Supplemental Digital Content is available in the text
Keywords: multimodal analgesia, safety, surgery
Abstract
Patient satisfaction measures and the opioid epidemic have highlighted the need for effective perioperative pain management. Multimodal analgesia, including non-steroidal anti-inflammatory drugs (NSAIDs), have been shown to maximize pain relief and reduce opioid consumption, but are also associated with potential perioperative bleeding risks.
A multidisciplinary panel conducted a clinical appraisal of bleeding risks associated with perioperative NSAID use. The appraisal consisted of review and assessment of the current published evidence related to the statement “In procedures with high bleeding risk, NSAIDs should always be avoided perioperatively.” We report the presented literature and proceedings of the subsequent panel discussion and national pilot survey results. The authors’ assessment of the statement based on current evidence was compared to the attempted national survey data, which revealed a wide range of opinions reflecting the ongoing debate around this issue in a small number of respondents.
The appraisal concluded that caution is warranted with respect to perioperative use of NSAIDs. However, summarily excluding NSAIDs from perioperative use based on potential bleeding risks would be imprudent. It is recommended that NSAID use be guided by known patient- and procedure-specific factors to minimize bleeding risks while providing effective pain relief.
1. Introduction
The major challenge with respect to postoperative pain management is to maximize pain relief while minimizing side effects associated with different analgesic classes.[1] Opioids, which selectively bind mu, kappa, and delta opioid receptors and may produce profound analgesia in the presence of severe pain, are the analgesic modality most commonly used in the perioperative setting.[2] While efficacious, there is growing appreciation for the limitations of using opioids as a monotherapy based on known risks for adverse events (AEs) such as nausea, vomiting, constipation, and cognitive effects that may delay recovery from surgery and, in some cases, have serious patient safety implications.[3,4] Consequently, limited opioid usage is being emphasized at the provider, legislative, and regulatory levels. A landmark article by Joshi et al cautions that complete elimination of opioids in the postsurgical setting would be impractical and inappropriate.[5] Instead, the article recommends implementation of multimodal analgesia and procedure- and population-specific pain management regimens. Currently, physicians have several options for managing postoperative pain,[3,6,7] and there is growing support for multimodal approaches tailored to patient needs.[1,7–9]
Multimodal analgesia involves the concurrent use of multiple agents with different mechanisms of action to maximize pain relief, permit use of lower opioid doses, and limit serious side effects attributable to opioids.[1,7,10] Drugs used for multimodal analgesia include local anesthetics, acetaminophen, and non-steroidal anti-inflammatory drugs (NSAIDs), among others.[3,6,7–11]
Although inhibition of prostaglandin synthesis has important analgesic and anti-inflammatory effects, NSAID use is also associated with potential safety concerns. For example, inhibiting cyclooxygenase (COX)-1 enzyme functionality may have unintended effects on the production of the prothrombotic AEs, which is essential for platelet aggregation and production.[17] Conversely, while COX-2 inhibition has a lesser effect on platelet function, the selective COX-2 inhibitors valdecoxib (BextraTM) and rofecoxib (VioxxTM) are no longer available in the US due to safety concerns related to cardiovascular events such as heart attack and stroke.[4,12–16,18–21]
As a surgical complication, bleeding is associated with increased mortality and morbidity.[22] Bleeding at the operative site or in the gastrointestinal tract may necessitate additional surgical intervention and lengthen recovery time.[23,24] Given the significance of these outcomes and the challenges associated with managing bleeding complications in surgical patients, it is necessary to identify circumstances associated with an elevated bleeding risk and employ evidence-based interventions to reduce this risk while simultaneously maximizing pain control. Factors that can increase bleeding risk with NSAID use include advanced patient age, high NSAID dosage, and concomitant anticoagulant use.[25,26] As a result, NSAID-containing product labels are required to include warnings regarding bleeding risks.[27,28] Still, many questions remain regarding the association between perioperative NSAID use and bleeding, in part because NSAID use is typically limited to a relatively brief postoperative period. Other factors contributing to this uncertainty include variability at the patient and procedure level and the variety of NSAIDs, administration routes, and dosing paradigms used in this setting. Given the large majority of bleeding studies have evaluated tonsillectomies, we limited our review primarily to this cohort of subjects when reviewing the available meta-analyses.
In light of
-
(1)
the importance of NSAIDs in surgical pain management (including their ability to be used preemptively and avoid opioid-related AEs), and
-
(2)
the potential bleeding risks that might limit their use in this setting, a clinical appraisal of the current evidence regarding bleeding outcomes following perioperative NSAID use was conducted. Specifically, the appraisal assessed the validity of the statement “In procedures with high bleeding risk, NSAIDs should always be avoided perioperatively.” Therefore, this work represents an evidence-based literature review by an expert panel and current clinical appraisal.
2. Methodology
2.1. National pilot survey
To gauge national perceptions regarding pain management in the perioperative setting, an electronic survey was distributed to 70,000 physicians registered with the American Medical Association. The survey sought current opinions regarding a number of distinct but related pain management issues that are the subject of current debate in the medical community. The study was approved as IRB exemption by The Ohio State University College of Medicine Institutional Review Board. The survey was distributed on June 10, 2015 and responses were collected through July 10, 2015. Physicians involved in pain management in the surgical setting (anesthesiologists, general surgeons, orthopedic surgeons) were asked to indicate their level of agreement (1 - Strongly agree; 2 - Mostly agree, but with minor reservations; 3 - Slightly agree, with major reservations; 4 - Slightly disagree, due to minor reservations; 5 - Mostly disagree, due to major reservations; 6 - Strongly disagree) with each of the statements in the survey. Respondents also had the opportunity to provide written comments regarding each survey statement. In total, 571 responses were obtained, with 565 included in the analysis for the statement evaluated in this appraisal (310 (54.8%) anesthesiologists; 75 (13.3%) orthopedic surgeons; 180 (31.9%) general surgeons).
Results were tabulated and stratified by clinical specialty (see Fig. 1). Expert panel and literature review. The authors met in July 2015 to discuss the current literature regarding NSAID use and bleeding risk in the perioperative setting as it relates to the statement “In procedures with high bleeding risk, NSAIDs should always be avoided perioperatively.” The first author was tasked with presentation of an unbiased review of the current literature, presenting both statement supporting and statement-refuting evidence. The goals of this appraisal were to discuss the evidence relevant to the statement and to assess how this evidence influences current perceptions, as demonstrated by the national survey results.
Figure 1.
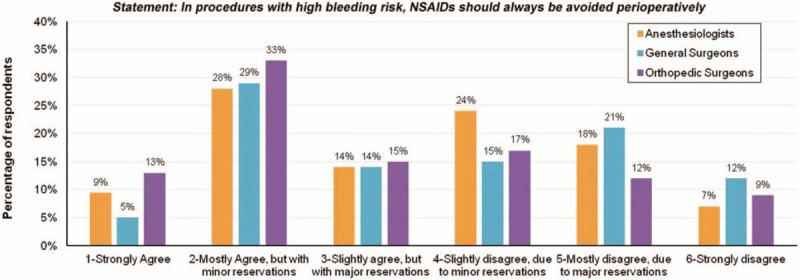
Physicians’ responses for the statement: In procedures with high risk bleeding, NSAIDs should be avoided perioperatively.
The literature search was conducted in June 2015, using the PubMed electronic database Advanced Search Builder. Search terms used were as follows: “NSAIDs bleeding,” “postoperative bleeding,” and “NSAIDs bleeding AND postoperative bleeding” (346 results); “NSAIDs AND bleeding risk” and “NSAIDs AND operative site bleeding risk” (120 results); “high bleeding risk operations” and “NSAIDs” (209 results); “NSAIDs” and “perioperative bleeding” (330 results). After excluding case reports, small cohort studies, and articles not reporting bleeding endpoints, ten articles [with an emphasis on randomized controlled trials (RCTs) and meta-analyses], were selected for the presentation (See Appendix 1 for 10 articles chosen for presentation to the panel and for expert review).
Prior to presentation and discussion of the selected studies, the authors anonymously indicated their level of agreement with the appraisal statement, using the same 6-point scale used by the national survey respondents. Following the presentation, the panel rated the quality of the evidence as follows:
-
(1)
Evidence obtained from meta-analysis, including at least 1 large RCT;
-
(2)
Evidence obtained from either meta-analysis, including at least 1 small RCT or from at least 1 well designed, large RCT;
-
(3)
Evidence obtained from well-designed cohort or case controlled studies;
-
(4)
Evidence obtained from case series, case reports, or flawed clinical trials;
-
(5)
Opinions of respected authorities based on clinical experience, descriptive studies, or reports of expert committees;
-
(6)
Insufficient evidence to form an opinion.
The authors subsequently indicated their level of agreement with the appraisal statement given the presented data, noting whether the presentation had an effect on their responses. The authors discussed how the evidence relates to current perioperative pain management practice and identified important areas of future research needed to inform perioperative NSAID use.
Given the large majority of bleeding studies have evaluated tonsillectomies, we limited our review primarily to this cohort of subjects when reviewing the available meta-analyses.
3. Survey results and literature review
3.1. National pilot survey results
While return rates of voluntary surveys are generally low, this survey has a return rate of less than 1% that may cause selection bias; therefore, the results of this part of the study should be interpreted with caution, and the authors chose not to base their recommendations on these results; however, they are presented for completeness.
The national pilot survey revealed a wide range in the level of support for the statement “In procedures with high bleeding risk, NSAIDs should always be avoided perioperatively” (Fig. 1), suggesting a lack of consensus among respondents. Illustrating the observed range of opinion, 9.4% (53/565) of respondents overall indicated strong agreement with the statement, while 9.2% (52/565) indicated strong disagreement. Respondent comments mirrored the survey results. In support of the statement, respondents indicated a lack of bleeding-related issues associated with NSAIDs in their experience, as well as a tendency for NSAID risks to be overemphasized.
Conversely, other respondents emphasized the bleeding risks associated with NSAIDs and indicated that COX inhibition in the surgical setting was inappropriate. Reflecting important considerations regarding NSAID use, respondents also acknowledged the importance of COX inhibition profile (COX-2 versus COX-1) and potential implications based on surgery type. This variability in physician perceptions is not entirely surprising, given the complex nature of perioperative pain management practice. Similar to the national survey, responses from the six-member panel's pre-presentation survey ranged the entire spectrum from strong agreement (1 author) to slight agreement with major reservations (3 authors), mostly disagreement due to major reservations (1 author), and strong disagreement (1 author) with the statement (Fig. 2).
Figure 2.
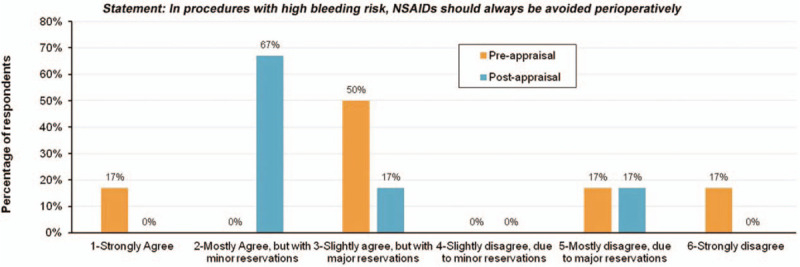
Responses from the 6-member panel's pre-presentation survey.
3.2. Past and present perspectives on perioperative NSAID use
Among the first reports to raise concerns about bleeding risks with perioperative NSAID use was a post-marketing surveillance cohort study by Strom et al. evaluating the incidence of gastrointestinal bleeding (GIB) and operative site bleeding (OSB) in > 20,000 surgical patients.[29] The average course of study drug was 2.6 days in length in both groups and data on outcomes was collected through the third day following the final dose.[29] This study found that patients who received ketorolac, administered intravenous (IV) or intramuscularly, had higher incidences of both GIB and OSB as compared to patients who received opiates, however, statistical significance was only observed with respect to GIB (multivariate odds ratio (OR) for ketorolac versus opiates: GIB = 1.30 [95% CI: 1.11;1.52]; OSB = 1.02 [0.95;1.10]). Thus, these early findings suggested some added GIB risk associated with ketorolac use in surgical patients. A more recent systematic review and meta-analysis by Gobble et al. examining outcomes in surgical patient receiving ketorolac encompassed 27 RCTs.[30] This analysis revealed no significant increase in the incidence of postoperative bleeding in patients receiving intra- or postoperative ketorolac in comparison to placebo, opioids, or acetaminophen (OR: 1.12 [95% CI: 0.61;2.06]; P = .72). (See Appendix for Summary of bleeding outcomes in reviewed studies).
A literature search was performed in June 2015 using the Cochrane Database with the search term “postoperative pain.” After initial review, a total of 17 articles were identified as being relevant based on external/internal validity criteria (study population, clinical similarities).
Looking more broadly at the NSAID class, a systematic literature review and meta-analysis of 25 RCTs by Moiniche et al. examined outcomes in a total of 970 tonsillectomy patients who received NSAIDs (ketorolac, diclofenac, and ibuprofen, among others) via various routes and 883 patients who received non-NSAIDs (eg, opioids) or placebo.[31] This analysis identified a significantly higher rate of re-operation due to bleeding in the NSAID group (Peto-modified Mantel-Haenszel odds ratio (Peto-OR): 2.33 [95% CI: 1.12;4.83]). Importantly, however, this analysis also revealed no significant difference with respect to 3 other bleeding-related outcomes, including volume of intraoperative blood loss [weighted mean difference: 0.38 mL/kg [95% CI:-0.06;-0.81]), postoperative bleeding (Peto-OR: 1.30 [0.89; 1.89]), and postoperative hospital admission or readmission due to bleeding (Peto-OR: 2.10 [0.85;5.19]). Thus, this study indicated increased risk of reoperation due to bleeding, justifying caution with respect to NSAID use in patients undergoing tonsillectomy.
In a similar study, Riggin et al[32] conducted a systematic review and meta-analysis of 26 studies (1747 children, 446 adults) that compared outcomes in tonsillectomy patients who received NSAIDs (via multiple routes) versus placebo or opioids. This study revealed no significant added risk of bleeding with NSAIDs. This was true when evaluating all patients (OR: 1.30 [95% CI: 0.90; 1.88]), as well as children specifically (OR: 1.06 [95% CI: 0.65; 1.74]), suggesting that NSAIDs may not pose an added safety risk with respect to bleeding in tonsillectomy patients.
Taken together, these meta-analyses indicate some potential bleeding risks associated with perioperative NSAID use, but also suggest the possibility of safe use with respect to bleeding. It is important to note, however, that these studies largely examined the NSAID class as a whole, as well as limited surgery types, indicating a need for additional data to provide a better understanding of how factors such as patient characteristics and surgery type must be considered in the decision-making process.
A Cochrane review and meta-analysis (15 studies involving 1,101 children undergoing elective tonsillectomy or adenosillectomy)[33] revealed that the odds of bleeding requiring surgical intervention were not significantly increased in patients who received NSAIDs versus placebo or a non-NSAID (Peto-OR: 1.69 [95% CI: 0.71;4.01]) and that NSAIDs did not significantly alter the number of perioperative bleeding events requiring non-surgical intervention (Peto-OR: 0.99 [0.41; 2.40]). Nevertheless, the authors of the review concluded that there was “insufficient evidence to exclude an increased risk of bleeding when NSAIDs are used in pediatric tonsillectomy” and called for additional research. This conservative stance is consistent with the prevailing culture of maximizing safety in the perioperative setting. It is worth noting that, in addition to examining bleeding outcomes, adverse effects frequently associated with opioid use were also examined in the reviewed studies. Opioid-related AEs, including potential gastrointestinal, respiratory, and cognitive effects, represent an important aspect of the safety picture that should be considered alongside bleeding outcomes, given the growing emphasis on strategic use of opioids in conjunction with non-opioids. The systematic review by Moiniche et al, which revealed a non-significant effect of NSAIDs with respect to 3 of 4 bleeding outcomes examined, found that the risk of emesis was significantly reduced in the NSAID group compared to the non-NSAID group (relative risk: 0.73 [95% CI 0.63; 0.85]).[31] Furthermore, the meta-analysis of tonsillectomy patients by Lewis et al revealed a lower risk of vomiting among patients receiving NSAIDs (versus nonNSAIDs or placebo).[33]
When evaluating other types of surgery, for example gastrointestinal surgery, additional risks of NSAIDs such as the risk of increased anastomotic leakage after NSAID application need to be evaluated as well.
3.3. Identifying bleeding risk groups
Despite the challenges associated with implementing optimal perioperative pain management regimens, there remains a need to define patient- or procedure-related risk factors to aid physicians in the process of determining if and when to use NSAIDs, which NSAID to use, and how best to administer it. High-risk patients need to be identifiable, and strategic NSAID use that minimizes bleeding risks is critical. Commonly cited general risk factors for bleeding with NSAID use include advanced age, concomitant use of anticoagulants, and prolonged exposure, [17,26,34] and NSAID use should be guided by an awareness of these key factors, among others.
In line with these accepted risk factors, Strom et al. observed a significantly elevated incidence of GIB (OR: 1.66 [95% CI: 1.23;2.25]) in patients ≥75 years old who received ketorolac versus opiates. Notably, GIB incidence increased with age in both comparator groups, but this increase was greatest in the ketorolac group.[29] This study also demonstrated that ketorolac (versus all doses of opiates) was associated with significantly greater GIB and OSB incidence at higher dosage, i.e. when the average daily dose exceeded 105 mg/d (GIB: OR: 2.87 [95% CI: 1.97; 4.18]); OSB: OR: 2.79 [95% CI: 2.29;3.40]).[29]
Adding to the complex perioperative pain management puzzle, physicians also need to weigh potential drug interactions. Using NSAIDs in anticoagulated patients, for example, is discouraged. However there is no clear consensus regarding the impact of anticoagulants on specific NSAIDs or whether there is a synergistic relationship negatively influencing bleeding outcomes. Forrest et al[35] conducted a prospective, randomized, multicenter trial that evaluated the relative bleeding risk associated with ketorolac versus ketoprofen and diclofenac (5634 surgical patients receiving ketorolac, 5611 receiving diclofenac or ketoprofen). Examining outcomes over 30 days, the study found that there was no overall difference between comparator groups with respect to OSB (OR: 1.07 [95% CI: 0.75;1.54]). Concomitant anticoagulant use increased the odds of surgical site bleeding in both the ketorolac (OR: 2.65 [1.51;4.67]) and comparator groups (OR: 3.58 [1.93;6.70]), with no significant difference observed when comparing patients who received anticoagulants between treatment groups.[35]
Recently, Devereaux et al[36] randomized 10,010 patients undergoing non-cardiac surgery and at risk for vascular complications to receive aspirin, which inhibits platelet aggregation and thrombus formation (200 mg prior to surgery, followed by 100 mg daily for 30 days post-surgery), or placebo. In this study, major bleeding was more common in the aspirin group compared to the placebo group (OR: 1.23 [95% CI: 1.01;1.49]).
Anticoagulant use is an important consideration when evaluating NSAID administration in the perioperative setting, particularly when growing numbers of surgical patients are receiving concomitant anticoagulants based on the presence of comorbid conditions or surgery type.[35] Still, it remains unclear whether the NSAIDs and anticoagulants act synergistically to increase bleeding risk. In addition, specifics regarding the impact of dosing and the relative order of NSAID-anticoagulant administration remain undefined in the current evidence-based literature.
3.4. Recommendations for future research
In addition to the challenge of defining which circumstances allow for the safest NSAID use, the decision about which NSAID(s) to use is also important. Pharmacodynamic heterogeneities within the NSAID class, such as degree of COX-2 selectivity, may explain differing bleeding risk-profiles among distinct NSAID subtypes. However, further understanding of these mechanistic variations and their influence on clinical outcomes is essential and therefore remains a focus in current research. Dirkmann et al[37] conducted a multicenter, double-blind RCT, assigning radical prostatectomy patients to receive an initial 40 mg IV dose of the selective COX-2 inhibitor parecoxib or placebo and subsequent doses of 20 mg parecoxib (parecoxib group) or placebo (placebo group) every 12 hour until 48 hour post-surgery, with availability of patient controlled analgesia (PCA) morphine. This study reported reduced opioid consumption and an improved benefit of analgesia (OBAS score, taking into account pain intensity, opioid-related AEs, and patient satisfaction) in patients receiving parecoxib, but also significantly greater blood loss (decrease in serum hemoglobin: 4.3 g/dL versus 3.2 g/dL for placebo; P = .02), suggesting potential bleeding risks associated with this COX-2-selective drug.
It has also been suggested that drugs with a balanced COX-1/COX-2 inhibition profile, such as ibuprofen, might carry less of a bleeding risk than highly COX-selective NSAIDs. A multicenter randomized, doubled blind, placebo-controlled trial in 161 tonsillectomy patients conducted by Moss et al showed no significant difference in the incidence of serious AEs, including surgical blood loss (P = .662), incidence of postoperative bleeding, or need for reoperation in patients receiving IV ibuprofen versus placebo.[38] Similarly, Gan et al conducted a phase 4, multicenter, open-label, clinical surveillance study in 21 US hospitals (300 patients undergoing any surgery with expected need for analgesia, excluding coronary artery bypass graft surgery) and reported no cases of perioperative bleeding in patients receiving IV ibuprofen preoperatively (800 mg dose, given at the time of induction of anesthesia).[39]
3.5. Quality and impact of the presented evidence
Following presentation and discussion of the literature, 4 of 6 authors assessed the evidence as being of very high or high quality (levels 1 and 2; see Methodology). One author assessed the evidence as being obtained from well-designed cohort or case-controlled studies (level 3), while the remaining author assessed the evidence as insufficient to form an opinion (level 6). Revisiting the statement “In procedures with high bleeding risk, NSAIDs should always be avoided perioperatively,” the post-discussion survey resulted in a range of responses similar to that observed in the pre-discussion survey, although there was a greater tendency to agree with the statement (ie, express more caution with respect to NSAID use in light of perioperative bleeding considerations). Overall, 5/6 authors indicated some level of agreement with the statement, with minor or major reservations, while 1 author indicated mostly disagreement due to major reservations (Fig. 2). The mean level of support for the statement, using the 1–6 scale, decreased from 3.50 to 2.67 following the literature review. No author indicated either complete agreement or disagreement with the statement following discussion of the evidence.
In retrospect, in reviewing the response rate (1%) of the attempted national survey, an appropriate sampling method would probably be more useful, and while impossible to revise the current study post-hoc, future evaluations will include a revised sampling methodology.
4. Summary and conclusions
Acute postoperative pain, if inadequately controlled, can be a major source of patient dissatisfaction.[40,41] It can also increase the risk of complications and lead to delays in recovery following surgery.[40–44] Providers may be further challenged to minimize opioid use by state regulatory agencies. It is necessary for all providers to be aware of the full armamentarium of analgesic options and to employ a multimodal strategy for effective pain surgical pain management. Furthermore, inadequately controlled acute postoperative pain can develop into chronic pain, which can have long-term impacts on patient well-being.[45–48] Physicians must weigh the need to control pain while acknowledging that different analgesic classes are associated with different potential safety risks.[1,3,4,17] Thus, the decision-making process with respect to surgical pain management is complex and requires that several factors be considered by the treating physician. These factors include expected pain levels and available analgesic options as well as patient history, procedure type, and concomitant medication use. Among the important considerations with respect to surgical patient management is the risk of perioperative bleeding, given its association with unfavorable postoperative outcomes.[22–24]
While NSAIDs present an important adjunct to opioids for management of surgical pain, this class is also associated with bleeding concerns in some patients, including individuals receiving concomitant anticoagulants, necessitating careful risk assessment before being implemented for a given patient. Additional caution is warranted in that besides bleeding, NSAID use may also be associated with other risk such as kidney injury, especially in elderly patient, patients with chronic kidney disease, dehydration, and/or when using in combination of other renal toxic medications/drugs such as contrast media, (angiotensin converting enzyme) angiotensin converting enzyme inhibitors, Vancomycin, and so on.
Different surgical procedure types inherently carry different bleeding risks. The definition of “high bleeding risk procedures” is not currently standardized in the literature, however there is agreement that procedures such as tonsillectomy, prostatectomy, cardiovascular surgery, major orthopedic surgery, and certain plastic surgery procedures can be particularly prone to bleeding complications.[31,49] While the studies included in this appraisal involved patients undergoing a range of procedures, they also used a wide array of NSAIDs, administration regimens, and dosages. Thus, relative NSAID-associated bleeding risks across surgery types remain to be elucidated. Given the numerous NSAID regimens used in current practice and the wide array of variables that can influence perioperative bleeding risk, studies to clarify this relationship will need to rely on large, inclusive data sets. Further, such studies would ideally weigh bleeding risks in light of potential effects on other serious outcomes.[50,51] These challenges highlight the need for, data-driven procedure-specific recommendations regarding postoperative NSAID.
As reflected by the authors’ post-presentation survey results, current evidence confirms multiple complex issues surrounding the use of NSAIDs in the perioperative setting as it relates to bleeding risk. Current literature provides evidence both in support of and against the use of NSAIDs. Overall, current published evidence is insufficient to definitively conclude either for or against the statement: “In procedures with high bleeding risk, NSAIDs should always be avoided perioperatively.” Published data supports an approach in which factors with the potential to influence bleeding risk are carefully considered before using NSAIDs in patients undergoing high-bleeding risk procedures (Fig. 3). Still, many questions remain unanswered. For example, NSAIDs with different COX inhibition profiles might be associated with a spectrum of associated bleeding risk.[52] Thus, while evaluation of the entire NSAID class in the context of this appraisal is highly informative, future studies and analyses focused on specific NSAIDs are likewise critical. In light of the efficacy and opioid-sparing effects of NSAIDs in the surgical setting, excluding NSAIDs from perioperative practice based on potential bleeding risks would be unwise. Instead, current evidence indicates that the use of NSAIDs should be guided by careful consideration of known risks. Ideally, a multimodal, patient- and procedure-specific approach that includes NSAIDs, when appropriate, should be implemented to maximize analgesia and limit unwanted side effects. The methodology applied in this clinical appraisal provides a unique model with which further investigations can be based.
Figure 3.
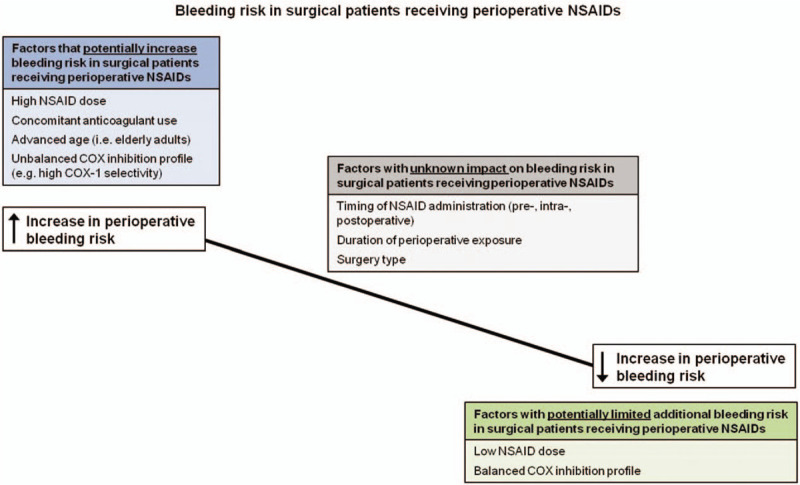
Bleeding risk in surgical patients receiving perioperative NSAIDs. NSAID = non-steroidal anti-inflammatory drugs.
Based on the modest number of papers cited and described, the authors hence have no possibility to answer the initial research question completely, as the cited papers only partially contain data regarding this issue.
Due to paucity of evidence, the authors are unable to make definite recommendations.
The majority of the manuscripts cited deal with NSAID use in patients after tonsillectomy. The reason for this possibly is the fact that tonsillectomy can easily be defined to be an operation with high bleeding risk and is a very consistent and hence comparable operation. Reviews regarding this distinct topic hence deliver more exact results. Also, bleeding risks vary depending on the surgery, so classifying the cases would have given more credence. However, the authors believe this model has been validated by a related work,[53] and represents an evidence-based literature review, and current clinical appraisal.
Acknowledgments
The authors wish to thank Hospira, a Pfizer company, for their willingness to support this project with minimal oversight and for allowing the authors to explore the use of NSAID in the perioperative space with academic freedom and without focusing on specific on-market products. The authors thank Laura A. Silver and Dr. Maria Thompson of LAS Communications, LLC for support in developing and conducting the clinical appraisal and editorial support, and Danielle Lozier and Dr. Scott M. Paluszkiewicz of Boston Strategic Partners, Inc. for editorial support. Hospira, a Pfizer company, funded LAS Communications for the conduct of the clinical appraisal and editorial support and Boston Strategic Partners for editorial support.
Author contributions
Conceptualization: Ketan R. Sheth, Nicholas M. Bernthal, Hung S. Ho, Sergio D. Bergese, Christian C. Apfel, Jonathan Jahr.
Data curation: Ketan R. Sheth, Sergio D. Bergese, Jonathan Jahr.
Formal analysis: Nicholas M. Bernthal, Hung S. Ho, Sergio D. Bergese, Christian C. Apfel, Nicoleta Stoicea, Jonathan Jahr.
Investigation: Ketan R. Sheth, Nicholas M. Bernthal, Hung S. Ho, Sergio D. Bergese, Christian C. Apfel, Jonathan Jahr.
Methodology: Ketan R. Sheth, Nicholas M. Bernthal, Hung S. Ho, Sergio D. Bergese, Christian C. Apfel, Jonathan Jahr.
Project administration: Jonathan Jahr.
Resources: Ketan R. Sheth, Sergio D. Bergese, Christian C. Apfel.
Supervision: Ketan R. Sheth, Sergio D. Bergese, Jonathan Jahr.
Validation: Nichola M. Bernthal.
Writing – original draft: Ketan R. Sheth, Nicholas M. Bernthal, Hung S. Ho, Sergio D. Bergese, Christian C. Apfel, Nicoleta Stoicea, Jonathan Jahr.
Writing – review & editing: Ketan R. Sheth, Nicholas M. Bernthal, Hung S. Ho, Sergio D. Bergese, Christian C. Apfel, Nicoleta Stoicea, Jonathan Jahr.
Supplementary Material
Footnotes
Abbreviations: AEs = adverse events, COX = cyclooxygenase, GIB = gastrointestinal bleeding, IV = intravenous, NSAID = non-steroidal anti-inflammatory drugs, OR = odd ratio, OSB = operative site bleeding, RCTs = randomized controlled trials.
How to cite this article: Sheth KR, Bernthal NM, Ho HS, Bergese SD, Apfel CC, Stoicea N, Jahr JS. Perioperative bleeding and non-steroidal anti-inflammatory drugs (NSAIDs): an evidence-based literature review, and current clinical appraisal. Medicine. 2020;99:31(e20042).
Disclosures: The survey and the expert panel which form the basis of this manuscript were sponsored by Hospira, Inc. (Lake Forest, IL), which was acquired by Pfizer in September 2015. All 6 authors ere members of a speakers’ bureau funded by Hospira. The authors received no compensation towards the development and writing of this manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630–41. [DOI] [PubMed] [Google Scholar]
- [2].Sadhasivam S, Chidambaran V. Pharmacogenomics of opioids and perioperative pain management. Pharmacogenomics 2012;13:1719–40. [DOI] [PubMed] [Google Scholar]
- [3].Becker DE. Pain Managment: Part 1: Managing Acute and Postoperative Dental Pain. Anesth Prog 2010;57:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marret E, Kurdi O, Zufferey P, et al. Effects of nonsteroidal anti-inflammatory drugs on patient-controlled analgesia morphine side effects. Anesthesiology 2005;102:1249–60. [DOI] [PubMed] [Google Scholar]
- [5].Joshi GP, Beck DE, Emerson RH, et al. Defining new directions for more effective management of surgical pain in the United States: highlights of the inaugural Surgical Pain Congress™. Am Surg 2014;80:219–28. [PubMed] [Google Scholar]
- [6].Carr DB, Goudas LC. Acute pain. Lancet 1999;353:2051–8. [DOI] [PubMed] [Google Scholar]
- [7].Macintyre P, Schug SA, Scott DA, et al. Acute Pain Management: Scientific Evidence. Melbourne: Australia Australian and New Zealand College of Anesthetists and Faculty of Pain Medicine; 2010. [Google Scholar]
- [8].American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248–73. [DOI] [PubMed] [Google Scholar]
- [9].Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesth 2002;13:524–39. [DOI] [PubMed] [Google Scholar]
- [10].McQuay HJ. Tramer M. Acute pain. Evidence-based resource in anaesthesia and analgesia. London: BMJ Books; 2000;87-106. [Google Scholar]
- [11].Yon JH, Choi GJ, Kang H, et al. Intraoperative systemic lidocaine for pre-emptive analgesics in subtotal gastrectomy: a prospective, randomized, double-blind, placebo-controlled study. Can J Surg 2014;57:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sharma CV, Mehta V. Paracetamol mechanisms and updates. Critical Care and Pain 2013;14:153–8. [Google Scholar]
- [13].Panchal SJ. Sinatra RS, Jahr JS, Watkins-Pitchford JM. Analgesic Gaps. The Essence of Analgesia and Analgesics. 1st ed.Cambridge, UK: Cambridge University Press; 2011. 51–6. [Google Scholar]
- [14].Elia N, Lysakowski C, Tramer MR. Does multimodal analgesia with acetaminophen, nonsteroidal anti-inflammatory drugs,;1; or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology 2005;103:1296–304. [DOI] [PubMed] [Google Scholar]
- [15].Krenzischek DA, Dunwoody CJ, Polomano RC, et al. Pharmacotherapy for acute pain: implications for practice. J Perianesth Nurs 2008;23(1A):S28–42. [DOI] [PubMed] [Google Scholar]
- [16].Lachiewicz PF. The role of intravenous acetaminophen in multimodal pain protocols for perioperative orthopedic patients. Orthopedics 2013;26: 2 Suppl: 15–9. [DOI] [PubMed] [Google Scholar]
- [17].Risser A, Donovan D, Heintzman J. NSAID prescribing precautions. Am Fam Physician 2009;80:1371–8. [PubMed] [Google Scholar]
- [18].Feldman HI, Kinman JL, Berlin JA, et al. Parenteral ketorolac: the risk for acute renal failure. Ann Intern Med 1997;126:193–9. [DOI] [PubMed] [Google Scholar]
- [19].Sun SX, Lee KY, Bertram CT, et al. Withdrawal of COX-2 selective inhibitors rofecoxib and valdecoxib: impact on NSAID and gastroprotective drug prescribing and utilization. Curr Med Res Opin 2007;23:1859–66. [DOI] [PubMed] [Google Scholar]
- [20].US Food and Drug Administration. Questions and Answers FDA Regulatory Actions for the COX-2 Selective and Non-Selective Non-Steroidal Anti-inflammatory drugs (NSAIDs). April 2005; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm106148.htm. Accessed December 9, 2015. [Google Scholar]
- [21].US Food and Drug Administration. FDA Issues Public Health Advisory on Vioxx as its Manufacturer Voluntarily Withdraws the Product. September 30, 2004; Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108361.htm. Accessed December 9, 2015. [Google Scholar]
- [22].Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res 2011;11(135): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. New Engl J Med 2009;361:1368–75. [DOI] [PubMed] [Google Scholar]
- [24].Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014;174:947–53. [DOI] [PubMed] [Google Scholar]
- [25].Frampton C, Quinlan J. Evidence for the use of non-steroidal anti-inflammatory drugs for acute pain in the post anaesthesia care unit. J Periop Pract 2009;19:418–23. [DOI] [PubMed] [Google Scholar]
- [26].Vonkeman HE, van de Laar MA. Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention. Semin Arthritis Rheum 2010;39:294–312. [DOI] [PubMed] [Google Scholar]
- [27].Kroll PB, Meadows L, Rock A, et al. A multicenter, randomized, double-blind, placebo-controlled trial of intravenous ibuprofen (IV-ibuprofen) in the management of postoperative pain following abdominal hysterectomy. Pain Pract 2011;11:23–32. [DOI] [PubMed] [Google Scholar]
- [28].US Food and Drug Administration. Department of Health and Human Services. Decision memorandum: analysis and recommendations for agency action: COX-2 selective and nonselective NSAIDs. 2005; Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm106201.pdf. Accessed September 20, 2013. [Google Scholar]
- [29].Strom BL, Berlin JA, Kinman JL, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding. J Am Med Assoc 1991;275:376–82. [PubMed] [Google Scholar]
- [30].Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg 2014;133:741–55. [DOI] [PubMed] [Google Scholar]
- [31].Moiniche S, Romsing J, Dahl JB, et al. Nonsteroidal anti-inflammatory drugs and the risk of operative site bleeding after tonsillectomy: a qunatitative sysyemic review. Anesth Analg 2003;96:68–77. [DOI] [PubMed] [Google Scholar]
- [32].Riggin L, Ramakrishna L, Sommer DD, et al. A 2013 updated systematic review & meta-analysis of 36 randomized controlled trials; no apparent effects of non-steroidal anti-inflammatory agents on the risk of bleeding after tonsillectomy. Clin Otolaryngol 2013;38:115–29. [DOI] [PubMed] [Google Scholar]
- [33].Lewis SR, Nicholson A, Cardwell ME, et al. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst Rev 2013;18:CD003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kroll PB, Meadows L, Rock A, et al. A multicenter, randomized, double-blind, placebo-controlled trial of intravenous ibuprofen (i.v.-ibuprofen) in the management of postoperative pain following abdominal hysterectomy. Pain Pract 2011;11:23–32. [DOI] [PubMed] [Google Scholar]
- [35].Forrest JB, Camu F, Greer IA, et al. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery. Br J Anaesth 2002;88:227–33. [DOI] [PubMed] [Google Scholar]
- [36].Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014;370:1494–503. [DOI] [PubMed] [Google Scholar]
- [37].Dirkmann D, Groeben H, Farhan H, et al. Effects of parecoxib on analgesia benefit and blood loss following open prostatectomy: a multicentre randomized trial. BMC Anesthesiol 2015;15(31.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moss JR, Watcha MF, Bendel LP, et al. A multicenter, randomized, double-blind placebo-controlled, single dose trial of the safety and efficacy of intravenous ibuprofen for treatment of pain in pediatric patients undergoing tonsillectomy. Paediatr Anaesth 2014;24:483–9. [DOI] [PubMed] [Google Scholar]
- [39].Gan TJ, Candiotti K, Turan A, et al. The shortened infusion time of intravenous ibuprofen, part 2: a multicenter, open-label, surgical surveillance trial to evaluate safety. Clin Ther 2015;37:368–75. [DOI] [PubMed] [Google Scholar]
- [40].Hutchison RW. Challenges in acute post-operative pain management. Am J Health Syst Pharm 2007;64: Suppl 4: 52–5. [DOI] [PubMed] [Google Scholar]
- [41].Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent perioperative pain. Anesthesiology clinics of North America 2005;23:21–36. [DOI] [PubMed] [Google Scholar]
- [42].Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain 2003;103:303–11. [DOI] [PubMed] [Google Scholar]
- [43].Argoff CE. Recent management advances in acute postoperative pain. Pain Pract 2014;14:477–87. [DOI] [PubMed] [Google Scholar]
- [44].Smith BH, Torrance N, Bennett MI, et al. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J Pain 2007;23:143–9. [DOI] [PubMed] [Google Scholar]
- [45].Cregg R, Anwar S, Farquhar-Smith P. Persistent postsurgical pain. Curr Opin Support Palliat Care 2013;7:144–52. [DOI] [PubMed] [Google Scholar]
- [46].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [47].Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. Anesthesiology 2000;93:1123–33. [DOI] [PubMed] [Google Scholar]
- [48].Shipton EA. The transition from acute to chronic post surgical pain. Anaesth Intensive Care 2011;39:824–36. [DOI] [PubMed] [Google Scholar]
- [49].University of Wisconsin - UW Health. Periprocedural and Regional Anesthesia Management with Antithrombotic Therapy: Adult. Inpatient and Ambulatory Clinical Practice Guideline August 2015; Available T: http://www.uwhealth.org/files/uwhealth/docs/anticoagulation/Periprocedural_Anticoagulation_Guideline.pdf. Accessed December 9, 2015. [Google Scholar]
- [50].Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- [51].Gukop P, Gutman N, Bilkhu R, et al. Who might benefit from early aspirin after coronary artery surgery? Interact Cardiovasc Thorac Surg 2015;19:505–11. [DOI] [PubMed] [Google Scholar]
- [52].Chaiamnuay S, Allison JJ, Curtis JR. Risks versus benefits of cyclooxygenase-2-selective nonsteroidal anti-inflammatory drugs. Am J Health Syst Pharm 2006;63:1837–51. [DOI] [PubMed] [Google Scholar]
- [53].Jahr JS, Bergese SD, Sheth KR, Bernthal NM, Ho HS, Stoicea N, Apfel CC. Current perspective on the use of opioids in perioperative practice: a multidisciplinary clinical appraisal. Pain Medicine 2017. epub pnx191, Available at: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


