Abstract
Background:
To comprehensively evaluate the association between the polymorphism of matrix metalloproteinase-9 (MMP-9)-C1562T (rs3918242) and susceptibility to chronic obstructive pulmonary disease (COPD) in middle-aged and elderly patients through Meta-analysis.
Methods:
PubMed, EMBASE, CNKI, Wanfang, VIP, and other databases were searched by computer in the inception to August 2019 to collect all the case-control studies that met the inclusion criteria in this literature. Meta-analysis was performed using Stata 15.0, including the OR value calculations of the association between the merged MMP-9-C1562T polymorphism and the COPD susceptibility. Subgroup analysis, sensitivity analysis, and publication bias test were also performed. A total of 13 literature were included in this Meta-analysis with a total of 2512 cases and 2716 controls.
Results:
The results have shown that the OR of MMP-9-C1562T T allele to C allele was 0.35 (95% confidence interval [CI]: 0.23–0.52, P < .01). The subgroup analysis of ethnicity result showed that the merged OR of MMP-9-C1562T T allele to C allele was 0.24 (95% CI: 0.17–0.34, P < .01) in Caucasian while the merged OR was 0.62 (95% CI: 0.22–1.70, P > .05) in Asian. However, there were no statistically significant models in the dominant, recessive, homozygote and heterozygote genetic models.
Conclusion:
The MMP-9-C1562T polymorphism was associated with the susceptibility to middle-aged and elderly COPD patients. Compared with T allele, C allele increased the risk of disease, especially in Caucasian, but not found in Asian.
Keywords: chronic obstructive pulmonary disease, meta-analysis, matrix metalloproteinase-9, polymorphism, rs3918242
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic airway disease involving chronic local and systemic inflammatory changes, clinically characterized by continuous and progressive airflow obstruction with airway remodeling and lung parenchyma destruction as pathological basis.[1] It is one of diseases that seriously affect human health and life quality worldwide. In 2013, COPD has become the third leading cause of death for residents in China, resulting in >900 thousand deaths.[2] A nationwide cross-sectional study based on 10 provinces (or autonomous regions) in mainland China that was recently published in Lancet magazine, have shown that the prevalence of COPD is 8.6% in people over 20 years old while 13.7% in people aged 40 and over.[3] To find out the pathogenesis of COPD and its corresponding treatment has become an important research direction. The existing research results indicate that COPD is the result of a combination of environmental and genetic factors. The former is mainly cigarette smoking, in addition to occupational dust exposure and indoor air pollution represented by fumes from kitchen and fuel combustion. 85% of COPD patients are long-term smokers, but only 10% to 20% will develop into symptomatic COPD patients, indicating that individuals have different susceptibility to COPD, which is the phenotype of gene polymorphism.[4] The genetic factor has been proved to be the α1-antitrypsin (α1-AT) deficiency, which is closely associated with early-onset emphysema and smoking increases the risk of its incidence. Researchers at home and abroad attach great importance to the role genetic factor plays in the occurrence and development of COPD, and many genes have been selected as candidate genes for COPC molecular genetic research. Matrix metalloproteinases (MMPs) is a family of calcium- and zinc-dependent proteinase. There are currently at least 26 subtypes that can degrade almost all extracellular matrix and basement membrane components.[5] Matrix metalloproteinase-9 (MMP-9) is one of the key members in the MMP family, mainly secreted by macrophages, neutrophils, and eosinophils in the alveoli and can also be produced by lung tissue cells under certain conditions.[6] The MMP-9 gene is located on human chromosome 16, including 13 exons and 12 introns and its regulation mainly occurs at the transcriptional level. In the pathogenesis of COPD, MMP-9 mainly degrades the extracellular matrix and basement membrane of alveolar wall, destroying the normal structure of lung tissue. At the same time, MMP-9 also repairs the extracellular matrix and participates in respiratory tract reconstruction.[7–9] In addition, MMP-9 can also participate in inflammatory response, causing inflammatory cells to accumulate in the airway, thus increasing airway responsiveness. Study found that MMP-9 is highly expressed in the lung tissues of COPD patients and leads to generation of sputum.[10] Therefore, the analysis of MMP-9 gene polymorphism is an important starting point to explore the susceptibility to COPD. It has been found that there is a mutation from C to T at site 1562 of promoter MMP-9, which may affect the expression level of MMP-9 gene. The MMP-9-C1562T polymorphism is an important reason for the abnormal increase of MMP-9 expression level. In the past few years, several studies have reported the relationship between MMP-9-C1562T and susceptibility to COPD in middle-aged and elderly patients, but the conclusions are very different and inconclusive. Therefore, it is necessary to apply Meta-analysis to collect and analyze the data of each independent study to quantitatively understand the correlation between the both. In this study, we further analyzed the relationship between MMP-9-C1562T polymorphism and susceptibility to COPD in middle-aged and elderly patients, so as to provide theoretical basis and corresponding evidence-based medical basis for clinical medicine and other professional researches.
2. Materials and methods
2.1. Literature retrieval
PubMed, EMBASE, CNKI, Wanfang, VIP, and other databases were searched by computer in the inception to August 2019, with “matrix metalloproteinase,” “matrix metalloproteinase 9,” “MMP-9,” “gene polymorphism,” “rs3918242,” “SNP,” “chronic obstructive pulmonary disease,” “COPD” as keywords. All the case-control studies that met the inclusion criteria were collected. The retrieval papers were limited to English and Chinese, and the unpublished papers were not included.
2.2. Inclusion and exclusion criteria
2.2.1. Inclusion criteria
Studies on the association between MMP-9-C1562T polymorphism and COPD susceptibility; case-control studies with the average age over 40 years old in the cases; complete data or statistical indicators OR (95% CI) provided directly or indirectly.
2.2.2. Exclusion criteria
Simple case studies, case reports, reviews, and comments; repeatedly published literature with incomplete information; controls were not in accordance with Hardy-Weinberg (H-W) genetic balance.
2.3. Data extraction
Two researchers screened and extracted data independently, and disagreement was resolved by discussion or assistance of the third researcher. The extracted information was included as following: first author, year of publication, country, race, sample size of cases and controls, age of cases, number of genotypes, sequencing method, source of controls. For the H-W balance test of the controls, cases with P < .05 were considered to be not in accordance with H-W balance.
2.4. Literature quality evaluation
Read the full text. According to the Newcastle-Ottawa scale (NOS),[11] the literature quality was evaluated. Literature awarded below 6 stars are low-quality literature, while 6 stars and above are high-quality ones. Only the latter ones were included in this study. According to the uniform quality standard, two assessors evaluated independently to extract data, and then performed cross-check. Discrepancies were resolved by discussion or assistance of the third researcher.
2.5. Statistical method
Meta-analysis was performed by Stata 15.0 statistical software (Texas, USA). Q test was used to test the heterogeneity of each research result. If I2 ≥ 50% or P ≤ .05 suggested heterogeneity, the random effect model (REM) can be used for data merging. If I2 < 50% and P > .05 suggested no heterogeneity, the fixed effect model (FEM) can be used. Z test was used to test the significance of the pooled OR, and subgroup analysis of ethnicity was performed. In this Meta-analysis, funnel plot and Egger test were used to evaluate publication bias. Funnel plot was drawn by using the standard error of log (OR) and OR. The asymmetric funnel plot indicates publication bias.
3. Results
3.1. Literature retrieval basic information
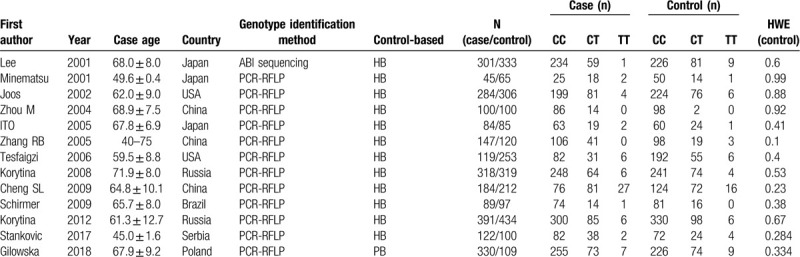
According to the criteria, a total of 13 literature was included,[12–24] including 7 in Europe and the United States and 7 in Asia. There were 2512 patients in COPD group and 2716 in the control group. The specific screening process is shown in Fig. 1. The study characteristics and genotype frequencies are shown in Table 1.
Figure 1.
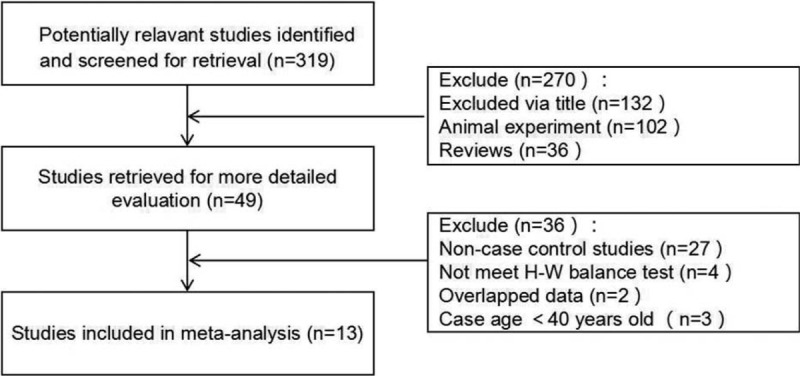
A flow diagram of the study selection process.
Table 1.
Characters of included studies.
3.2. Meta-analysis results
3.2.1. Allele comparison
The result of Meta-analysis is shown in Table 2 and Fig. 2. T allele was compared with C allele, there was statistically significant heterogeneity among studies (I2 = 93.8%, P < .05), so the REM was used. The final result showed that there was statistically significant difference (pooled OR = 0.35, 95% CI: 0.23–0.52, P < .01). According to the subgroup analysis of ethnicity, the results showed that there was statistically significant heterogeneity in Caucasian (OR = 0.24, 95% CI: 0.17–0.34, P < .01) but not in Asian (OR = 0.62, 95% CI: 0.23–1.70, P > .05). Forest plot is shown in Fig. 2A. This suggested that MMP-9-C1562T polymorphism was associated with susceptibility to COPD in middle-aged and elderly patients, and allele C is a risk factor for COPD patients. The symmetry deviation of funnel plot (Fig. 3A) and Egger test indicated that there was a certain publication bias (P < .05).
Table 2.
Results of meta-analysis for MMP-9-C1562T polymorphism and COPD.
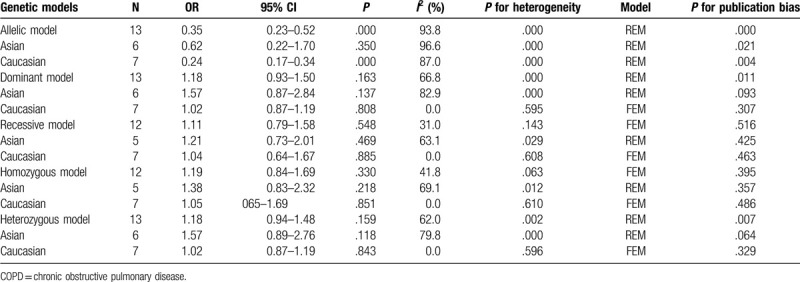
Figure 2.
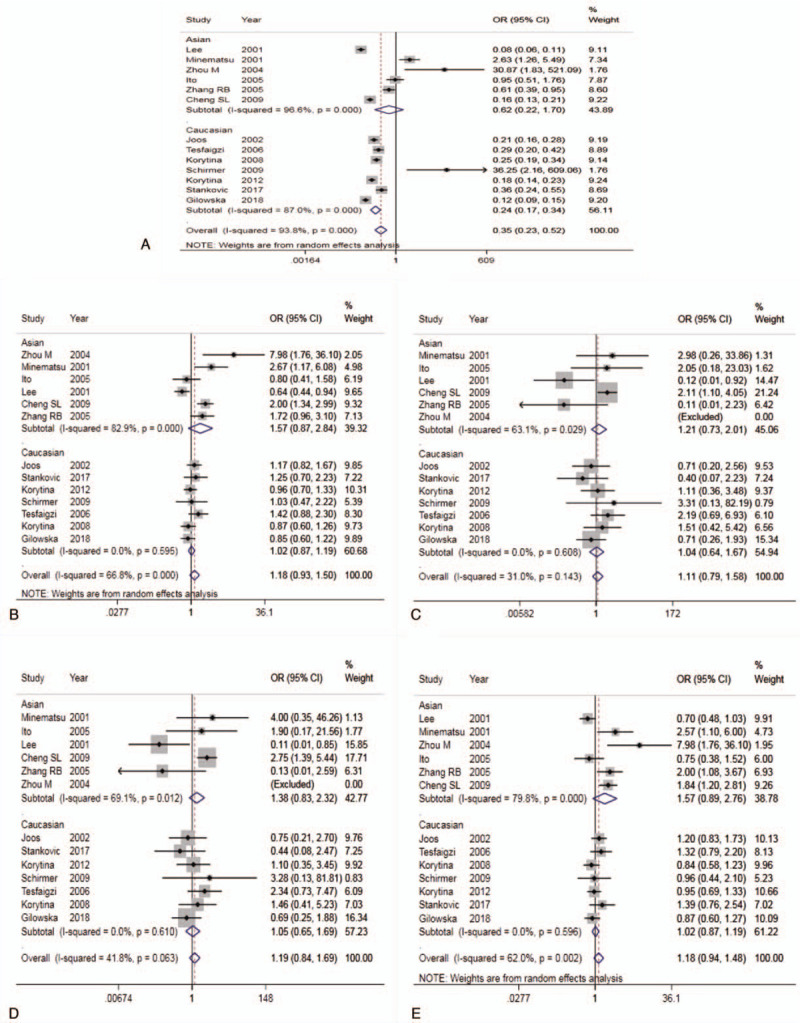
Forest plot for the association between MMP-9-C1562T and COPD (A: Allelic model; B: Dominant model; C: Recessive model; D: Homozygous model; E: Heterozygote model).
Figure 3.
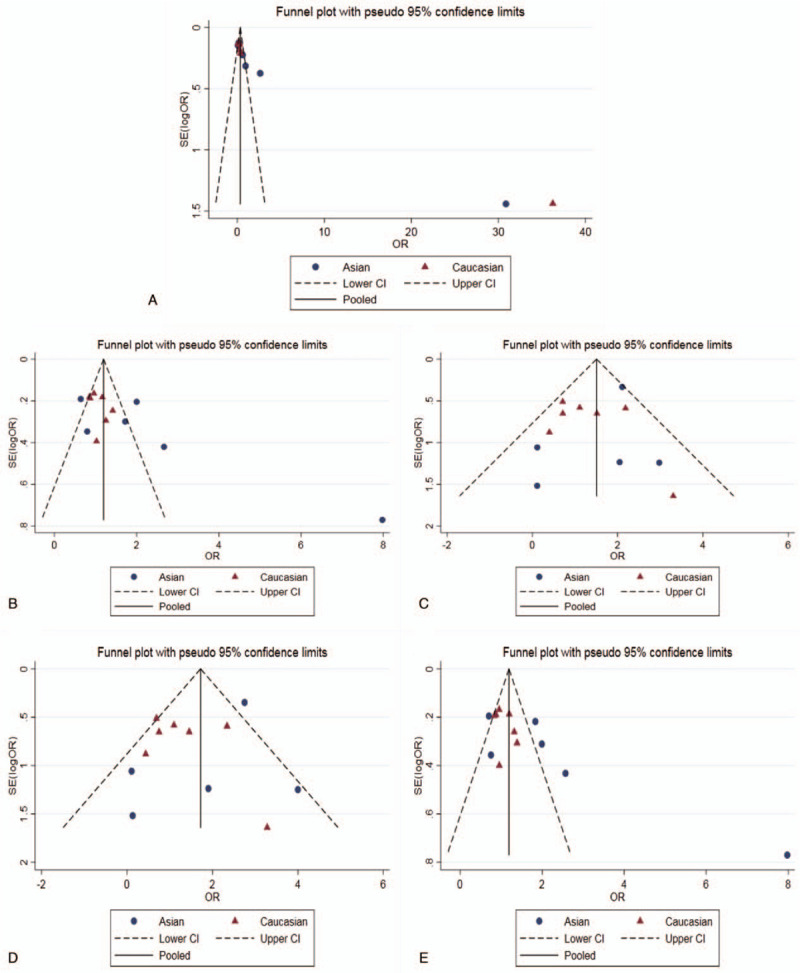
Funnel plot for the assessment of publication bias (A: Allelic model; B: Dominant model; C: Recessive model; D: Homozygous model; E: Heterozygote model).
3.2.2. Dominant genetic model
TT+TC genotype was compared with CC genotype. There was statistically significant heterogeneity among studies (I2 = 66.8%, P < .05), so the REM was used. The result showed that there was no statistically significant difference (pooled OR = 1.18, 95% CI: 0.93–1.50, P > .05). The subgroup analysis showed the same results (Fig. 2B). The Caucasian and Asian dominant genetic models were not statistically significant. This suggested that MMP-9-C1562T polymorphism could not be considered to be associated with susceptibility to COPD in middle-aged and elderly patients. The funnel plot was basically symmetrical (Fig. 3B), and Egger test showed that P value was slightly <.05. After subgroup analysis, the Egger test of Caucasian and Asian population showed that the publication bias was well controlled (P > .05).
3.2.3. Recessive genetic model
TT genotype was compared with TC+CC genotype. There was no statistically significant heterogeneity among studies (I2 = 31.0%, P > .05), so the FEM was used. The result showed that there was no statistically significant difference (pooled OR = 1.11, 95% CI: 0.79–1.58, P > .05). The result of subgroup analysis was the same (Fig. 2C). The Caucasian and Asian dominant genetic models were not statistically significant. This suggested that MMP-9-C1562T polymorphism could not be considered to be associated with susceptibility to COPD in middle-aged and elderly patients. The funnel plot was basically symmetrical (Fig. 3C) and Egger test showed that the publication bias was well controlled (P > .05).
3.2.4. Homozygote model
TT genotype was compared with CC genotype. There was no statistically significant heterogeneity among studies (I2 = 41.8%, P > .05), so the FEM was used. The result showed that there was no statistically significant difference (pooled OR = 1.19, 95% CI: 0.84–1.69, P > .05). The result of subgroup analysis was the same (Fig. 2D). The Caucasian and Asian dominant genetic models were not statistically significant. This suggested that MMP-9-C1562T polymorphism could not be considered to be associated with COPD susceptibility in middle-aged and elderly patients. The funnel plot was basically symmetrical (Fig. 3D) and Egger test showed that the publication bias was well controlled (P > .05).
3.2.5. Heterozygote model
TC genotype was compared with CC genotype. There was statistically significant heterogeneity among studies (I2 = 62.0%, P < .05), so the REM was used. The result showed that there was no statistically significant difference (pooled OR = 1.18, 95% CI: 0.94–1.48, P > .05). The result of subgroup analysis was the same (Fig. 3E). The Caucasian and Asian dominant genetic models were not statistically significant. This suggested that MMP-9-C1562T polymorphism could not be considered to be associated with susceptibility to COPD in middle-aged and elderly patients. The funnel plot was basically symmetrical (Fig. 3E), but the Egger test showed P < .05. However, the Egger test of Asian and Caucasian showed that there was no significant difference, which indicated that the publication bias was well controlled.
3.3. Sensitivity analysis
The result of sensitivity analysis is shown in Fig. 4. Each study was excluded one by one and Meta-analysis was performed. The result showed that in dominant genetic model, the OR value was statistically significant after excluding the result of Lee et al,[24] indicating that it has a great influence on the result of dominant genetic model. The combined effect size of other genetic models did not change significantly, indicating that 14 included literature were stable.
Figure 4.
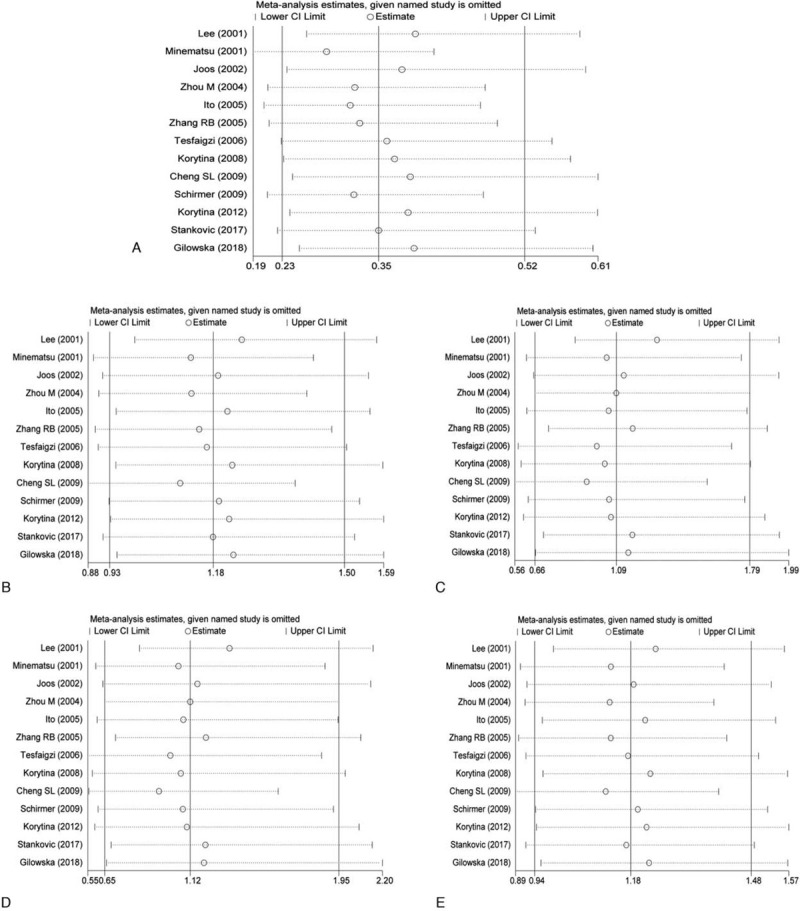
Sensitivity analysis results (A: Allelic model; B: Dominant model; C: Recessive model; D: Homozygous model; E: Heterozygote model).
4. Discussion
COPD is a common and frequently occurring disease of human respiratory system. It is recognized as one of the main diseases threatening human health, clinically characterized by cough, expectoration, shortness of breath and decreased activity endurance, and complicated with progressive airflow obstruction and decreased lung function. COPD now with extremely high morbidity and mortality worldwide and has become an important public health problem.[25] Its important pathogenesis includes protease–antiprotease imbalance, chronic inflammation, and oxidation–antioxidant imbalance, but lung tissue destruction has not been fully studied in cellular and molecular level. MMPs are part of a large metalloendopeptidase superfamily. They typically consist of a pro-domain and a catalytic domain. The latter contains a zinc ion in the active site, as well as a characteristic methionine turn, which is caused by a conserved methionine residue downstream of the zinc binding site.[26] MMP-9 is a member of metalloproteinases family, which can be synthesized and released by various cells including alveolar macrophages. MMP-9 is 1 of the 2 gelatinases and the other is MMP-2. Gelatinase not only contains the original structure of MMPs, but fibronectin type II-like repeats in its catalytic region, leading to its higher binding affinity to gelatin and elastin.[27] MMP-9 contains collagen fiber type V-like region with high glycosylation, which may affect the specificity and anti-degradation of the substrate. MMP-9 is released by cells in the form of zymogens. In vitro, MMP-9 can be activated by organic mercury while in vivo, by a variety of proteases,[28] including MMP-3 (matrix degrading enzyme), MMP-2, or hypochloric acid.[29] Among them, MMP-3 may be the most effective activator.[30] The most effective inhibitor in the cycle is the α 2-macroglobulin. The activity of MMP-9 is closely related to the regulation of specific endogenous inhibitors: tissue inhibitor of metalloproteinases (TIMPs). TIMP1,2,3,4 are now known has affinity to MMP-9.[31,32] Although environmental factors, including smoking, are the main risk factors for COPD, genetic factors are also closely related to its incidence.[33,34] The combination of these 2 factors leads to COPD, which involves multiple pathogenesis, such as inflammatory response, protease-antiprotease, and oxidation–antioxidation imbalances. Each pathogenesis is interrelated and interact with each other.[35,36] Previous studies on the pathogenesis of COPD have found many COPD susceptible genes and their single nucleotide polymorphisms (SNP). At present, there are many studies related to the relationship between MMP-9-C1562T and susceptibility to COPD, but the conclusions are inconsistent. Minematsu et al[12] have suggested that people who carry the C allele in MMP-9-C1562T polymorphism have a lower risk of COPD than those with T allele, indicating that TT genotype may be a high risk genetic factor for COPD. However, some studies have come to the opposite conclusion. Gilowska et al[23] have believed that people who carry the T allele have a lower risk of COPD, and MMP-9-C1562T polymorphism can be used as a predictor of genetic susceptibility to COPD. Therefore, in order to comprehensively evaluate the relationship between MMP-9-C1562T polymorphism and the risk of COPD, this study conducted a Meta-analysis of MMP-9-C1562T polymorphism and susceptibility to COPD in middle-aged and elderly patients.
A total of 13 literature[12–24] were included in this study, among which 6 from Asia and 7 from Europe and America. The results showed that there was a strong correlation between MMP-9-C1562T gene polymorphism and susceptibility to COPD in middle-aged and elderly patients. According to the subgroup analysis of ethnicity, compared with C allele, the T allele showed that there was statistical significance in Caucasian (OR = 0.24, 95% CI: 0.17–0.34, P < .01) but not in Asian (OR = 0.62, 95% CI: 0.23–1.70, P > .05). It suggested that MMP-9-C1562T gene polymorphism was associated with susceptibility to COPD in middle-aged and elderly patients, especially in Caucasian, but not in Asian. The results of funnel plot of allele model and Egger test indicated that there was a certain publication bias (P < .05). The results heterogeneity test showed that there was heterogeneity among the studies (I2 > 50%). The result of sensitivity analysis after excluding a single literature showed that there was no significant change in the combined effect size of allele model, indicating that the included literature have good stability. Therefore, it could be considered that there was a correlation between MMP-9-C1562T polymorphism and susceptibility to COPD in middle-aged and elderly patients, and those with C alleles were more susceptible to COPD than those with T alleles. In the dominant genetic model, there was no significant difference (pooled OR = 1.18, 95% CI: 0.93–1.50, P > .05). The same result was found in subgroup analysis of ethnicity, and there was no statistical significance in Caucasian and Asian in dominant genetic models. However, the result of sensitivity showed that the result of Lee et al[24] has a great influence on the result in dominant genetic model. The difference was statistically significant after excluding it. But in the recessive, homozygote and heterozygote genetic model, there was no statistical significance, indicating that publication bias was well controlled and the result of sensitivity analysis was stable. A previous Meta-analysis of MMP-9 gene polymorphism and susceptibility to COPD in middle-aged and elderly patients by Chen et al[37] has showed that compared with C allele, T allele was a high risk factor for COPD patients. The dominant genetic model in their Meta-analysis has statistical significance, and TT genotype is one of the risk genotypes. The results of the dominant genetic model are related to the results of the sensitivity analysis in this study. Therefore, the conclusion of the dominant genetic model should be careful. However, their conclusion of alleles is contrary to our study, the reason of which laying on the inclusion of the literature. The latest studies were included in this study, and the control groups that were not in accordance with Hardy Weinberg Equilibrium (HWE) were excluded. Therefore, to a certain extent, the conclusion of this study is reliable.
Certainly, this study also has some limitations: there was great heterogeneity among alleles for both Asian and Caucasian; the results of sensitivity analysis of dominant genetic model showed that the conclusion was unstable to some extent; there was a certain publication bias in allele model and dominant genetic model; the effects of gene linkage and gene–environment interaction in middle-aged and elderly COPD patients were not analyzed.
To sum up, MMP-9-C1562T polymorphism was associated with susceptibility to COPD in middle-aged and elderly patients, and the risk of C allele was higher than that of T allele, especially in Caucasian, but not in Asian. However, in view of the limitations of the study, more profound research need to be conducted in the future.
Author contributions
Study concept and design: All authors; Acquisition of data: All authors; Analysis and interpretation of data: All authors; Drafting of the manuscript: All authors; Critical revision of the manuscript for important intellectual content: All authors; Statistical analysis: All authors; Administrative, technical, and material support: All authors; Study supervision: All authors; all authors have read and approved the manuscript.
Footnotes
Abbreviations: α1-AT = α1-antitrypsin, COPD = chronic obstructive pulmonary disease, MMP-9 = matrix metalloproteinase-9, MMPs = matrix metalloproteinases, NOS = Newcastle-Ottawa scale, TIMPs = tissue inhibitor of metalloproteinases.
How to cite this article: Zhao R, Zhou H, Zhu J. MMP-9-C1562T polymorphism and susceptibility to COPD: a meta-analysis. Medicine. 2020;99:31(e21479).
RZ and HXZ have contributed equally to this work.
Ethical approval: Because this study is a meta-analysis, it does not involve moral approval and patient consent.
The Social Science Planning Project of Chongqing (2015PY37). The Humanities and Social science foundation of Third Military Medical University (2016XRW07).
The authors have no conflicts of interest to disclose..
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Bidan CM, Veldsink AC, Meurs H, et al. Airway and extracellular matrix mechanics in COPD. Front Physiol 2015;6:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 2016;387:251–72. [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Xu J, Lan Y, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706–17. [DOI] [PubMed] [Google Scholar]
- [4].Golpe R, Sanjuán-López P, Martín-Robles I, et al. Cardiovascular studies in patients with chronic obstructive pulmonary disease due to biomass smoke or tobacco. Lung 2018;196:195–200. [DOI] [PubMed] [Google Scholar]
- [5].Navratilova Z, Kolek V, Petrek M. Matrix metalloproteinases and their inhibitors in chronic obstructive pulmonary disease. Arch Immunol Ther Exp (Warsz) 2016;64:177–93. [DOI] [PubMed] [Google Scholar]
- [6].Zhou L, Le Y, Tian J, et al. Cigarette smoke-induced RANKL expression enhances MMP-9 production by alveolar macrophages. Int J Chron Obstruct Pulmon Dis 2018;14:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hendrix AY, Kheradmand F. The role of matrix metalloproteinases in development, repair, and destruction of the lungs. Prog Mol Biol Transl Sci 2017;148:1–29. [DOI] [PubMed] [Google Scholar]
- [8].Joos L, He JQ, Shepherdson MB, et al. The role of matrix metalloproteinase polymorphisms in the rate of decline in lung function. Hum Mol Genet 2002;11:569–76. [DOI] [PubMed] [Google Scholar]
- [9].Wert SE, Yoshida M, LeVine AM, et al. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci USA 2000;97:5972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hui-Ping MA, Wei L, Xiao-Min L. Matrix metalloproteinase 9 is involved in airway inflammation in cough variant asthma. Exp Ther Med 2014;8:1197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Andreas S. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [12].Minematsu N, Nakamura H, Tateno H, et al. Genetic polymorphism in matrix metalloproteinase-9 and pulmonary emphysema. Biochem Biophys Res Commun 2001;289:116–9. [DOI] [PubMed] [Google Scholar]
- [13].Ladina J, Jian-Qing H, Shepherdson MB, et al. The role of matrix metalloproteinase polymorphisms in the rate of decline in lung function. Hum Mol Genet 2002;11:569–76. [DOI] [PubMed] [Google Scholar]
- [14].Zhou M, Huang SG, Wan HY, et al. Genetic polymorphism in matrix metalloproteinase-9and the susceptibility to chronic obstructive pulmonary disease in Han population of south China. Chin Med J (Engl) 2004;117:1481–4. [PubMed] [Google Scholar]
- [15].Ito I, Nagai S, Handa T, et al. Matrix metalloproteinase-9 promoter polymorphism associated with upper lung dominant emphysema. Am J Respir Crit Care Med 2005;172:1378–82. [DOI] [PubMed] [Google Scholar]
- [16].Zhang RB, He QY, Yang RH, et al. Study on matrix metalloproteinase 1, 9, 12 polymorphisms and susceptibility to chronic obstructive pulmonary disease among Han nationality in Northern China. Chinese J Epidemiol 2005;26:907–10. [PubMed] [Google Scholar]
- [17].Tesfaigzi Y, Myers OB, Stidley CA, et al. Genotypes in matrix metalloproteinase 9 are a risk factor for COPD. Int J Chron Obstruct Pulmon Dis 2006;1:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Korytina GF, Akhmadishina LZ, Yanbaeva DG, et al. Polymorphism in promoter regions of matrix metalloproteinases (MMP1, MMP9, and MMP12) in chronic obstructive pulmonary disease patients. Genetika 2008;44:242–9. [PubMed] [Google Scholar]
- [19].Cheng SL, Yu CJ, Yang PC. Genetic polymorphisms of Cytochrome P450 and matrix metalloproteinase in chronic obstructive pulmonary disease. Biochem Genet 2009;47:591–601. [DOI] [PubMed] [Google Scholar]
- [20].Schirmer H, Basso DSL, Teixeira PJ, et al. Matrix metalloproteinase gene polymorphisms: lack of association with chronic obstructive pulmonary disease in a Brazilian population. Genet Mol Res 2009;8:1028–34. [DOI] [PubMed] [Google Scholar]
- [21].Korytina GF, Akhmadishina LZ, Viktorova EV, et al. Association of MMP3, MMP9, ADAM33, and TIMP3 polymorphisms with chronic obstructive pulmonary disease and its progression. Mol Biol 2012;46:438–49. [PubMed] [Google Scholar]
- [22].Stankovic M, Nikolic A, Nagorniobradovic L, et al. Gene–Gene interactions between glutathione S-transferase M1 and matrix metalloproteinases 1, 9, and 12 in chronic obstructive pulmonary disease in serbians. COPD 2017;14:581–9. [DOI] [PubMed] [Google Scholar]
- [23].Gilowska I, Kasper Ł, Bogacz K, et al. Impact of matrix metalloproteinase 9 on COPD development in Polish patients: genetic polymorphism, protein level, and their relationship with lung function. Biomed Res Int 2018;2018:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee S, Kim M, Kang H. Polymorphisms in matrix metalloproteinase-1, -9 and -12 genes and the risk of chronic obstructive pulmonary disease in a Korean population. Basic Sci Investig 2010;80:133–8. [DOI] [PubMed] [Google Scholar]
- [25].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [26].Demedts IK, Brusselle GG, Bracke KR, et al. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol 2005;5:257–63. [DOI] [PubMed] [Google Scholar]
- [27].Shipley JM, Doyle GA, Fliszar CJ, et al. The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. Role of the fibronectin type II-like repeats. J Biol Chem 1996;271:4335–41. [DOI] [PubMed] [Google Scholar]
- [28].Koh SA, Lee KH. HGF mediated upregulation of lipocalin 2 regulates MMP9 through nuclear factor-κB activation. Oncol Rep 2015;34:2179–87. [DOI] [PubMed] [Google Scholar]
- [29].Peppin GJ, Weiss SJ. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc Natl Acad Sci USA 1986;83:4322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vempati P, Karagiannis ED, Popel AS. A biochemical model of matrix metalloproteinase 9 activation and inhibition. J Biol Chem 2007;282:37585–96. [DOI] [PubMed] [Google Scholar]
- [31].Ni WJ, Ding HH, Zhou H, et al. Renoprotective effects of berberine through regulation of the MMPs/TIMPs system in streptozocin-induced diabetic nephropathy in rats. Eur J Pharmacol 2015;764:448–56. [DOI] [PubMed] [Google Scholar]
- [32].Pietruszewska W, Bojanowska-Poźniak K, Kobos J. Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: an immunohistochemical study. Otolaryngol Pol 2016;70:32–43. [DOI] [PubMed] [Google Scholar]
- [33].Busch R, Hobbs BD, Zhou J, et al. Genetic association and risk scores in a chronic obstructive pulmonary disease meta-analysis of 16,707 subjects. Am J Respir Cell Mol Biol 2017;57:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cho J, Lee C-H, Huang S-S, et al. Risk of acute exacerbations in chronic obstructive pulmonary disease associated with biomass smoke compared with tobacco smoke. BMC Pulm Med 2019;19:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aggarwal T, Wadhwa R, Rohil V, et al. Biomarkers of oxidative stress and protein-protein interaction in chronic obstructive pulmonary disease. Arch Physiol Biochem 2018;124:226–31. [DOI] [PubMed] [Google Scholar]
- [36].Xia S, Qu J, Jia H, et al. Overexpression of Forkhead box C1 attenuates oxidative stress, inflammation and apoptosis in chronic obstructive pulmonary disease. Life Sci 2019;216:75–84. [DOI] [PubMed] [Google Scholar]
- [37].Lei C, Tao W, Lian L, et al. Matrix metalloproteinase-9 -1562C/T promoter polymorphism confers risk for COPD: a meta-analysis. PLoS One 2013;8:e60523. [DOI] [PMC free article] [PubMed] [Google Scholar]


