Abstract
Paraquat (PQ) poisoning is associated with high mortality rate. Therefore, an accurate method for predicting the survival of patients with PQ poisoning is required. This study evaluated the value of serum anion gap (AG) at admission in predicting the survival of such patients.
Cases of patients with PQ poisoning admitted to Cangzhou Central Hospital between May 2012 and March 2019 were retrospectively analyzed. The patients were classified into survival and nonsurvival groups on the basis of their 90-day prognosis. Correlation analysis, Cox regression analysis, and receiver operating characteristic and Kaplan–Meier curve analyses were performed to assess the value of AG in predicting the 90-day survival of patients with PQ poisoning.
Only 44 of the 108 patients with PQ poisoning survived; thus, the 90-day survival was 40.74%. AG levels at admission were significantly higher in nonsurvivors (26.53 ± 4.93 mmol/L) than in survivors (20.88 ± 2.74 mmol/L) (P < .001) and negatively correlated with 90-day survival (r = −0.557; P < .001). Cox regression analysis revealed that AG at admission is an independent prognostic marker of the 90-day survival of patients with PQ poisoning. AG level at admission had an area under the receiver operating characteristic curve of 0.836 (95% confidence interval: 0.763–0.909) and an optimal cut-off value of 25.5 mmol/L (59.4% sensitivity and 95.5% specificity).
AG level at admission may serve as a candidate marker for predicting the survival of patients with PQ poisoning.
Keywords: anion gap, paraquat, prediction
1. Introduction
Paraquat (PQ) has an excellent weeding effect, but it is highly toxic to humans and animals. Its misuse or intentional self-use can induce acute poisoning and has become a common cause of death. The lethal ingestion dose of PQ for humans is approximately 35 mg/kg, which is equivalent to 10 to 15 mL of a 20% solution.[1,2] Suicidal or accidental ingestion of PQ results in rapid multiple organ failure with a mortality rate that exceeds 60%.[3–6] These poor outcomes are attributed to the severe toxicity of PQ and the lack of effective detoxification therapies. Management of PQ poisoning is mainly directed at the following aspects: reduced absorption, increased excretion, anti-inflammation, anti-oxidation, and supportive treatment.[6–9] The serious clinical problem of PQ poisoning has prompted comprehensive research on its pathogenesis, prevention, and treatment. However, no relevant breakthrough has been achieved to date.
The current assessment of patients with PQ poisoning primarily depends on blood PQ concentration with relatively good sensitivity and specificity.[10,11] Unfortunately, PQ assays are not widely available, particularly in developing countries. PQ level detection often requires expensive devices, professional operators, and high cost and is thus limited for clinical use. Therefore, the most effective and appropriate therapy for PQ poisoning must be determined.
Anion gap (AG) can be easily and rapidly determined and is therefore suitable in clinical situations, such as sepsis,[12] acute pancreatitis,[13] and kidney diseases.[14,15] However, to the best of our knowledge, the correlation of AG level at admission with the prognosis of PQ poisoning remains unclear. The relevance of AG level at admission in terms of PQ toxicity has not been fully investigated. Therefore, this study aimed to evaluate the clinical predictive value of AG level at admission in patients with PQ poisoning.
2. Materials and methods
2.1. Ethics, consent, and setting
This retrospective clinical study was approved by the ethics committee of Cangzhou Central Hospital (no: 2017-090-01). Informed consent was waived because demographic, clinical, and biologic data were collected from medical records. All information on patients with PQ poisoning was analyzed anonymously and securely protected. All data were available to the investigators only.
2.2. Patients
Data were obtained from all patients admitted to the emergency department from May 2012 and March 2019 for intentional or accidental ingestion of PQ as verified by plasma PQ concentration test. Patients with acute oral PQ poisoning were selected according to the following criteria: the time from poison ingestion to hospital admission was within 12 hours and the age of the patients was >14 years. The exclusion criteria were as follows: PQ was ingested in combination with any other poison; the patients had a history of a chronic disease or an end-stage disease; known pregnancy; lost to follow-up; and time of exposure was unknown.
2.3. Protocol for PQ detoxification
The PQ detoxification protocol involved gastric lavage with a large amount of 0.9% saline, followed by 1 g/kg activated charcoal, 1 g/kg Fuller earth, and 250 mL of 20% mannitol through a nasogastric tube.[16] All patients received 1 to 3 courses of 3 hours active charcoal hemoperfusion therapy based on the results of urine PQ detection and clinical condition.[17,18] The protocol also included pulse therapies of methylprednisolone (0.375–1 g/d for 3 days), vitamin C (3.0 g/d for 10–14 days), and glutathione (2.4 g/d for 10–14 days).[1,18] Hemofiltration was performed when acute renal failure occurred.
2.4. Data collection
All data obtained from the patients were recorded and standardized in a Microsoft Excel spreadsheet by 2 physicians who were unaware of the purpose of this investigation. Information on patient demographics, clinical presentations, laboratory findings, and outcomes upon patient admission were collected by reviewing their medical records. The primary endpoint of this study was 90-day mortality. Patients were categorized into survivor or nonsurvivor groups on the basis of whether they survived after the 90-day follow-up period.
2.5. Statistical analysis
Statistical analysis was performed using the SPSS 13.0 software (SPSS Inc, Chicago, IL). Student t test or a Wilcoxon rank-sum test was performed on the numerical data between the groups. Chi-squared test was used for categorical data. Spearman correlation was performed to analyze the relationship between 2 metabolites. Correlation analysis, Cox regression analysis, and receiver operating characteristic (ROC) and Kaplan–Meier curve analyses were used to assess the predictive value of AG in mortality. P < .05 indicated a statistically significant difference.
3. Results
3.1. Patient characteristics
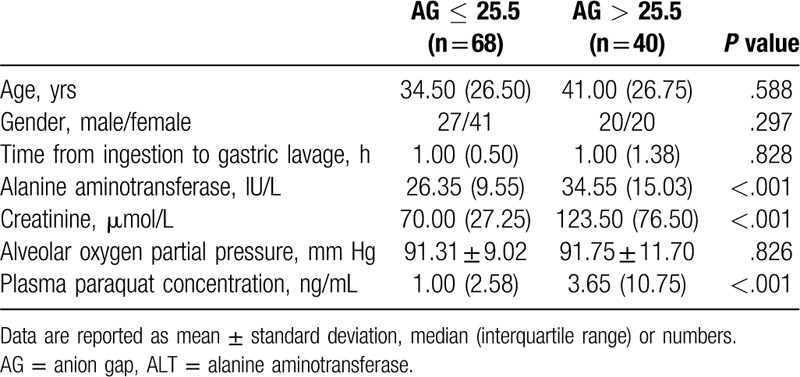
A total of 123 patients were admitted to our hospital from May 2012 to March 2019 because of PQ poisoning. Fifteen of the patients were excluded because of incomplete data (n = 7), unsuccessful follow-up (n = 3), transfer to another hospital (n = 2), and incomplete blood gas analysis (n = 3). A total of 108 patients met the inclusion criteria and were finally included in this study. The total survival rate was 40.74% (44/108) after the 90-day follow-up period. The clinical characteristics and biologic data upon admission of the nonsurvival and survival groups are presented in Table 1. Nonsurvivors had high AG, alanine aminotransferase (ALT), creatinine, and plasma PQ concentration but low alveolar oxygen partial pressure. Clinical characteristics and biologic data upon admission were stratified on the basis of AG level at admission, as shown in Table 2. Patients with AG >25.5 mmol/L had high ALT, creatinine, and plasma PQ concentration.
Table 1.
Clinical characteristics and biologic data at admission of the nonsurvival and survival groups.
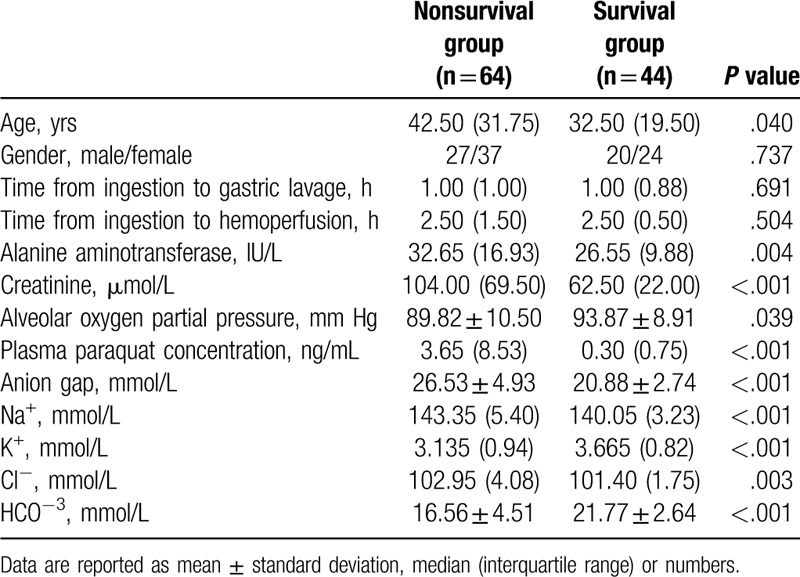
Table 2.
Clinical characteristics and biologic data stratified on the basis of AG level at admission.
3.2. Correlation analysis
Bivariate analysis revealed that AG was positively associated with plasma PQ concentration (r = 0.337; P < .001) and negatively correlated with survival time (r = −0.512; P < .001) and 90-day survival (r = −0.557; P < .001).
3.3. Cox proportional hazard regression analysis
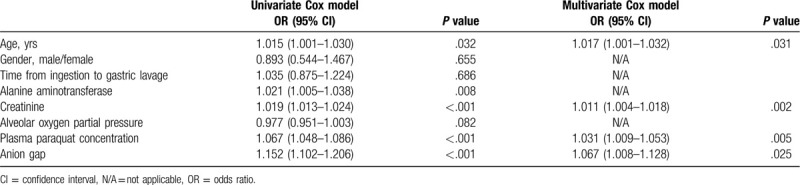
Univariate and multivariate Cox proportional hazard analyses (Table 3) showed that the high AG level at admission was an independent risk factor for 90-day survival (hazard ratio: 1.152, 95% confidence interval [CI]: 1.102–1.206, P < .001; hazard ratio: 1.067, 95% CI: 1.008–1.128, P = .025, respectively).
Table 3.
Cox regression model.
3.4. ROC curve analysis for 90-day mortality
Table 4 shows that the area under the ROC curve (AUC) was 0.836 for AG (59.4% sensitivity and 95.5% specificity), 0.845 for creatinine (75.0% sensitivity and 88.6% specificity), and 0.965 for plasma PQ concentration (89.1% sensitivity and 93.2% specificity). Pairwise comparison using Hanley and McNeil method (Fig. 1) showed that the predictive power of AG was lower than that of plasma PQ concentration (Z = 0.189; P = .001) and similar to that of creatinine (Z = 3.304; P = .851).
Table 4.
ROC curve analysis.
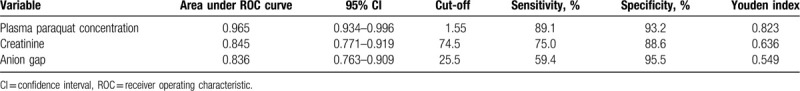
Figure 1.
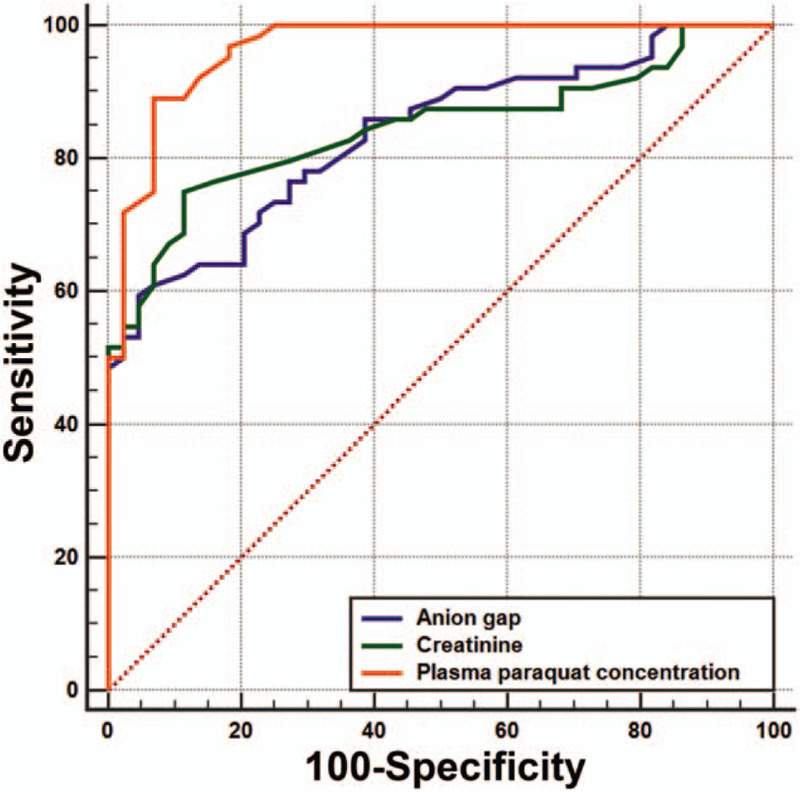
Area under the receiver operating characteristic curve analysis.
3.5. Kaplan–Meier survival analysis
The Kaplan–Meier curve during the 90-day follow-up period showed that survival was significantly higher in the low AG group than in the high AG group (log rank test, P < .001; Fig. 2).
Figure 2.
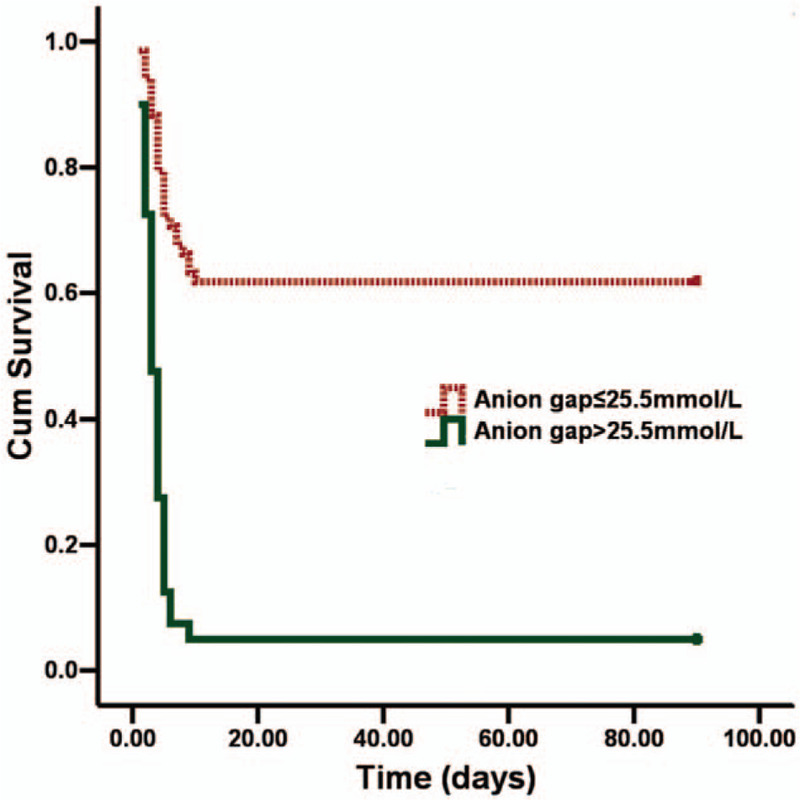
Kaplan–Meier curve analysis of survival curves for the groups stratified according to anion gap level at admission.
4. Discussion
Patients with PQ poisoning have a low survival rate of 40.74%; thus, accurate prognostic indicators must be identified. Appropriate prognostic indicators can aid physicians in evaluating the severity of poisoning and predicting overall outcomes. Furthermore, the early determination of prognosis can prevent patients with inevitable deaths to undergo inappropriate treatments.
Several parameters have been suggested to predict clinical outcomes after PQ ingestion. Plasma PQ levels are an excellent prognostic indicator for patient outcome.[10,11] However, most hospitals cannot readily measure plasma PQ levels. Another important predictor of mortality is the oral dose of the poison.[5] Unfortunately, estimates on the amount ingested are often unobtainable or unreliable in a number of intoxicated patients. The use of Acute Physiology and Chronic Health Evaluation II,[5,19] Sequential Organ Failure Assessment,[3] and machine-learning methods[20] to prognosticate patients with PQ poisoning by combining coagulation, liver, and kidney indices can provide a perfect predictive value for the outcome of PQ poisoning. However, many variables are required in using these scores. The extent of lung injury as shown by computed tomography scans and the presence of pulmonary emphysema are also correlated with the prognosis of PQ poisoning.[21–23] Lung injury in computed tomography scans and pulmonary emphysema may take 24 hours or more to determine; thus, these diagnostic methods would prevent the early application of treatment.
This study examined for the 1st time the predictive value of AG on the 90-day survival of patients with PQ poisoning. Results suggested that elevated AG was present in nonsurviving patients, and the AUC of AG was 0.836 (95% CI: 0.763–0.909); thus, the diagnostic accuracy of AG was relatively high.
High AG in patients with PQ poisoning possibly has a complicated mechanism. First, an increased amount of lactate acid accounts for a considerable proportion in the increments in AG.[24,25] Deteriorated circulation[26,27] is also common in moderate and severe PQ poisoning and causes lactic acid accumulation. Second, kidneys, as the main detoxification organ, encounter extremely high concentrations of PQ during the body's process of eliminating the poison; hence, proximal tubular cells undergo vacuolization, which ultimately results in acute tubular necrosis.[28] The loss of tubular function decreases the clearance of various organic anions,[29] and the accumulation of organic anion contributes to the high AG in patients with PQ poisoning.
The limitations of our study must be acknowledged. First, a severely high triglyceride level may underestimate Na+ and Cl− concentrations; thus, AG level at admission might also be underestimated. Second, potential residual confounders owing to unavailable information in the dataset used may be present because of the retrospective design of this study. Third, the study was conducted in a single center, and almost the entire population was native. Therefore, the findings may not be generalizable to all countries.
In conclusion, acute PQ poisoning is a highly fatal condition that requires rapid and precise assessment. AG and creatinine can be used as prognosis predictors of PQ poisoning if plasma PQ concentration cannot be determined in real time because of the unavailability of testing materials.
Author contributions
Conceptualization: Yong Zhao, Shun Yi Feng, Yong Li.
Data curation: Yong Zhao.
Software: Shun Yi Feng.
Writing – original draft: Yong Zhao.
Writing – review & editing: Yong Li.
Footnotes
Abbreviations: AG = anion gap, AUC = area under the curve, CI = confidence interval, OR = odds ratio, PQ = paraquat.
How to cite this article: Zhao Y, Feng SY, Li Y. Serum anion gap at admission as a predictor of the survival of patients with paraquat poisoning: a retrospective analysis. Medicine. 2020;99:31(e21351).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol 2011;72:745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang L, Li Q, Liu W, et al. Mesenchymal stem cells alleviate acute lung injury and inflammatory responses induced by paraquat poisoning. Med Sci Monit 2019;25:2623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weng CH, Hu CC, Lin JL, et al. Sequential organ failure assessment score can predict mortality in patients with paraquat intoxication. PLoS One 2012;7:e51743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Myung W, Lee GH, Won HH, et al. Paraquat prohibition and change in the suicide rate and methods in South Korea. PLoS One 2015;10:e0128980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu XW, Ma T, Li LL, et al. Predictive values of urine paraquat concentration, dose of poison, arterial blood lactate and APACHE II score in the prognosis of patients with acute paraquat poisoning. Exp Ther Med 2017;14:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Y, Wu Y, Shen F, et al. Clinical effect of haemoperfusion combined with continuous veno-veno haemofiltration in treatment of paraquat poisoning: a Meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019;31:214–20. [DOI] [PubMed] [Google Scholar]
- [7].Hong SY, Yang JO, Lee EY, et al. Effect of haemoperfusion on plasma paraquat concentration in vitro and in vivo. Toxicol Ind Health 2003;19:17–23. [DOI] [PubMed] [Google Scholar]
- [8].Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, et al. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008;38:13–71. [DOI] [PubMed] [Google Scholar]
- [9].Wu MR, Hsiao CY, Cheng CH, et al. Is endotracheal intubation a non-beneficial treatment in patients with respiratory failure due to paraquat poisoning? PLoS One 2018;13:e0195071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Proudfoot AT, Stewart MS, Levitt T, et al. Paraquat poisoning: significance of plasma-paraquat concentrations. Lancet 1979;2:330–2. [DOI] [PubMed] [Google Scholar]
- [11].Hart TB, Nevitt A, Whitehead A. A new statistical approach to the prognostic significance of plasma paraquat concentrations. Lancet 1984;2:1222–3. [DOI] [PubMed] [Google Scholar]
- [12].Mitra B, Roman C, Charters KE, et al. Lactate, bicarbonate and anion gap for evaluation of patients presenting with sepsis to the emergency department: a prospective cohort study. Emerg Med Australas 2019;32:20–4. [DOI] [PubMed] [Google Scholar]
- [13].Shen X, Ke L, Yang D, et al. The prognostic value of the strong ion gap in acute pancreatitis. J Crit Care 2016;36:140–5. [DOI] [PubMed] [Google Scholar]
- [14].Abramowitz MK, Hostetter TH, Melamed ML. The serum anion gap is altered in early kidney disease and associates with mortality. Kidney Int 2012;82:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Passos RDH, Caldas JR, Ramos JGR, et al. Acid base variables predict survival early in the course of treatment with continuous venovenous hemodiafiltration. Medicine (Baltimore) 2018;97:e12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Weng CH, Chen HH, Hu CC, et al. Predictors of acute kidney injury after paraquat intoxication. Oncotarget 2017;8:51345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun L, Yan PB, Zhang Y, et al. Effect of activated charcoal hemoperfusion on renal function in patients with paraquat poisoning. Exp Ther Med 2018;15:2688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu WP, Lai MN, Lin CH, et al. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PLoS One 2014;9:e87568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu S, Hu H, Jiang Z, et al. APACHE score, severity index of paraquat poisoning, and serum lactic acid concentration in the prognosis of paraquat poisoning of Chinese patients. Pediatr Emerg Care 2015;31:117–21. [DOI] [PubMed] [Google Scholar]
- [20].Hu L, Li H, Cai Z, et al. A new machine-learning method to prognosticate paraquat poisoned patients by combining coagulation, liver, and kidney indices. PLoS One 2017;12:e0186427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou CY, Kang X, Li CB, et al. Pneumomediastinum predicts early mortality in acute paraquat poisoning. Clin Toxicol (Phila) 2015;53:551–6. [DOI] [PubMed] [Google Scholar]
- [22].Li J, Zhao J, Zhang Q, et al. The value of assessment of area of ground glass opacity in lungs cast by high-resolution computed tomography on the prognosis of patients with acute paraquat intoxication. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27:270–3. [DOI] [PubMed] [Google Scholar]
- [23].Kim YT, Jou SS, Lee HS, et al. The area of ground glass opacities of the lungs as a predictive factor in acute paraquat intoxication. J Korean Med Sci 2009;24:636–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gabow PA, Kaehny WD, Fennessey PV, et al. Diagnostic importance of an increased serum anion gap. N Engl J Med 1980;303:854–8. [DOI] [PubMed] [Google Scholar]
- [25].Balasubramanyan N, Havens PL, Hoffman GM. Unmeasured anions identified by the Fencl-Stewart method predict mortality better than base excess, anion gap, and lactate in patients in the pediatric intensive care unit. Crit Care Med 1999;27:1577–81. [DOI] [PubMed] [Google Scholar]
- [26].Cai Q, Liu Z. An analysis of relevant factors of early death in acute paraquat poisoning. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014;26:379–82. [DOI] [PubMed] [Google Scholar]
- [27].Lee Y, Lee JH, Seong AJ, et al. Arterial lactate as a predictor of mortality in emergency department patients with paraquat intoxication. Clin Toxicol (Phila) 2012;50:52–6. [DOI] [PubMed] [Google Scholar]
- [28].Sittipunt C. Paraquat poisoning. Respir Care 2005;50:383–5. [PubMed] [Google Scholar]
- [29].Eraly SA, Vallon V, Vaughn DA, et al. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem 2006;281:5072–83. [DOI] [PubMed] [Google Scholar]


