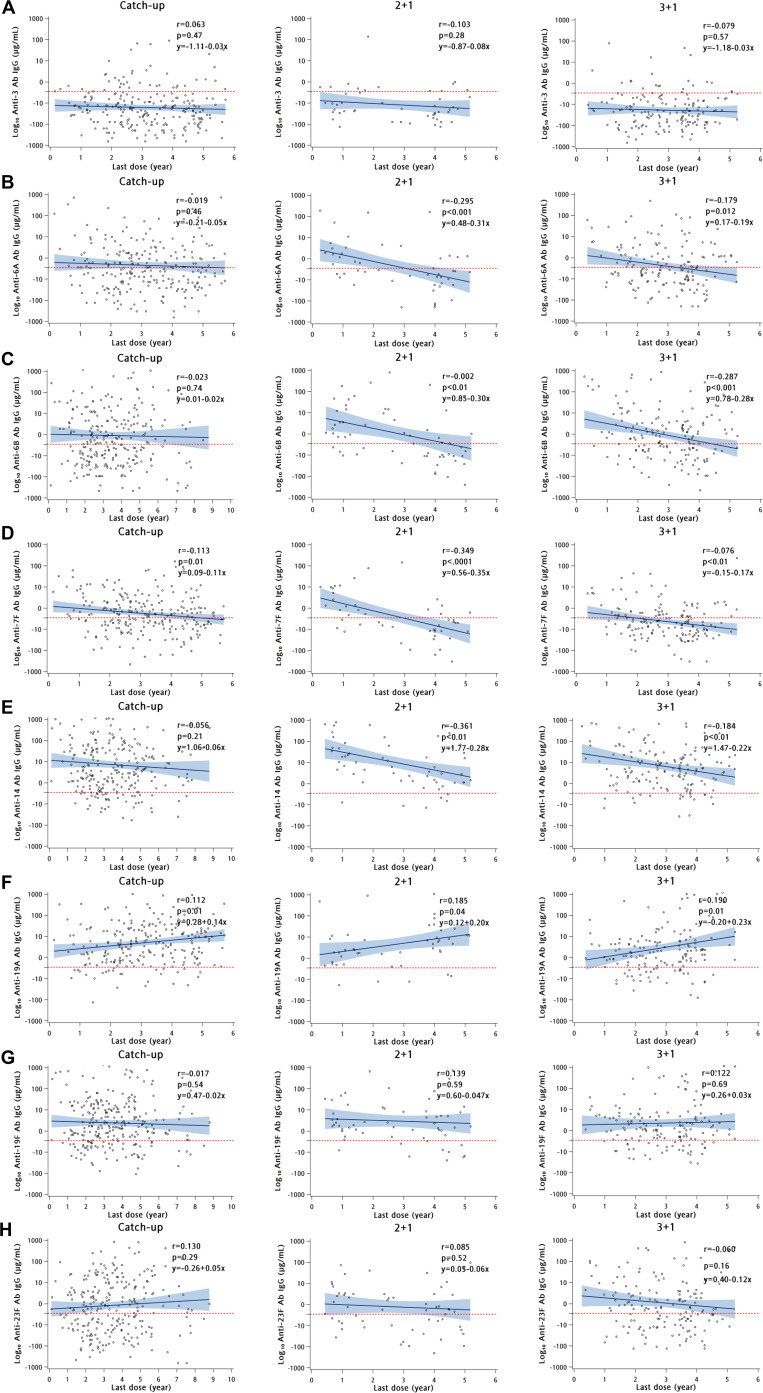
Four to six years after the PCV13 booster dose, IgG levels remained significantly high (p<0.05) for serotype 19A and significantly low (p<0.05) for 7F in all the groups (see online supplementary table S3). Regression lines and equation predicted that IgG titres against 19A and 19F serotypes could persist above the IPD protective threshold for >10 years in all the study groups (figure 1), in contrast a higher proportion of serotype 3-specific IgG titres was persistently below the threshold. There was a gradual decrease in serotypes 6A-specific, 6B-specific, 7F-specific and 14-specific IgG over time in all groups (figure 1). Only 19A-specific IgG titres showed a positive correlation (r=0.117–0.216) with time after vaccination in all the three groups, and there were >89% of children with IgG levels above the IPD protective threshold in all groups (figure 1, online supplementary figure S3 and table S3).
Figure 1.
Scatter plots showing linear regression lines and correlation coefficients between log IgG antibody titres with time (years) after PCV13 vaccination in children with catch-up (1–2 doses), 2+1 and 3+1 schedules. The individual titres are indicated by dots. Correlation coefficients were determined with the Pearson correlation test. r, Pearson correlation coefficient. P values <0.05 were regarded as statistically significant. Solid blue line is the regression line and the shaded blue region represents 95% CI of the regression line. Panels A–H refer to serotypes 3, 6A, 6B, 7F, 14, 19A, 19F and 23F, respectively. Time since last PCV13 dose ranged from 1 month to 9 years. Serotype 19A epidemic peaked in the years 2011 and 2012 in Taiwan; this time period corresponds to 4–6 years post last PCV13 dose. IgG, immunoglobulin G; PCV13, 13-valent pneumococcal conjugate vaccine.
thoraxjnl-2019-213878supp006.pdf (55.4KB, pdf)
thoraxjnl-2019-213878supp007.pdf (110.5KB, pdf)
The proportion of subjects reaching both the seroprotective thresholds (IgG ≥0.35 µg/mL and OPA ≥8) for serotypes 1, 3, 5 and 19A were 30.4, 36.2, 67.4 and 92%, respectively (see online supplementary figure S4A). Overall, for serotype 3, a high percentage (53.2%; 25/47) of subjects could not reach the IgG level of ≥0.35 µg/mL; however, among them, 31.9% (15/47) attained functional OPA titre of ≥8 and the geometric mean titre was 36.64 (see online supplementary figure S4B). Serotype 19A-specific functional OPA titres were found to be elevated and stable for 2–5 years in 66% of the subjects (see online supplementary figure S4C).
thoraxjnl-2019-213878supp008.pdf (607.8KB, pdf)
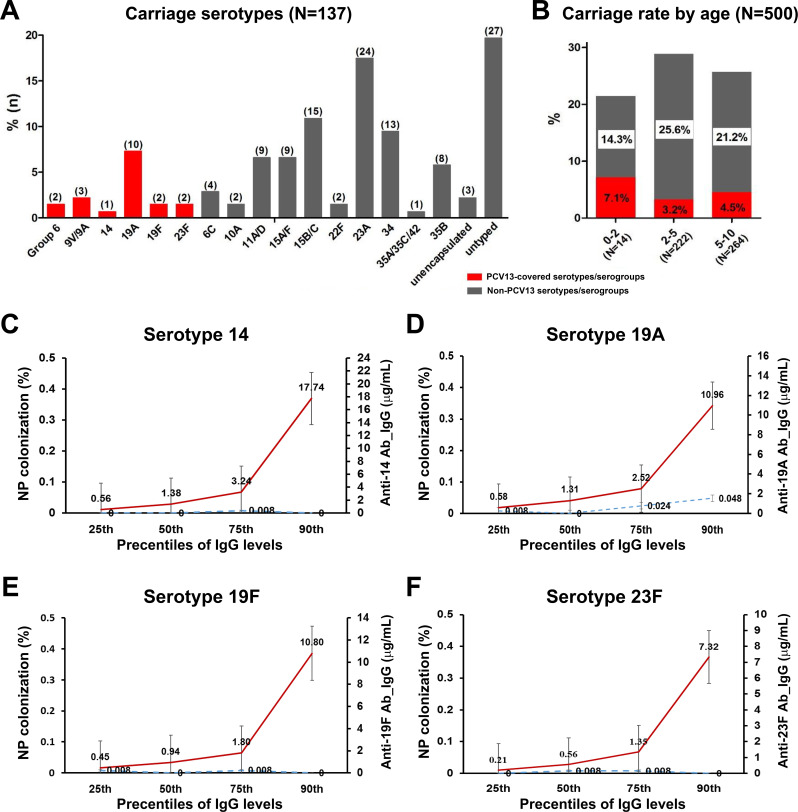
Among the 500 children, 135 (27.0%) were positive for pneumococcal carriage and overall 20 (4%) carried PCV13 serotypes. The predominant vaccine serotype was 19A and the most prevalent non-vaccine serotypes were 23A, 15B/C and 34 (figure 2A, B). None of the children were colonised with PCV13 serotypes 1, 3, 4, 5, 7F, 9V and 18C.
Figure 2.
Nasopharyngeal carriage of Streptococcus pneumoniae in children vaccinated with PCV13 in 2016–2017. Panel A displays serotype distribution of S. pneumoniae isolates. Number of isolates per serotype is in parentheses. PCV13 (red bars) and non-PCV13 serotypes (black bars). Panel B displays carriage rate of S. pneumoniae isolates of PCV13 serotypes (red bars) and non-PCV13 serotypes (black bars) by age group. The carriage rate was computed by dividing the number of positive samples by the total number of samples collected. Panels C–F display predicted point-prevalence carriage rates for serotypes 14, 19A, 19F and 23F corresponding to IgG concentration after PCV13 vaccination in 500 children. For each serotype, subjects were tested for IgG antibody levels and nasopharyngeal carriage of S. pneumoniae. The predicted changes in point-prevalence nasopharyngeal carriage rate corresponding to the antibody concentration threshold of ≥0.35 µg/mL and to selected percentiles of IgG concentration were calculated for the total population. Red line represents the IgG antibody levels in the secondary axis, and the blue dotted line represents the percent of nasopharyngeal carriage in the primary axis. IgG, immunoglobulin G; PCV13, 13-valent pneumococcal conjugate vaccine.
The carriage rates of PCV13 serotypes were low and there was no evidence of a statistically significant relationship between IgG concentrations and carriage rates, except for serotype 19A (figure 2C–F). Despite the fact that no difference was found for serotypes 14, 19F and 23F, the point-prevalence nasopharyngeal carriage rates were 0 at the 90th percentile of IgG titres for the three serotypes. The carriage rate for 19A slightly increased with an increase in serum IgG titres. The odds of carriage were similar for all serotypes, whereas the odds were statistically significant for 19A only (p=0.0061, online supplementary table S4). Comparison of pneumococcal carriage rates with IgG percentiles revealed that a serum IgG level >10.96 µg/mL is associated with a low point-prevalence nasopharyngeal carriage rate of serotype 19A (figure 2D).
thoraxjnl-2019-213878supp009.pdf (38.7KB, pdf)
Discussion
Long-term immunogenicity studies on CRM197-based PCVs have been largely based on PCV7, indicating that serum IgG levels against vaccine serotypes remained ≥0.35 µg/mL in the majority of children for at least 4 years after the booster.5 6 In this study, trend lines predicted that IgG titres against the predominant nasopharyngeal isolates in 2010–2014 (19A and 19F) in this region could persist above 0.35 µg/mL for >10 years in both the 2+1 and 3+1 immunisation groups, but the antibody concentrations and the persistence for the rare serotype 7F were lower (<3 years) in both groups.
Interestingly, trend lines revealed that IgG levels could persist for ≥10 years for the prevalent PCV13 serotypes in the catch-up group. Since the age (2.97±0.12 years) at the administration of the last dose in the catch-up group was >20 months, compared with 1.22±0.15 years in the 2+1 group and 1.08±0.03 years in the 3+1 group, administration of PCV13 to older children could induce a stronger immune response. This observation was consistent with a previous case–control study that found that the incidence of vaccine-type (84%, 19A) severe pneumococcal pneumonia was significantly less in children with catch-up schedules (7.1%) compared with that in children with the 2+1/3+1 schedule (24.1%) and in age-inappropriate, under-immunised children (28.6%).7 Some clinical trials of PCVs also observed a robust immune response to one dose of PCV13 in previously immunised children aged 2–5 years.5 8
In a subset of 50 subjects from the three groups, serotype 3-specific IgG titres that reached the protective level (≥0.35 µg/mL) were found only in 46% of the children, whereas the OPA titres that achieved the threshold (≥1:8) were found in 68.1% of the children, suggesting that some sera with lower IgG titres were OPA-positive. Despite the low levels of serotype 3-specific IgG titres in the study population, none of the children were colonised with serotype 3 isolates. Together, these observations support the notion that functional OPA may be a better predictor of protection than serum IgG for some serotypes such as serotype 3.
Among PCV13 serotypes, 19A carriage rate in our earlier study (5.0%, 2014–2015) was not different from this study (7.4%, 2016–2017); the persistent carriage might partially explain the 19A residual infections occurring in Taiwan. After the introduction of PCV13 initially as a catch-up schedule in 2013–2014, the incidence of IPDs caused by 19A in children aged 0–5 years has decreased significantly.1 In Australia, after introduction of the 3+0 schedule, the incidence of 19A IPDs decreased significantly, but 19A persisted as the predominant PCV13 serotype causing breakthrough IPD in the second year of life.9 Our data also demonstrated that a single ‘19A-like’ epidemic serotype did not emerge from the non-vaccine serotypes in the post-PCV13 era, but isolates belonging to serogroup 15 and serotypes 23A, 11A, 35B and 34 have emerged with high prevalence. However, these non-vaccine serotypes tended to exhibit less invasive potential, thereby contributing to the overall decline of IPD.1 6
Both colonisation and residual diseases caused by serotypes 19A and 19F contributed to the natural boosting of serotype-specific antibodies that subsequently could persist for ≥10 years.1 6 8Given the current carriage rate of 2.0% for serotype 19A, very high concentration of serum IgG (>10.96 µg/mL) will be required to eliminate the carriage. Alternatively, we could also hypothesise that after the last dose of PCV13 a new acquisition and colonisation of vaccine serotype is still possible in some children despite the presence of high-level serotype-specific IgG. These conclusions reflect this cross-sectional study conducted in the PCV13 era without the baseline carriage rates in the same population for comparison. Previous studies also showed that natural exposure to circulating serotypes increases antibody titres, but higher antibody titres are required to prevent colonisation and infections by frequently carried serotypes, including 19F and 19A.10 11 Therefore, a common correlate of protection against colonisation for all serotypes could not be established and the predicted correlates varied among studies in children and adults.3 12Further studies are necessary to discover better correlates of protection derived from memory B cells or nasal wash IgG titres.11 These new models for Streptococcus pneumoniae acquisition and carriage could give us better prediction results.
Acknowledgments
The authors thank the children and their families for participating in this research with prior consent. The authors thank for the statistical assistance and acknowledge the support from the Maintenance Project of the Center for Big Data Analytics and Statistics at Chang Gung Memorial Hospital for statistical consultation and data analysis.
Footnotes
Contributors: C-HC conceived and designed the study. M-HH, S-HW and M-JY carried out the experiments. RPJ, M-HH, C-LC, S-HW, M-JY, L-HS, T-YL and C-HC performed the data analysis and figure/table preparation. RPJ and C-HC reviewed the literature, interpreted data and wrote the manuscript.
Funding: This work was supported by Chang Gung Memorial Hospital (CMRPG3H0041, CMRPG3G1971, CMRPG3F1143 and CMRPG3F0083) and Ministry of Science and Technology (105-3011-F-182A-001), Taiwan.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Lu C-Y, Chiang C-S, Chiu C-H, et al. . Successful control of Streptococcus pneumoniae 19A replacement with a catch-up primary vaccination program in Taiwan. Clin Infect Dis 2019;69:1581–7. 10.1093/cid/ciy1127 [DOI] [PubMed] [Google Scholar]
- 2. Huang L-M, Lin T-Y, Juergens C. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine given with routine pediatric vaccines in Taiwan. Vaccine 2012;30:2054–9. 10.1016/j.vaccine.2011.12.054 [DOI] [PubMed] [Google Scholar]
- 3. Goldblatt D, Southern J, Andrews NJ, et al. . Pneumococcal conjugate vaccine 13 delivered as one primary and one booster dose (1 + 1) compared with two primary doses and a booster (2 + 1) in UK infants: a multicentre, parallel group randomised controlled trial. Lancet Infect Dis 2018;18:171–9. 10.1016/S1473-3099(17)30654-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Su L-H, Kuo A-J, Chia J-H, et al. . Evolving pneumococcal serotypes and sequence types in relation to high antibiotic stress and conditional pneumococcal immunization. Sci Rep 2015;5:15843. 10.1038/srep15843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trück J, Snape MD, Tatangeli F, et al. . Pneumococcal serotype-specific antibodies persist through early childhood after infant immunization: follow-up from a randomized controlled trial. PLoS One 2014;9:e91413. 10.1371/journal.pone.0091413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dagan R, Juergens C, Trammel J, et al. . Modeling pneumococcal nasopharyngeal acquisition as a function of anticapsular serum antibody concentrations after pneumococcal conjugate vaccine administration. Vaccine 2016;34:4313–20. 10.1016/j.vaccine.2016.06.075 [DOI] [PubMed] [Google Scholar]
- 7. Lee H-Y, Hsieh Y-C, Liu C-C, et al. . Invasive pneumococcal pneumonia caused by 13-valent pneumococcal conjugate vaccine types in children with different schedules. J Microbiol Immunol Infect 2018;51:199–206. 10.1016/j.jmii.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 8. Frenck R, Thompson A, Yeh SH, et al. . Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in children previously immunized with 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2011;30:1086–91. 10.1097/INF.0b013e3182372c6a [DOI] [PubMed] [Google Scholar]
- 9. Jayasinghe S, Chiu C, Quinn H, et al. . Effectiveness of 7- and 13-valent pneumococcal conjugate vaccines in a schedule without a booster dose: a 10-year observational study. Clin Infect Dis 2018;67:367–74. 10.1093/cid/ciy129 [DOI] [PubMed] [Google Scholar]
- 10. Ekström N, Ahman H, Palmu A, et al. . Concentration and high avidity of pneumococcal antibodies persist at least 4 years after immunization with pneumococcal conjugate vaccine in infancy. Clin Vaccine Immunol 2013;20:1034–40. 10.1128/CVI.00039-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pennington SH, Pojar S, Mitsi E, et al. . Polysaccharide-specific memory B cells predict protection against experimental human pneumococcal carriage. Am J Respir Crit Care Med 2016;194:1523–31. 10.1164/rccm.201512-2467OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andrews NJ, Waight PA, Burbidge P, et al. . Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 2014;14:839–46. 10.1016/S1473-3099(14)70822-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2019-213878supp001.pdf (170.5KB, pdf)
thoraxjnl-2019-213878supp002.pdf (47KB, pdf)
thoraxjnl-2019-213878supp003.pdf (112.4KB, pdf)
thoraxjnl-2019-213878supp004.pdf (102KB, pdf)
thoraxjnl-2019-213878supp005.pdf (483KB, pdf)
thoraxjnl-2019-213878supp006.pdf (55.4KB, pdf)
thoraxjnl-2019-213878supp007.pdf (110.5KB, pdf)
thoraxjnl-2019-213878supp008.pdf (607.8KB, pdf)
thoraxjnl-2019-213878supp009.pdf (38.7KB, pdf)