Abstract
Rationale:
The use of autologous hematopoietic stem cell transplantation (AHSCT) for autoimmune diseases has become the first indication for transplant in nonmalignant disease. Mucormycosis is a rare invasive infection with increasing incidence in patients treated with AHSCT. We report the first case of pulmonary mucormycosis following AHSCT for systemic sclerosis (SSc).
Patient concerns:
A 24-year-old woman with rapidly progressive diffuse cutaneous SSc presented with an acute respiratory distress syndrome 6 days after AHSCT.
Diagnoses:
The results of clinical and computed tomography scan were consistent with pulmonary mucormycosis and the diagnosis was confirmed by a positive Mucorales Polymerase Chain Reaction on a peripheral blood sample.
Interventions and Outcomes:
Early antifungal therapy by intravenous amphotericin B provided rapid improvement within 4 days and sustained recovery after 2 years of follow-up.
Lessons:
With the progressively increasing use of AHSCT and other stem cell therapy for treatment of severe SSc and other autoimmune diseases, the potential onset of rare post-transplant fungal infections, such as mucormycosis, requires careful patient monitoring and better awareness of early initiation of adequate therapy.
Keywords: autologous stem cells, mucormycosis, systemic sclerosis
1. Introduction
Mucormycosis is a rare severe invasive fungal infection, caused by filamentous fungi of the class zygomycetes order Mucorales, primarily encountered in transplanted or diabetic patients.[1–4] This severe disease occurs in highly immunosuppressed patients and is associated with a mortality rate ranging from 35% to 60%, depending on the associated comorbidities.[5]
Over the past 20 years the use of autologous hematopoietic stem cell transplantation (AHSCT) to treat severe rapidly progressive systemic sclerosis (SSc) has expanded progressively and this procedure is now recommended as a first-line option,[6–8] on condition that the procedure is performed in an expert center, according to European Society for Blood and Marrow Transplantation guidelines.[9–12] In such circumstances, careful patient pretransplant evaluation and close posttransplant follow-up are recommended,[11,12] especially during the first 2 years after transplant while progressive immune reconstitution occurs. Mucormycosis has never been reported following AHSCT for autoimmune diseases or in SSc patients.
In this article we report on the first case of pulmonary mucormycosis after AHSCT for rapidly progressive diffuse cutaneous SSc.
2. Case
A 24-year-old woman diagnosed with SSc 2 years ago and who had no other past medical history, was initially treated with low-dose corticosteroids and mycophenolate mofetil for 9 months then with methotrexate for 6 months and 2 courses of rituximab. She was then referred for AHSCT due to a rapidly progressive disease with extensive skin fibrosis (modified Rodnan skin score 25/51) and pulmonary involvement [decreased diffusing capacity of the lung for carbon monoxide at 56% of the theoretical values on lung function tests and the presence of ground-glass infiltrate and diffuse bronchial wall thickening on chest computerized tomography (CT) scan]. Right heart catheterization with fluid overload, echocardiography, cardiac magnetic imaging, and myocardial scintigraphy were all normal.
Peripheral blood hematopoietic stem cells (PBSCs) were mobilized with intravenous (IV) cyclophosphamide (CPM) (total of 2 g/m2 administered over 2 consecutive days), and filgrastim (10 μ/kg/day for 7 days), allowing collection of 25 × 106 CD34+ cells/kg in 2 cytaphereses.
Forty days later, a conditioning regimen with a total dose of 200 mg/kg IV CPM over 4 consecutive days with 2 L of 0.9% IV saline/day, plus IV rabbit antithymocyte globulins (2.5 mg/kg/day for 5 consecutive days) was administered followed by a reinjection of non-selected PBSC (day 0).
On day 0, the patient had leukopenia of less than 0.1 g/L and aplasia was sustained until day 12. She experienced her first febrile peak (39°C) the day after PBSC reinfusion while physical examination was otherwise normal and peripheral and port-a-cath blood cultures remained sterile. An empiric IV antibiotic therapy by piperacillin-tazobactam and amikacin was started. The patient remained afebrile until day 4 after PBSC reinjection, when she had a new onset of fever with a progressive increase in C-reactive protein level (278 mg/L). Repeated peripheral and port-a-cath blood cultures were sterile. The IV antibiotic therapy was switched to meropenem, ciprofloxacin, and vancomycin.
The patient remained febrile (39°C) and 2 days later developed a dry cough. The chest CT scan revealed massive bilateral pleural effusion, upper right lobe condensation, and less extensive upper left lobe condensation (Fig. 1).
Figure 1.
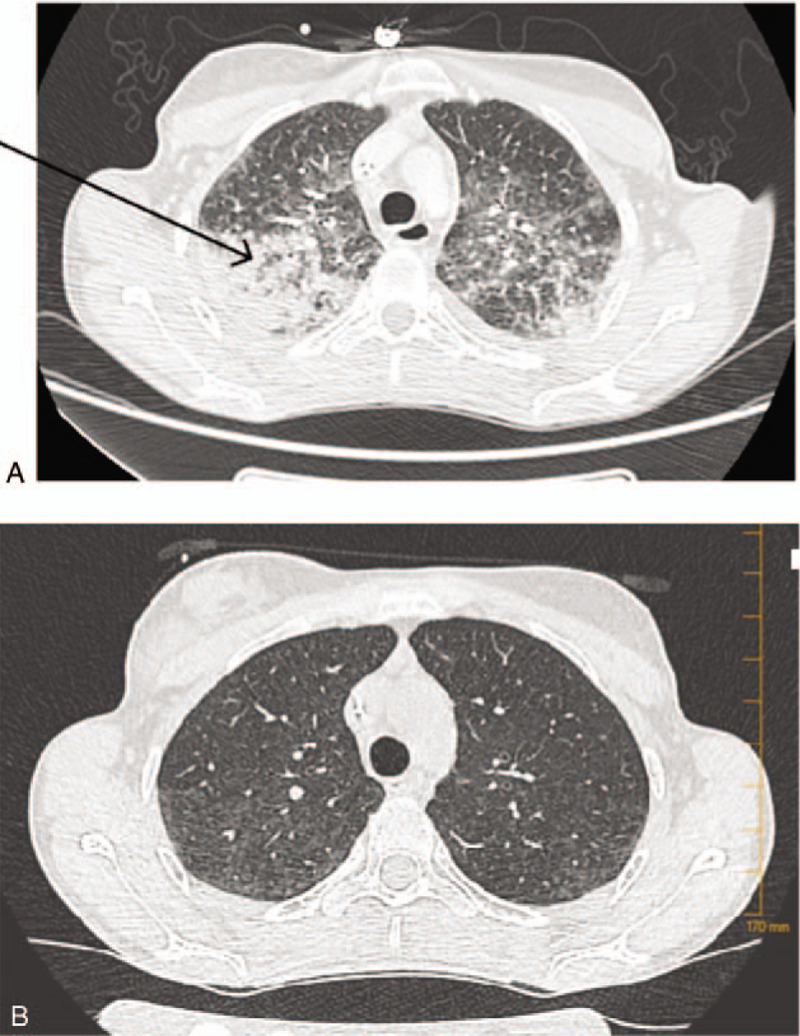
Pulmonary mucormycosis appearing as a mass with the halo sign on computerized tomography (CT) in 24-year-old woman after autologous hematopoietic stem cell transplantation for rapidly progressive diffuse cutaneous systemic sclerosis. A, CT scan on day 6, showing a bilateral parenchymatous condensation within the right upper lobe the reverse halo sign. B, CT scan on day 20 showing showed a nearly complete regression of bilateral parenchymatous condensation and disappearance of the reverse halo sign.
On day 6, an acute respiratory distress syndrome with hypoxemia at 60 mm Hg required endotracheal intubation and mechanical ventilation. Acyclovir (10 mg/kg/dose/8 h IV) and amphotericin B (5 mg/kg/dose/24 h IV) were added. Repeated CT scan showed bilateral parenchymal condensation with a focal ground-glass opacity surrounded by a consolidation ring in the right upper lobe, consistent with the reverse halo sign (Fig. 1A).
Bronchoalveolar lavage (BAL) was performed and tests for cytomegalovirus, herpes simplex virus 1, herpes simplex virus 2, varicella zoster virus, adenovirus, other respiratory viruses, as well as for Aspergillus sp and mucormycoses (species tested for were Mucor sp., Rhizopus sp., Lichtheimia sp, and Rhizomucor sp.) by polymerase chain reaction (PCR) were all negative. Blood, Aspergillus antigenemia was negative, whereas Mucor sp. and/or Rhizopus sp PCR were positive.
The diagnosis of pulmonary mucormycosis was established on day 10 after AHSCT, and IV amphotericin B, increased to 7.5 mg/kg, with the addition of isavuconazole (200 mg/dose/8 h IV for 48 h and then 200 mg/24 h) were started. Antibiotics were discontinued and sulfamethoxazole-trimethoprim and valacyclovir were resumed at prophylactic doses. Rapid clinical improvement was observed, the patient was extubated on day 14 and oxygen therapy was stopped on day 17. Blood PCR for mucormycosis was negative on day 18.
The follow-up chest CT scan (on day 20) showed almost complete regression of bilateral alveolar parenchymal condensation and resolution of the reverse halo sign (Fig. 1B). Isavuconazole was stopped on day 21. Amphotericin B was discontinued on day 22 because of refractory hypokalemia due to tubular injury and loss of potassium. Maintenance therapy with posaconazole per os was administered from day 21 until day 28 (patient's decision) and the patient was discharged on day 22 after AHSCT.
After 1 year of follow-up, the patient is still in clinical and CT scan remission from mucormycosis.
3. Discussion
Invasive mucormycosis has a high mortality rate of 76% and the lungs are the second most commonly reported site after the sinuses.[5] The clinical features of pulmonary mucormycosis are nonspecific and cannot be distinguished from pulmonary aspergillosis. High-grade fever and a nonproductive cough are the most common symptoms.[5]
Most of the signs of pulmonary mucormycosis on CT scan are indistinguishable from other invasive pulmonary fungal infections, with the presence of infiltrates, consolidation, nodules, cavitations, atelectasis, effusion, posterior tracheal band thickening and hilar or mediastinal lymphadenopathies. Only the reversed halo sign, a focal round area of ground-glass attenuation surrounded by a ring of consolidation, appears more specific and is highly suggestive of pulmonary mucormycosis.[13]
Evidence of mucormycosis on cultures or on histopathological examination is required for a definitive diagnosis.[14] PCR detection of Mucorales DNA in blood samples is now recognized as the earliest biological indication of mucormycoses[15–17] and could even precede the diagnosis of proven mucormycosis by an average of 9 days.[16]
Mucormycosis tends to occur late in autologous stem cells recipients for various diseases (median interval, 412 days; range, 190–2254 days).[18] In this case, the patient presented with acute respiratory distress only 6 days after PBSC reinjection, which underscores the crucial role of prior immunosuppression, especially prolonged use of corticosteroids, which was the case for our patient.
Management guidelines suggest amphotericin B as the first-line treatment (daily dose 5 mg/kg) combined with a surgical procedure, when necessary, and then a switch to posaconazole as a maintenance treatment.[19] Even so, mortality rates are still high (61%).[20]
Early antifungal therapy is essential, as soon as the diagnosis is suspected, without waiting for biological evidence. In fact, Chamilos et al[21] showed that delaying the administration of amphotericin B–based regimens by 5 days is associated with a 2-fold increase in mortality.
In hematological patients, the risk of mucormycosis after stem cell transplantation is well-documented, with a postallograft incidence estimated at 0.3%,[3] which represents approximately 8% of the postallograft invasive fungal infections.[18] Less data is available on the occurrence of mucormycosis after AHSCT, but the incidence appears to be lower with 7.8% of the post-autograft invasive fungal infections.[4] In systemic autoimmune diseases, the onset of mucormycosis remains uncommon and appears to be related to the use of corticosteroids.[5,22] In a series of 24 cases of autoimmune disease patients with mucormycosis,[23] 83% had systemic lupus erythematosus. All the subjects had been exposed to corticosteroids and 6 had received additional CPM. In autoimmune diseases, mucormycosis symptoms can mimic a flare of the underlying disease.
In France, between 1996 and 2018, 75 AHSCT for severe SSc were performed[11] with no report of increased risk for post-transplantation invasive fungal infection. In the various other trials, which showed the benefit of AHSCT in SSc patients,[6–8] a higher proportion of viral infections were described but no increased risk for fungal infection has been noted.
4. Conclusion
In light of the increase in the availability of AHSCT for severe SSc clinicians should be aware of this rare post-transplant fungal infection and that early initiation of adequate therapy for patients with clinical and radiological features consistent with mucormycosis could change the outcome.
Author contributions
Conceptualization: Grégory Pugnet.
Supervision: Grégory Pugnet.
Validation: Grégory Pugnet.
Writing – original draft: Xavier Boumaza.
Writing – review & editing: Xavier Boumaza, Lucie Lelievre, Sarah Guenounou, Cécile Borel, Anne Huynh, Guillaume Beziat, Karen Delavigne, Damien Guinault, Marie Garric, Marie Piel-Julian, Kim Paricaud, Guillaume Moulis, Leonardo Astudillo, Laurent Sailler, Dominique Farge, Grégory Pugnet.
Footnotes
Abbreviations: AHSCT = autologous hematopoietic stem cell transplantation, CPM = cyclophosphamide, CT = computerized tomography, EBMT = European Society for Blood and Marrow Transplantation, IV = intravenous, PBSC = peripheral blood hematopoietic stem cell, PCR = polymerase chain reaction, SSc = systemic sclerosis, VZV = varicella zoster virus.
How to cite this article: Boumaza X, Lelièvre L, Guenounou S, Borel C, Huynh A, Beziat G, Delavigne K, Guinault D, Garric M, Piel-Julian M, Paricaud K, Moulis G, Astudillo L, Sailler L, Farge D, Pugnet G. Pulmonary mucormycosis following autologous hematopoietic stem cell transplantation for rapidly progressive diffuse cutaneous systemic sclerosis: a case report. Medicine. 2020;99:31(e21431).
The authors have no funding and conflicts of interest to disclose.
Patient has provided informed consent for publication of the case.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010;50:1101–11. [DOI] [PubMed] [Google Scholar]
- [2].Robin C, Alanio A, Cordonnier C. Mucormycosis: a new concern in the transplant ward? Curr Opin Hematol 2014;21:482–90. [DOI] [PubMed] [Google Scholar]
- [3].Lanternier F, Sun H-Y, Ribaud P, et al. Mucormycosis in organ and stem cell transplant recipients. Clin Infect Dis 2012;54:1–8. [DOI] [PubMed] [Google Scholar]
- [4].Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter Prospective Antifungal Therapy (PATH) Alliance Registry. Clin Infect Dis 2009;48:265–73. [DOI] [PubMed] [Google Scholar]
- [5].Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012;54: suppl_1: S23–34. [DOI] [PubMed] [Google Scholar]
- [6].Burt RK, Shah SJ, Dill K, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet 2011;378:498–506. [DOI] [PubMed] [Google Scholar]
- [7].Van Laar JM, Farge D, Sont JK, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 2014;311:2490–8. [DOI] [PubMed] [Google Scholar]
- [8].Sullivan KM, Goldmuntz EA, Keyes-Elstein L, et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N Engl J Med 2018;378:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017;76:1327–39. [DOI] [PubMed] [Google Scholar]
- [10].Snowden JA, Saccardi R, Allez M, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2012;47:770–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pugnet G, Castilla-Llorente C, Puyade M, et al. Indications and follow-up for autologous hematopoietic stem cell transplantation in autoimmune and autoinflammatory diseases: guidelines from the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC). Bull Cancer 2017;104:S169–80. [DOI] [PubMed] [Google Scholar]
- [12].Farge D, Burt RK, Oliveira M-C, et al. Cardiopulmonary assessment of patients with systemic sclerosis for hematopoietic stem cell transplantation: recommendations from the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party and collaborating partners. Bone Marrow Transplant 2017;52:1495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Legouge C, Caillot D, Chrétien ML, et al. The reversed halo sign: pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin Infect Dis 2014;58:672–8. [DOI] [PubMed] [Google Scholar]
- [14].Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 2014;20: Suppl 3: 5–26. [DOI] [PubMed] [Google Scholar]
- [15].Millon L, Larosa F, Lepiller Q, et al. Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis 2013;56:e95–101. [DOI] [PubMed] [Google Scholar]
- [16].Millon L, Herbrecht R, Grenouillet F, et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect 2016;22:810.e1–8. [DOI] [PubMed] [Google Scholar]
- [17].Dadwal SS, Kontoyiannis DP. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn 2018;18:1–0. [DOI] [PubMed] [Google Scholar]
- [18].Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001-2006. Emerg Infect Dis 2011;17:1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017;102:433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634–53. [DOI] [PubMed] [Google Scholar]
- [21].Chamilos G, Lewis RE, Kontoyiannis DP, et al. Amphotericin B–based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis 2008;47:503–9. [DOI] [PubMed] [Google Scholar]
- [22].Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000;13:236–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Royer M, Puéchal X. Mucormycosis in systemic autoimmune diseases. Joint Bone Spine 2014;81:303–7. [DOI] [PubMed] [Google Scholar]


