Abstract
Whether periodontitis is a risk factor for developing bipolar disorders (BD) has not been investigated. We aimed to determine whether periodontitis is associated with the subsequent development of BD and examine the risk factors for BD among patients with periodontitis.
Using ambulatory and inpatient claims data from the National Health Insurance Research Database (NHIRD), we identified 12,337 patients who were aged at least 20 years and newly diagnosed with periodontitis between 2000 and 2004. The date of the first claim with a periodontitis diagnosis was set as the index date. For each patient with periodontitis, 4 subjects without a history of periodontitis were randomly selected from the NHIRD and frequency-matched with the patients with periodontitis according to sex, age (in 5-year bands), and index year.
The periodontitis group had a mean age of 44.0 ± 13.7 years and slight predominance of men (51.3%). Compared with the subjects without periodontitis, the patients with periodontitis had higher prevalence of diabetes mellitus, hyperlipidemia, hypertension, ischemic heart disease, stroke, head injury, major depressive disorder, chronic obstructive pulmonary disease (COPD), and asthma (P < .001). The incidence rate of BD was higher in the periodontitis group than in the non-periodontitis group (2.74 vs 1.46 per 1000 person-year), with an adjusted hazard ratio of 1.82 (95% confidence interval = 1.59–2.08) after adjustment for sex, age, and comorbidities.
The patients with periodontitis exhibited a significantly higher risk of developing BD. Keep the better oral hygiene to reduce periodontitis might be a preventive strategy for BD.
Keywords: bipolar disorder, hazard ratio, National Health Insurance Research Database, periodontitis
1. Introduction
Periodontitis is initiated by bacterial plaque biofilm[1] and can be caused by gingivitis affecting soft tissues near the teeth, resulting in the destruction of the tissue supporting the teeth.[2] Periodontal tissues respond to bacterial invasion by mobilizing defense cells and releasing inflammatory cytokines, such as interleukins (ILs),[3–5] tumor necrosis factor alpha (TNF-α),[6,7] and prostaglandin E2 (PGE2),[7] which may cause tissue destruction by stimulating the production of enzymes such as matrix metalloproteinase.[8] Evidence is mounting for possible associations between periodontitis and other diseases such as,[9,10] depression,[11] diabetes mellitus (DM),[12] atherosclerotic cardiovascular disease,[13–15] and rheumatoid arthritis.[16–18]
Bipolar disorder (BD) is a disabling, recurrent mental illness that varies widely in severity. The onset of BD is typically observed in late childhood or early adolescence.[19] Patients with BD have higher rates of comorbidities of psychiatric disorders and other medical conditions, which might entail an increased medical burden and multiple physical abnormalities.[20] Early detection and treatment of BD can improve patient outcomes.[19] One study suggested that patients with BD are at high risk of dental diseases.[21] When patients are in depressive episodes, they pay less attention to oral hygiene, leading to an increase in dental caries, and periodontal disease.[22] By contrast, when patients are in a manic period, they may overuse oral health aids, which has been correlated with the increased incidence and severity of cervical injuries and occasional mucosal or gingival wounds.[23] Furthermore, drug treatments, such as antidepressants and antipsychotics, have been demonstrated to cause moderate-to-severe xerostomia, which can exacerbate dental diseases.[24]
Although the association between periodontitis and psychiatric conditions, such as major depressive disorders and cognitive decline, remains controversial,[25,26] periodontitis has been suggested as a risk factor for dementia.[9,10] Whether periodontitis is a risk factor for developing subsequent BD has not been investigated. In this study, we hypothesized that periodontitis increases the risk of BD. To test our hypothesis, we conducted a nationwide population-based study to investigate the incidence and risks of BD among patients with or without periodontitis.
2. Patients and methods
2.1. Data source
The Taiwan National Health Insurance (NHI) program was implemented in 1995. At the end of 2014, the program was providing health care to approximately 99% of the Taiwan population (23.75 million people). The NHI is a mandatory health insurance program that offers comprehensive medical care coverage to all residents of Taiwan. The National Health Insurance Research Database (NHIRD) is managed and maintained by the National Health Research Institutes (NHRI) according to the directives of the National Health Insurance Administration. Our study used the Longitudinal Health Insurance Database (LHID2000), which contains the data of 1 million enrollees sampled from the medical claim records of the NHI from 1996 to 2011. The LHID2000 contains comprehensive outpatient and inpatient data, including demographic, clinical visit, and prescription information, and diagnostic codes based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). To ensure privacy protection, the NHRI encrypts and converts the identification numbers of all NHIRD records before releasing them for research. Our study was exempted from review by the Institutional Research Ethics Committee of China Medical University (CMU-REC-101-012), Taiwan.
2.2. Study population
Using the ambulatory and inpatient claims data sets, the inclusion criteria for this study of patients with periodontitis included being 20 years old or older and newly diagnosed with periodontitis (ICD-9-CM 523.4x and 523.5x) between 2000 and 2004. The date of the first claim with a periodontitis diagnosis was considered as the index date. For each patient with periodontitis, 4 subjects with no history of periodontitis (ICD-9-CM 523.xx) were randomly selected from the NHIRD and frequency-matched according to sex, age (in 5-year bands), and index year. The definition of BD patients was based on the following criteria: patients with a diagnosis of BD (ICD-9-CM code 296.xx) at least 3 times before the index date. However, patients who developed BD within 1 month after the index date were excluded. Finally, the periodontitis and non-periodontitis groups comprised of 12,337 and 49,348 patients, respectively. The ICD-9 code 523.4 includes the symptoms of chronic periodontitis, including chronic pericoronitis, chronic pericementitis, periodontitis (no otherwise specific, complex, and simplex), but excludes chronic apical periodontitis (ICD 9 code: 522.6). ICD-9 code 523.5 refers to the type of periodontitis with diffuse atrophy of the alveolar bone.
Demographic data included sex and age (20–34 years, 35–49 years, 50–64 years, and ≥65 years). We also recorded claims data on comorbidities before the index date on DM (ICD-9-CM 250.xx), hyperlipidemia (ICD-9-CM 272.xx), hypertension (ICD-9-CM 401.xx-405.xx), ischemic heart disease (IHD, ICD-9-CM 410.xx-414.xx), stroke (ICD-9-CM 430.xx-438.xx), head injury (ICD-9-CM 850.xx-854.xx and 959.01), alcohol abuse and dependence (ICD-9-CM 303.xx, 305.0x, and V11.3), major depressive disorder (ICD-9-CM 296.2x, 296.3x, 311. xx, 300.4x, and 309.0x). Smoking status and alcohol consumption were not available in NHIRD. Thus, we performed the multivariate analysis by adjusting for tobacco related diseases (including tobacco dependence [ICD-9-CM codes 305.1], chronic obstructive pulmonary disease [ICD-9-CM codes 490–492, 494, and 496], and asthma [ICD-9-CM code 493]).
The primary outcome was a diagnosis of BD (ICD-9-CM code 296.xx), which was determined by linking the NHIRD ambulatory and inpatient data. All study participants were observed from the index date to BD diagnosis, withdrawal from the NHI program, or the end of 2011.
2.3. Statistical analysis
Summary statistics are expressed as frequencies and percentages for categorical data and means and standard deviations (SDs) for continuous variables. The Pearson chi-square test and Student t test were used to compare categorical and continuous variables, respectively, between the patients with and without periodontitis. The sex-, age-, and comorbidity-specific incidence rates of BD were measured for both groups. The Kaplan–Meier method was used to depict the cumulative incidence of BD for the groups. The log-rank test was used to test the difference between the curves. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for determining whether periodontitis is a risk factor for the development of BD, and the models were adjusted for sex, age, DM, hyperlipidemia, hypertension, IHD, stroke, head injury, alcohol abuse/dependence, and major depressive disorder. We also performed sex-, age-, and comorbidity-stratified analysis to investigate the association between periodontitis and the risk of BD. All the data processing and statistical analyses were performed using SAS Version 9.3 (SAS Institute, Inc., Cary, NC). A 2-sided P value of <.05 was considered statistically significant.
3. Results
We identified 12,337 patients with periodontitis from 2000 to 2004 as the periodontitis group and frequency-matched them with 49,348 subjects without periodontitis according to sex, age, and year of periodontitis diagnosis. Table 1 contains the demographics and comorbidities of the patients with and without periodontitis. The mean age of the periodontitis group was 44.0 years (SD = 13.7 years), with a slight predominance of men (51.3%). The patients with periodontitis had higher prevalence of DM, hyperlipidemia, hypertension, IHD, stroke, head injury, and major depressive disorder, chronic obstructive pulmonary disease (COPD), and asthma than the subjects without periodontitis (all P < .001).
Table 1.
Baseline demographic factors and comorbidities of patients according to periodontitis status.
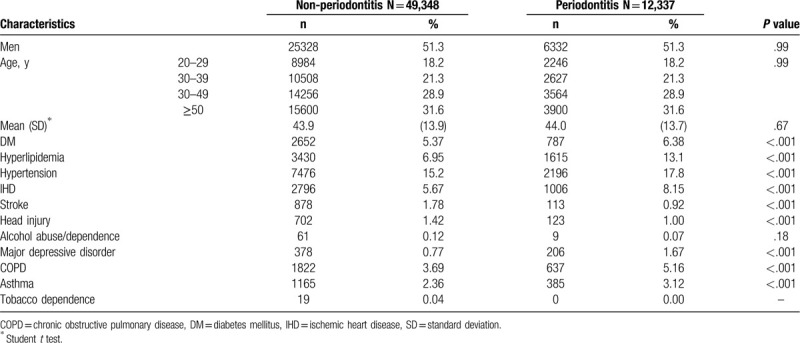
Figure 1 showed the results of the log-rank test and the cumulative incidences of BD. The Kaplan–Meier analysis was used to determine the risk of BD during follow-up in both groups. The cumulative incidence of BD was significantly higher in the periodontitis group than in the non-periodontitis group (P < .001).
Figure 1.
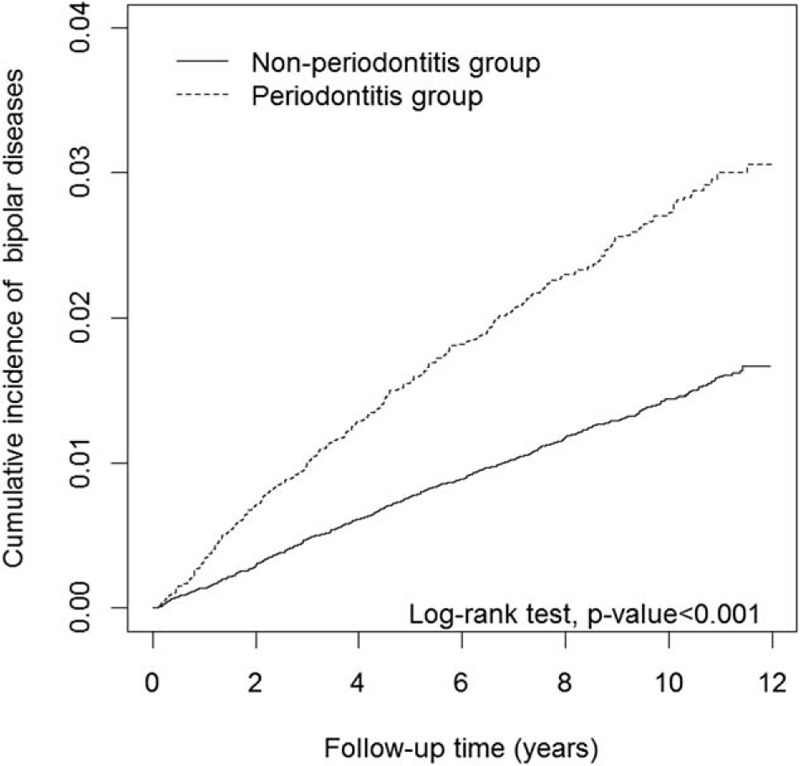
Comparison of the cumulative incidence of bipolar in the periodontitis cohort and non-periodontitis group.
During the average follow-up of 8.79 years, 339 (2.76%) patients in the periodontitis group and 611 (1.46%) patients in the non-periodontitis group developed BD. The incidence rate of BD was higher in the periodontitis group than in the non-periodontitis group (2.76 vs 1.46 per 1000 person-year), with an adjusted HR (aHR) of 1.82 (95% CI = 1.59–2.08) after adjustment for sex, age, and comorbidities (Table 2). In a multivariate Cox regression analysis, men exhibited a lower risk of BD than women did (aHR = 0.59, 95% CI = 0.52–0.68). Compared with the patients without counterpart comorbidities, higher risks of BD were observed in those with comorbidities of hyperlipidemia (aHR = 1.42, 95% CI = 1.16–1.74), hypertension (aHR = 1.34, 95% CI = 1.11–1.62), IHD (aHR = 1.52, 95% CI = 1.22–1.89), head injury (aHR = 1.85, 95% CI = 1.23–2.79), alcohol abuse or dependence (aHR = 9.43, 95% CI = 4.66–19.1), major depressive disorder (aHR = 7.42, 95% CI = 5.86–9.41), and COPD (aHR = 1.36, 95% CI = 1.04–1.77).
Table 2.
Cox model measured hazards ratios and 95% confidence intervals of bipolar disorder associated with periodontitis and covariates.
Stratified by sex, the higher risks of BD in patients with periodontitis were exhibited by both women (aHR = 1.68, 95% CI = 1.42–2.00) and men (aHR = 1.87, 95% CI = 1.51–2.32) compared with the subjects without periodontitis. Stratified by age group, the patients with periodontitis had a significantly higher risk of BD compared with the subjects without periodontitis in all age categories. The aHRs of BD were 1.76 (95% CI = 1.26–2.45) for those aged 20 to 29 years, 1.80 (95% CI = 1.32–2.47) for those aged 30 to 39 years, 1.74 (95% CI = 1.33–2.27) for those aged 40 to 49 years, and 1.68 (95% CI = 1.35–2.08) for those aged 50 years or older. Regardless of the subjects’ comorbidity status, patients with periodontitis had a higher risk of BD than subjects without periodontitis (aHR = 1.80, 95% CI = 1.50–2.16 for those without comorbidity and aHR = 1.78, 95% CI = 1.47–2.17 for those with comorbidity) (Table 3).
Table 3.
Incidence rates and hazard ratios of bipolar disorder according to periodontitis status and stratified by sex, age, and comorbidities.
4. Discussion
Periodontitis is a highly prevalent oral disease initiated by a bacterial plaque biofilm[1] around the teeth resulting in chronic inflammation in adjacent soft tissue. In routine dental procedures, even tooth brushing, these bacteria and their components, such as endotoxin, can be easily disseminated into the systemic circulation through minor or major gingival injuries. Notably, in immunocompromised people or patients with preexisting pathologic oral conditions, bacteremia may lead to the bacterial infection of distant organs, which may elicit immunological responses. Oral bacteria and endotoxins have also been associated with the occurrence of lung infection, sepsis, liver disease, and infective endocarditis,[1] but not with BD.
Our results revealed that the patients with periodontitis were at a significantly increased risk of BD. According to our analysis of the risk factors for BD in patients with periodontitis, we suggested that a possible mechanism is the interaction between chronic inflammation and the hypothalamic–pituitary–adrenal (HPA) axis. The key structures comprising the HPA axis are the paraventricular nucleus (PVN) of the hypothalamus, anterior lobe of the pituitary gland, and adrenal gland.[27,28] In addition, recent studies have demonstrated that chronic inflammation is associated with BD.[29–32] The immune reaction and proinflammatory cytokines, such as ILs and TNF-α, could induce neuroinflammation.[33] Lipopolysaccharide, a membrane component of Gram-negative bacteria, is an endotoxin and has been shown to stimulate microglia to produce numerous proinflammatory cytokines in the brain, such as TNF-α, interleukin-1 (IL-1), and interleukin-6 (IL-6).[34] Likewise, inflammatory cytokines, such as TNF-α, IL-1, IL-6, and IL-17 have been shown to be increased in patients with chronic periodontitis. Elevated secretion of these cytokines contributes to acute and chronic inflammation and tissue injury, leading to increased risk of systemic diseases such as cardiovascular diseases, DM, cancer, and chronic respiratory diseases.[35–37] Interestingly, the serum levels of anti-inflammatory cytokines, IL-4, and IL-10 were reduced in patients with chronic periodontitis.[37,38] Therefore, periodontitis may result in a local infection and thereafter induce inflammatory cascades, thus increasing the susceptibility to other severe pathological conditions such as cardiovascular disease[39,40] and DM.[41] Notably, it has been shown that proinflammatory cytokines, such as IL-1β, can be detected in PVN. The upregulation of IL-6 and COX-2 has also been detected in the adrenal glands. These findings provide novel insight into the relationship between proinflammatory cytokines within key structures comprising the HPA axis.[27] Furthermore, chronic inflammation may disturb the HPA axis and induce hypercortisolemia and neuroinflammation through a proinflammatory cascade.[27,42,43] In addition to inducing neuroinflammation, proinflammatory cytokines could also induce indoleamine 2,3-dioxygenase, thus reducing the availability of tryptophan and disturbing serotonin synthesis.[44] Immune-inflammatory pathways and cytokine changes in BD have been linked to changes in oxidative stress, nitrosative stress, and tryptophan catabolites.[44] As a result, the risk of BD was increased among patients with periodontitis.
It has been reported that periodontitis is a risk factor of dementia. That is not a concrete causality research, instead that is an association study. Two research designs have been used on the similar topics, namely case-control study[10] and retrospective cohort study.[45] For the case-control study, they enrolled the cases with (experimental group) or without (control group) cognitive impairment or dementia, and analyzed the association with periodontitis to evaluate its risk to dementia.[10] For the retrospective cohort study, they enrolled the patients diagnosed with periodontitis during 2003 to 2004, followed up for overall dementia, Alzheimer disease, and vascular dementia until 2015, and retrospectively analyzed the adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) of dementia according to chronic periodontitis.[45] The experimental design of our current study was similar to the later one as a retrospective cohort study. Periodontitis represented a source of systemic inflammation,[46] and chronic inflammation is associated with dementia,[9,10,47] as well as BD,[29–32] suggesting that the mechanism of periodontitis increasing the risk to dementia and BD might share similar factors, at least in part, including inflammation.
In addition to inflammation, smoking is also a common risk factor for both periodontitis and BD. A previous study demonstrated that smoking and oral pain are factors related to the prevalence and risk of periodontitis among adults with or without DM.[48] Similarly, smoking is about 2 to 3 times common in adults with BD compared with the general population.[49] The associations may be related to lower levels of serotonin, which contributes to brain serotonergic function[50,51] in smokers. The associations between smoke-related diseases such as COPD, asthma, and tobacco dependence with periodontitis and BD were also evaluated in the current study. The results showed that the patients with periodontitis had higher prevalence of COPD and asthma compared with those without periodontitis (Table 1), and the patients with BD had higher risk of COPD (aHR 1.36, 95% CI 1.04–1.77) (Table 2). However, we could not examine the effect of tobacco dependence due to the limited patients being tobacco dependent (only 19 in the non-periodontitis group and no patient in the periodontitis group).
This population-based study specifically examined periodontitis as a risk factor for BD by using matched cohorts. The major finding of our study is the higher incidence of subsequent BD among patients with periodontitis. Furthermore, the patients with periodontitis exhibited higher prevalence of DM, hyperlipidemia, hypertension, IHD, stroke, head injury, and major depressive disorder than the patients without periodontitis (all P < .001) (Table 1). Notably, the male patients exhibited a lower risk of BD than the female patients did (aHR = 0.60, 95% CI = 0.52–0.68). Women have been demonstrated as having a higher rate of thyroid hormone disturbances as well as thyroid diseases and autoimmunity, which hinder BD treatments using lithium.[52] Thyroid hormones have been shown to regulate the functions and regeneration of the adult central nervous system.[53] Thyroid hormones were also suggested as worsening periodontal diseases, and disturbance of thyroid hormones may lead to the destruction of the periodontium.[54]
On the other hand, the mean age of the patients with periodontitis was around 44 years old and the incidence of BD increases at 50 years old in our study. Considering the onset of BD was usually in adolescence,[55] our finding was different from primary BD. However, our study showed the temporal association and long-term influence between periodontitis and BD. Therefore, we considered the BD after periodontitis may be secondary rather than primary in nature. We supposed this finding raises further concern in future study or clinical practice.
The large population-based cohort of patients constituted a strength of our study; however, several limitations should be considered when interpreting our results. First, the diagnosis of periodontitis and BD in the NHIRD was based on ICD-9-CM codes. Thus, the role of periodontitis severity in the risk of BD was not explored. Also, we could not confirm whether those patients with periodontitis having some hypomanic symptoms but not reaching or receiving the diagnosis of BD. Second, the causal relationship was assessed mainly according to the chronological order in which these 2 conditions were diagnosed; the possibility that periodontitis causes BD cannot be excluded. Third, the possibility of bias should be carefully considered because we could not exclude the possibility that BD was diagnosed soon after periodontitis, or BD was undiagnosed while the patient suffered from periodontitis. BD was reported to be genetically heterogeneous.[56] Besides, dental anxiety or fear was prevalent among patients undertaking dental procedure.[57,58] Also, dental anxiety was a highly anxious condition.[58,59] Other than anxiety, evidence had shown that affective disturbance including mood and irritability was noted as early manifestation of medical illness.[60] Therefore, those conditions may make clinical practitioners confused about whether those symptoms were reaching the diagnosis of BD. Fourth, we attempted to distinguish different subgroups, such as patients with depression, from within the bipolar cohort prior to BD diagnosis. However, BD is commonly diagnosed using only ICD-9-CM 296 in the Registry for Catastrophic Illness Patients database; therefore, clearly distinguishing these subgroups is difficult. Fifth, even though our study demonstrated the association between periodontitis and BD, whether having other factors independently cause periodontitis and BD was not investigated in our study. Finally, numerous demographic variables that might have provided useful information regarding factors possibly associated with BD and periodontitis, such as socioeconomic status, lifestyle, and family history, were unavailable. Although the prevalence of stroke in the periodontitis group was lower than the non-periodontitis group, the prevalence of HT and IHD in the periodontitis group was still higher. This might be due to the shorter follow-up period in the periodontitis group. Both HT and IHD usually occur before the onset of stroke. Because the periodontitis patients had higher risk of comorbidities, the non-periodontitis group was more active. This resulted in higher risk of head injury caused by vehicle accidents. Moreover, the patients with periodontitis might be more likely to stop drinking alcohol which may have contributed to the slightly higher (but not statistically significant) prevalence of alcohol abuse in the non-periodontitis group.
5. Conclusion
We propose that patients with periodontitis exhibit a significantly increased risk of developing BD. Accordingly, we suggest that, following the diagnosis of periodontitis, practitioners could notice the occurrence of the symptoms of BD, and associated prevention. Additional prospective studies investigating the relationship between periodontitis and BD are warranted.
Acknowledgments
The authors thank the grants from the Ministry of Science and Technology (MOST) of Taiwan Government (MOST 107-2314-B-715 -004 -MY3, MOST103-2314-B-715-001-MY2, MOST104-2314-B-715 -003 -MY3, MOST 105-2320-B-039-059-MY3, MOST 108-2320-B-039-013), intramural research grants from Mackay Medical College (1052B07, 1051B23, 1061B09, 1071B12, 1081E03), and from China Medical University (CMU108-MF-49). This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Drug Development Center, China Medical University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE), Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039 -005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Author contributions
Collection and assembly of data: Kuang-Hsi Chang, Yi-Chao Hsu, Ing-Ming Chiu, Lih-Chyang Chen, Chih-Chao Hsu, Chang-Yin Lee, Hueng-Chuen Fan, Hsuan-Ju Chen, Ruey-Hwang Chou.
Conception/Design: Yi-Chao Hsu, Ruey-Hwang Chou.
Data analysis and interpretation: Kuang-Hsi Chang, Yi-Chao Hsu, Ing-Ming Chiu, Lih-Chyang Chen, Chih-Chao Hsu, Chang-Yin Lee, Hueng-Chuen Fan, Hsuan-Ju Chen, Ruey-Hwang Chou.
Final approval of manuscript: Kuang-Hsi Chang, Yi-Chao Hsu, Ing-Ming Chiu, Lih-Chyang Chen, Chih-Chao Hsu, Chang-Yin Lee, Hueng-Chuen Fan, Hsuan-Ju Chen, Ruey-Hwang Chou.
Manuscript preparation: Kuang-Hsi Chang, Yi-Chao Hsu, Ing-Ming Chiu, Lih-Chyang Chen, Chih-Chao Hsu, Chang-Yin Lee, Hueng-Chuen Fan, Hsuan-Ju Chen, Ruey-Hwang Chou.
Provision of study materials and patients: Kuang-Hsi Chang, Yi-Chao Hsu.
Footnotes
Abbreviations: BD = bipolar disorders, CIs = confidence intervals, DM = diabetes mellitus, HPA = hypothalamic–pituitary–adrenal, HRs = hazard ratios, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IHD = ischemic heart disease, ILs = interleukins, LHID2000 = Longitudinal Health Insurance Database, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, PGE2 = prostaglandin E2, PVN = paraventricular nucleus, SDs = standard deviations, TNF-α = tumor necrosis factor alpha.
How to cite this article: Chang KH, Hsu YC, Chiu IM, Chen LC, Hsu CC, Lee CY, Fan HC, Chen HJ, Chou RH. Association between periodontitis and bipolar disorder: a nationwide cohort study. Medicine. 2020;99:31(e21423).
K-H C and Y-C H have contributed equally to this work.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and materials: The datasets during and/or analyses during the current study are available from the corresponding author upon reasonable request.
The authors declare that they have no competing financial interests.
Funding from the Ministry of Science and Technology (MOST) of Taiwan Government (MOST 107-2314-B-715 -004 -MY3, MOST103-2314-B-715-001-MY2, MOST104-2314-B-715 -003 -MY3, MOST 105-2320-B-039-059-MY3, MOST 108-2320-B-039-013), intramural research grants from Mackay Medical College (1052B07, 1051B23, 1061B09, 1071B12, 1081E03) and from China Medical University (CMU108-MF-49). This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Drug Development Center, China Medical University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE), Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039 -005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Data Availability: The authors confirm that all data underlying the findings are fully available without restriction. The dataset is owned by the Taiwan National Health Research Institutes (NHRI). Requests for the data set may be sent an e-mail to the NHRI at nhird@nhri.org.tw or call at +886-037-246166 ext. 33603 for immediate service. Office Hour: Monday-Friday 8:00-17:30 (UTC+8).
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
References
- [1].Hirschfeld J, Kawai T. Oral inflammation and bacteremia: implications for chronic and acute systemic diseases involving major organs. Cardiovasc Hematol Disord Drug Targets 2015;15:70–84. [DOI] [PubMed] [Google Scholar]
- [2].Xiong X, Buekens P, Vastardis S, et al. Periodontal disease and gestational diabetes mellitus. Am J Obstet Gynecol 2006;195:1086–9. [DOI] [PubMed] [Google Scholar]
- [3].Armingohar Z, Jorgensen JJ, Kristoffersen AK, et al. Polymorphisms in the interleukin-1 gene locus and chronic periodontitis in patients with atherosclerotic and aortic aneurysmal vascular diseases. Scand J Immunol 2014;79:338–45. [DOI] [PubMed] [Google Scholar]
- [4].Gupta A, Govila V, Saini A. Proteomics - the research frontier in periodontics. J Oral Biol Craniofac Res 2015;5:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen XT, Tan JY, Lei LH, et al. Cytokine levels in plasma and gingival crevicular fluid in chronic periodontitis. Am J Dent 2015;28:9–12. [PubMed] [Google Scholar]
- [6].Kato Y, Hagiwara M, Ishihara Y, et al. TNF-alpha augmented Porphyromonas gingivalis invasion in human gingival epithelial cells through Rab5 and ICAM-1. BMC Microbiol 2014;14:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liao CH, Fei W, Shen ZH, et al. Expression and distribution of TNF-alpha and PGE2 of periodontal tissues in rat periodontitis model. Asian Pac J Trop Med 2014;7:412–6. [DOI] [PubMed] [Google Scholar]
- [8].Deo V, Bhongade ML. Pathogenesis of periodontitis: role of cytokines in host response. Dent Today 2010;29:60–2. 64–66; quiz 68–9. [PubMed] [Google Scholar]
- [9].Gaur S, Agnihotri R. Alzheimer's disease and chronic periodontitis: is there an association? Geriatr Gerontol Int 2015;15:391–404. [DOI] [PubMed] [Google Scholar]
- [10].Gil-Montoya JA, Sanchez-Lara I, Carnero-Pardo C, et al. Is periodontitis a risk factor for cognitive impairment and dementia? A case-control study. J Periodontol 2015;86:244–53. [DOI] [PubMed] [Google Scholar]
- [11].Hsu CC, Hsu YC, Chen HJ, et al. Association of periodontitis and subsequent depression: a nationwide population-based study. Medicine (Baltimore) 2015;94:e2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhou X, Zhang W, Liu X, et al. Interrelationship between diabetes and periodontitis: role of hyperlipidemia. Arch Oral Biol 2015;60:667–74. [DOI] [PubMed] [Google Scholar]
- [13].Kholy KE, Genco RJ, Van Dyke TE. Oral infections and cardiovascular disease. Trends Endocrinol Metab 2015;26:315–21. [DOI] [PubMed] [Google Scholar]
- [14].Saffi MA, Furtado MV, Polanczyk CA, et al. Relationship between vascular endothelium and periodontal disease in atherosclerotic lesions: review article. World J Cardiol 2015;7:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Andrukhov O, Steiner I, Liu S, et al. Different effects of Porphyromonas gingivalis lipopolysaccharide and TLR2 agonist Pam3CSK4 on the adhesion molecules expression in endothelial cells. Odontology 2015;103:19–26. [DOI] [PubMed] [Google Scholar]
- [16].Hashimoto M, Yamazaki T, Hamaguchi M, et al. Periodontitis and porphyromonas gingivalis in preclinical stage of arthritis patients. PLoS One 2015;10:e0122121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kobayashi T, Yoshie H. Host responses in the link between periodontitis and rheumatoid arthritis. Curr Oral Health Rep 2015;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Silosi I, Cojocaru M, Foia L, et al. Significance of circulating and crevicular matrix metalloproteinase-9 in rheumatoid arthritis-chronic periodontitis association. J Immunol Res 2015;2015:218060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Price AL, Marzani-Nissen GR. Bipolar disorders: a review. Am Fam Physician 2012;85:483–93. [PubMed] [Google Scholar]
- [20].Maina G, Bechon E, Rigardetto S, et al. General medical conditions are associated with delay to treatment in patients with bipolar disorder. Psychosomatics 2013;54:437–42. [DOI] [PubMed] [Google Scholar]
- [21].Clark DB. Dental care for the patient with bipolar disorder. J Can Dent Assoc 2003;69:20–4. [PubMed] [Google Scholar]
- [22].Sjogren R, Nordstrom G. Oral health status of psychiatric patients. J Clin Nurs 2000;9:632–8. [DOI] [PubMed] [Google Scholar]
- [23].Friedlander AH, Friedlander IK, Marder SR. Bipolar I disorder: psychopathology, medical management and dental implications. J Am Dent Assoc 2002;133:1209–17. [DOI] [PubMed] [Google Scholar]
- [24].Shetty SR, Bhowmick S, Castelino R, et al. Drug induced xerostomia in elderly individuals: an institutional study. Contemp Clin Dent 2012;3:173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Solis AC, Marques AH, Pannuti CM, et al. Evaluation of periodontitis in hospital outpatients with major depressive disorder. J Periodontal Res 2014;49:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stewart R, Weyant RJ, Garcia ME, et al. Adverse oral health and cognitive decline: the health, aging and body composition study. J Am Geriatr Soc 2013;61:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hueston CM, Deak T. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol Behav 2014;124:77–91. [DOI] [PubMed] [Google Scholar]
- [28].Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 2006;8:383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barbosa IG, Rocha NP, Assis F, et al. Monocyte and lymphocyte activation in bipolar disorder: a new piece in the puzzle of immune dysfunction in mood disorders. Int J Neuropsychopharmacol 2015;18:pyu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Frey BN, Andreazza AC, Houenou J, et al. Biomarkers in bipolar disorder: a positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust N Z J Psychiatry 2013;47:321–32. [DOI] [PubMed] [Google Scholar]
- [31].Hope S, Dieset I, Agartz I, et al. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res 2011;45:1608–16. [DOI] [PubMed] [Google Scholar]
- [32].Hsu CC, Chen SC, Liu CJ, et al. Rheumatoid arthritis and the risk of bipolar disorder: a nationwide population-based study. PLoS One 2014;9:e107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Singhal G, Jaehne EJ, Corrigan F, et al. Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 2014;8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Weinstein JR, Swarts S, Bishop C, et al. Lipopolysaccharide is a frequent and significant contaminant in microglia-activating factors. Glia 2008;56:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cardoso EM, Reis C, Manzanares-Cespedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med 2018;130:98–104. [DOI] [PubMed] [Google Scholar]
- [36].Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res 2010;89:1349–63. [DOI] [PubMed] [Google Scholar]
- [37].Passoja A, Puijola I, Knuuttila M, et al. Serum levels of interleukin-10 and tumour necrosis factor-alpha in chronic periodontitis. J Clin Periodontol 2010;37:881–7. [DOI] [PubMed] [Google Scholar]
- [38].Buhlin K, Hultin M, Norderyd O, et al. Risk factors for atherosclerosis in cases with severe periodontitis. J Clin Periodontol 2009;36:541–9. [DOI] [PubMed] [Google Scholar]
- [39].El Fadl KA, Ragy N, El Batran M, et al. Periodontitis and cardiovascular disease: floss and reduce a potential risk factor for CVD. Angiology 2011;62:62–7. [DOI] [PubMed] [Google Scholar]
- [40].Jeftha A, Holmes H. Periodontitis and cardiovascular disease. SADJ 2013;68:60.62–63. [PubMed] [Google Scholar]
- [41].Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia 2012;55:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Garcia-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev 2008;32:1136–51. [DOI] [PubMed] [Google Scholar]
- [43].Choi DC, Furay AR, Evanson NK, et al. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology 2008;33:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Anderson G, Maes M. Bipolar disorder: role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Curr Psychiatry Rep 2015;17:8. [DOI] [PubMed] [Google Scholar]
- [45].Choi S, Kim K, Chang J, et al. Association of chronic periodontitis on alzheimer's disease or vascular dementia. J Am Geriatr Soc 2019;67:1234–9. [DOI] [PubMed] [Google Scholar]
- [46].Kamer AR, Craig RG, Dasanayake AP, et al. Inflammation and Alzheimer's disease: possible role of periodontal diseases. Alzheimers Dement 2008;4:242–50. [DOI] [PubMed] [Google Scholar]
- [47].Daly B, Thompsell A, Sharpling J, et al. Evidence summary: the relationship between oral health and dementia. Br Dent J 2018;223:846–53. [DOI] [PubMed] [Google Scholar]
- [48].Hong M, Kim HY, Seok H, et al. Prevalence and risk factors of periodontitis among adults with or without diabetes mellitus. Korean J Intern Med 2016;31:910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Heffner JL, Strawn JR, DelBello MP, et al. The co-occurrence of cigarette smoking and bipolar disorder: phenomenology and treatment considerations. Bipolar Disord 2011;13:439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Malone KM, Waternaux C, Haas GL, et al. Cigarette smoking, suicidal behavior, and serotonin function in major psychiatric disorders. Am J Psychiatry 2003;160:773–9. [DOI] [PubMed] [Google Scholar]
- [51].Thomson D, Berk M, Dodd S, et al. Tobacco use in bipolar disorder. Clin Psychopharmacol Neurosci 2015;13:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bauer M, Glenn T, Pilhatsch M, et al. Gender differences in thyroid system function: relevance to bipolar disorder and its treatment. Bipolar Disord 2014;16:58–71. [DOI] [PubMed] [Google Scholar]
- [53].Bhumika S, Darras VM. Role of thyroid hormones in different aspects of nervous system regeneration in vertebrates. Gen Comp Endocrinol 2014;203:86–94. [DOI] [PubMed] [Google Scholar]
- [54].Zahid TM, Wang BY, Cohen RE. The effects of thyroid hormone abnormalities on periodontal disease status. J Int Acad Periodontol 2011;13:80–5. [PubMed] [Google Scholar]
- [55].Marangoni C, De Chiara L, Faedda GL. Bipolar disorder and ADHD: comorbidity and diagnostic distinctions. Curr Psychiatry Rep 2015;17:604. [DOI] [PubMed] [Google Scholar]
- [56].Douglas LN, McGuire AB, Manzardo AM, et al. High-resolution chromosome ideogram representation of recognized genes for bipolar disorder. Gene 2016;586:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hofer D, Thoma MV, Schmidlin PR, et al. Pre-treatment anxiety in a dental hygiene recall population: a cross-sectional pilot study. BMC Oral Health 2016;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rayman S, Dincer E, Almas K. Managing dental fear and anxiety. N Y State Dent J 2013;79:25–9. [PubMed] [Google Scholar]
- [59].Armfield JM, Heaton LJ. Management of fear and anxiety in the dental clinic: a review. Aust Dent J 2013;58:390–407. quiz 531. [DOI] [PubMed] [Google Scholar]
- [60].Cosci F, Fava GA, Sonino N. Mood and anxiety disorders as early manifestations of medical illness: a systematic review. Psychother Psychosom 2015;84:22–9. [DOI] [PubMed] [Google Scholar]


