Abstract
Anti-N-methyl-d-aspartate receptor encephalitis (NMDARe) can coexist with myelin oligodendrocyte glycoprotein antibody (MOG-ab) disease.
To characterize MOG-ab disease during NMDARe, we analyzed all the patients with MOG-ab disease and NMDARe from our hospital from December 2018 to December 2019 and data from a systematical review of previously published reports. Details of the patients identified were summarized and literature was reviewed.
Four of thirty (14.2%) patients with anti-NMDARe had overlapping MOG-ab disease in our department. Analyze together with previously reported cases. Thirty-two NMDARe patients had overlapping MOG-ab disease. The onset age ranged from 3 to 48 years. Twenty-four patients (74%) developed abnormal behavior or cognitive dysfunction during the episodes of anti-NMDARe. None of these patients had tumors. 84% (27/32) patients received high doses of steroids as first-line immunotherapy and 28% (9/32) received mycophenolate mofetil (MMF) to prevent relapse. Twenty-six of twenty-seven (96%) had a good outcome.
Steroids are the most common first-line immunotherapies in NMDARe overlapping MOG-ab disease. Most of the NMDARe patients overlapping MOG-ab disease have a good prognosis.
Keywords: anti-N-methyl-d-aspartate receptor encephalitis, myelin oligodendrocyte glycoprotein
1. Introduction
Anti-N-methyl-d-aspartate receptor encephalitis (NMDARe) is a well-characterized immune-mediated encephalitis induced by the presence of IgG autoantibodies directed against the GluN1 subunit of the NMDAR [NMDAR antibody (NMDAR-ab)] in cerebrospinal fluid (CSF) and/or serum and predominantly affects young women.[1] Characteristic clinical features of NMDARe include psychiatric symptoms, seizures, dyskinesias, movement disorders, memory dysfunction, speech disorders, decreased levels of consciousness, autonomic dysfunctions, and central hypoventilation.[1,2] NMDAR are membrane receptors widely expressed in the central nervous system on various cells such as neurons, oligodendrocytes but also astrocytes.[3] An overlap has been recognized in NMDARe and demyelinating diseases, such as acute disseminated demyelinating encephalomyelitis,[4] myelitis,[5,6] neuromyelitis optical spectrum disorder (NMOSD),[3,7–11] optic neuritis (ON),[12,13] and multiple sclerosis.[3,14–18] Some of the patients have demyelinating-like lesions which are usually transient.[2]
Myelin oligodendrocyte glycoprotein (MOG) is a membrane protein expressed on the surface of oligodendrocyte cell and myelin sheaths.[19] Recently, myelin oligodendrocyte glycoprotein antibody (MOG-ab) has been found in a subset of patients with NMOSD who are seronegative for aquaporin4-ab (AQP4-ab).[20,21] MOG-ab is now becoming a potential biomarker of inflammatory diseases of the central nervous system (CNS). It is associated with wider clinical phenotypes, not merely limited to NMOSD, but also with ON, encephalitis with brain demyelinating lesions, and/or myelitis.[22] In the current study, we aimed to further characterize the clinical features of MOG-ab diseases overlapping NMDARe and review literature.
2. Methods
2.1. Study subjects
In this retrospective observational study, we reviewed the patients from our department diagnosed as NMDARe overlapping MOG-ab disease from December 2012 to December 2019. Written consent was obtained from all the patients and the study was approved by the Ethics Committee of Beijing Tiantan Hospital affiliated with Capital Medical University (Beijing, China).
2.2. AQP4-ab, MOG-ab, and anti-NMDAR-ab detection
AQP4-ab and MOG-ab were detected by using indirect immunofluorescence on a commercial assay (Euro-immune) according to the manufacturer's instructions. CSF and serum NMDAR (GluN1 subunit), leucine-richglioma-inactivated protein 1 LGI1, contactin-associated protein-like 2 receptor (CASPR2), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), dipeptide-peptidase like protein 6 (DPPX), and γ-aminobutyric acid-B receptor (GABABR) antibodies were also examined using cell-based assays (Euro-immune).
2.3. Literature review
We searched PubMed through December 2019 for articles published in the English language with the search string (“Receptors, N-Methyl-D-Aspartate” [Mesh Terms]) AND (“Myelin Oligodendrocyte Glycoprotein” [Mesh Terms] OR “MOG” OR “Demyelinating Diseases” [MeSH Terms] OR “Demyelinating Autoimmune Diseases, CNS” [MeSH Terms]). We also searched the references for related published articles. All obtained articles were reviewed to identify the cases of overlapping MOG-ab disease and NMDARe.
3. Results
3.1. Results from our cases
Five of thirty (14%) NMDARe patients coexist with demyelinating disorders. Of them, 2 were males, 3 were females. MOG-ab was positive in 4 of the 5 patients. No patients were positive for the GABABR, AMPAR, CASPR2, LGI1, and DPPX antibodies. All of the 4 patients fulfilled the diagnostic criteria for definite NMDARe and we diagnosed the NMDARe mainly depending on the CSF NMDAR-ab. The onset age ranged from 25 to 48 years. No signs of malignancy were detected on extensive exploration with serum tumor markers, ultrasound, CT, and magnetic resonance imaging (MRI) in the 4 patients. Brain and spinal cord MRIs were performed in all our patients (Figs. 1–4). NMDARe occurred prior to the episode of MOG-ab disease in 2 patients (cases 1 and 2, 4–70 months), simultaneously in 1 patient (case 4), and after the episode of MOG-ab disease in 1 patient (case 3, 2 months).
Figure 1.
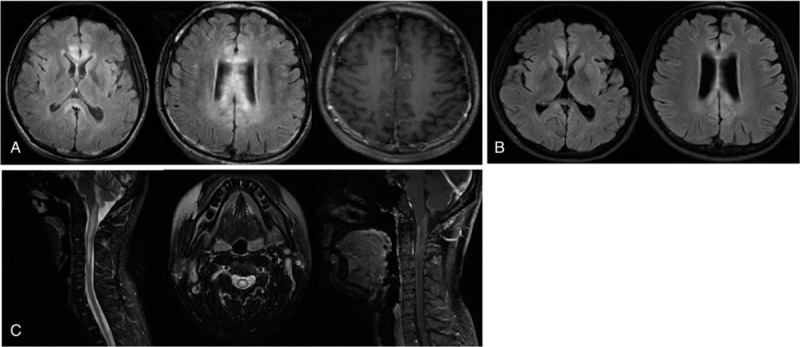
Brain MRI (sagittal T2 FLAIR images) showed high signal in the white matter and peri-ventricular with contrast-enhancing lesions at T1 (A). Brain MRI showed a high signal reduced after immunotherapy (B). MRI of the cervical cord showed T2 hyperintensities from the dorsal medulla to C4, including contrast-enhancing lesions (C).
Figure 4.
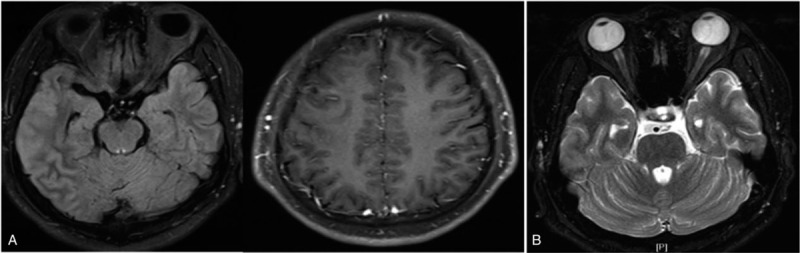
Brain MRI revealed the shallowness of the right frontal temporal lobe sulcus with contrast enhancement of the meninges (A) and orbital MRI showed T2 hyperintensities within the anterior of the bilateral optic nerve (B).
All 4 patients had supratentorial and infratentorial lesions. Two of the 4 patients had spinal cord lesions (cases 1 and 2). Tables 1 and 2 summarize the demographic data and laboratory data of our 4 patients, respectively. MRI findings of the 4 patients were described in Figures 1–4, respectively. High doses of steroids were the main treatment for attacks in our patients.
Table 1.
Demographic, clinical, and neuroimaging findings in our 4 patients of NMDARe overlapping MOG-ab disease.
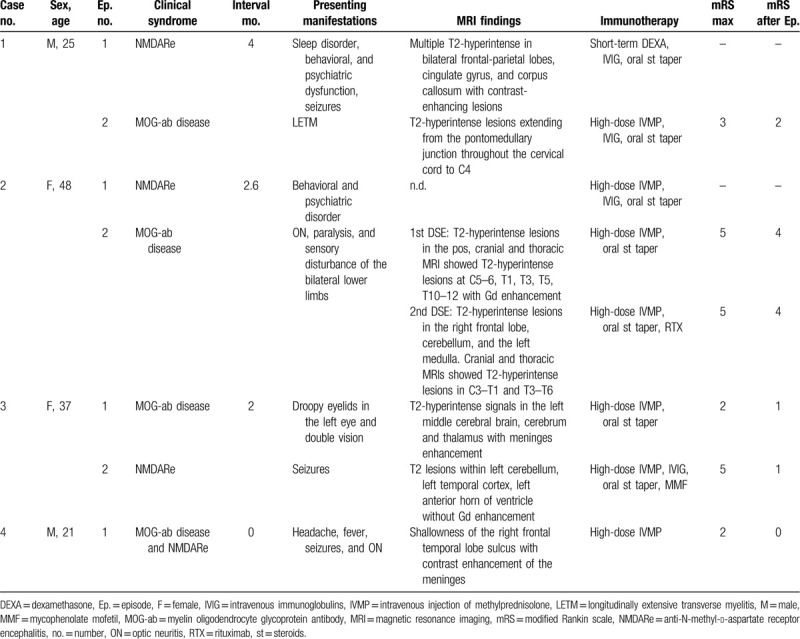
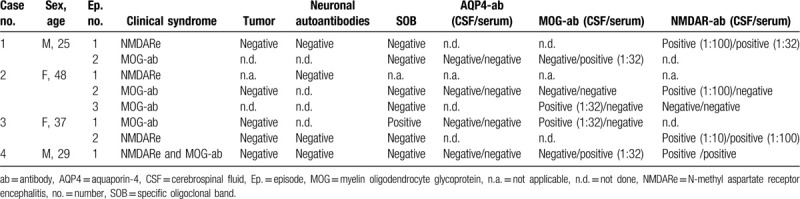
Table 2.
Laboratory findings in our patients.
3.1.1. Patient 1
A 25-year-old male complained of somnolent, behavioral, and psychiatric dysfunction for 1 month, seizures, and reduced level of consciousness for 12 days. He had presented with fever before admission. Brain MRI showed multiple T2-hyperintense lesions in bilateral frontal-parietal lobes, cingulate gyrus, and corpus callosum, some of which showed contrast enhancement (Fig. 1A). Lumbar puncture showed pleocytosis (18 × 106/L) and elevated protein level (22.41 mg/dL). The tests for AQP4-ab and MOG-ab were negative in his serum. Anti-NMDAR-ab (IgG isotype) titer was 1:32 in CSF and 1:100 in serum. At the same time, orofacial involuntary movements were noted. His symptoms were improved by intravenous dexamethasone (20 mg/day) and 5 days of intravenous immunoglobulin (IVIG) regimen (0.4 g/kg/day). A second MRI performed after 20 days of treatment indicated resolved lesions of the above-mentioned cortex (Fig. 1B). After discharge, he started on prednisone orally and titrated to 5 mg/day.
Four months later, he developed numbness and weakness in his right arm extremities. The numbness gradually progressed to his right arm, shoulder, chest, lower limb, and then left upper limb. Cranial MRI revealed T2-hyperintense lesions extending from the pontomedullary junction to C4 accompanied by spinal cord swelling and some contrast enhancement (Fig. 1C). The test for AQP4-ab was negative, but MOG-ab was positive in CSF (1:32). On day 12 after admission, the patient complained of itching from the face to the upper limbs. Five-day intravenously administered immunoglobulin was given without any improvement. His symptoms resolved 2 weeks after methylprednisolone (1000 mg/day) delivered intravenously and a tapering course of oral prednisolone. He was also treated with mycophenolate mofetil (MMF) and remained free of relapse.
3.1.2. Patient 2
A 48-year-old woman presented initially with a 70-day history of behavioral and psychiatric disorder (illusion, impulse, nonsense, self-harm). Ten days later, she developed vision loss in the right eye, paralysis, urinary retention, and sensory disturbance of the bilateral lower limbs. He had a history of a psychiatric disorder for 20 years. Brain MRI disclosed T2-hyperintense lesions in the pos without gadolinium (Gd) enhancement. Cranial and thoracic MRI showed T2-hyperintense lesions at C5–6, T1, T3, T5, T10–12 with Gd enhancement (Fig. 2A). The tests for AQP4-ab and MOG-ab were negative in serum and CSF. Anti-NMDAR-abs were positive in CSF (titer 1:100) and negative in serum. Besides, serum antinuclear antigen antibodies were positive, with a titer of 1:320. The patient's condition deteriorated despite the use of IVIG and high-dose pulse IV methylprednisolone (IVMP). Lumbar MRI showed long T1 and long T2 signals in spinal conus with Gd enhancement (Fig. 2B). Cranial showed T2-hyperintense lesions between C3 and T3 with Gd enhancement (Fig. 2C). Given that the patient only used hormones for 3 days, we retain the same treatment. Thereafter, her symptoms relieved gradually. However, the test for anti-NMDAR-ab became negative and MOG-IgG became positive in CSF after discharge.
Figure 2.
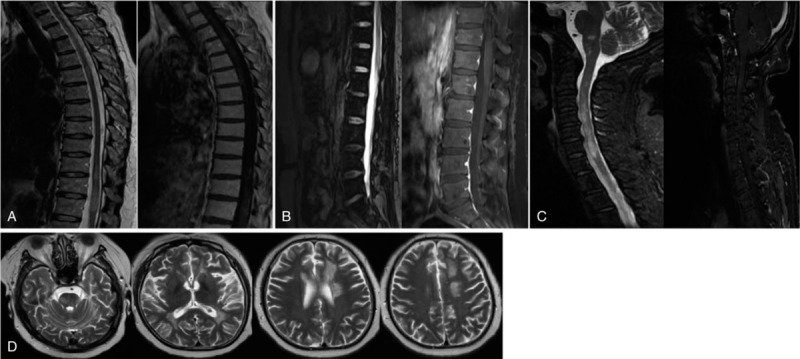
Thoracic MRI showed an extension of T2 lesions in T1, T3, T5, T10–12 with gadolinium (Gd) enhancement (A). Lumbar MRI showed long T1 long T2 signal in spinal conus with Gd enhancement (B). MR FLAIR imaging demonstrated multiple hyperintense lesions. Several lesions were found to be Gd enhancing on T1 (C). Brain MRI showed multiple hyperintense T2 lesions on the brain stem, bilateral ventricles, the left corona radiata, the centrum semiovale, and the white matter bilateral frontal with non-contrast enhancement (D).
Four months later, she exhibited lower limb weakness and dysuria, and the symptoms gradually aggravated with intermittent cognitive impairment and gibberish. Repeat cranial and thoracic MRIs showed T2-hyperintense lesions in C3-T1 and T3-T6 (not shown). She received IVMP only and relapsed 6 months later with bilateral lower extremity weakness, decreased binocular vision, and abnormal mental behavior (intermittent unfamiliarity with relatives, gibberish, mood fluctuations). Brain MRI showed T2-hyperintense lesions on the brain with non-contrast enhancement (Fig. 2D). Rituximab was administered. After a 21-day hospitalization, the paraparesis improved significantly. So far, no recurrence has been found.
3.1.3. Patient 3
A 37-year-old female manifested droopy eyelids in the left eye and double vision. MRI follow-up showed T2-hyperintense signals in the left middle cerebral brain, cerebrum, and thalamus with meninges enhancement. Her CSF demonstrated an increase in white blood cell numbers (158/mm3), mainly mononuclear cells. Protein was 0.51 mg/dL. MOG-ab was positive in CSF. After treatment with methylprednisolone (500 mg per day intravenously for 5 days), ceftriaxone (2 g intravenously) and aciclovir (750 mg, 3 times per day intravenously), her symptoms improved.
About 2 months later, she developed daily headaches and 1 generalized seizure. Brain MRI showed T2 lesions within the left cerebellum, left temporal cortex, left anterior horn of ventricle without contrast (Fig. 3). On day 15 of admission, the patient experienced shouting, gibberish, and 1 generalized seizure. The NMDAR-ab was found in both CSF (titer: 1:10) and serum (titer: 1:100). As soon as anti-NMDAR-ab was detected, she received IVIG and IVIP followed by a weaning course of oral prednisolone. Steroid-pulse therapy ameliorated her symptoms. To prevent relapse, we also gave her MMF.
Figure 3.
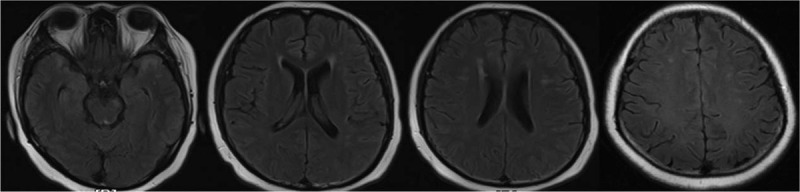
Brain MRI showing T2 lesions in the left cerebellum, bilateral temporal lobe, frontal lobe, lateral ventricles, and center of semioval without Gd enhancement.
3.1.4. Patient 4
A 21-year-old male presented with headache, fever, and generalized seizures. Initial MRI revealed the shallowness of the right frontal temporal lobe sulcus with contrast enhancement of the meninges (Fig. 4). His serum and CSF were tested for NMDAR-abs; the CSF was positive (1:32). We initially thought it was viral meningoencephalitis. After treatment with aciclovir and anti-epileptic for 15 days, the patient complained of a loss of vision in the right eye and could only see the fingers moving 1 m in front of his eyes. Orbital MRI showed T2 hyperintensities within the anterior of the bilateral optic nerve (Fig. 4). Repeat CSF analysis showed serum AQP4-ab was negative, while MOG-ab was positive (1:32). Anti-NMDAR-ab was weakly positive in CSF. Visual evoked potentials and EEG are normal. His symptoms improved following IVMP, IVIG, and a tapering course of oral prednisolone. In subsequent MRI studies, these lesions were no longer observed.
Three months later, the patient had another seizure. No lesions were found in brain MRI. Muscle biopsy and mitochondrial gene screening were negative, which excluded the possibility of metabolic encephalopathy and mitochondrial encephalopathy. This led us to consider the possibility of anti-NMDARe in our patient.
3.2. Results from a literature review
The initial literature search yielded 143 published reports. We reviewed the reports and their references for related published reports and identified other patients with MOG-ab disease. One case was excluded because the symptoms of anti-NMDARe were absent, although anti-NMDAR-ab was positive.
Of 30 patients with anti-NMDARe and MOG-ab disease (excluding 2 patients with unknown information), 12 anti-NMDARe patients occurred concurrently with MOG-ab disease, 14 preceded and 4 followed by independent episodes of MOG-ab disease. The onset age of these 32 patients ranged from 3 to 48 years. Twenty-four patients (74%) developed abnormal behavior or cognitive dysfunction during the episodes of anti-NMDARe. Abnormal behavior or cognitive dysfunction was the most common manifestation during attacks associated with anti-NMDARe in the above-mentioned 3 conditions, and brainstem demyelination was the most common onset manifestations of MOG-ab disease when anti-NMDARe occurred concurrently or preceded with MOG-ab disease (50%, 42%, respectively). None of the 32 patients had tumors. 84% (27/32) patients received high doses of steroids as first-line immunotherapy and 28% (9/32) received MMF to prevent relapse. Twenty-six of twenty-seven (96%) had a good outcome. The characteristics of these previously reported patients along with our 4 patients are summarized in Tables 3 and 4.
Table 3.
The characteristics of MOG-ab overlapping anti-NMDARe from these previously reported patients along with our 4 patients.
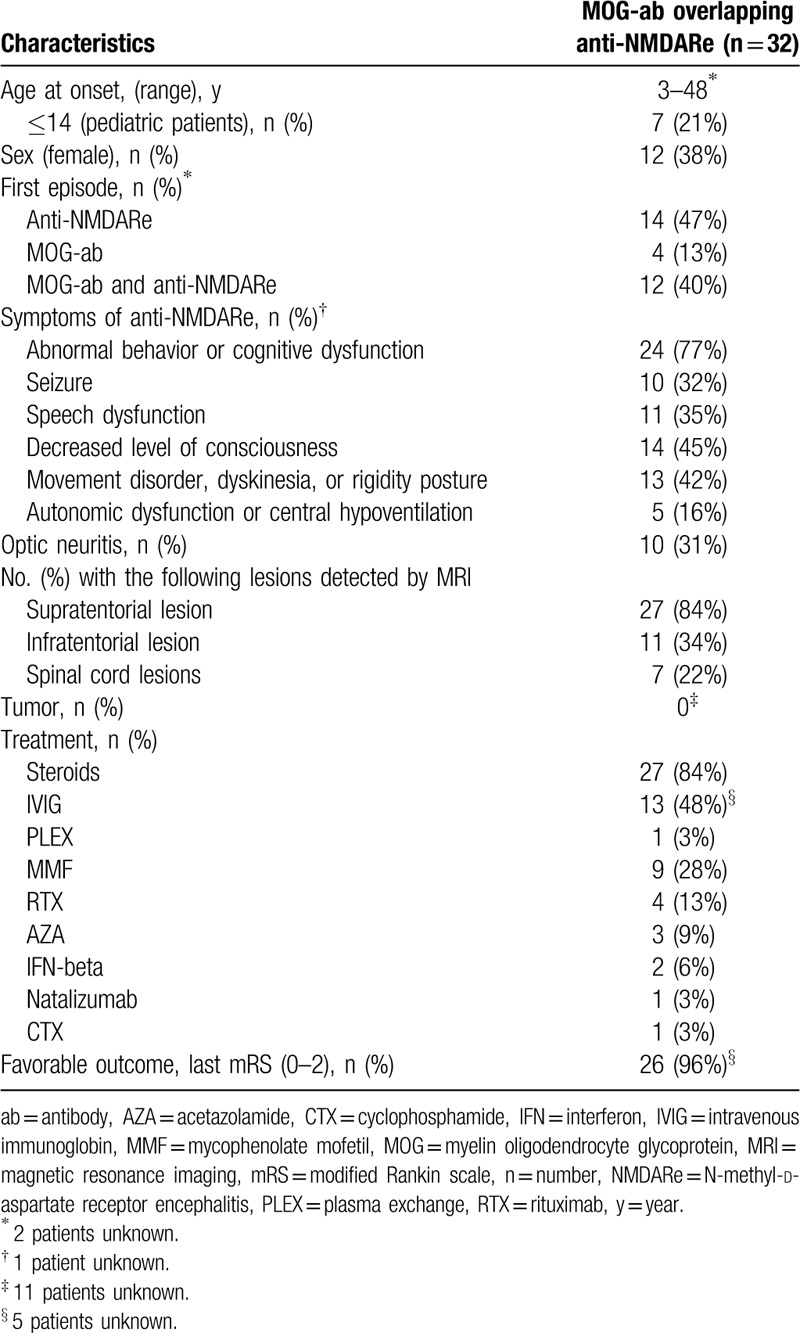
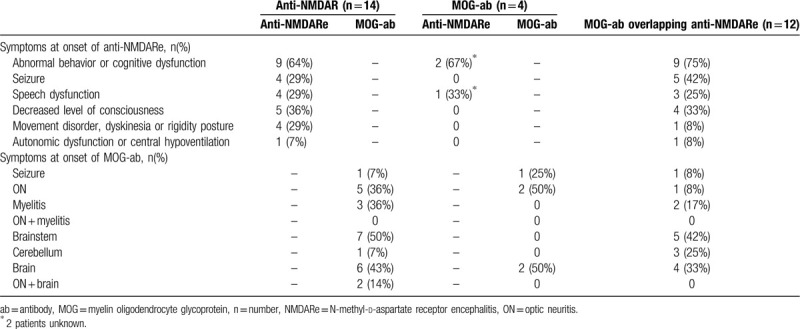
Table 4.
The symptoms at onset of anti-NMDARe coexisting with MOG-ab disease from these previously reported patients along with our 4 patients.
4. Discussion
Anti-NMDARe is the most common autoimmune encephalitis of the limbic system.[1] It is now recognized that the range of anti-NMDAR encephalitis is wider, as there are many cases in women, men, and children.[23] 33% of patients with anti-NMDARe have mild, or transient abnormal brain MRI[9] and the lesions can occur in the hippocampi, corpus callosum, cerebellar or cerebral cortex, frontobasal and insular regions, basal ganglia, brainstem, and the spinal cord.[1,2] The MOG-ab disease is an inflammatory demyelinating syndrome of CNS. When the clinical manifestations of the 2 overlap, especially when the brain stem is involved, it is extremely difficult to identify.[24,25]
There are few reports on the overlap of MOG antibodies and NMDAR antibodies. Titulaer et al[3] firstly reported that 23 of 691 patients with anti-NMDAR encephalitis have a demyelinating disorder and most had AQP4-IgG or MOG-IgG antibodies. Thereafter, cases of anti-NMDARe combined with MOG-ab disease have been reported.[13,25–30] According to these previously reported patients and our 4 patients, we found that the clinical manifestations of the overlap of these 2 syndromes may have the following characteristics:
-
(1)
Consistent with previous studies,[3,25,28,30] symptoms of anti-NMDARe and MOG-ab disease exist independently or overlap sometimes.
-
(2)
Abnormal behavior or cognitive dysfunction is both the most common and the most common initial symptoms during attacks associated with anti-NMDARe, and brain demyelination was the most common initial manifestations of MOG-ab disease.
-
(3)
High-dose steroids were the most commonly used first-line immunotherapy.
-
(4)
No tumors were detected in these patients, and most patients demonstrated good recoveries, this is in line with previously reported cases.[12,31]
Viral studies for influenza viruses B IgM were positive in patient 1. We think influenza B viruses may be an infectious etiology. A study by the California Encephalitis Project found that the frequency of anti-NMDARe surpassed that of any individual viral etiology in young individuals.[32] Nakamura et al[33] described MOG-IgG-positive ADEM after infectious mononucleosis and Amano et al[34] reported MOG-IgG-positive longitudinally extensive transverse myelitis after influenza infection. Herpes simplex encephalitis (HSE) has been reported as a possible trigger of NMDAR-ab formation.[35–39] Whether this infectious entity is caused by molecular mimicry or breakdown of immunologic tolerance towards the NMDAR expressed by damaged neurons in an inflamed environment is unknown. Synthesis of NMDAR-ab may begin after HSE.[35] However, CSF PCR assays for viral pathogens [HSV-1, HSV-2, HSV (1 + 2)] were all negative in our patients. There is also a possibility that pregnancy and/or delivery could trigger anti-NMDAR encephalitis, as several patients developed this disorder during pregnancy or in the postpartum period.[40–42] However, none of our patients suffer from this condition.
Several retinal neurons express NMDAR and might be affected directly in acute anti-NMDARe; however, the retinal structure appears unaltered in those acute anti-NMDARe who have mild visual dysfunction in these patients.[43] Patient 4 presented with decreased vision. Since visual dysfunction is not among the common symptoms of acute NMDARe.[43] Our hypothesis was ON as the first event of demyelinating disorders. Interestingly, MOG-ab was positive in CSF. Optical coherence tomography revealed an increase in the thickness of the binocular retinal nerve fibre layer in the right eye. These confirmed our diagnosis.
Autoantibodies against NMDAR might be closely related to clustered seizures. Seizures can occur at any time during the disease. MOG-ab disease has also been recently described in association with seizures.[44] As in our study, 3 of 32 patients presented with onset seizures during MOG-ab disease. Hamid et al reported that patients with MOG-IgG antibodies were more likely to have seizures or an encephalitis-like illness than AQP4-IgG-positive NMOSD patients.[19] Nabbout et al[45] reported that some epilepsy patients who are not sensitive to routine anticonvulsants may have immune-mediated causes. The contribution of NMDAR-abs and other antibodies (such as AQP4, MOG) to demyelination is unknown, but it is noteworthy that oligodendrocytes express NMDAR,[46] and that the immune attack targeting myelin may simultaneously involve NMDAR and vice versa.
There are some limitations to our study. First, the number of reported cases available for evaluation is small. Second, the retrospective study is also a limitation. Finally, detection methods of MOG-ab are not uniform in each literature.
5. Conclusions
The results of these case studies suggest that patients with anti-NMDARe may develop concurrent or separate episodes of MOG-ab disorders. Anti-NMDARe patients with atypical episodes (especially ON or myelitis) or brain demyelination (especially brainstem) may have MOG-ab disease, and MOG-ab disease with unusual symptoms (such as psychiatric manifestations or cognitive dysfunction, decreased level of consciousness, seizure) may have anti-NMDARe. The tests for anti-NMDAR-ab and MOG-ab should be considered in these patients. Early diagnosis and treatment can improve the patient's outcome.
Author contributions
Conceptualization: Li Du, Huabing Wang, Xinghu Zhang, Linlin Yin.
Data curation: Heng Zhou, Haoxiao Chang, Yuzhen Wei, Hengri Cong, Wangshu Xu, Yuetao Ma, Tian Song.
Formal analysis: Li Du.
Methodology: Heng Zhou, Haoxiao Chang, Yuzhen Wei, Hengri Cong, Wangshu Xu, Yuetao Ma, Tian Song.
Supervision: Xinghu Zhang, Linlin Yin.
Writing – original draft: Li Du, Huabing Wang.
Footnotes
Abbreviations: AMPAR = alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, AQP4 = aquaporin 4, CASPR2 = contactin-associated protein-like 2 receptor, CNS = central nervous system, CSF = cerebrospinal fluid, DPPX = dipeptide-peptidase like protein 6, GABABR = γ-aminobutyric acid-B receptor, HSE = Herpes simplex encephalitis, LGI1 = leucine-richglioma-inactivated protein 1, MOG-ab = myelin oligodendrocyte glycoprotein antibody, NMDAR-ab = NMDAR antibody, NMDARe = anti-N-methyl-d-aspartate receptor encephalitis, NMOSD = neuromyelitis optical spectrum disorder, ON = optic neuritis.
How to cite this article: Du L, Wang H, Zhou H, Chang H, Wei Y, Cong H, Xu W, Ma Y, Song T, Zhang X, Yin L. Anti-NMDA receptor encephalitis concomitant with myelin oligodendrocyte glycoprotein antibody diseases: a retrospective observational study. Medicine. 2020;99:31(e21238).
The authors have no conflicts of interests to disclose.
This work was supported by the the Beijing Natural Science Foundation [7162208] and the Advanced Innovation Center for Human Brain Protection (Neuroimmune 01).
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Titulaer MJ, Hoftberge R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;75:411–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lekoubou A, Viaccoz A, Didelot A, et al. Anti-N-methyl-D-aspartate receptor encephalitis with acute disseminated encephalomyelitis-like MRI features. Eur J Neurol 2012;19:e16–7. [DOI] [PubMed] [Google Scholar]
- [5].Pennington C, Livingstone S, Santosh C, et al. N-methyl D-aspartate receptor antibody encephalitis associated with myelitis. J Neurol Sci 2012;317:151–3. [DOI] [PubMed] [Google Scholar]
- [6].Olivier Outteryck GB, Jérôme H, Marianne G, et al. Extensive myelitis associated with anti-NMDA receptor antibodies. BMC Neurol 2013;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kruer MC, Koch T, Bourdette D, et al. NMDA receptor encephalitis mimicking seronegative neuromyelitis optica. Neurology 2010;74:1473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zoccarato M, Saddi MV, Serra G, et al. Aquaporin-4 antibody neuromyelitis optica following anti-NMDA receptor encephalitis. J Neurol 2013;260:3185–7. [DOI] [PubMed] [Google Scholar]
- [9].Luo JJ, Lv H, Sun W, et al. Anti-N-methyl-d-aspartate receptor encephalitis in a patient with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2016;8:74–7. [DOI] [PubMed] [Google Scholar]
- [10].Qin K, Wu W, Huang Y, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) antibody encephalitis presents in atypical types and coexists with neuromyelitis optica spectrum disorder or neurosyphilis. BMC Neurol 2017;17:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ran Y, Wang L, Zhang F, et al. Anti-NMDAR encephalitis followed by seropositive neuromyelitis optica spectrum disorder: a case report and literature review. Clin Neurol Neurosurg 2017;155:75–82. [DOI] [PubMed] [Google Scholar]
- [12].Ishikawa N, Tajima G, Hyodo S, et al. Detection of autoantibodies against NMDA-type glutamate receptor in a patient with recurrent optic neuritis and transient cerebral lesions. Neuropediatrics 2007;38:257–60. [DOI] [PubMed] [Google Scholar]
- [13].Zhou L, ZhangBao J, Li H, et al. Cerebral cortical encephalitis followed by recurrent CNS demyelination in a patient with concomitant anti-MOG and anti-NMDA receptor antibodies. Mult Scler Relat Disord 2017;18:90–2. [DOI] [PubMed] [Google Scholar]
- [14].Takeda A, Shimada H, Tamura A, et al. A case of anti-N-methyl-d-aspartate receptor encephalitis with multiple sclerosis-like demyelinated lesions. Mult Scler Relat Disord 2014;3:391–7. [DOI] [PubMed] [Google Scholar]
- [15].Hacohen Y, Absoud M, Hemingway C, et al. NMDA receptor antibodies associated with distinct white matter syndromes. Neurol Neuroimmunol Neuroinflamm 2014;1:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fleischmann R, Prüss H, Rosche B, et al. Severe cognitive impairment associated with intrathecal antibodies to the NR1 subunit of the N-methyl-D-aspartate receptor in a patient with multiple sclerosis. JAMA Neurol 2015;72:96–9. [DOI] [PubMed] [Google Scholar]
- [17].Baheerathan A, Brownlee WJ, Chard DT, et al. Antecedent anti-NMDA receptor encephalitis in two patients with multiple sclerosis. Mult Scler Relat Disord 2017;12:20–2. [DOI] [PubMed] [Google Scholar]
- [18].Suleman S, Javed Q. NMDAR (N-methyl-D-aspartate receptor) encephalitis in a patient with MS (multiple sclerosis): a rare and challenging case. BMJ Case Rep 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hamid SHM, Whittam D, Saviour M, et al. Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease vs aquaporin 4 IgG disease. JAMA Neurol 2018;75:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mader S, Gredler V, Schanda K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflamm 2011;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 2012;79:1273–7. [DOI] [PubMed] [Google Scholar]
- [22].Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflamm 2018;15:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis, autoimmunity, and psychosis. Schizophr Res 2016;176:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol 2018;83:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hou C, Wu W, Tian Y, et al. Clinical analysis of anti-NMDAR encephalitis combined with MOG antibody in children. Mult Scler Relat Disord 2020;42:102018. [DOI] [PubMed] [Google Scholar]
- [26].Fan S, Xu Y, Ren H, et al. Comparison of myelin oligodendrocyte glycoprotein (MOG)-antibody disease and AQP4-IgG-positive neuromyelitis optica spectrum disorder (NMOSD) when they co-exist with anti-NMDA (N-methyl-D-aspartate) receptor encephalitis. Mult Scler Relat Disord 2018;20:144–52. [DOI] [PubMed] [Google Scholar]
- [27].Zhou J, Winwen T, Tan SE, et al. An unusual case of anti-MOG CNS demyelination with concomitant mild anti-NMDAR encephalitis. J Neuroimmunol 2018;320:107–10. [DOI] [PubMed] [Google Scholar]
- [28].Ren Y, Chen X, He Q, et al. Co-occurrence of anti-N-methyl-D-aspartate receptor encephalitis and anti-myelin oligodendrocyte glycoprotein inflammatory demyelinating diseases: a clinical phenomenon to be taken seriously. Front Neurol 2019;10:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kaneko K, Sato DK, Misu T, et al. Anti-N-methyl-D-aspartate receptor encephalitis with multiphasic demyelination. Ann Neurol 2014;76:462–4. [DOI] [PubMed] [Google Scholar]
- [30].Wang L, ZhangBao J, Zhou L, et al. Encephalitis is an important clinical component of myelin oligodendrocyte glycoprotein antibody associated demyelination: a single-center cohort study in Shanghai, China. Eur J Neurol 2019;26:168–74. [DOI] [PubMed] [Google Scholar]
- [31].Honda K, Yuasa T. A case of anti-aquaporin-4 and anti-glutamate receptor antibodies positive myelitis presented with modest clinical signs. Magn Reson Med Sci 2008;7:55–8. [DOI] [PubMed] [Google Scholar]
- [32].Gable MS, Sheriff H, Dalmau J, et al. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis 2012;54:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nakamura Y, Nakajima H, Tani H, et al. Anti-MOG antibody-positive ADEM following infectious mononucleosis due to a primary EBV infection: a case report. BMC Neurol 2017;17:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Amano H, Miyamoto N, Shimura H, et al. Influenza-associated MOG antibody-positive longitudinally extensive transverse myelitis: a case report. BMC Neurol 2014;14:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pruss H, Finke C, Höltje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012;72:902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013;81:1637–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hacohen Y, Deiva K, Pettingill P, et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord 2014;29:90–6. [DOI] [PubMed] [Google Scholar]
- [38].DeSena A, Graves D, Warnack W, et al. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis report of 2 cases. JAMA Neurol 2014;71:344–6. [DOI] [PubMed] [Google Scholar]
- [39].Gilbert GJ, Leypoldt F, Dalmau J. Herpes simplex virus-1 encephalitis can trigger anti-Nmda receptor encephalitis: case report. Neurology 2014;82:2041–12041. [DOI] [PubMed] [Google Scholar]
- [40].McCarthy A, Dineen J, McKenna P, et al. Anti-NMDA receptor encephalitis with associated catatonia during pregnancy. J Neurol 2012;259:2632–5. [DOI] [PubMed] [Google Scholar]
- [41].Mathis S, Pin JC, Pierre F, et al. Anti-NMDA receptor encephalitis during pregnancy: a case report. Medicine (Baltimore) 2015;94:e1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shahani L. Steroid unresponsive anti-NMDA receptor encephalitis during pregnancy successfully treated with plasmapheresis. BMJ Case Rep 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brandt AU, Oberwahrenbrock T, Mikolajczak J, et al. Visual dysfunction, but not retinal thinning, following anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm 2016;3:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm 2017;4:e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nabbout R. Autoimmune and inflammatory epilepsies. Epilepsia 2012;53: Suppl 4: 58–62. [DOI] [PubMed] [Google Scholar]
- [46].Höftberger R. Neuroimmunology: an expanding frontier in autoimmunity. Front Immunol 2015;6:206. [DOI] [PMC free article] [PubMed] [Google Scholar]


