Abstract
The incidence of eosinophilic esophagitis (EoE) and pollen-food allergy syndrome (PFAS) is increasing worldwide, and coexistence of these 2 diseases has been reported in adults. In children, however, these conditions have not been reported as comorbidities probably because sensitization to aeroallergens occurs at an older age. We report the case of a boy with EoE and PFAS. He had had intermittent vomiting since 2 years of age. At 7 years of age, he experienced an episode of itchiness of the lips and throat for the first time, followed by vomiting, immediately after ingesting some raw fruits. We diagnosed PFAS based on the skin prick test at 8 years of age and diagnosed EoE by esophagogastroduodenoscopy 11 months after the diagnose of PFAS. His digestive symptoms did not disappear despite eliminating the fruits responsible for PFAS, but esomeprazole improved his symptoms. The incidence of EoE and PFAS as comorbidities in children might increase in the future.
Keywords: Case report, Eosinophilic esophagitis, Pollen-food allergy syndrome
INTRODUCTION
Eosinophilic esophagitis (EoE) is characterized by eosinophilic infiltration of the esophageal mucosa, which induces various digestive symptoms. The mechanism of development of EoE is not yet completely clear [1]. Recent reports have indicated a rapid increase in the incidence of EoE worldwide [2,3].
EoE is often observed with other allergic diseases [1,2]. Coexistence of EoE and pollen-food allergy syndrome (PFAS) as comorbidities have been reported in adult patients [4]. PFAS provokes oral symptoms after ingesting particular fruits that have cross-reactivity to pollen [5]. The number of young children with PFAS is also increasing [6].
However, data on the correlation between the 2 diseases is insufficient. A previous report suggested that an association between PFAS and EoE might be present only in adults [7]. We herein report the case of an 8-year-old boy with EoE and PFAS.
CASE REPORT
The patient was a Japanese boy with a family history of allergic rhinitis (AR) in his father and atopic dermatitis in his mother. He had had intermittent vomiting of unknown etiology since the age of 2 years. He underwent tonsillectomy at the age of 4 years because his treating physician considered tonsillar hypertrophy as the possible cause of his vomiting. However, his vomiting did not resolve after the surgery. His growth and development were appropriate for his age, but he experienced rhinorrhea and nasal congestion in winter and spring from the age of 5 years. At 7 years of age, he experienced his first episode of itchiness of the lips and throat, followed by vomiting, immediately after ingesting raw kiwifruit, cherry, and apple. When he was 8 years old, he was referred to the National Center for Child Health and Development in order to evaluate the necessity for eliminating fruits from his diet.
At this stage, he was 125.7 cm tall (–0.1 standard deviation [SD]) and weighed 25.6 kg (–0.2 SD). Physical examination was unremarkable, with no signs of eczema, wheezing, swollen lips, etc. Blood tests showed elevation of IgE antibodies specific for birch, apple, kiwifruit, peaches, and walnuts, the levels of all of which were above 0.35 UA/mL. Slightly elevated total IgE (215 IU/mL) levels were also noted, but hypereosinophilia was not detected (288/µL). He underwent skin prick testing for fruits and nuts, including apple, kiwifruit, cherry, and peach [8]. Positive results (wheal ≥ 3 mm in diameter) were observed with raw kiwifruit, raw and heated peach, raw cherry, walnut, and hazelnut (Table 1). Raw apple resulted in a wheal-like rash less than 3 mm in diameter. Testing with cooked apple and kiwifruit did not produce significant wheals. Based on these results and his clinical course, the patient was diagnosed with PFAS for apple, kiwifruit, and cherries. Only for peach, which is a cause of lipid transfer protein syndrome [9], both raw and heated antigens caused positive results. These findings led us to consider the possibility of different sensitization routes for apple, kiwifruit, and cherry, versus peach. Both the patient and his mother did not agree to oral food challenges with nuts and peach. Based on these results, we recommended elimination of apple, kiwifruits, cherry, peach, and foods that demonstrated positive results in the skin prick test. However, his vomiting did not resolve despite complete elimination of the above foods.
Table 1. Results of allergic tests within one month of the patient’s first visit to our department.
| Antigen | Antigen-specific-IgE level (UA/mL) | Skin prick testing wheal diameter (mm) | |
|---|---|---|---|
| Raw antigens | Heated antigens | ||
| Japanese cedar | 17.50 | NA | NA |
| Ragweed | 0.31 | NA | NA |
| Birch | 7.77 | NA | NA |
| Orchard grass | 0.27 | NA | NA |
| Kiwifruit | 1.25 | 7 × 6 | 0 × 0 |
| Apple | 3.34 | 2 × 2 | 0 × 0 |
| Peach | 1.51 | 3 × 3 | 5 × 4 |
| Cherry | NA | 3 × 3 | NA |
| Mango | <0.10 | 0 × 0 | NA |
| Banana | 0.20 | NA | NA |
| Orange | 0.14 | 0 × 0 | NA |
| Melon | <0.10 | NA | NA |
| Watermelon | 0.10 | NA | NA |
| Walnut | 1.69 | 15 × 7 | NA |
| Hazelnut | NA | 15 × 7 | NA |
| Cashew | NA | 0 × 0 | NA |
NA, not applicable.
Numbers in bold: sensitization, as indicated by specific IgE levels and positivity on skin prick tests. In terms of antigen-specific IgE levels, a level of 0.35 UA/mL or more was defined as sensitization. In the skin prick test, the diameter of the wheal was expressed as the longest diameter × shortest diameter (mm) and positivity was defined as a wheal diameter above 3 mm × 3 mm. Wheal diameter was expressed relative to the wheal diameter with normal saline (as a negative control) of 0 mm × 0 mm, and with histamine extract (10 mg/mL) (as a positive control) of 5 mm × 6 mm.
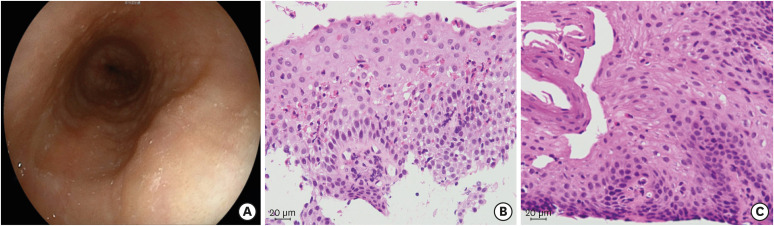
Eleven months after PFAS was diagnosed, an esophagogastroduodenoscopy was performed to evaluate the cause of his occasional vomiting. Longitudinal furrows and esophageal rings in the mid to lower esophagus, along with marked lymphoid follicles in the lower esophagus, were noted (Fig. 1A). There were no noticeable abnormal findings in the stomach and duodenum. Eosinophilic infiltration (39 per high-power field [HPF]) was observed in the stratified squamous epithelium of the mid esophagus (Fig. 1B), with no significant eosinophilic infiltration of the stomach and duodenum. Ileo-colonoscopy was not performed as the patient did not have diarrhea or hematochezia. Based on the findings, his condition was diagnosed as EoE and oral esomeprazole (20 mg/day) therapy was initiated [10]. Within a month, his vomiting had resolved, and 8 weeks later, he underwent another esophagogastroduodenoscopy to evaluate the effects of esomeprazole. This examination revealed improvement in his EoE, with very few eosinophils (0–1/HPF) in the esophageal epithelium (Fig. 1C).
Fig. 1. Histologic examination of esophageal tissue samples (hematoxylin and eosin staining). (A) Upper gastrointestinal endoscopy showed longitudinal furrows and esophageal rings in the mid to lower esophagus, and lymphoid follicles in the lower esophagus. (B) Histological studies before initiating esomeprazole treatment showed eosinophilic infiltration of 39/high-power field (HPF) in the stratified squamous layer of the mid esophagus. (C) Eosinophilia and inflammation resolved 2 months after the start of esomeprazole (eosinophils 0–1/HPF).
This study was reviewed and approved by the review board of National Center for Child Health and Development (#2019-008). Oral informed consent, including consent to publish the findings, was obtained from the study participant and his mother.
DISCUSSION
We report here the case of a boy with PFAS complicated with EoE. An association between PFAS and EoE has been described in adults [7,11]. Mahdavinia et al. [7] showed that adults with EoE and AR had a higher prevalence of PFAS compared to AR patients without EoE (50.9% vs. 10.2%, p < 0.001). Two possible mechanisms were proposed for this association. One hypothesis is that EoE occurs as a result of esophageal inflammation secondary to PFAS. There is a report showing an adult case of PFAS and EoE that might have been caused by continuous ingestion of the PFAS-related food; elimination of the related food improved the symptoms and histological findings of EoE [11]. Another hypothesis is that PFAS is caused by sensitization secondary to epithelial barrier disruption in EoE. It is possible that gastroesophageal reflux, which causes eosinophilic infiltration similar to EoE, also contributes to sensitization to aeroallergens [12]. EoE might also contribute to the development of PFAS by amplifying sensitization to aeroallergens.
We opined that our case might have been caused by the latter mechanism, since his long-term vomiting resolved with the use of a proton pump inhibitor, but not by elimination of PFAS-related foods. Our case and other reports suggest the possibility that EoE, AR, and PFAS can become causal risk factors for each other. Future prospective clinical studies are needed to reveal the complex interactions of these diseases.
This report is limited by the fact that the diagnosis of PFAS was not based on component-resolved diagnosis [5]. However, measuring component-specific IgE levels was not required for the diagnosis, since his symptoms, medical history, and test results all supported the diagnosis of PFAS.
With recent increases in various allergic disorders, pediatric cases of comorbid PFAS and EoE might increase in the near future [2,6]. Performance of esophagogastroduodenoscopy with mucosal biopsies should be considered in atypical PFAS cases with digestive symptoms.
ACKNOWLEDGEMENTS
The authors would like to express their deepest appreciation to all those who enabled the completion of this report, including the patient and his family. We would additionally like to thank our colleagues (Hiroya Ogita, Tomoyuki Kiguchi, Yoshitsune Miyagi, Yusuke Inuzuka, Kenji Toyokuni, Koji Nishimura, Fumi Ishikawa, Mayako Saito-Abe, Miori Sato, Shigenori Kabashima and Yumiko Miyaji) for their valuable advice, and the physicians of the gastroenterology team (Natsuki Ito, Masaaki Usami, and Takuro Sato) who were instrumental in performing endoscopy.
Footnotes
- Conceptualization: Makoto Irahara.
- Data curation: Makoto Irahara.
- Formal analysis: Makoto Irahara, Ichiro Nomura.
- Funding acquisition: Yukihiro Ohya.
- Methodology: Makoto Irahara, Ichiro Nomura.
- Project administration: Makoto Irahara, Ichiro Nomura, Takako Yoshioka, Katsuhiro Arai, Yukihiro Ohya.
- Visualization: Makoto Irahara, Katsuhiro Arai, Takako Yoshioka.
- Writing - original draft: Makoto Irahara.
- Writing - review & editing: Makoto Irahara, Ichiro Nomura, Ichiro Takeuchi, Kiwako Yamamoto-Hanada, Hirotaka Shimizu, Tatsuki Fukuie, Takako Yoshioka, Katsuhiro Arai, Yukihiro Ohya.
References
- 1.Durrani SR, Mukkada VA, Guilbert TW. Eosinophilic Esophagitis: an Important Comorbid Condition of Asthma? Clin Rev Allergy Immunol. 2018;55:56–64. doi: 10.1007/s12016-018-8670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinoshita Y, Ishimura N, Oshima N, Ishihara S. Systematic review: Eosinophilic esophagitis in Asian countries. World J Gastroenterol. 2015;21:8433–8440. doi: 10.3748/wjg.v21.i27.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, Locke GR, 3rd, Talley NJ. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–1061. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letner D, Farris A, Khalili H, Garber J. Pollen-food allergy syndrome is a common allergic comorbidity in adults with eosinophilic esophagitis. Dis Esophagus. 2018;31 doi: 10.1093/dote/dox122. [DOI] [PubMed] [Google Scholar]
- 5.Yagami A, Ebisawa M. New findings, pathophysiology, and antigen analysis in pollen-food allergy syndrome. Curr Opin Allergy Clin Immunol. 2019;19:218–223. doi: 10.1097/ACI.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 6.Brown CE, Katelaris CH. The prevalence of the oral allergy syndrome and pollen-food syndrome in an atopic paediatric population in south-west Sydney. J Paediatr Child Health. 2014;50:795–800. doi: 10.1111/jpc.12658. [DOI] [PubMed] [Google Scholar]
- 7.Mahdavinia M, Bishehsari F, Hayat W, Elhassan A, Tobin MC, Ditto AM. Association of eosinophilic esophagitis and food pollen allergy syndrome. Ann Allergy Asthma Immunol. 2017;118:116–117. doi: 10.1016/j.anai.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Natstume O, Fukuie T, Yasuoka R, Tajima I, Suzuki M, Taguchi T, Kitazawa H, Futamura M, Narita M, Ogata T, Ohya Y. “Kanetsu kudamono wo heiyoushita hihu purikkutesuto no yuuyousei” [Usefulness of prick test by heated fruit for pollen-food allergy syndrome] Jpn J Latex Allergy. 2014;17:19–24. (in Japanese) [Google Scholar]
- 9.Pascal M, Muñoz-Cano R, Reina Z, Palacín A, Vilella R, Picado C, Juan M, Sánchez-López J, Rueda M, Salcedo G, Valero A, Yagüe J, Bartra J. Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin Exp Allergy. 2012;42:1529–1539. doi: 10.1111/j.1365-2222.2012.04071.x. [DOI] [PubMed] [Google Scholar]
- 10.Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, Spechler SJ, Attwood SE, Straumann A, Aceves SS, Alexander JA, Atkins D, Arva NC, Blanchard C, Bonis PA, Book WM, Capocelli KE, Chehade M, Cheng E, Collins MH, Davis CM, Dias JA, Di Lorenzo C, Dohil R, Dupont C, Falk GW, Ferreira CT, Fox A, Gonsalves NP, Gupta SK, Katzka DA, Kinoshita Y, Menard-Katcher C, Kodroff E, Metz DC, Miehlke S, Muir AB, Mukkada VA, Murch S, Nurko S, Ohtsuka Y, Orel R, Papadopoulou A, Peterson KA, Philpott H, Putnam PE, Richter JE, Rosen R, Rothenberg ME, Schoepfer A, Scott MM, Shah N, Sheikh J, Souza RF, Strobel MJ, Talley NJ, Vaezi MF, Vandenplas Y, Vieira MC, Walker MM, Wechsler JB, Wershil BK, Wen T, Yang GY, Hirano I, Bredenoord AJ. Updated International Consensus Diagnostic Criteria for eosinophilic esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155:1022–1033. doi: 10.1053/j.gastro.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter H, Wong T, Winstanley A, Till SJ. Eosinophilic esophagitis linked to pollen food syndrome. J Allergy Clin Immunol Pract. 2018;6:667–668. doi: 10.1016/j.jaip.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Hait EJ, McDonald DR. Impact of gastroesophageal reflux disease on mucosal immunity and atopic disorders. Clin Rev Allergy Immunol. 2019;57:213–225. doi: 10.1007/s12016-018-8701-4. [DOI] [PubMed] [Google Scholar]