Abstract
Objectives:
To evaluate the efficacy and safety of caudal dexmedetomidine in pediatric caudal anesthesia (CA).
Methods:
We searched PubMed, Embased, and Cochrane Library (from inception to June 2019) for eligible studies. The primary outcomes were the time to first analgesia, time of postoperative eye opening, intraoperative endtidal sevoflurane concentration, and postoperative sedation score. We calculated pooled risk ratios (RR) and 95% CIs using random- or fixed-effects models.
Results:
Thirteen trials involving 793 patients were found. Meta-analysis showed that the time to first rescue pain medication and the time from the end of anesthesia to eye opening in the CA+dexmedetomidine group were significantly longer than in the CA group (P < .00001). The intraoperative end-tidal sevoflurane concentration in the CA+dexmedetomidine group was significantly decreased (P < .00001). Dexmedetomidine appeared to increase the rate of bradycardia in the CA+dexmedetomidine group (P = .04). Additionally, the sedation score in the CA+ dexmedetomidine group was significantly higher at 2 hours after the operation compared with the CA group (P < .00001 at 2 hours).
Conclusions:
Caudally administered dexmedetomidine is a good alternative for prolonging postoperative analgesia with less pain, decreased intraoperative end-tidal sevoflurane concentration, and full postoperative sedation.
Keywords: dexmedetomidine, meta-analysis, pediatric caudal
1. Introduction
Caudal anesthesia (CA) is one of the most popular, reliable, and safe techniques in pediatric analgesia that can provide analgesia for a variety of infra- and supra-umbilical surgical procedures. The main disadvantage of a caudal epidural block is the short duration of action after a single injection.[1,2] The effectiveness of local anesthetics used in caudal blocks depends on the dose, volume, and concentration of the local anesthetic solution, but high-concentration local anesthetics can increase the incidence of motor weakness, delayed micturition, or urinary retention.[3] In children undergoing ambulatory surgery, such adverse effects can prolong the discharge time and might result in inadvertent admission.
Dexmedetomidine is a highly selective α2 agonist with sedative and analgesic properties. Dexmedetomidine possesses anxiolytic, sedative, sympatholytic, and analgesic properties without respiratory depressant effects.[4] In addition, dexmedetomidine has the ability to reduce both the anesthetic and opioid analgesic requirements during the perioperative period.[5] Our study aimed to investigate the effect of dexmedetomidine added to caudal anesthesia in children.
2. Methods
This systematic review was conducted according to the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA).[6] We prospectively registered our system review at PROSPERO. (Registration number: CRD42015025393). The proposed study will utilize published data, as such there is no requirement for ethical approval.
2.1. Data sources and search strategy
The PubMed, Embase, and Cochrane Library databases were searched from inception to June 2019 for relevant studies investigating the effect of dexmedetomidine in pediatric caudal anesthesia. The following search terms were used: (((“Anesthesia, Caudal” [Mesh] OR “Caudal Anesthesia” OR “Anesthesia, Sacral Epidural” OR “Epidural Anesthesia, Sacral” OR “Sacral Epidural Anesthesia”)) OR (“Anesthesia, Epidural” [Mesh] OR “Anesthesia, Peridural” OR “Peridural Anesthesia” OR “Anesthesia, Extradural” OR “Extradural Anesthesia” OR “Epidural Anesthesia”))) AND (“Dexmedetomidine”[Mesh] OR MPV-1440 OR “MPV 1440” OR “MPV1440” OR Precedex OR “Hospira Brand of Dexmedetomidine Hydrochloride” OR “Dexmedetomidine Hydrochloride” OR “Hydrochloride, Dexmedetomidine”). A manual search of the reference sections of included trials, published meta-analyses, and relevant review articles was conducted to identify additional articles. If duplicated data were presented in several studies, only the most recent, largest, or most complete study was included.
2.2. Study selection
Original studies included were based on PICOS (patient, intervention, comparison, outcome, and study design) as follows: P: American Society of Anesthesiologists (ASA) grade I/ II pediatric patient undergoing caudal anesthesia; I and C: caudal anesthesia with and without dexmedetomidine respectively; O: time to first rescue pain medication, time from the end of anesthesia to eye opening, end-tidal concentration of sevoflurane, sedation score and postoperative side effects; S: only randomized controlled trials (RCTs) were included. Only English-language studies were selected.
2.3. Data extraction
Patient characteristics (number of patients, ASA rating, age, gender, type of surgery, and anesthesia) and trial design (intervention, follow-up time, and reported outcomes) were recorded. If the data mentioned above were unavailable in the article, the corresponding authors were contacted for missing information. If the mean and variance in studies was not offered, we would have estimated it from the median, range, and the size of the sample based on Hozo's study.[7] All of the data were independently extracted using a standard data collection form by 2 reviewers (X-XW and H-JG), and then the collected data were checked and entered into Review Manager analysis software (RevMan) Version 5.3. All discrepancies were checked, and a consensus was reached by discussion with a third author (D-BP). A record of reasons for excluding studies was kept.
2.4. Assessment of study quality and risk of bias
A critical evaluation of the quality of the included studies was performed by 2 reviewers (X-XW and H-JG) by using a 5-point Jadad scale.[8] The main categories of the Jadad scale consist of the following 5 areas of evaluation: “was the study described as randomized? ”, “was the method used to generate the sequence of randomization described and appropriate (random numbers, computer-generated, etc.)? ”, “was the study described as double-blind?”, “was the method of double-blinding described and appropriate (identical placebo, active placebo, dummy, etc.)?”, and “was there a description of withdrawals and drop-outs?”. A score of 4 to 5 was considered high methodological quality.
Two reviewers (X-XW and H-JG) independently evaluated the risk of bias according to the recommendations from the Cochrane Collaboration.[9] The main categories consisted of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting and other bias. Each domain was assessed as “high risk,” “low risk,” or “unclear risk.” A designation of “low risk” was for items with sufficient and correct information and a designation of “high risk” was for incorrectly reported items. If the information of an item was insufficient or unsanctioned, it was designated as “unclear risk.” An “unclear risk” judgement was also made if the item was reported, but the risk of bias was unknown. The disagreement was solved by a senior reviewer (D-BP).
2.5. Statistical analysis
The weighted mean difference (WMD) with 95% CI was used as a common measure of the effect between 2 groups. The meta-analysis was conducted using Review Manager, version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK). Statistical heterogeneity across studies was usually investigated using the I2 statistic. When I2 values of less than 50% were determined, heterogeneity could be accepted, and the fixed-effects model was adopted. Otherwise, 1 analytical approach is to incorporate it into a random effects model. A random effects meta-analysis model involves an assumption that the effects being estimated in the different studies are not identical, but follow some distribution. We investigated the influence of a single study on the overall pooled estimate by omitting 1 study in each turn. Subgroup analysis was also conducted to investigate potential sources of between-study heterogeneity. Publication bias was assessed by funnel plot. A P value of < 0.05 was considered statistically significant.
2.6. Trial sequential analysis
In a meta-analysis, random errors increase the risk of type I error due to sparse data and repetitive testing of accumulating data. In a single trial, monitoring boundaries are used to examine how a single randomized trial could be terminated to avoid increasing the risk of type I error, because the outcome shows the anticipated effect, or for futility. Similarly, trial sequential monitoring boundaries are applied for a meta-analysis. This method of meta-analysis is called trial sequential analysis (TSA). It can determine whether the evidence in a meta-analysis is reliable and conclusive. If the cumulative Z-curve crosses the trial sequential monitoring boundary, no further trials are needed to reach the anticipated effect. Otherwise, the conclusion is insufficient and a larger dataset is required.
We assessed the required information size adjusted for diversity because the heterogeneity adjustment with I2 might undervalue the required information size. The TSA was performed to maintain an overall 5% risk of a type I error (α = 0.05) and 20% risk of a type II error (a power of 80%). We used software Trial Sequential Analysis (version 0.9) and provide the 95% confidence intervals adjusted for sparse data or repetitive testing.[10–12]
3. Results
3.1. Identification of eligible studies
In total, 211 potentially relevant abstracts were identified. After duplicates were removed, 191 unique abstracts remained. After reviewing the abstracts, 17 publications seemed to meet the inclusion criteria. Of these, 5 were excluded for the following reasons: unpublished studies,[13] no available data on the outcome of interest in references,[14,15] non-English language,[16] and caudal anesthesia with lidocaine.[17] Finally, the remaining 12 studies[2,3,18–27] with existing data met our selection criteria and were included in the systematic review. Data of 5 studies,[18,20,21,24,25] which mainly involved in sedation score, was estimated from the median, range, and the size of the sample. A flow diagram of the search strategy and study selection is shown in Figure 1.
Figure 1.
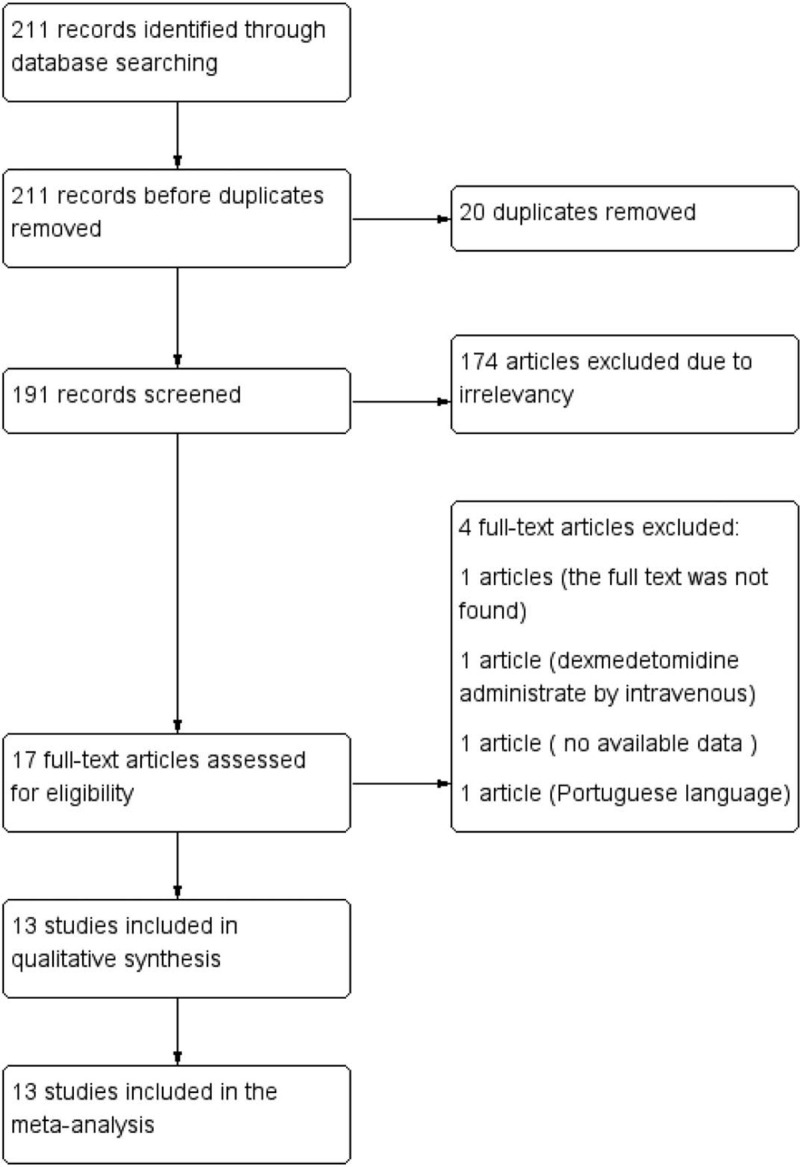
Flow diagram of the search strategy and study selection.
3.2. Study characteristics
The characteristics of all the included studies are shown in Table 1. All subjects were pediatric patients undergoing caudal anesthesia. The quality of the included studies was evaluated by a Jadad score. There are 9 high-quality studies (a score of 4 or 5), and the mean score was 4 (from 2 to 5). A quality assessment of the 12 RCTs is presented. The baseline characteristics of patients were reported in all trials, and 9 trials mentioned the method of random selection (Fig. 2).
Table 1.
The characteristics of all included studies.
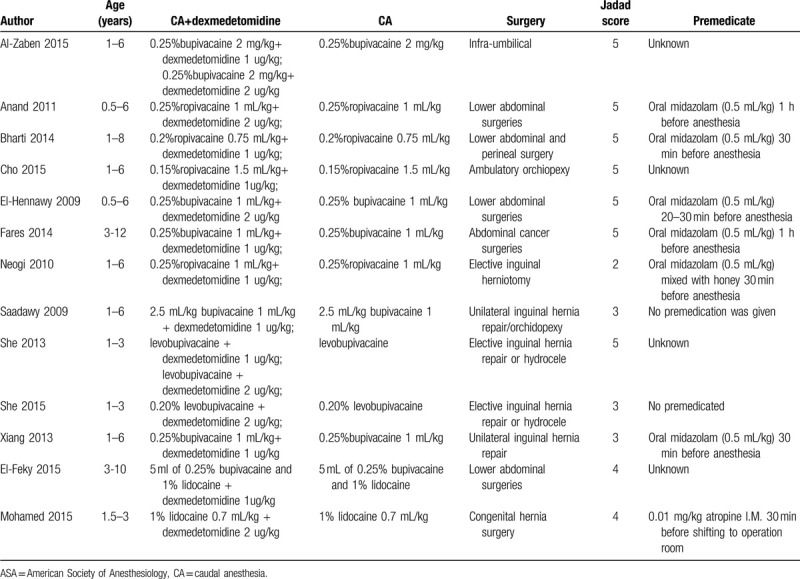
Figure 2.
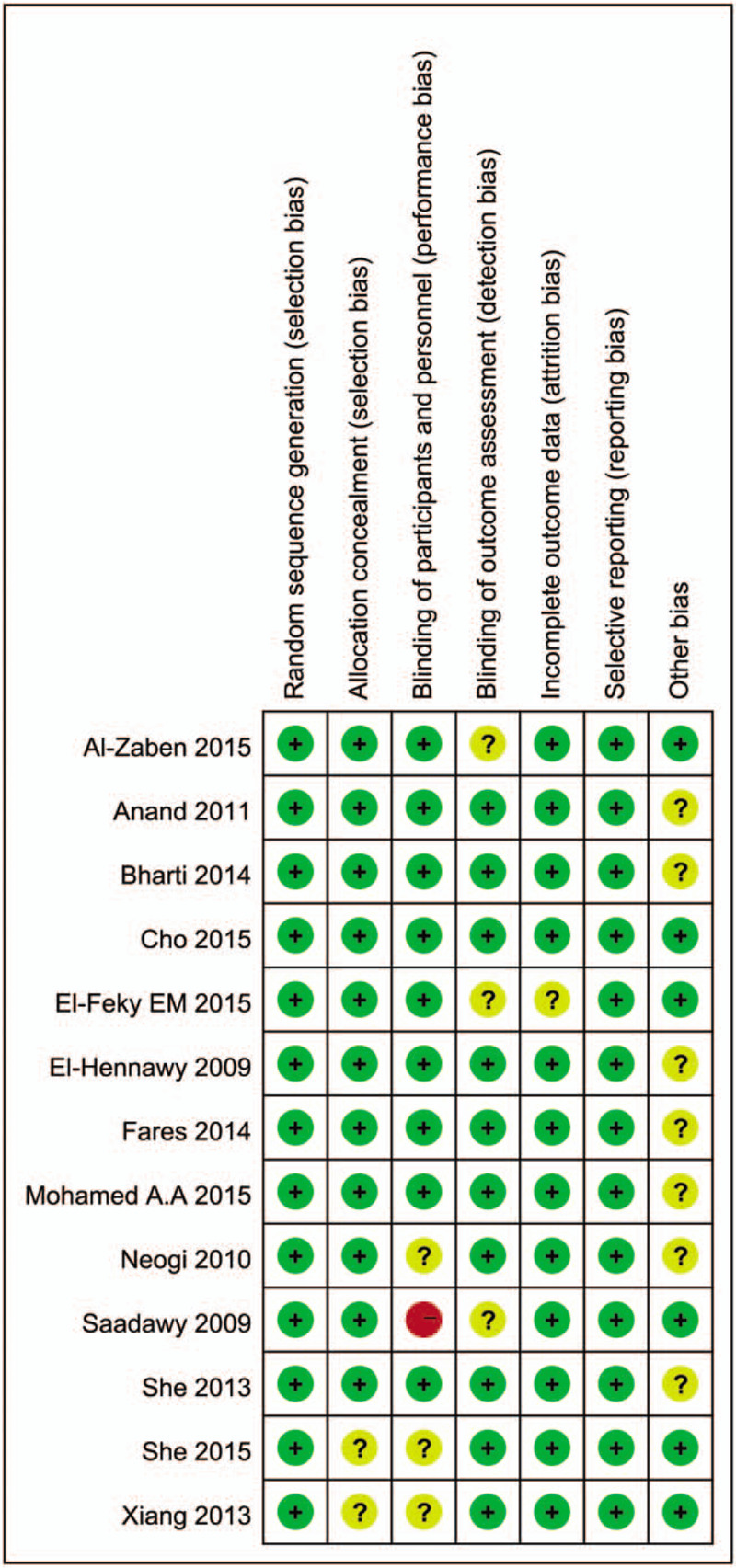
Summary of review authors’ assessments of risk of bias for each Cochrane item and each included study.
These studies were published between 2009 and 2015. The sample size of the included studies ranged from 20 to 70. All were randomized controlled trials, and the primary end points were time to first rescue pain medication, time to eye opening from the end of anesthesia, end-tidal concentration of sevoflurane, sedation score. All the others involved bupivacaine or ropivacaine.
3.3. Meta-analyses of primary outcomes
3.3.1. Time to first rescue pain medication
The aggregated results were studied in 11 trials[2,3,18–24,26,27] and shown in Figure 3. The results suggest that the time to first rescue pain medication in CA+dexmedetomidine group was significantly longer than in the CA group (WMD = 7.32, 95% CI: 5.74–8.91, P < .00001). Heterogeneity was noted among the studies (I2 = 99%; P < .00001), and a random effect model was selected. There were 2 different doses of dexmedetomidine in these trials (1 μg/kg and 2 μg/kg). We performed a subgroup analysis according to these 2 doses, and found that the time to first rescue pain medication in the 1 μg/kg dexmedetomidine group and in the 2 μg/kg dexmedetomidine group were both significantly longer compared with that for the CA group [WMD: 7.06, 95% CI: 5.33–8.79, P < .00001 for 1 μg/kg dexmedetomidine, and WMD: 7.72, 95% CI: 2.94–12.51, P = .002 for 2 μg/kg dexmedetomidine]. Heterogeneity was the same in the subgroup (I2 = 99%; P < .00001), and a random effect model was selected. In addition, a funnel plot was used to assess publication bias; the result suggested that publication bias probably had some effect on summary estimates (Fig. 4). TSA showed that cumulative Z-curves surpassed the futility boundary and crossed the trial sequential monitoring boundary (Fig. 5). The TSA adjusted 95% CI was 5.74–8.91 hours.
Figure 3.
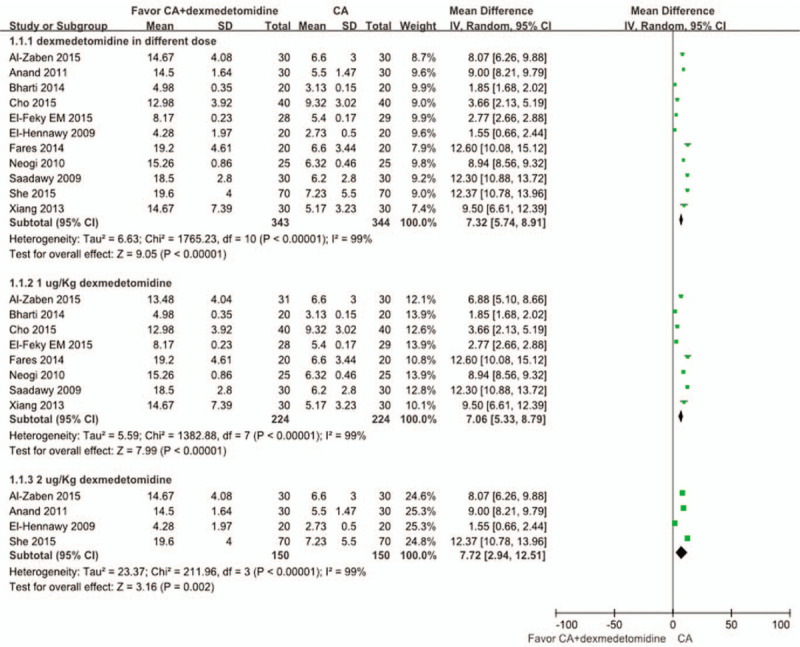
Meta-analysis of the net change in time to first rescue pain medication associated with dexmedetomidine intervention. A subgroup analysis was performed based on different doses of dexmedetomidine. The area of each square is proportional to the inverse of the variance of the WMD. Diamonds represent pooled estimates from inverse variance (IV) weighted random effect model. WMD = weighted mean difference.
Figure 4.
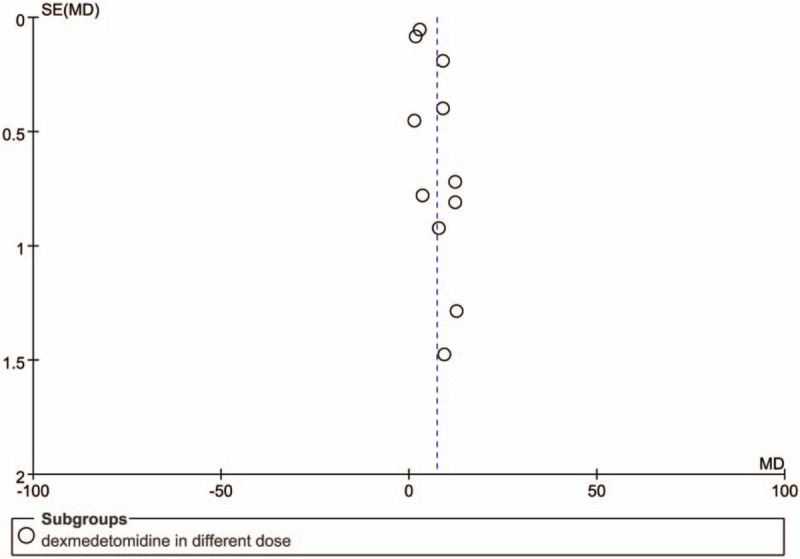
The funnel plot of the time to first rescue pain medication associated with dexmedetomidine intervention. Each circle represents a single study. We could assess the publication bias of each study by observing whether the round deviated from the central dotted line.
Figure 5.
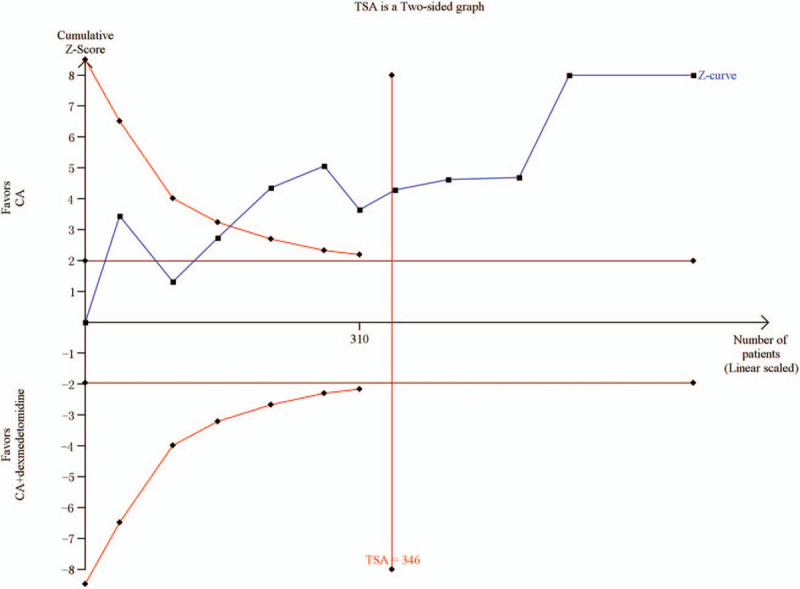
Trial sequential analysis of the time to first rescue pain medication associated with dexmedetomidine intervention.
3.3.2. Time to eye opening from the end of anesthesia
Six studies[18–20,24,26,27] with a total of 420 children reported time to eye opening from the end of anesthesia. Compared with the CA group, the time to eye opening from the end of anesthesia was significantly longer in the CA+dexmedetomidine group (WMD: 17.18, 95% CI: 12.58–21.77, P < .00001). Heterogeneity was noted among the studies (I2 = 99%; P < .00001), and a random effect model was selected (Fig. 6). Among 6 studies, four[16,18,23,25] reported the time of postoperative spontaneous eye opening. Meta-analysis showed that the time of postoperative spontaneous eye opening between the 2 groups was significantly different, and it was longer in the CA+dexmedetomidine group (WMD: 32.44, 95% CI: 21.01–42.86, P < .00001). TSA showed that cumulative Z-curves surpassed the futility boundary and crossed the trial sequential monitoring boundary (Fig. 7). The TSA adjusted 95% CI was 2.25 to 2.85 hours.
Figure 6.
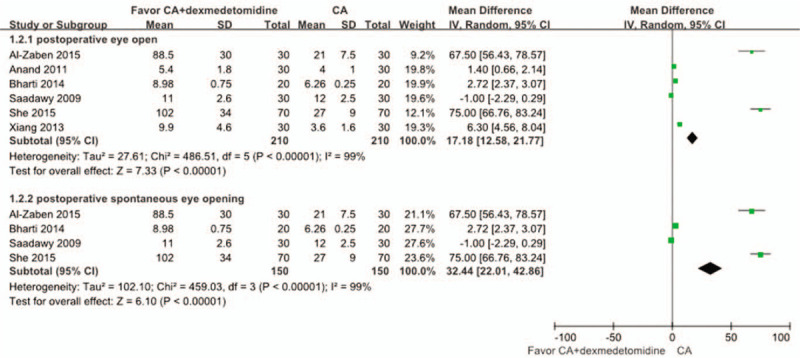
Meta-analysis of the net change in time to eye opening from the end of anesthesia associated with dexmedetomidine intervention. A subgroup analysis was performed. The area of each square is proportional to the inverse of the variance of the WMD. Diamonds represent pooled estimates from inverse variance (IV) weighted random effect model.
Figure 7.
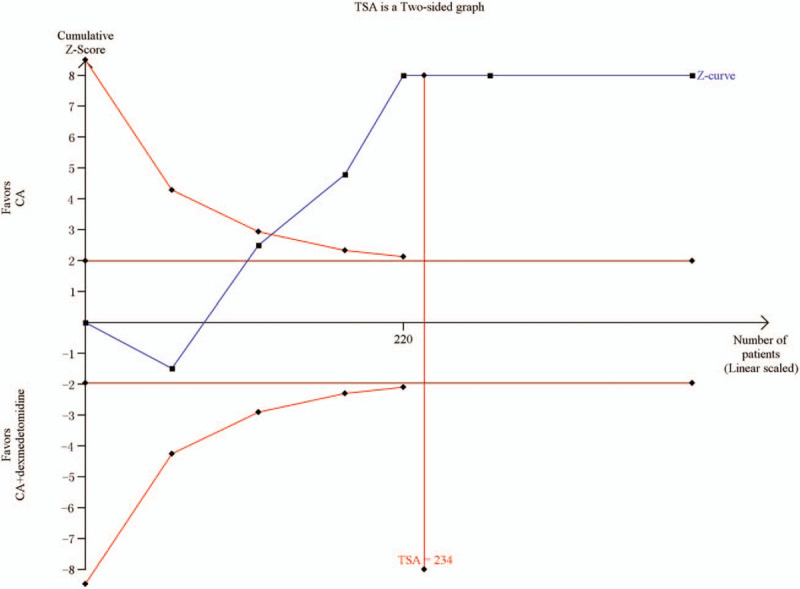
Trial sequential analysis of time to eye opening from the end of anesthesia.
3.4. The intraoperative end-tidal sevoflurane concentration
Four of the studies[2,3,18,24] reported the intraoperative endtidal sevoflurane concentration for pediatric patients in the CA+dexmedetomidine group and in the CA group. One study[3] did not supply the available data, but stated that the end-tidal sevoflurane concentration was significantly lower in the CA+dexmedetomidine group compared with the CA group during anesthesia (P < .05). The remaining 3 studies were examined by meta-analysis, and we also found that the end-tidal sevoflurane concentration in the CA+dexmedetomidine group was significantly lower compared with the CA group (WMD: −0.71, 95% CI: −0.91 to −0.50, P < .00001). Heterogeneity was noted among the studies (I2 = 72%; P = .03), and a random effect model was selected (Fig. 8).
Figure 8.
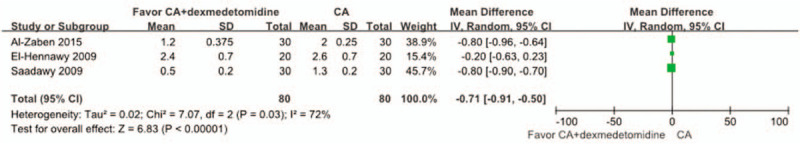
Meta-analysis of the net change in intraoperative end-tidal sevoflurane concentration associated with dexmedetomidine intervention. The area of each square is proportional to the inverse of the variance of the WMD. Diamonds represent pooled estimates from inverse variance (IV) weighted random effect model.
3.5. Sedation score in 2 hours of postoperative
Three studies[21,24,25] compared the sedation score between 2 groups after 2 hours of the postoperation. The sedation score in the CA+dexmedetomidine group was significantly higher at 2 hours after the operation compared with the CA group: WMD: 1.30, 95% CI: 0.82 to 1.78, P < .00001 at 2 hours (Fig. 9).
Figure 9.
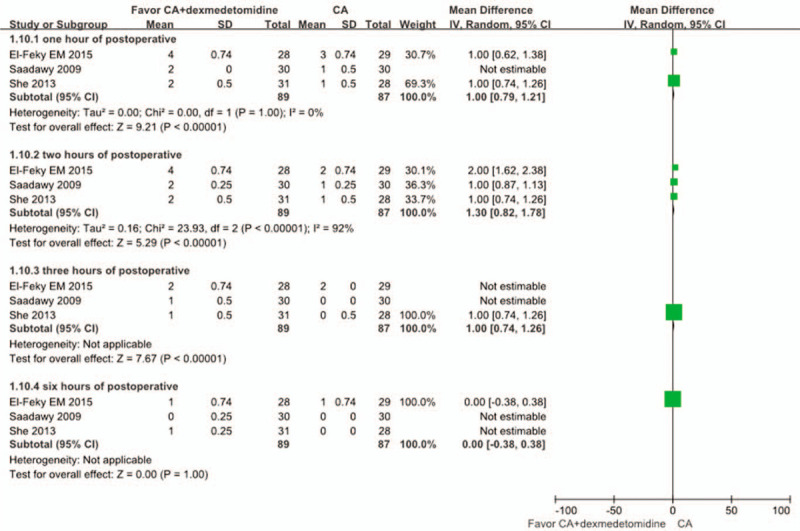
Meta-analysis of the net change in the postoperative sedation score at 2 hours of postoperative associated with dexmedetomidine intervention. The area of each square is proportional to the inverse of the variance of the WMD. Diamonds represent pooled estimates from inverse variance (IV) weighted random effect model.
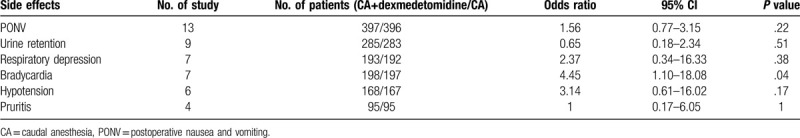
3.6. Adverse events
There were 12 trials[2,3,18–27] that reported the number of pediatric patients who experienced various side effects after caudal anesthesia (Table 2). The most common side effect was postoperative nausea and vomiting (PONV). Twelve trials[2,3,18–27] reported the incidence of PONV and showed no significant difference between the CA+ dexmedetomidine group and the CA group (OR: 1.56; 95% CI: 0.73–3.32; P = .25). Bradycardia was assessed in 7 trials[18–21,24,25,27] and the rate of bradycardia was significantly higher in the CA+ dexmedetomidine group than in the CA group (OR: 4.45; 95% CI: 1.10–18.08; P = .04). Others side effects, such as respiratory depression,[17,18,20–24] urinary retention,[2,18–20,23–27] hypotension,[18–21,25,27] and pruritis,[2,19,20,23] were not significantly different between the CA+ dexmedetomidine group and CA group (Table 2).
Table 2.
Side effects of included studies.
4. Discussion
This study is an additional meta-analysis that evaluates the effect of dexmedetomidine in pediatric caudal anesthesia. Compared with a previous meta-analysis,[28] the present meta-analysis included an additional 6 recent RCTs.[3,18,20–22,26] Our meta-analysis showed that dexmedetomidine can significantly prolong the time to first rescue pain medication, which is in line with the results of previous meta-analysis. In addition, dexmedetomidine can decrease the intraoperative end-tidal sevoflurane concentration and prolong the time to eye opening from the end of anesthesia. Trial sequential analysis provided firm evidence of prolonged first analgesia and postoperative eye opening associated with caudal anesthesia plus dexmedetomidine compared with caudal anesthesia without dexmedetomidine.
In our study, we identified heterogeneity in all results, which might be due to factors such as age, region, race, and other patient characteristics. After omitting 1 study at a time to determine the influence of a single study on the overall pooled estimate, heterogeneity remained. We performed subgroup analyses to locate the source of heterogeneity, but the heterogeneity persisted regardless of changes made. Factors influencing heterogeneity might be associated with age, gender, race, perioperative situation, sample size of a single study, and so on.
Our meta-analysis expands on an earlier meta-analysis to provide a better characterization of the evidence for dexmedetomidine use in caudal anesthesia. First, in our analysis, we included more studies with larger sample sizes than in the previous analysis, giving greater power to evaluate the effect of dexmedetomidine in pediatric caudal anesthesia. Second, we evaluated the time to eye opening from the end of anesthesia, the intraoperative end-tidal sevoflurane concentration, and the postoperative sedation score, which were not shown in the previous meta-analysis. In addition, the rate of bradycardia was found to be significantly higher in the CA+ dexmedetomidine group compared with the CA group in our meta-analysis, which was inconsistent with the results of previous meta-analysis. Finally, we applied trial sequential analysis to identify whether the outcomes reach a conclusive conclusion. To our knowledge, this is the first time that trial sequential analysis has been applied for caudal dexmedetomidine.
Nowadays, the use of opioids as additives has decreased from 36% to 18% due to the higher incidence of side-effects such as nausea and vomiting, itching, and respiratory depression especially in children.[29,30] A wide range of additives has been used in combination with local anesthetics to promote analgesia,[15,31] which was contained dexmedetomidine. The effect of dexmedetomidine is due to local vasoconstriction and, increased potassium conductance in Aδ and C fibers. Dexmedetomidine enters the central nervous system either via systemic absorption or by diffusion into the cerebrospinal fluid and reaches α2 receptors in the superficial laminae of the spinal cord and brainstem or indirectly activates spinal cholinergic neurons.[2,24,32,33] As shown in our studies, dexmedetomidine can significantly prolong the time to first analgesia, which is the primary analgesic effect of dexmedetomidine.
Dexmedetomidine has unique sedative properties caused by hyperpolarization of excitable cells in the locus coeruleus.[34] Dexmedetomidine produces a unique form of sedation, in which patients become responsive as well as calm and cooperative when aroused, and then back to sleep when not stimulated. A certain degree of sedation after pediatric surgery might be a desired effect for parents. A calm and sedated child during the early postoperative period could decrease the parent's anxiety.[24,35] The duration of sedation was prolonged in the CA+ dexmedetomidine group compared with the CA group in our meta-analysis.
Dexmedetomidine was also associated with a significant decrease in the end-tidal sevoflurane requirements compared with the CA group. The sedative and analgesic effects of dexmedetomidine probably account for the anesthesia-sparing effect.
Bradycardia is considered to be a prominent adverse effect of α2-adrenoreceptor agonists, and is caused by both an increase in vagal tone resulting from central stimulation of parasympathetic outflow, and reduced sympathetic drive.[36] In our meta-analysis, we found that the rate of bradycardia in the CA+ dexmedetomidine group was significantly higher compared with that of the CA group. Other adverse effects between the 2 groups were not statistically significant. The heart rate becomes slower within a certain range with increasing age. The age composition of the studies included in our meta-analysis was not known, and this might have caused bias. In addition, premedication can cause changes in the heart rate, and the use of premedication was different among the included studies (Table 1). Although these 2 phenomena might have influenced the results, further studies are required to confirm this.
In our meta-analysis, the patients enrolled were relatively homogeneous. Nine studies had Jadad scores of ≥ 4 and were of high quality. The participants in all studies were well matched (e.g., sex, age, ASA grade, administration time, method of surgery/anesthesia, etc.). However, the results in this current meta-analysis should be interpreted with careful consideration given the limitations inherent to the design of the study. First, some of the major outcomes had small sample sizes, which might result in a small-study effect. Second, this meta-analysis included 3 types of local anesthetics which might have influenced the postoperative analgesic effects and adverse events. Third, this meta-analysis was based on studies published in English, which might have generated bias. Fourth, the dose of caudal dexmedetomidine applied among the studies was inconsistent and the missing outcome data in 3 studies[19,20,26] was not described. Five, the data in some studies was shown in median and range about the time measurement, which may lead to skew. Final, we selected published studies, and many studies were not registered in clinical trial databases.
Nonetheless, our study provides useful evidence for future studies of dexmedetomidine in pediatric caudal anesthesia. With an increased sample size, the present study found that the rate of bradycardia in the CA+dexmedetomidine group was significantly higher compared with the CA group, and this finding was not present in the previous meta-analysis. Therefore, attention should continue to focus on the adverse events associated with dexmedetomidine use in pediatric caudal anesthesia. Moreover, the optimal dose of caudal dexmedetomidine is not known. Further studies are recommended to assess the effect of different doses of caudal dexmedetomidine as an adjunct to caudal epidural block in the pediatric population.
5. Conclusions
In conclusion, caudally administered dexmedetomidine is a good alternative for the prolongation of postoperative analgesia with less pain, decreased intraoperative end-tidal sevoflurane concentration, and full sedation postoperatively. However, some of the results in our meta-analysis should be interpreted carefully because of the clinical heterogeneity and insufficient data.
Author contributions
XXW and DBP conceived the study, participated in the study design, collected the data, and drafted the manuscript. XXW and HJG participated in the study design, collected the data, performed the statistical analysis and contributed to drafting the manuscript. JD, LD, AGZ and HJG helped to perform the statistical analysis and revised the manuscript critically to ensure all important intellectual content was present. All authors read and approved the final manuscript.
Footnotes
Abbreviations: ASA = American Society of Anesthesiology, CA = caudal anesthesia, PICOS = patient, intervention, comparison, outcome, and study design, PONV = postoperative nausea and vomiting, RCTs = randomized controlled trials, RR = risk ratios, WMD = weighted mean difference.
How to cite this article: Wang Xx, Dai J, Dai L, Guo Hj, Zhou Ag, Pan Db. Caudal dexmedetomidine in pediatric caudal anesthesia: A systematic-review and meta-analysis of randomized controlled trials. Medicine. 2020;99:31(e21397).
No additional unpublished data are available.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Lloyd-Thomas AR. Pain management in paediatric patients. Br J Anaesth 1990;64:85–104. [DOI] [PubMed] [Google Scholar]
- [2].El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, et al. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth 2009;103:268–74. [DOI] [PubMed] [Google Scholar]
- [3].Cho JE, Kim JY, Park SJ, et al. The effect of 1 (g/kg dexmedetomidine combined with high-volume/low-concentration caudal ropivacaine in children undergoing ambulatory orchiopexy. Biol Pharm Bull 2015;38:1020–5. [DOI] [PubMed] [Google Scholar]
- [4].Hall JE, Uhrich TD, Barney JA, et al. Sedative, amnestic and analgesic properties of small-dose Dex infusions. Anesth Analg 2000;90:699–705. [DOI] [PubMed] [Google Scholar]
- [5].Gmbet A, Basagan-Mogol E, Turker G, et al. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anesth 2006;53:646–52. [DOI] [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [7].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a simple. BMC Med Res Methodol 2015;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [9].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brok J, Thorlund K, Gluud C, et al. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008;61:763–9. [DOI] [PubMed] [Google Scholar]
- [11].Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- [12].Li S, Zeng XT, Ruan XL, et al. Holmium laser enucleation versus transurethral resection in patients with benign prostate hyperplasia: an updated systematic review with meta-analysis and trial sequential analysis. PLoS One 2014;9:e101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cho JE, Kil HK. Effect of caudal dexmedetomidine with 0.15% ropivacaine on sevoflurane requirement, emergence agitation, and postoperative analgesia in children undergoing ambulatory surgery. Region Anesth Pain M. Conference: 31st Annual European Society of Regional Anaeathesial, ESRA Congress 2012 Bordeaux France. [Google Scholar]
- [14].Asaad OM, Hafez M, Mohamed MY, et al. Comparative study between prophylactic single dose of fentanyl and dexmedetomidine in the management of agitation after sevoflurane anesthesia in children. Egyptian J Anaesth 2011;27:31–7. [Google Scholar]
- [15].El Shamaa HA, Ibrahim M. A comparative study of the effect of caudal dexmedetomidine versus morphine added to bupivacaine in pediatric infra-umbilical surgery. Saudi J Anaesth 2014;8:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Salgado PFS, Sabbag AT, Da Silva PC, et al. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anesthesia. Rev Associacao Med Brasileira 2008;54:110–5. [DOI] [PubMed] [Google Scholar]
- [17].Mohamed AA. Prevention of sevoflurane agitation in children undergoing congenital hernia repair, impact of adding dexmedetomidine to caudal analgesia. Egyptian J Anaesth 2015;31:227–31. [Google Scholar]
- [18].Al-Zaben KR, Qudaisat IY, Abu-Halaweh SA, et al. Comparison of caudal bupivacaine alone with bupivacaine plus two doses of dexmedetomidine for postoperative analgesia in pediatric patients undergoing infra-umbilical surgery: a randomized controlled double-blinded study. Paediatr Anaesth 2015;25:883–90. [DOI] [PubMed] [Google Scholar]
- [19].Anand VG, Kannan M, Thavamani A, et al. Effects of dexmedetomidine added to caudal ropivacaine in paediatric lower abdominal surgeries. Indian J Anaesth 2011;55:340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bharti N, Praveen R, Bala I. A dose–response study of caudal dexmedetomidine with ropivacaine in pediatric day care patients undergoing lower abdominal and perineal surgeries: a randomized controlled trial. Paediatr Anaesth 2014;24:1158–63. [DOI] [PubMed] [Google Scholar]
- [21].El-Feky EM, Abd El Aziz AA. Fentanyl, dexmedetomidine, dexamethasone as adjuvant to local anesthetics in caudal analgesia in pediatrics: a comparative study. Egyptian J Anaesth 2015;31:175–80. [Google Scholar]
- [22].Fares KM, Othman AH, Alieldin NH. Efficacy and safety of dexmedetomidine added to caudal bupivacaine in pediatric major abdominal cancer surgery. Pain Physician 2014;17:393–400. [PubMed] [Google Scholar]
- [23].Neogi M, Bhattacharjee DP, Dawn S, et al. A comparative study between clonidine and dexmedetomidine used as adjuncts to ropivacaine for caudal analgesia in paediatric patients. J Anaesth Clin Pharmacol 2010;26:149–53. [Google Scholar]
- [24].Saadawy I, Boker A, Elshahawy MA. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand 2009;53:251–6. [DOI] [PubMed] [Google Scholar]
- [25].She YJ, Zhang ZY, Song XR. Caudal dexmedetomidine decreases the required concentration of levobupivacaine for caudal block in pediatric patients: a randomized trial. Paediatr Anaesth 2013;23:1205–12. [DOI] [PubMed] [Google Scholar]
- [26].She YJ, Xie GT, Tan YH, et al. A prospective study comparing the onset and analgesic efficacy of different concentrations of levobupivacaine with/without dexmedetomidine in young children undergoing caudal blockade. J Clin Anesth 2015;27:17–22. [DOI] [PubMed] [Google Scholar]
- [27].Xiang Q, Huang DY, Zhao YL, et al. Caudal dexmedetomidine combined with bupivacaine inhibit the response to hernial sac traction in children undergoing inguinal hernia repair. Br J Anaesth 2013;110:420–4. [DOI] [PubMed] [Google Scholar]
- [28].Tong Y, Ren H, Ding X, et al. Analgesic effect and adverse events of dexmedetomidine as additive for pediatric caudal anesthesia: a meta-analysis. Paediatr Anaesth 2014;24:1224–30. [DOI] [PubMed] [Google Scholar]
- [29].Lönnqvist PA, Ivani G, Moriarty T. Use of caudal-epidural opioids in children: Still state of the art or the beginning of the end? Paediatr Anaesth 2002;12:747–9. [DOI] [PubMed] [Google Scholar]
- [30].Lönnqvist PA. Adjuncts to caudal block in children — Quo vadis? Br J Anaesth 2005;95:431–3. [DOI] [PubMed] [Google Scholar]
- [31].Peutrell JM, Mather SJ. Regional Anesthesia for Babies and Children. 1997;Oxford: Oxford University Press, 187–233. [Google Scholar]
- [32].Gertler R, Brown HC, Mitchell DH, et al. Dexmedetomidine: a novel sedative–analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Konakci S, Adanir T, Yilmaz G, et al. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol 2008;25:403–9. [DOI] [PubMed] [Google Scholar]
- [34].Berridge CW, Waterhouse BD. The locus coeruleus-nora-drenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 2003;42:33–84. [DOI] [PubMed] [Google Scholar]
- [35].Lerman J. Anxiolysis – by the parent or for the parent? Anesthesiology 2000;92:939–46. [DOI] [PubMed] [Google Scholar]
- [36].Talke P, Chen R, Thomas B, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg 2000;90:834–9. [DOI] [PubMed] [Google Scholar]


