Abstract
Background
Panax notoginseng saponins (PNS) are bioactive substances extracted from P. notoginseng that are widely used to treat cardiovascular and cerebrovascular diseases and interstitial diseases. PNS have the functions of scavenging free radicals, anti-inflammation, improving blood supply for tissue and so on.
Objectives
The aim of this study was to investigate the effects of PNS on the oxidative stress of immune cells induced by porcine circovirus 2 (PCV2) infection in vitro and in vivo.
Methods
Using an oxidative stress model of PCV2 infection in a porcine lung cell line (3D4/2 cells) and mice, the levels of nitric oxide (NO), reactive oxygen species (ROS), total glutathione (T-GSH), reduced glutathione (GSH), and oxidized glutathione (GSSG) and the activities of xanthine oxidase (XOD), myeloperoxidase (MPO) and inducible nitric oxide synthetase (iNOS) were determined to evaluate the regulatory effects of PNS on oxidative stress.
Results
PNS treatment significantly reduced the levels of NO and ROS, the content of GSSG and the activities of XOD, MPO, and iNOS (p < 0.05), while significantly increasing GSH and the ratio of GSH/GSSG in infected 3D4/2 cells (p < 0.05).Similarly, in the in vivo study, PNS treatment significantly decreased the level of ROS in spleen lymphocytes of infected mice (p < 0.05), increased the levels of GSH and T-GSH (p < 0.05), significantly decreased the GSSG level (p < 0.05), and decreased the activities of XOD, MPO, and iNOS.
Conclusions
PNS could regulate the oxidative stress of immune cells induced by PCV2 infection in vitro and in vivo.
Keywords: Panax notoginseng, saponin, porcine circovirus 2, oxidative stress
INTRODUCTION
Porcine circovirus 2 (PCV2) has been identified as a main pathogen causing postweaning multisystemic wasting syndrome, which has been traced back to 1969 and is clinically characterized by progressive emaciation, respiratory distress and jaundice, causing enormous economic loss in the pig industry worldwide [1,2,3,4]. Vaccine immunization is a routine way to prevent PCV2 infection, but the efficacy of vaccine protection is limited because of the emergence of new viral strains.
As a second messenger, reactive oxygen species (ROS) play a vital role in many intracellular signaling cascades aimed at maintaining the intracellular environmental balance. In cells, the major mechanisms of ROS scavenging include glutathione (GSH), superoxide dismutase (SOD), ascorbate peroxidase and catalase. However, oxidative stress arises when excessive ROS are not effectively eliminated by antioxidants [5]. The imbalance between oxidation and antioxidation causes damage to many important biological molecules, cells and organs, leading to various diseases [6,7]. Host phagocytes produced ROS after infection with the Sendai virus and the level of GSH in mouse lungs decreased after influenza virus infection, so oxidative stress is associated with viral infection [8]. It has been reported that many viral infections are accompanied by intracellular redox state changes [9], and the ROS levels in porcine kidney 15 (PK-15) cells increased after infection with PCV2; in addition, the increase in ROS promotes PCV2 replication [10].
For a long time, antioxidants extracted from natural plants for food and medicine have been a hot topic, and the antioxidant mechanisms of traditional Chinese medicine are an interesting area. Panax notoginseng is a traditional Chinese medicinal herb used for promoting blood circulation and is mainly distributed in Southwest China [11]. P. notoginseng saponins (PNS) includes a variety of saponin monomers, such as Rb1, Rg1, Re, Rd, and R1, which have the functions of improving cell energy metabolism, scavenging free radicals and antioxidant stress, regulating apoptotic signaling pathways, anti-inflammation, balancing ion metabolism, improving blood supply for tissue and so on. In the clinic, P. notoginseng and Salvia miltiorrhiza are usually combined to treat and prevent cardiovascular diseases, which may be based on the complementation of antioxidant mechanisms: the extract of P. notoginseng exhibits high ferrous ion chelating activity and strong scavenging activities for hydrogen peroxide (H2O2) and hydroxyl radicals, while the extract of S. miltiorrhiza possesses powerful reducing ability and high scavenging activities of hydroxyl, superoxide anion and DPPH radicals [12]. In addition, antioxidant compounds have been proposed for neuroprotection because of improved endogenous antioxidant defenses. It was reported that PNS presented neuroprotection on H2O2-induced brain cells by reducing ROS accumulation in vitro [13]. In addition, PNS can attenuate platelet aggregation through the peroxisome proliferator-activated receptor-γ pathway [14]. These data suggest that PNS is an effective antioxidant.
In our previous studies, oxidative stress models induced by PCV2 infection in RAW264.7 cells, 3D4/2 cells and primary porcine splenic lymphocytes were established. In the present study, we aimed to investigate the regulatory effects of PNS on the oxidative stress induced by PCV2 in 3D4/2 cells and mice.
MATERIALS AND METHODS
Materials
Dichlorodihydrofluorescein diacetate (DCFH-DA) was purchased from Applygen, China. Commercial test kits for xanthine oxidase (XOD), myeloperoxidase (MPO), and inducible nitric oxide synthetase (iNOS) were purchased from Nang Jingcheng Bioengineering Institute, China. Enhanced chemiluminescence equipment was obtained from Millipore, USA. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), o-phthalaldehyde (OPA), GSH, oxidized glutathione (GSSG), naphthalene ethylene diamine and p-aminobenzene sulfonic acid were of analytical grade, and standards Rb1, Rg1, Re, Rd, and R1 were purchased from the China National Institute for the Control of Pharmaceutical and Biological Products.
Preparation of PNS
P. notoginseng was produced in Yunnan Province and was purchased from the Nan Ning Xiang Dai chemist shop of the Guangxi Zhuang Autonomous Region in China. PNS was prepared using ethanol extracted and purified by macroporous adsorption resin, and the content was measured by high-performance liquid chromatography (Waters Alliance e2695; Waters, USA). Briefly, the powder of P. notoginseng was soaked in 70% ethanol for 1 h, extracted by the reflux method for 2 h, and then the extract was filtered. The residue of the filter was extracted again for 1.5 h, the filtrates were mixed and concentrated by vacuum rotary evaporation, and the concentrate was freeze-dried to a powder. The powder was then dissolved in methanol and immersed in a D-101 resin layer, and the eluate was collected until an adsorption equilibrium was reached. Then, the column was washed with distilled water, and the Molish reaction was used to judge the end point of washing. Furthermore, elution was carried out with 80% ethanol, and the eluate was collected. The eluent was concentrated by a rotary evaporator, and the refined PNS was obtained by freeze-drying. The purity of PNS was measured by HPLC according to the Pharmacopoeia of the People's Republic of China [15], using standards Rb1, Rg1, Re, Rd, and R1 as controls.
Cells and viruses
PK-15 cells and 3D4/2 cells were provided by the Laboratory of Animal Infectious Disease at the College of Animal Science and Technology at Guangxi University.PK-15 cells were cultured in Dulbecco's Modified Eagle Medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), penicillin (100 IU/mL), and streptomycin (100 mg/mL) at 37°C in an atmosphere of 5% CO2. 3D4/2 cells were seeded in RPMI-1640 medium (Gibco) supplemented with 10% FBS (Gibco), penicillin (100 IU/mL), and streptomycin (100 mg/mL) at 37°C in an atmosphere of 5% CO2.
PCV2 was provided by the Key Laboratory of Animal Diseases Diagnostic and Immunology of Ministry of Agriculture at Nanjing Agricultural University and amplified using PK-15 cells. The viral titer was determined to be 104TCID50/0.1 mL using the Reed-Muench assay.
PCV2 infection of 3D4/2 cells and treatment
3D4/2 cells with a density of 1 × 105 cells/mL were seeded in 24- or 96-well plates and cultured for 2 h. Then, the supernatant was removed, and the cells were washed three times with 0.1 mol/L phosphate-buffered saline (PBS). Except for the negative control, the cells were adsorbed with PCV2 at a dilution of 10−1 for 2 h, then the inoculum was removed, and the cells were washed three times with 0.1 mol/L PBS. The infected cells were grown in complete RPMI-1640 medium supplemented with100 µg/mL, 200 µg/mL, or 400 µg/mL PNS and 200 µM vitamin C (VC), while the negative and PCV2-infected control cells were cultured in complete RPMI-1640 medium.
Animals and treatment
Kunming inbred mice of either sex weighing 20 ± 2 g were purchased from the Experimental Animal Center of Guangxi Medical University (animal study approval number: SCXK Gui 2014-0003).The protocol and procedures employed were ethically reviewed and approved by the ethical committee of Guangxi University. The experiments were performed in accordance with Guangxi University guidelines and regulations for the care and use of laboratory animals. Mice were housed in an environment of 22 ± 2°C, given adequate feed and water ad libitum for 5 days before experimentation. Mice were randomly divided into seven groups with 12 mice per group according to Table 1. Mice were administered 1 mL of physiological saline (PS, 0.9% sodium chloride solution) or PCV2 for three days via intragastric, intranasal dropping or intraperitoneal injection. From the fourth day to the sixth day, the mice were administered PS, VC, or PNS by intraperitoneal injection, as shown in Table 1. On the seventh day, these mice were weighed and sacrificed according to the rules of animal experimental ethics of Guangxi University. Splenic lymphocytes were isolated for experiments. Spleen and thymus tissues were collected for further analysis.
Table 1. Groupings and treatments of mice.
| Group | Day 1–3 | Day 4–6 |
|---|---|---|
| Negative control | PS 1 mL/mouse | PS 0.02 mL/kg bw |
| PNS | PS 1 mL/mouse | PNS 200 mg/kg bw |
| PCV2 + VC | PCV2 1 mL/mouse | VC 100 mg/kg bw |
| PCV2 | PCV2 1 mL/mouse | PS 0.02 mL/kg bw |
| PCV2 + PNS 50 | PCV2 1 mL/mouse | PNS 50 mg/kg bw |
| PCV2 + PNS 100 | PCV2 1 mL/mouse | PNS 100 mg/kg bw |
| PCV2 + PNS 200 | PCV2 1 mL/mouse | PNS 200 mg/kg bw |
PNS, Panax notoginseng saponins; PCV2, porcine circovirus type 2; VC, vitamin C; PS, physiological saline.
Isolation and culture of splenic lymphocytes
After the mice were sacrificed, the spleens were removed from the abdominal cavities and washed two times with PBS. Then, each spleen was polished using a plunger and filtered through a nylon sieve with a size of 80 μm. The erythrocytes in the suspension were lysed using Tris-NH4Cl, and the splenic lymphocytes were resuspended and washed twice with PBS and seeded in 24-well plates with 10% FBS-RPMI-1640 medium.
Cell viability assay
After the cells were treated and subcultured for 20 h or 44 h, MTT was added to the cell wells at a final concentration of 5 mg/mL for 4 h, and then the medium was replaced with 100 μL of dimethyl sulfoxide and incubated for 10 min. The optical density was measured by an automatic enzyme-linked immunosorbent assay (ELISA) plate reader at 450 nm.
Nitrite assay
After the cells were treated and subcultured for 4 h, 8 h, 12 h, or 24 h, culture supernatants (100 μL) were mixed with 100 μL of Griess reagent (0.5% sulfanilamide,0.05% naphthylethylenediamine dihydrochloride, 2.5% phosphoric acid), incubated for 10 min at room temperature, and the absorbance was measured at 450 nm using an ELISA plate reader.
DCFH-DA detection for ROS
ROS in 3D4/2 cells and splenic lymphocytes were detected using the fluorescent probe DCFH-DA (Applygen). Briefly, after 3D4/2 cells were treated and cultured for 4 h,8 h, 12 h, 24 h, or 48 h, the cultured supernatant was removed, and DCFH-DA (10 µM) was added for incubation in the dark at 37°C and 5% CO2 for 30 min. Then, the cells were washed three times with 0.01 M PBS. Splenic lymphocytes were mixed with 500 µL of DCFH-DA (10 µM) and incubated in a dark environment at 37°C and 5% CO2 for 60 min. Then, the supernatant was discarded, and the cells were washed three times with 0.01M PBS, and then the pellets were resuspended in 2,000 μL of PBS. The cells were imaged using a laser scanning confocal microscope (Zeiss LSM 510; Carl Zeiss AG, Germany) at a wavelength of 488 nm for excitation and 525 nm for emission. Ten random fields of vision were observed for each well. The data are expressed as fluorescence values.
Determination of intracellular GSH and GSSG
The cells were collected and centrifuged after pretreatment for 4 h, 8 h, 12 h, 24 h, and 48 h, and the pellets were treated with 0.4 mL of 5% trichloroacetic acid and cracked by ultrasonic decomposition for 1 min in an ice water bath. Following centrifugation at 12,000 × g at 4°C for 15 min, the supernatant was used for GSH and GSSG detection. The method refers to previous report [16]; briefly, for GSH determination, 200 μL of the supernatant was mixed with 3.6 mL of PBS-ethylenediaminetetraacetic acid and 200 μL of o-Phthalaldehyde and incubated at room temperature for 40 min. The fluorescence of the mixtures was measured in a spectrophotometer at excitation and emission wavelengths of 350 nm and 425 nm, respectively, and the levels of GSH were calculated according to a standard curve. For the GSSG analysis, 100 μL of supernatant was mixed with 40 μL of N-ethylmaleimide (0.04 mol/L) and incubated at room temperature for 30 min. Then, 1.9 mL of NaOH (0.1 mol/L)and 100 μL of o-Phthalaldehyde were added, the mixture was incubated at room temperature for 15 min, and the fluorescence was measured with a spectrophotometer at excitation and emission wavelengths of 337.8 nm and 421.6 nm, respectively. The levels of GSSG were calculated according to a standard curve.
Determination of the activity of intracellular XOD, MPO and iNOS
The cells were collected by centrifugation at 2,000 × g for 5 min after pretreatment for 4 h, 8 h, 12 h, 24 h, and 48 h, and the cell pellets were washed three times with PBS followed by 10 min of ultrasonic decomposition. After centrifugation at 10,000 ×g for 10 min, the supernatant was used for the XOD, MPO, and iNOS activity assays using commercial test kits in accordance with the manufacturer's instructions.
Calculation of the spleen and thymus indices
The spleen and thymus were collected after the mice were sacrificed, and the surface moisture of the organs was removed with filter paper. The spleen and thymus indices of the mice were measured according to the formulas: spleen or thymus weight (mg)/bodyweight (g), respectively [17].
Determination of the contents of total glutathione (T-GSH), GSH, GSSG and the activities of XOD, MPO and iNOS
The spleens of the mice were homogenized by adding precooled PS at the ratio of weight (g): volume (mL) = 1:10 in a homogenizer tube in an ice water bath. The homogenate was centrifuged at 4°C and 5,000 rpm for 10 min, and then the supernatant was collected for analysis. The contents of T-GSH, GSH, and GSSG and the activities of XOD, MPO, and iNOS were detected according to the instructions of commercial test kits.
Statistical analysis
Statistical analyses were performed using one-way analysis of variance followed by Duncan's test and were analyzed with SPSS 21.0 software (IBM Corp., USA). The data are shown as the mean ± SD. Bars with different letters are significantly different (p < 0.05).
RESULTS
The content of PNS
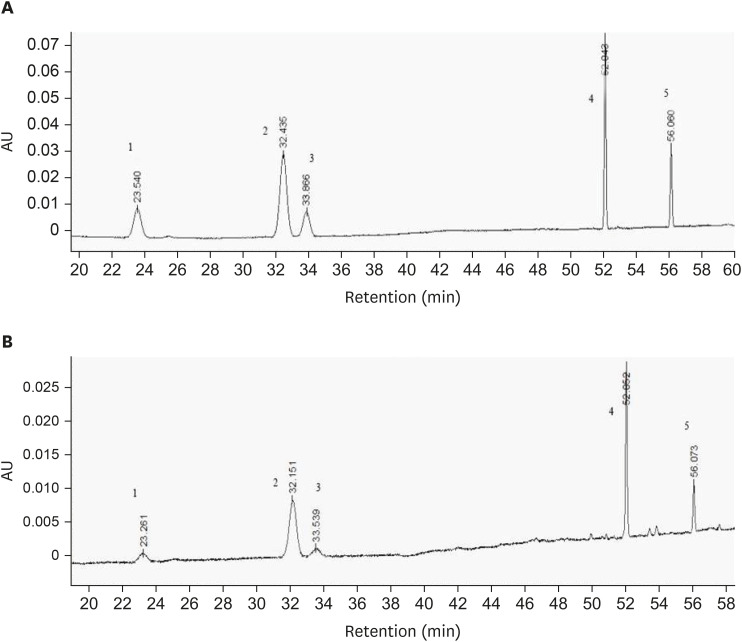
From Fig. 1, the constituents of PNS were separated. The standard curves for R1, R1, Rg1, Re, Rb1, and Rd were y = 5,421.1x + 1,629.8 (R2 = 0.9996, linear range: 48.6–1.529 μg/mL); y = 6,456.8x + 9,280.1 (R2 = 0.9998, linear range: 42.48–1.229 μg/mL); y = 5,222.1x + 3553.1 (R2 = 0.9995, linear range: 42.48–1.298 μg/mL); y = 4,744.9x + 4,168.6 (R2 = 0.9999, linear range: 137.75–4.301 μg/mL); and y = 5,530.9x + 2,051.1 (R2 = 0.9999, linear range: 63.45–1.99 μg/mL), respectively, and the contents of R1, Rg1, Re, Rb1, and Rd were determined to be 61.79 mg/g, 274.61 mg/g, 13.73 mg/g, 343.26 mg/g, 68.65 mg/g, respectively, according to the standard curves. The total content of notoginsenoside was 76.205%.
Fig. 1. HPLC chromatograms of PNS. (A) Mixture of R1, Rg1, Re, Rb1, Rd standards; (B) Analysis of components in PNS. Elution peaks: 1, R1; 2, Rg1; 3, Re; 4, Rb1; 5, Rd.
PNS, Panax notoginseng saponins.
Effect of PNS on 3D4/2 cell viability
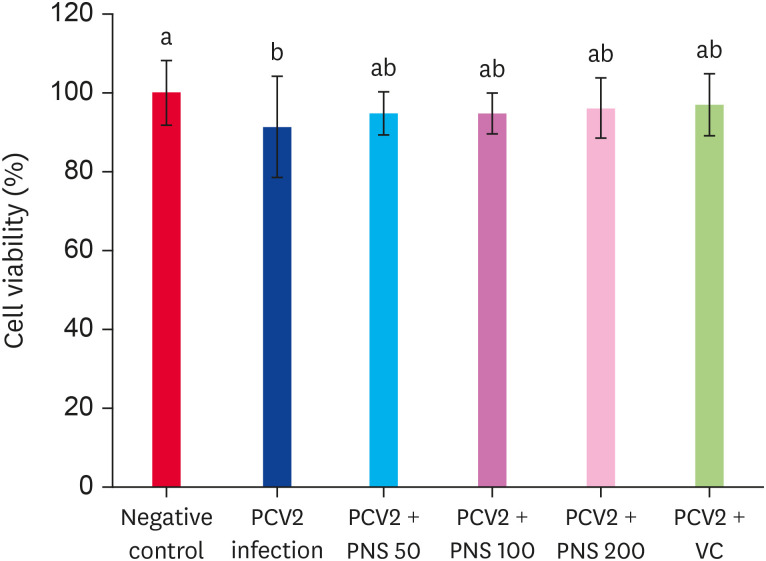
After infection with PCV2 for 48 h, the viability of 3D4/2 cells was significantly decreased compared with the negative control (p < 0.05), while treatment with VC and PNS attenuated the decrease in cell viability, but there was no significant difference compared with the PCV2 infection group (Fig. 2).
Fig. 2. The cell viabilities in PNS treated 3D4/2 cells with PCV2 infection. Treatment with PNS for 48 h post PCV2 infection, the cell viabilities were detected. Negative control: the cells were not infected with PCV2 and not treated with PNS and VC; PCV2: the cells were infected with 104TCID50 PCV2; PCV2 + PNS 50–200: the cells were treated with PNS at concentration of 50, 100, or 200 µg/mL after 104TCID50 PCV2 infection respectively; PCV2 + VC: the cells were treated with VC at concentration of 200 µM after 104TCID50 PCV2 infection. The values are represented mean ± SD. Bars with different letters are statistically different (p < 0.05).
PCV2, porcine circovirus type 2; PNS, Panax notoginseng saponins; VC, vitamin C; TCID50, median tissue culture infectious dose.
Nitric oxide (NO) contents
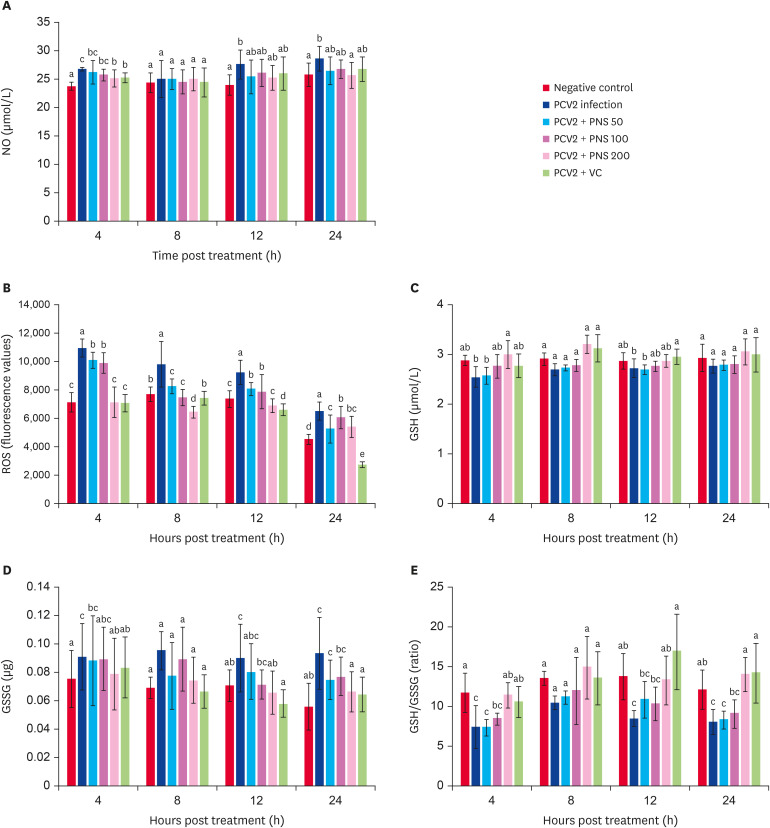
PCV2 infection significantly increased the levels of NO in 3D4/2 cells at 4 h, 12 h, and 24 h (p < 0.05). PNS at 200 μg/mL significantly decreased the NO level at 4 h and 24 h postinfection compared with PCV2 infection alone (p < 0.05), but there was no significant difference between the VC treatment group and the infection group (Fig. 3A).
Fig. 3. The levels of NO, ROS, GSH, GSSG, and GSH/GSSG in PNS treated 3D4/2 cells at 4 h, 8 h, 12 h and 24 h post PCV2 infection. Negative control: the cells were not infected with PCV2 and not treated with PNS and VC; PCV2: the cells were infected with 104TCID50 PCV2; PCV2 + PNS 50–200: the cells were treated with PNS at concentration of 50, 100 or 200 µg/mL after 104TCID50 PCV2 infection respectively; PCV2 +VC: the cells were treated with VC at concentration of 200 µM after 104TCID50 PCV2 infection. The values are represented mean ± SD. Bars with different letters are statistically different (p < 0.05).
NO, nitric oxide; PCV2, porcine circovirus type 2; PNS, Panax notoginseng saponins; VC, vitamin C; ROS, reactive oxygen species; GSH, glutathione; GSSG, oxidized glutathione; TCID50, median tissue culture infectious dose.
The production of ROS
As Fig. 3B shows, a dramatically increased ROS level was observed in PCV2-infected cells compared to the negative control group (p < 0.05). However, the level of ROS in PCV2-infected cells was decreased after treatment with different concentrations of PNS, and no significant difference was observed compared with the negative control group.
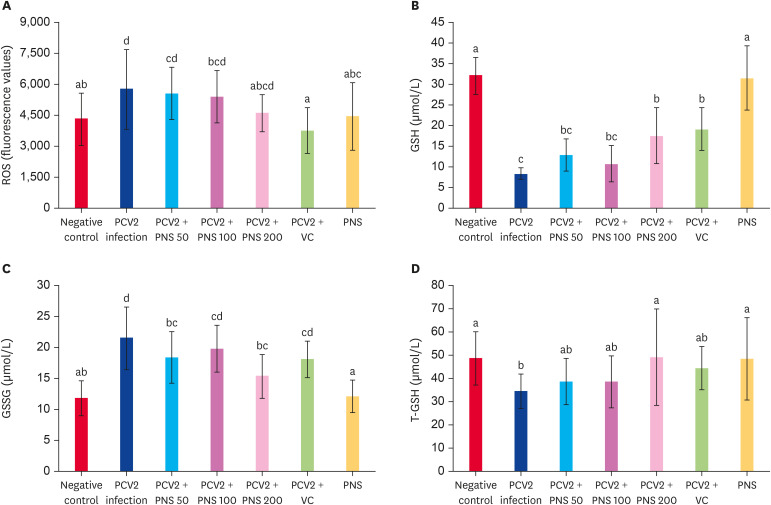
ROS levels in splenic lymphocytes were detected, and the levels were significantly increased 7 days after PCV2 infection (p < 0.05). Different concentrations of PNS decreased the ROS levels, especially at the concentration of 200 mg/kg bw, which significantly decreased the ROS level compared with the PCV2-infected group (p < 0.05), and there were no significant differences compared with the negative control (Fig. 4A).
Fig. 4. Effect of PNS on levels of ROS, GSH, GSSH, and T-GSH in the spleen of PCV2-infected mice. Negative control: the mice were not infected with PCV2 and not treated with PNS and VC. PCV2: the mice were infected with 104TCID50 PCV2; PCV2 + PNS 50–200 mg/kg bw: the mice were treated with PNS at concentration of 50, 100, or 200 mg/kg bw after 104TCID50 PCV2 infection respectively; PCV2 + VC: the mice were treated with VC at concentration of 200 µM after 104TCID50 PCV2 infection. PNS: the mice were treated with 200 mg/kg bw of PNS without PCV2 infection. The values are represented mean ± SD. Bars with different letters are statistically different (p < 0.05).
ROS, reactive oxygen species; PCV2, porcine circovirus type 2; PNS, Panax notoginseng saponins; VC, vitamin C; GSH, glutathione; GSSG, oxidized glutathione; T-GSH, total glutathione; TCID50, median tissue culture infectious dose.
Effects of PNS on redox status in vitro and in vivo
Fig. 3C-E shows that PCV2 infection decreased the level of GSH in 3D4/2 cells, and PNS at a concentration of 200 μg/mL significantly increased the GSH level compared with PCV2 infection alone (p < 0.05). GSSG content was significantly increased at 4 h, 12 h, and 24 h after PCV2 infection (p < 0.05). Treatment with PNS (200 μg/mL) at 4 h, 12 h, and 24 h after PCV2 infection notably decreased the GSSG level (p < 0.05), and the efficacy was similar to that of VC. Infection with PCV2 significantly reduced the ratio of GSH/GSSG at 4 h,12 h, and 24 h after PCV2 infection (p < 0.05), while PNS (200 μg/mL) significantly upregulated the ratio of GSH/GSSG compared with PCV2 infection alone (p < 0.05) and with no significant difference compared with the VC treatment group.
Changes in T-GSH, GSH, and GSSG in the mouse spleens were also detected. On the seventh day after infection, the levels of GSH and T-GSH significantly decreased, and the GSSG content was markedly increased (p < 0.05).Different concentrations of PNS promoted the levels of GSH and T-GSH, especially PNS at a concentration of 200 mg/kg bw, which significantly increased the levels of GSH and T-GSH compared with the PCV2 infection alone group (p < 0.05), but the GSH level was still lower than that of the negative control. PNS at concentrations of 200 mg/kg bw and 50 mg/kg bw significantly downregulated the GSSG level compared with PCV2 infection alone (p < 0.05) but still significantly exceeded that of the negative control (p < 0.05). The levels of T-GSH, GSH and GSSG were not obviously changed by treatment with PNS alone (200 mg/kg bw) (Fig. 4B-D).
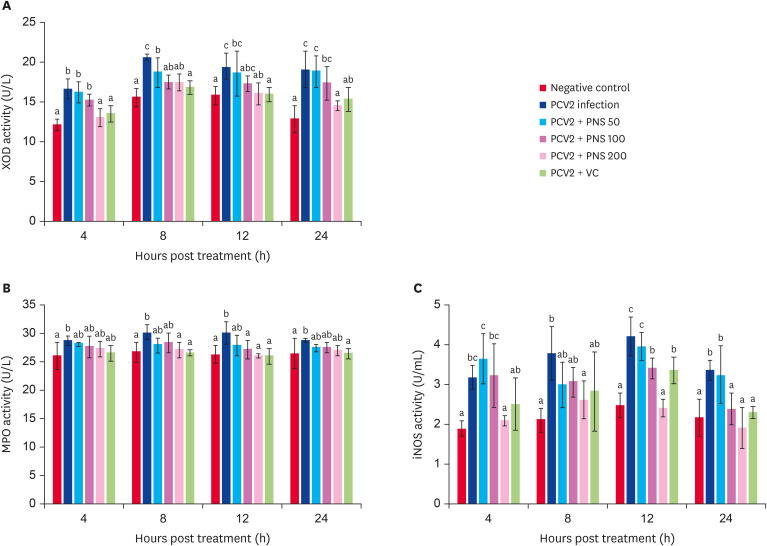
Effects of PNS on the activities of XOD, MPO and iNOS in vitro and in vivo
The activities of XOD, MPO, and iNOS in 3D4/2 cells was significantly promoted after PCV2 infection (p < 0.05), as shown in Fig. 5. PNS at 200 μg/mL, 50 μg/mL, and 100 μg/mL (treatment for 8 h) significantly decreased XOD activity (p < 0.05). The cells were treated with PNS at concentrations of 200 μg/mL and100 μg/mL for 12 h, and treatment with 200 μg/mL PNS for 8 h significantly decreased MPO activity (p < 0.05). iNOS activity was significantly downregulated by PNS (200 μg/mL), and PNS (100 μg/mL) significantly decreased iNOS activity at 12 h and 24 h after PCV2 infection (p < 0.05).
Fig. 5. The activities of XOD, MPO, and iNOS in PNS treated 3D4/2 cells with PCV2 infection. Negative control: the cells were not infected with PCV2 and not treated with PNS and VC; PCV2: the cells were infected with 104TCID50 PCV2; PCV2 + PNS 50–200: the cells were treated with PNS at concentration of 50, 100, or 200 µg/mL after 104TCID50 PCV2 infection respectively; PCV2 + VC: the cells were treated with VC at concentration of 200 µM after 104TCID50 PCV2 infection. The values are represented mean ± SD. Bars with different letters are statistically different (p < 0.05).
XOD, xanthine oxidase; PCV2, porcine circovirus type 2; PNS, Panax notoginseng saponins; VC, vitamin C; MPO, myeloperoxidase; iNOS, inducible nitric oxide synthetase; TCID50, median tissue culture infectious dose.
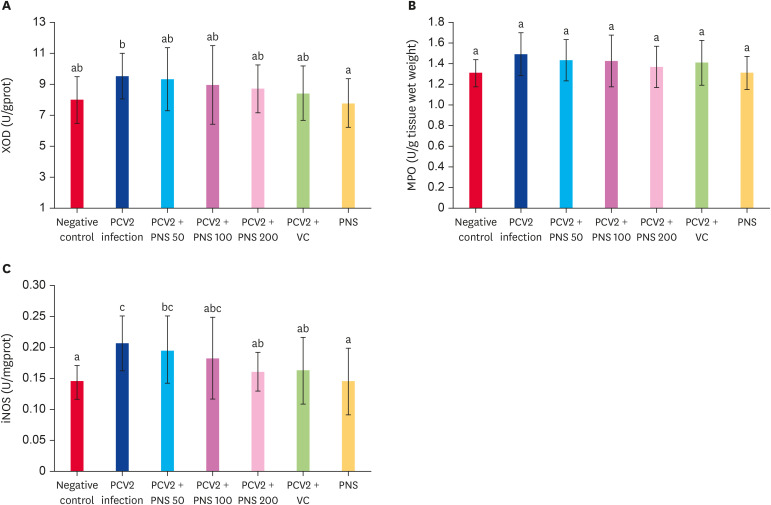
In vivo, PCV2 infection increased the activities of XOD, MPO, and iNOS, and the iNOS activity was significantly higher than that of the negative control (p < 0.05). VC decreased the activities of XOD, MPO, and iNOS in the spleens of PCV2-infected mice, but there were no significant differences compared with the negative control, although the iNOS activity was significantly lower than that in the PCV2 infection group. Different concentrations of PNS decreased the activities of XOD, MPO, and iNOS in infected mice, but no significant differences were observed compared with the negative control; however, the iNOS activity in the group treated with 200 mg/kg bw PNS was significantly lower than that in the PCV2 infection group. The activities of XOD, MPO, and iNOS were not obviously changed by treatment with 200 mg/kg bw PNS alone (Fig. 6).
Fig. 6. Effect of PNS on the activities of XOD, MPO, and iNOS in the spleen of PCV2-infected mice. Negative control: the mice were not infected with PCV2 and not treated with PNS and VC. PCV2: the mice were infected with 104TCID50 PCV2; PCV2 + PNS 50–200 mg/kg bw: the mice were treated with PNS at concentration of 50, 100, or 200 mg/kg bw after 104TCID50 PCV2 infection respectively; PCV2 +VC: the mice were treated with VC at concentration of 200 µM after 104TCID50 PCV2 infection. PNS: the mice were treated with 200 mg/kg bw of PNS without PCV2 infection. The values are represented mean ± SD. Bars with different letters are statistically different (p < 0.05).
XOD, xanthine oxidase; PCV2, porcine circovirus type 2; PNS, Panax notoginseng saponins; VC, vitamin C; MPO, myeloperoxidase; iNOS, inducible nitric oxide synthetase; TCID50, median tissue culture infectious dose.
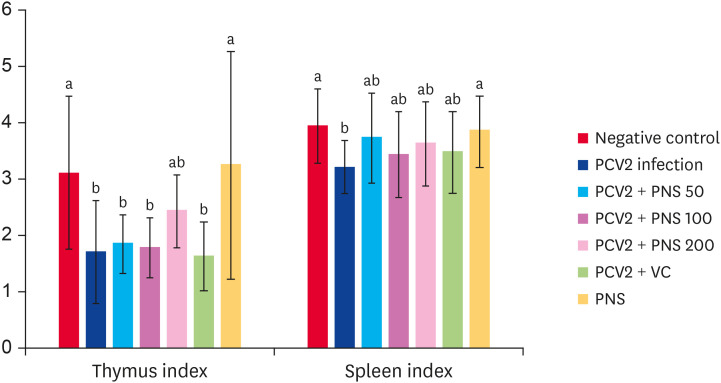
Effects of PNS on the spleen and thymus indices of PCV2-infected mice
On the seventh day postinfection with PCV2, the mouse spleen index, but not the thymus index, significantly decreased. To some extent, different concentrations of PNS increased the spleen and thymus indices but no significant differences were observed compared with the PCV2-infected group. Treatment with 200 mg/kg bw PNS alone without infection significantly increased the spleen and thymus indices compared with the PCV2-infected group (p < 0.05), and there were no differences compared with the negative control group (Fig. 7).
Fig. 7. Effect of PNS on the spleen and thymus indices of PCV2-infected mice (mg/g). Negative control: the mice were not infected with PCV2 and not treated with PNS and VC. PCV2: the mice were infected with 104TCID50 PCV2; PCV2 + PNS 50–200 mg/kg bw: the mice were treated with PNS at concentration of 50, 100, or 200 mg/kg bw after 104TCID50 PCV2 infection respectively; PCV2 + VC: the mice were treated with VC at concentration of 200 µM after 104TCID50 PCV2 infection. PNS: the mice were treated with 200 mg/kg bw of PNS without PCV2 infection. The values are represented mean ± SD. Bars with different letters are statistically different (p < 0.05).
PCV2, porcine circovirus type 2; PNS, Panax notoginseng saponins; VC, vitamin C; TCID50, median tissue culture infectious dose.
DISCUSSION
PCV2 is a virus that causes immune suppression in animals and oxidative damage to cells. Moreover, intracellular redox status could influence the replication of PCV2. Studies have shown that PCV2 infection reduces the proliferative activity of RAW264.7 cells or splenic T and B lymphocytes [16,18]. GSH and SOD concentrations decreased and the MDA concentration increased after PCV2 infection, and the replication of PCV2 in PK15 cells was impaired after increasing intracellular GSH; in contrast, PCV2 replication was promoted after the reduction of GSH [19]. Under normal physiological conditions, intracellular ROS are used as a second messenger to regulate gene expression, activate receptors and nuclear transcription factors, and induce an adaptive response. However, once endogenous or exogenous ROS reaches an excessive level, it will lead to damage to cell membranes, tissues and enzymes, resulting in disorders of body function and inducing diseases [20]. In the early stage of viral infection, viruses induce oxidative stress in host cells and release large amounts of ROS. On the one hand, production of the superoxide anion (O2·−) effectively kills invasive microorganisms to resist infection; on the other hand, the strong oxidative activity of ROS causes oxidative damage to cells. Furthermore, ROS, as a second messenger, promote viral replication [21].As some studies have shown, ROS production is increased when animals or animal cells are infected by a virus in vitro or in vivo, and the accumulation of ROS promotes viral replication, leading to immune suppression [22,23,24,25]. Therefore, some compounds that affect the excessive production of ROS can inhibit the replication of viruses in cells, such as taurine attenuating PCV2 replication by blocking ROS-dependent autophagy [26] and selenizing astragalus polysaccharide attenuating the PCV2 replication enhancement caused by oxidative stress [27]. In our study, the results showed that 50 µg/mL, 100 µg/mL, and 200 µg/mL PNS and 200 µM vitamin C significantly increased the proliferative activity of PCV2-infected 3D4/2 cells; ROS levels of 3D4/2 cells and splenic lymphocytes in mice increased after infection with PCV2. This result was consistent with the report that PCV2 infection increased ROS levels in PK-15 cells [28]. After treatment with PNS, the ROS levels in 3D4/2 cells infected by PCV2 significantly decreased, and the effects were similar to the action of vitamin C; in addition, PNS significantly reduced the increase in ROS levels in spleen lymphocytes from mice induced by PCV2 infection, and the antioxidant effects were dose-dependent with the concentration of PNS. These results suggested that PNS reduced the oxidative stress induced by PCV2 infection and increased the activity of immune cells, restoring the redox state of cells by reducing the production of ROS.
NO is an important regulating mediator in cells, which is not only an antimicrobial, tumoricidal, and tissue-damaging effector molecule working in the innate immune system but also an effective molecule for the adaptive immune response and cytoprotection [29]. NO is synthesized by nitric oxide synthase (NOS) from nicotinamide adenine dinucleotide phosphate, L-arginine, and oxygen. In immune cells, NO is mainly synthesized by iNOS under stimulation by pathogenic microorganisms and inflammation [30]. It was observed that NOS expression was upregulated and the NO level increased in models of influenza A virus H3N2-infected mice or human respiratory epithelial cells [31,32,33]. In our study, PCV2 significantly increased the activity of iNOS in 3D4/2 cells and mouse spleen lymphocytes and increased the level of NO in 3D4/2 cells. PNS downregulated the activity of iNOS and inhibited the over secretion of NO. In particular, a PNS concentration of 200 mg/kg bw was the most effective in the PCV2-infected mouse group. The results show that PNS regulated NO secretion to enhance cellular immune activity.
As one of the most important and abundant endogenous antioxidants in mammalian cells, reduced GSH is a nonenzymatic antioxidant that regulates physiological processes in many cells under normal physiological conditions, and the concentration of GSH in cells is an important index to measure the degree of oxidative stress in cells [34,35]. The decrease in GSH concentration is one of the manifestations of oxidative stress [36]. Under normal physiological conditions, the contents of GSH and GSSG are in a dynamic equilibrium state. When cells are in a state of peroxidation, the GSSG concentration increases or the GSH/GSSG ratio decreases. Therefore, the GSH/GSSG value is often used as an important indicator to evaluate the dynamic equilibrium between oxidants and antioxidants. In our experiment, PCV2 significantly increased the level of GSSG in 3D4/2 cells and decreased both the level of GSH and the value of GSH/GSSG. PNS downregulated the GSSG level and upregulated the GSH level, indicating that PNS improves the redox status of cells. Moreover, after infection with PCV2, the GSH, and T-GSH levels of splenic lymphocytes from mice were significantly decreased, and the GSSG level significantly increased, consistent with human immunodeficiency viruses infection significantly reducing intracellular T-GSH levels [37], indicating that the antioxidant capacity of splenic lymphocytes from mice was significantly decreased under oxidative stress. In addition, treatment with PNS significantly antagonized the increase in GSSG levels and the decrease in GSH and T-GSH levels in the spleen of mice infected with PCV2; in particular, the 100 mg/kg bw dose of PNS had the best antioxidant effects. This result suggests that PNS exerted antioxidant activity by promoting the synthesis of T-GSH and enhancing the free radical-scavenging capacity of reduced GSH.
MPO is a cationic protein that is mainly secreted by neutrophils and monocytes and catalyzes H2O2 to produce hypochlorous acid and other deleterious ROS that kill pathogens during infections [38,39]. However, MPO and MPO-derived oxidants are also thought to promote inflammation and cause tissue damage [40]. XOD is a molybdenum iron–sulfur flavin hydroxylase that catalyzes the oxidation of hypoxanthine to xanthine and then to uric acid and plays an important role in purine catabolism in the body [41]. During XOD reoxidation, endogenous ROS, including H2O2 species and superoxide radicals, are generated, causing oxidative damage to living tissues. In this study, PCV2 significantly increased the activity of MPO and XOD in 3D4/2 cells and in spleen lymphocytes of mice, resulting in oxidative stress. PNS significantly decreased the activity of MPO and XOD, particularly PNS at 200 mg/kg bw. The results showed that PNS effectively regulated oxidative stress induced by the PCV2 virus and resisted viral infection.
The immune organ index is often used to evaluate animal immune function. In this experiment, the thymus and spleen indices of the mice were significantly reduced after PCV2 infection for 7 days, and treatment with PNS increased the indices but they were still lower than those of the negative control group. The results show that PNS can improve the immune deficiency caused by PCV2 infection, but the immune function was not completely recovered, which may be related to the short duration of PNS treatment.
In conclusion, we concluded that PNS could regulate oxidative stress in immune cells induced by PCV2 in vitro and in vivo by decreasing the levels of ROS, NO, GSSG, and H2O2 and the activity of XOD, MPO, and iNOS, increasing the GSH level and the value of GSH/GSSG, promoting the capacity to inhibit ·OH and resist O2·−, and decreasing the production of free radicals or eliminating free radicals.
ACKNOWLEDGMENTS
We thank Dr. Huang KH in the College of Veterinary Medicine at Nanjing Agricultural University, for his kindness of providing PCV2.
Footnotes
Funding: This work was financially supported by the National Natural Science Fund of China (grant numbers: 31260619 and 31560708); and the Innovation Driven Development Fund of Guangxi (grant number: AA17204081-2); The Guangxi innovation team building project of the national modern agricultural industry technology system (grant number: nycytxgxcxtd-14-02).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Hu TJ, Wang QH, Kuang N.
- Data curation: Wang QH, Yin D, Hu WY.
- Formal analysis: Hu TJ, Wang QH, Kuang N, Hu WY.
- Investigation: Hu TJ, Wang QH, Kuang N.
- Writing - original draft: Wang QH, Kuang N, Hu WY, Wei YY.
- Writing - review & editing: Wang QH, Kuang N, Hu WY, Yin D, Wei YY, Hu TJ.
References
- 1.Yang J, Tan HL, Gu LY, Song ML, Wu YY, Peng JB, et al. Sophora subprosrate polysaccharide inhibited cytokine/chemokine secretion via suppression of histone acetylation modification and NF-κb activation in PCV2 infected swine alveolar macrophage. Int J Biol Macromol. 2017;104(Pt A):900–908. doi: 10.1016/j.ijbiomac.2017.06.102. [DOI] [PubMed] [Google Scholar]
- 2.Ouardani M, Wilson L, Jetté R, Montpetit C, Dea S. Multiplex PCR for detection and typing of porcine circoviruses. J Clin Microbiol. 1999;37(12):3917–3924. doi: 10.1128/jcm.37.12.3917-3924.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segalés J. Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res. 2012;164(1-2):10–19. doi: 10.1016/j.virusres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19(6):591–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- 5.Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 7.Duracková Z. Some current insights into oxidative stress. Physiol Res. 2010;59(4):459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 8.Peterhans E, Grob M, Bürge T, Zanoni R. Virus-induced formation of reactive oxygen intermediates in phagocytic cells. Free Radic Res Commun. 1987;3(1-5):39–46. doi: 10.3109/10715768709069768. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz KB. Oxidative stress during viral infection: a review. Free Radic Biol Med. 1996;21(5):641–649. doi: 10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Ren F, Hesketh J, Shi X, Li J, Gan F, et al. Reactive oxygen species regulate the replication of porcine circovirus type 2 via NF-κB pathway. Virology. 2012;426(1):66–72. doi: 10.1016/j.virol.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Han LF, Sakah KJ, Liu LL, Kojo A, Wang T, Zhang Y. Saponins from roots of Panax notoginseng . Chin Herb Med. 2014;6(2):159–163. [Google Scholar]
- 12.Zhao GR, Xiang ZJ, Ye TX, Yuan YJ, Guo ZX. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng . Food Chem. 2006;99(4):767–774. [Google Scholar]
- 13.Zhou N, Tang Y, Keep RF, Ma X, Xiang J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine. 2014;21(10):1189–1195. doi: 10.1016/j.phymed.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Q, Li J, Zhang C, Wang P, Mohammed A, Ni S, et al. Panax notoginseng saponins reduce high-risk factors for thrombosis through peroxisome proliferator-activated receptor -γ pathway. Biomed Pharmacother. 2017;96:1163–1169. doi: 10.1016/j.biopha.2017.11.106. [DOI] [PubMed] [Google Scholar]
- 15.Chinese Veterinary Pharmacopoeia Commission. Chinese Veterinary Pharmacopoeia. Beijing: China Medical Science and Technology Press; 2015. p. 11. [Google Scholar]
- 16.Su ZJ, Wei YY, Yin D, Shuai XH, Zeng Y, Hu TJ. Effect of Sophora subprosrate polysaccharide on oxidative stress induced by PCV2 infection in RAW264.7 cells. Int J Biol Macromol. 2013;62:457–464. doi: 10.1016/j.ijbiomac.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Wang Z, Wu H, Jia W, Teng L, Song J, et al. Sarcodon imbricatus polysaccharides protect against cyclophosphamide-induced immunosuppression via regulating Nrf2-mediated oxidative stress. Int J Biol Macromol. 2018;120(Pt A):736–744. doi: 10.1016/j.ijbiomac.2018.08.157. [DOI] [PubMed] [Google Scholar]
- 18.Wei YY, Hu TJ, Su ZJ, Zeng Y, Wei XJ, Zhang SX. Immunomodulatory and antioxidant effects of carboxymethylpachymaran on the mice infected with PCV2. Int J Biol Macromol. 2012;50(3):713–719. doi: 10.1016/j.ijbiomac.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Ren F, Hesketh J, Shi X, Li J, Gan F, et al. Interaction of porcine circovirus type 2 replication with intracellular redox status in vitro . Redox Rep. 2013;18(5):186–192. doi: 10.1179/1351000213Y.0000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niki E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 2012;586(21):3767–3770. doi: 10.1016/j.febslet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, et al. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156(3):274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 22.Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofalo RP. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus. Role in viral-induced interferon regulatory factor activation. J Biol Chem. 2001;276(23):19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- 23.Jamaluddin M, Tian B, Boldogh I, Garofalo RP, Brasier AR. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol. 2009;83(20):10605–10615. doi: 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, et al. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280(45):37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 25.Waris G, Turkson J, Hassanein T, Siddiqui A. Hepatitis C virus (HCV) constitutively activates STAT-3 via oxidative stress: role of STAT-3 in HCV replication. J Virol. 2005;79(3):1569–1580. doi: 10.1128/JVI.79.3.1569-1580.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Zhai N, Wang H, Chen Y, Li H, Viktor K, Huang K, et al. Taurine attenuates OTA-promoted PCV2 replication through blocking ROS-dependent autophagy via inhibiting AMPK/mTOR signaling pathway. Chem Biol Interact. 2018;296:220–228. doi: 10.1016/j.cbi.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Liu D, Xu J, Qian G, Hamid M, Gan F, Chen X, et al. Selenizing astragalus polysaccharide attenuates PCV2 replication promotion caused by oxidative stress through autophagy inhibition via PI3K/AKT activation. Int J Biol Macromol. 2018;108:350–359. doi: 10.1016/j.ijbiomac.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Xue H, Gan F, Zhang Z, Hu J, Chen X, Huang K. Astragalus polysaccharides inhibits PCV2 replication by inhibiting oxidative stress and blocking NF-κB pathway. Int J Biol Macromol. 2015;81:22–30. doi: 10.1016/j.ijbiomac.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi S, Hamashima S, Homma T, Sato M, Kusumi R, Bannai S, et al. Cystine/glutamate transporter, system Xc -, is involved in nitric oxide production in mouse peritoneal macrophages. Nitric Oxide. 2018;78:32–40. doi: 10.1016/j.niox.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Kawakami T, Koike A, Amano F. Sodium bicarbonate regulates nitric oxide production in mouse macrophage cell lines stimulated with lipopolysaccharide and interferon γ. Nitric Oxide. 2018;79:45–50. doi: 10.1016/j.niox.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Uetani K, Der SD, Zamanian-Daryoush M, de La Motte C, Lieberman BY, Williams BR, et al. Central role of double-stranded RNA-activated protein kinase in microbial induction of nitric oxide synthase. J Immunol. 2000;165(2):988–996. doi: 10.4049/jimmunol.165.2.988. [DOI] [PubMed] [Google Scholar]
- 32.Stark JM, Khan AM, Chiappetta CL, Xue H, Alcorn JL, Colasurdo GN. Immune and functional role of nitric oxide in a mouse model of respiratory syncytial virus infection. J Infect Dis. 2005;191(3):387–395. doi: 10.1086/427241. [DOI] [PubMed] [Google Scholar]
- 33.Lalle E, Bordi L, Castilletti C, Meschi S, Selleri M, Carletti F, et al. Design and clinical application of a molecular method for detection and typing of the influenza A/H1N1pdm virus. J Virol Methods. 2010;163(2):486–488. doi: 10.1016/j.jviromet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 35.Biswas SK, Newby DE, Rahman I, Megson IL. Depressed glutathione synthesis precedes oxidative stress and atherogenesis in Apo-E(-/-) mice. Biochem Biophys Res Commun. 2005;338(3):1368–1373. doi: 10.1016/j.bbrc.2005.10.098. [DOI] [PubMed] [Google Scholar]
- 36.Hosakote YM, Liu T, Castro SM, Garofalo RP, Casola A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol. 2009;41(3):348–357. doi: 10.1165/rcmb.2008-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura H, Masutani H, Yodoi J. Redox imbalance and its control in HIV infection. Antioxid Redox Signal. 2002;4(3):455–464. doi: 10.1089/15230860260196245. [DOI] [PubMed] [Google Scholar]
- 38.Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Guilpain P, Servettaz A, Batteux F, Guillevin L, Mouthon L. Natural and disease associated anti-myeloperoxidase (MPO) autoantibodies. Autoimmun Rev. 2008;7(6):421–425. doi: 10.1016/j.autrev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Strzepa A, Pritchard KA, Dittel BN. Myeloperoxidase: a new player in autoimmunity. Cell Immunol. 2017;317:1–8. doi: 10.1016/j.cellimm.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan Y, Zou B, Zeng H, Zhang L, Chen M, Fu G. Inhibitory effect of verbascoside on xanthine oxidase activity. Int J Biol Macromol. 2016;93(Pt A):609–614. doi: 10.1016/j.ijbiomac.2016.09.022. [DOI] [PubMed] [Google Scholar]