Abstract
Traditional open surgery (OS) is usually necessary when testicular torsion (TT) cannot be excluded by scrotal ultrasound. Scrotoscopy has been used as a minimally invasive technique to diagnose or treat scrotal diseases, and it may also play a role in diagnosing TT.
A retrospective analysis was performed for patients with TT to evaluate the consistency of scrotoscopy and OS in the diagnosis of TT. In the cases where preoperational Color Doppler ultrasonography was performed, scrotoscopy, open surgery, and confirmed TT were included for future analysis.
A total of 43 patients were studied. Twisted testes were retained in 11 cases (25.59%), and the remaining 32 patients (74.41%) underwent orchiectomy. There were significant differences in the diagnostic value between the grading of scrotoscopy and ultrasound, as well as between ultrasound grading and blood supply grading (BSG) (both P < .05). However, no significant difference was observed between the grading of scrotoscopy and BSG in traditional OS (P > .05), but a high degree of consistency existed between scrotoscopy grading and BSG in traditional OS (Kappa = 0.733, P ≤ .001).
Our limited data indicate that the diagnosis of testicular torsion by scrotoscopy is highly consistent with that of traditional surgical exploration. Therefore, further studies are necessary to confirm its application value in the future. Scrotoscopy may have potential application value for the patients whom testicular torsion are insufficiently diagnosed but cannot be excluded.
Keywords: Color Doppler Ultrasound, orchiectomy, scrotoscopy, testicular torsion
1. Introduction
Testicular torsion (TT) is one of the most common urological emergencies. The condition is often dangerous, progresses quickly, and its outcomes change rapidly. Therefore, it is necessary to confirm diagnosis and administer effective treatment as soon as possible.[1,2] Color Doppler Ultrasound (CDU) is the preferred method for its diagnosis, as this can help visualize the position of the testis and the characteristics of the blood flow.[3] Presently, the diagnostic accuracy of ultrasonography for TT is as high as 90%. However, some patients are misdiagnosed, their diagnoses are missed or delayed, or their treatment is delayed, resulting in testicular atrophy and risk of testicular necrosis or other serious adverse consequences. In such situations, traditional open surgery (OS) is usually necessary when TT cannot be excluded by scrotal ultrasound. However, OS offers a non-minimal invasion to scrotum, and brings postoperative discomfort and complications.[4]
Compared with OS, scrotoscopy is a minimally invasive diagnostic and treatment technique for scrotal diseases.[5] It can provide more elaborate diagnostic information than ultrasound and other imaging techniques. In some cases, the condition can even be treated using a scrotoscope.[5–7] In this study, we performed a retrospective analysis by including all patients with TT who underwent scrotoscopy, and evaluated the diagnostic value of scrotoscopy in TT.
2. Materials and methods
2.1. Patients included
A retrospective analysis was performed for patients hospitalized between January 2010 and May 2018. The patients were admitted to Second Xiangya Hospital of the Central South University and Fujian Provincial Hospital. The inclusion criteria were: all cases were finally diagnosed as TT; all cases were performed preoperational CDU, scrotoscopy, and open surgery; patients were aged 14 years old and above. The following information was recorded for the included cases: time of onset, results of ultrasound, imaging findings of scrotoscopy, operation time, intraoperative testicular blood supply and TT status, and short- and long-term postoperative complications. Our exclusion criteria were: patients finally free of TT, above information unavailable, did not perform CDU/scrotoscopy/open surgery, or unwilling to participate in this study.
2.2. Scrotoscopy technique
First, genital skin was shaved and intravenous antibiotics were preoperatively administered. Epidural anesthesia, spinal anesthesia, or general anesthesia was administered. Then, the patient was made to lie in the lithotomy position, and a routine vulvar disinfection was performed. The plastic incise drape was pasted at the surgical site. The surgical instrument set—such as an endoscope, cystoscope (17 Fr. or 22 Fr., 12° or 30°, 27026 A, Karl Storz), or bipolar resectoscope (26 Fr., 12° or 30°, TUVis, Olympus)—was prepared.
The first step was to establish a small incision. At this point, an assistant immobilized the affected testicle. A 1-cm incision was made on the anterior wall of the scrotum, and the tissue of the scrotum was separated layer by layer, until the cavity of perididymis was reached. Then, the entire layer of the scrotum wall was clamped with 2 Allies clamps. A small amount of dark-red or dark-black non-coagulable fluid spilled out when the cavity of perididymis was reached (Fig. 1A), which was the first typical performance of TT under scrotoscopy, usually indicating testicular necrosis happened. In the second step, the assembled endoscope was placed under direct view via the abovementioned incision. Sterile 0.9% NaCl solution was used as perfusion fluid, and the perfusion pressure was maintained at about 60 cmH2O. After washing the cavity of perididymis several times with saline at a continuous low flow rate, the bloody effusion was rinsed, and the contents of the scrotum were observed carefully. The path of the perfusate into the cavity was the operation channel of the cystoscope or resectoscope, and the outlet channel was the gap between the scope sheath and the edge of the incision. Typically, TT presented with a high testicular position in a horizontal plane, and a spiral spermatic cord could be observed, which was the second typical but the most important performance of TT under scrotoscopy (Fig. 1B). The testis and epididymis appeared dark purple or dark black (Fig. 1C), which was the third typical performance of TT under scrotoscopy, also a sign of different degrees of testicular necrosis. In some patients’ epididymis, erosions associated with necrosis were observed. For patients who admitted quickly (usually <3 hours) after TT occurred, the testis and epididymis were dark gray, and the albuginea was a dull red. Based on these findings, TT was confirmed.
Figure 1.
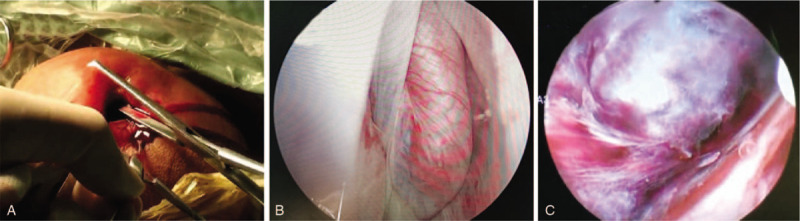
Key signs of scrotoscopy in diagnosing TT. A: When reaching into the cavity of perididymis, a slight dark red, non-coagulable fluid flows out, which is the first typical performance of TT under scrotoscopy, usually indicating testicular necrosis. B: TT is in a position of horizontal plane, and a spiral spermatic cord is observed, the second typical but the most important performance of TT under scrotoscopy. C: Under the scrotoscope, the testis and epididymis appear dark black, also a sign of different degrees of testicular necrosis. TT = testicular torsion.
After confirmed diagnosis, the third step was to extend the original small incision to perform traditional OS. Then, testicular detorsion was performed, its blood supply observed, and blood supply grading evaluated. The affected testis and epididymis were retained or removed, depending on the observation. Orchioechtomy could also be performed under the abovementioned small incision. If it is necessary for the testicle to be removed, the spermatic cord and the vas deferens were then conformally sutured. Additionally, after the scrotal incision was sutured, contralateral orchiopexy was performed. A rubber drainage membrane was conventionally indwelled into the cavity of perididymis. Then, conventional catheterization was performed, scrotal support provided, and local compression given. For an organ-sparing approach for the testis, the scrotum was pressed slightly or not at all, so as not to affect the testicular blood supply. The catheter and drainage membrane were removed between 24 and 48 hours after surgery.
2.3. Evaluation method
2.3.1. Color Doppler ultrasonography grading
Preoperative emergency ultrasound was used to assess testicular blood supply and testicular position, comparing them with the healthy side. The likelihood of diagnosis of TT based on ultrasound reports was graded as follows:
Grade I: reliable blood supply with or without good testicular position; TT is under excluded.
Grade II: unreliable blood supply with or without poor testicular position; TT is probable.
Grade III: no blood supply with or without testicular transverse position; the TT is confirmed or highly suspected.
2.3.2. Scrotoscope grading
The performance status using scrotoscopy was graded as follows:
Grade I: testis with or without epididymis showed a state of burgundy wine alternating with a reddish color.
Grade II: the overall testis showed dark red, with black strip vascular thrombosis visible in some areas.
Grade III testicles appeared black with extensive thrombosis in the blood vessels; erosion and necrosis could be observed.
2.3.3. Blood supply grading during OS after testicular detorsion
After the testis were reset and active arterial bleeding (bright red blood) was observed, the blood supply of the twisted testis was assessed according to Marcello et al's method.[8] The incision was performed traditionally, and subsequent detorsion was performed immediately to observe the blood supply time. The blood-supply grading (BSG) was classified as follows:
Grade I: bright red blood flowing immediately from the edge of the cut.
Grade II: bright red blood flowing out within 10 minutes of the cut.
Grade III: bleeding absent after 10 minutes.
2.4. Follow-up data
Follow-up visits were conducted at least twice, in the first and sixth months after surgery. The follow-up data included surgical complications such as wound infection, edema, and hematoma. Ultrasound examination of the testis was performed to assess testicular blood supply and testicular size. The testicular volume was calculated according to the formula 0.52 × length × width × thickness (cm) of the testicular ellipsoid, and was then compared with the contralateral testis. If the testicular volume was <50% of the contralateral side, the possibility of testicular atrophy was considered.[8] The sensitivity, specificity, and safety of scrotoscopy for TT were then evaluated.
2.5. Statistical analysis
All statistical analyses were performed with SPSS 24.0 for Windows student version (SPSS, Chicago, IL). The McNemar–Bowker paired chi-square test and the Kappa consistency test were performed to analyze the difference and consistency of scrotoscopy in diagnosing TT, compared with ultrasound and BSG. The difference was considered significant when a P value of <.05 was obtained.
3. Results
3.1. General characteristics
Finally, 43 patients (mean age of 18.42 years; age range 14–23 years) were confirmed as having TT, and were thus included in the study. The average time from onset to surgery was 16.02 hours (range, 3–38 hours). Among the patients, TT was on the left side in 30 cases (69.77%) and the right in 13 cases (30.23%). The average twist angle was 426.98° (range: 180°–1080°). The average operation time was 64.79 ± 12.08 minutes (range: 47–88 minutes). The average time of scrotal exploration by scrotoscopy was 6.84 ± 2.45 minutes (range: 2–11 minutes), which accounted for 10.56% of the total surgery time. The average time of blood supply observation during OS was 25.89 ± 9.51 minutes (range: 5–45 minutes), accounting for 39.96% of the total surgery time. The twisted testes were retained in 11 cases (25.59%), and the remaining 32 patients (74.41%) underwent orchiectomy.
3.2. Evaluating the diagnostic value of scrotoscopy compared with ultrasound and OS
TT was excluded by ultrasound in 7 cases (16.28%) (Grade I). It was considered possible, highly suspected, or diagnosed by ultrasound in 36 cases (83.72%), including 25 (58.14%) for Grade II and 11 (25.58%) for Grade III. Thus, the accuracy rate of ultrasound-based diagnosis of TT was 83.72%. All patients were confirmed as having TT by scrotoscopy. Thus, the accuracy, sensitivity, and specificity of scrotoscope were all 100%. Among these cases, 6 (13.95) were classified as Grade I, 11 (25.58%) as Grade II, and 26 (60.47%) as Grade III. Additionally, according to BSG during OS, there were 5 (11.63%) Grade I cases, 9 (20.93%) Grade II cases, and 29 (67.44%) Grade III cases.
McNemar–Bowker's paired chi-square test proved that for TT, there were significant differences in the diagnostic value between the grading established using scrotoscopy and ultrasound, as well as between the grading established using ultrasound and blood supply during OS. However, no significant difference was observed between the grading established using scrotoscopy and blood supply. Further, the Kappa consistency test found that the consistency between grading established using scrotoscopy and blood supply during OS was significantly high (Kappa = 0.733, P ≤ .001). (See Tables 1–3 for details). These results suggest scrotoscopy and traditional OS show a high degree of consistency in the diagnosis of TT. As BSG was the key basis for intraoperative determination of testicular retention or resection,[8] scrotoscopy could help decide whether the testes should be removed or preserved. The predictive value may be comparable to that of BSG during OS.
Table 1.
Results of paired chi-square test: differences and consistencies between grading of CDU and scrotoscopy.
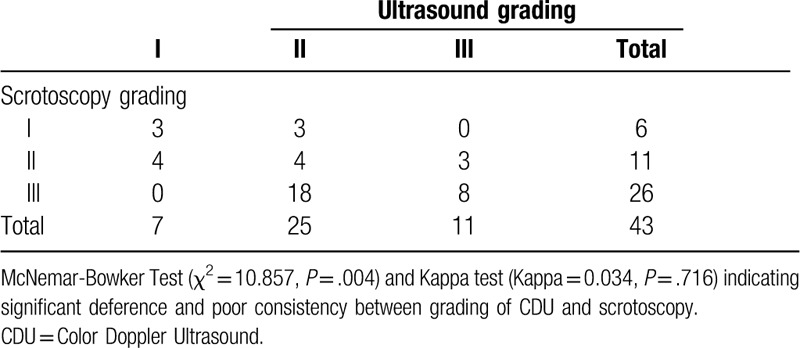
Table 3.
Results of paired chi-square test: differences and consistencies between grading of scrotoscopy and blood supply.
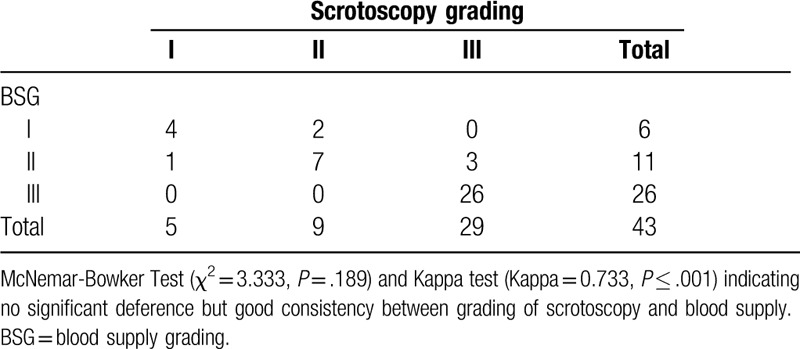
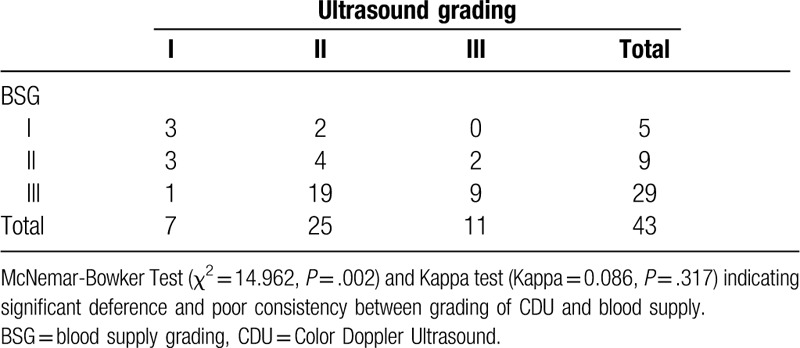
Table 2.
Results of paired chi-square test: differences and consistencies between grading of CDU and blood supply.
3.3. Outcomes of cases undiagnosed by ultrasound
The above 7 patients were all graded I in ultrasound. Among them, 3 cases and 4 other cases were classified as Grade I and Grade II in scrotoscopy, respectively. Six of the 7 patients successfully retained their testes, while 1 patient who had Grade II in scrotoscopy but BSG Grade III in OS underwent orchiectomy.
3.4. Postoperative complications
Although scrotal edema was found in 9 cases (20.93%) after surgery, no wound infection occurred, and all patients were significantly relieved within 24 to 48 hours. Three patients (6.98%) who underwent orchiectomy developed scrotal hematoma, and the hematoma was absorbed after applying a local compression dressing on the scrotum for 3 days. Four out of 9 patients (36.3%) were confirmed as having testicular atrophy. Among these 43 cases, only about 10 patients came back at 2 to 3 months, and only 2 of them complained a little scrotal discomfort who finally reported covered at the sixth month follow-up; and 1 worried the status of his teste on the healthy side and was finally proved to be a little anxious. No other complications were observed during the sixth month follow-up. All these complications were considered Grade I or II according to the classification of surgical complications by Dindo et al.[9]
4. Discussion
Emergency surgical exploration remains the primary treatment for TT. Rapid diagnosis and treatment of TT is essential to preserve the testis and minimize the need for orchiectomy. Once the possibility of TT is clinically considered, surgical exploration is recommended to restore the testicular blood supply in the shortest time, to save the affected testicle.[10,11] It is necessary to distinguish TT followed by necrosis from a rare clinical entity of testicular infarction and necrosis in clinical practice. The latter is rare, usually resulting in bacterial embolism, thrombosis, or tumor embolism.[12–14] Except for endoscopic changes in the location of the testis, the other expected extent of testicular necrosis may be similar to that of TT. In addition to orchiectomy or organ sparing, the causes must be discovered and treated.
Scrotoscopy for the diagnosis of TT is a safe and minimally invasive method. The traditional open exploration is performed by making a 3 to 5 cm incision in the ipsilateral scrotum and then pulling the testis, epididymis, and spermatic cord completely out of the scrotum for detorsion and subsequent treatment. In line with the extensive application of minimally invasive techniques to various fields of urology, scrotoscopy has also been applied to treat scrotal diseases. Scrotoscopy has been applied to the diagnosis and treatment of multiple scrotal diseases, such as epididymal cyst resection, auxiliary hydrocele sheath resection,[5,7] assisting scrotal wall vascular myxoma resection,[15] assisting testicular rupture, and even idiopathic testicular pain diagnosis and treatment.[6,16,17] These have achieved good results.
Scrotoscopy has obvious advantages in the diagnosis and treatment of scrotal diseases, such as a shorter operation time, the need for only small incisions, and rapid recovery after surgery. All of these meet the definition of minimally invasive.[18] Further, scrotoscopy does not cause extra trauma because the cavity of perididymis is a natural human cavity.[5–7,15] The diagnosis of TT by scrotoscopy also requires a small incision. Unlike for some of the abovementioned scrotal diseases, in TT treatment the extension of the incision still needs to be enough to restore the testicles and epididymis to the orthotopic condition. Therefore, for patients for whom it is uncertain whether the testicle has become necrotic, the advantage of the small incision is not very evident. Testicular necrosis has been confirmed by scrotoscopy. The advantage of a small incision is that it can be used to perform testicular epididymectomy. Among the 43 patients studied here, Grade III performance under scrotoscope appeared in 26 cases (60.47%). The scrotoscope grading presented high consistency with BSG during OS, suggesting that orchidectomy could be considered directly and conducted using a smaller incision than traditional OS. In this case, minimally invasive surgery presented obvious advantages. Of course, there is a certain risk, particularly as the current diagnostic value of scrotoscope in TT has not been fully verified. Therefore, in actual clinical applications, it may still be necessary to extend the incision, and reset and observe the blood supply before proceeding further.
In terms of safety, scrotal exploration under a scrotoscope does not significantly increase intraoperative or postoperative complications. The overall incidence of complications in our cases was 37.21% (16/43). Scrotal edema is a unique complication of scrotoscope, which is usually resolved within 24 to 48 hours without any special treatment. Scrotal hematoma and testicular atrophy occurred in 3 (6.98%) and 4 (9.30%) cases, respectively. The hematoma significantly resolved only after treatment by scrotal pressure. The occurrence of testicular atrophy is the outcome of TT to a large extent, which is not directly related to the use of a scrotoscope. All of these complications were Grade I–II according to the classification of surgical complications.[9] Therefore, scrotoscopy is generally safe for the diagnosis of TT.
Scrotoscope exploration has a high diagnostic value for TT. A review of the literature found that the diagnostic sensitivity and specificity of CDU in TT were 63% to 99% and 97% to 100%, respectively. The lack of bloodstream supply in the twisted testis was positively found by ultrasound in 76% of cases.[19] Thus, a considerable number of cases still do not have an accurate diagnosis by ultrasound examination, which may delay subsequent treatment and lead to serious adverse consequences, such as infection and delayed testicular resection.[11,19] In clinical practice, we found it was not uncommon to misdiagnose TT as epididymitis and/or orchitis by a combination of CDU and physical examination. Conversely, scrotoscopy seems to have a diagnostic value beyond ultrasound. The diagnosis of TT by scrotoscopy has earlier been reported in China[20]; their conclusion agrees with those of this study, both reporting 100% sensitivity and specificity of scrotoscopy for diagnosing TT. The difference between these 2 reports was that in the case of this study, we tried to grade the status of TT for the first time using a scrotoscope, and then performed a chi-square test to find the differences and consistency between scrotoscope grading and ultrasound/BSG during OS after testicular detorsion. No evident difference was found in the diagnosis in these 3 grading groups. As the scrotoscope grading was highly and significantly consistent with BSG, it may be considered an alternative to the latter.
A potential clinical benefit of scrotoscope grading over BSG is that the testis may be retained or removed according to the performance of the scrotoscope, thereby shortening unnecessary waiting time for blood supply observation during surgery. However, this decision needs to be very cautious. We do not advice to make the decision based on scrotoscopy alone but it should be confirmed by OS, because of limitations of the current application of scrotoscopy. From this study, we found that the average time for scrotal exploration in scrotoscopy was 6.84 minutes, accounting for 10.56% of the whole operation time. Conversely, the average time for blood supply observation was 25.89 minutes, accounting for 39.96% of the operation time. Therefore, if the resection or retention is judged based on the observation results of scrotoscopy, operation time can be reduced to under 40 minutes. Of course, there is a risk that it may not be possible to perform testicular detorsion by scrotoscopy alone. Further, there is a possibility of bias based on a surgeon's experience and judgment. Therefore, patients with uncertain blood supply conditions, particularly those with a short time of onset of <6 hours, need to be handled with more care.
Finally, it should be noted that even diagnostic value of scrotoscopy in TT may be better than emergency scrotal ultrasound and seems similar to traditional OS. But, it cannot be a replacement for ultrasound as an initial assessment for patients with sudden scrotal pain. Scrotoscopy presents an application value especially in cases where TT cannot be excluded by ultrasound.
5. Limitations
At present, scrotoscopy cannot replace ultrasound examination and traditional open surgery, but it may provide an alliterative new method and possibility in addition to the existing methods for testicular torsion. The limitations of this study should be obvious. First, this is a retrospective study with a small sample size and no control group. More case control studies or prospective randomized studies are needed to further evaluate the value of scrotoscopy in the diagnosis and treatment of TT. Additionally, the reversal of the twisted testis and the decision to completely retain or remove it under the scrotoscope needs to be learned via trial and error, to achieve a goal of minimal invasiveness and shortened operation time. Importantly, cases without TT were excluded from this study, as the main potential advantage of scrotoscopy should be excluding TT when CDU fails to do so. Further studies should include all cases with clinical suspicions of TT, and also patients with acute scrotum excluding torsion, in order to evaluate the specificity and sensitivity of scrotoscopy in the future. Furthermore, scrotoscope should be improved in order to bring more benefit for these kinds of patients with scrotal diseases, such as a small flexible scope may be designed to directly observe the contents of scrotum under local anesthesia.
6. Conclusion
The limited data of this study confirmed that the diagnosis of testicular torsion by scrotoscopy was highly consistent with that of traditional surgical exploration. In our future clinical practice, for those patients whom TT are insufficiently diagnosed but cannot be excluded combining symptoms, signs and results of emergency scrotal CDU, in order to reduce possible of misdiagnosis that may delay the best time to retain testes, we may spend a few minutes to do a minimally invasive scrotoscopy under local anesthesia etc, which may helpful to make determinate diagnose or exclude TT, and may avoid complications of conventional OS. While for those who have been clinically confirmed having TT, it may not be necessary to perform scrotoscopy, but directly perform OS to achieve detorsion as soon as possible in order to reduce the ischemic time of the lesion testis. However, we emphasize that as this is only a preliminary study it is still unknown whether the diagnostic value of scrotoscopy for TT can withstand more cases during future clinical practice. Therefore, we caution that for patients who cannot diagnose TT or exclude TT in scrotoscopy, open surgical exploration should be conduct to avoid irreparable consequences. More studies are needed to confirm its application value in TT cases in the future.
Author contributions
Huaishan Hong and Xiang Wu prepared the draft of manuscript. Le Lin, Tao Li, and Qingguo Zhu collected and analyzed the data. Wanghai Cai, Jinfeng Wu, Liefu Ye, and Yunliang Gao participated in the cases diagnosis and management and follow-up. Jinrui Yang and Yongbao Wei sponsored the study and took part in the paper editing. All authors read and approved the final manuscript.
Footnotes
Abbreviations: BSG = blood supply grading, CDU = Color Doppler Ultrasound, OS = open surgery, TT = testicular torsion.
How to cite this article: Hong H, Cai W, Wu J, Wu X, Lin L, Li T, Zhu Q, Gao Y, Ye L, Wei Y, Yang J. Scrotoscopy and traditional open surgery shows a high degree of consistency in the diagnosis of testicular torsion: an initial report. Medicine. 2020;99:31(e21545).
YW and JY have contributed equally to the study.
Additional information: As a case series report, all data generated or analyzed are included in this paper.
This study was supported by Joint Funds for the innovation of science and Technology, Fujian province (2017Y9064) and high-level hospital foster grants from Fujian Provincial Hospital, Fujian province, China (2019HSJJ29).
Ethics Statement: The approval for this study was obtained from the Fujian Provincial Hospital. Written informed consents were obtained from the guardians of these patients. Identifying information was removed from the study. All data were kept by only the administrator of the study in a confidential manner and was not used by any other purposes. The methods were performed in accordance with the relevant guidelines and regulations.
Competing interests: None declared.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Lodwick DL, Cooper JN, Minneci PC, et al. Factors affecting pediatric patient transfer in testicular torsion. J Surg Res 2016;203:40–6. [DOI] [PubMed] [Google Scholar]
- [2].Dias FA, Oliveira RR, Riccetto CL, et al. Improving organ salvage in testicular torsion: comparative study of patients undergoing vs not undergoing preoperative manual detorsion. J Urol 2016;197:811–7. [DOI] [PubMed] [Google Scholar]
- [3].Xiao H, Gao Y, Li Y, et al. Ultrasound assessment of perinatal testicular torsion. Br J Radiol 2016;89:20151077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Onol SY, Ilbey YO, Onol FF, et al. A novel pull-through technique for the surgical management of idiopathic hydrocele. J Urol 2009;181:1201–5. [DOI] [PubMed] [Google Scholar]
- [5].Yang JR, Wei YB, Yan B, et al. Comparison between open epididymal cystectomy and minimal resection of epididymal cysts using a scrotoscope: a clinical trial for the evaluation of a new surgical technique. Urology 2015;85:1510–4. [DOI] [PubMed] [Google Scholar]
- [6].Wang Z, Yang JR, Huang YM, et al. Diagnosis and management of testicular rupture after blunt scrotal trauma: a literature review. Int Urol Nephrol 2016;48:1967–76. [DOI] [PubMed] [Google Scholar]
- [7].Bin Y, Yong-Bao W, Zhuo Y, et al. Minimal hydrocelectomy with the aid of scrotoscope: a ten-year experience. Int Braz J Urol 2014;40:384–9. [DOI] [PubMed] [Google Scholar]
- [8].Cimador M, DiPace MR, Castagnetti M, et al. Predictors of testicular viability in testicular torsion. J Pediatr Urol 2007;3:387–90. [DOI] [PubMed] [Google Scholar]
- [9].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sessions AE, Rabinowitz R, Hulbert WC, et al. Testicular torsion: direction, degree, duration and disinformation. J Urol 2003;169:663–5. [DOI] [PubMed] [Google Scholar]
- [11].Chidi NM, Bhaskar KS, Chris MG. Outcomes of scrotal exploration for acute scrotal pain suspicious of testicular torsion: a consecutive case series of 173 patients. BJU Int 2011;107:990–3. [DOI] [PubMed] [Google Scholar]
- [12].Lyon TD, Ferroni MC, Casella DP, et al. Segmental testicular infarction due to minocycline-induced antineutrophil cytoplasmic antibody--positive vasculitis. Urology 2014;84:e1–2. [DOI] [PubMed] [Google Scholar]
- [13].Fossum BD, Woods JC, Blight EJ. Cavernous hemangioma of testis causing acute testicular infarction. Urology 1981;18:277–8. [DOI] [PubMed] [Google Scholar]
- [14].Madaan S, Joniau S, Klockaerts K, et al. Segmental testicular infarction: conservative management is feasible and safe. Eur Urol 2008;53:441–5. [DOI] [PubMed] [Google Scholar]
- [15].Wang Z, Wei YB, Yin Z, et al. Diagnosis and management of scrotal superficial angiomyxoma with the aid of a scrotoscope: case report and literature review. Clin Genitourin Cancer 2015;13:e311–3. [DOI] [PubMed] [Google Scholar]
- [16].Wei Y, Yang J, Hong H, et al. Scrotoscopy exploration of testicular rupture: a pilot study. Medicine (Baltimore) 2019;98:e17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lei J, Luo C, Su X, et al. How to treat chronic idiopathic testicular pain? Scrotoscopy with a novel percutaneous endoscopy equipment. Biomed Res Int 2018;2018:9808152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Darzi A, Mackay S. Recent advances in minimal access surgery. BMJ 2002;324:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fehér ÁM, Bajory Z. A review of main controversial aspects of acute testicular torsion. J Acute Dis 2016;5:1–8. [Google Scholar]
- [20].Ye H, Liu Z, Wang H, et al. A minimally invasive method in diagnosing testicular torsion: the initial experience of scrotoscope. J Endourol 2016;30:704–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


